Introduction
Stroke is a heterogeneous disease with high
prevalence worldwide. It has been estimated that >200 million
patients suffer from stroke annually leading to ~150 million deaths
per year (1). Cerebral ischemia
accounts for more than 80% of all stroke patients and it is a major
cause of morbidity and mortality and is the third cause of
disability worldwide (2–4). It is characterized by a severe
reduction in cerebral blood flow accompanying with cerebral edema,
formation of free radicals and a rapid and substantial local
inflammatory reaction (5).
Although thrombolytic therapy is now well-established treatment for
ischemic stroke, few patients are able to benefit from thrombolytic
therapy due to the limited time window (6). Therefore, it is essential to develop
effective therapeutic strategies to ameliorate neural damage.
FTY720 and vitamin E have gained interest in the
treatment of cerebral ischemia due to their unique pharmacological
effects. FTY720, an agonist of sphingosine-1-phosphate (S1P)
receptor-1 (S1P1), −3, −4 and −5 (7), exerts immunomodulatory actions by
inhibiting the production, egress, trafficking and apoptosis of
lymphocytes (8–10). In addition to the immunosuppressive
activities, FTY720 has been reported to have the ability of
enhancing the integrity of the blood brain barrier (11) and to alleviate infarctions and
promote cell regeneration in the central nervous system (CNS)
(12). Researchers have recently
suggested that FTY720 possesses a neuroprotective effect via S1P1
in ischemic stroke (13,14). In addition, early research has
demonstrated that administration of vitamin E serves a protective
role in cerebral ischemia due to its both antioxidant activity and
non-antioxidant effects (15).
However, little information is available concerning the effects of
combination of FTY720 and vitamin E on cerebral ischemia.
Therefore, the present study investigated the
combined effects of FTY720 and vitamin E on simulated cerebral
ischemia, in addition to the underlying mechanisms. This involved
pretreating astrocytes with or without FTY720, vitamin E or
combination of both. Following exposure to oxygen-glucose
deprivation (OGD) to simulate an ischemic model, pre-treatment with
FTY720 and vitamin E was found to exert synergistic neuroprotective
effects in the simulated cerebral ischemia in vitro.
Materials and methods
Primary culture of astrocytes
Astrocytes were isolated from newborn Sprague-Dawley
rat pups (1-day-old; weigh 5–6 g; n=10) that were purchased from
the Shanghai Laboratory Animal Center (Shanghai, China). The rats
were kept under normal animal-house illumination with a 12 h
light/dark cycle and access to food and water. The animal
experiments were performed according to the National Institutes of
Health Guide for Care and Use of Laboratory Animals (Bethesda, MA,
USA). Briefly, the cortex of newborn rats was isolated, and the
meninges were removed under sterile conditions. The tissues were
dissociated mechanically in Dulbecco's modified Eagle medium (DMEM;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), homogenized
and incubated in 0.125% trypsin at 37°C for 20 min. The DMEM medium
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added to stop
trypsinization. The suspension was then filtered, centrifuged at
300 × g for 10 min at 4°C and re-suspended in neurobasal
medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10%
FBS, B-27, 1.0 mM glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin (all Sigma-Aldrich; Merck Millipore). The cells
(1×106 cells/ml) were added to 25 cm2 flasks,
incubated in DMEM/F-12 containing 10% FBS at 37°C in a 5%
CO2 incubator. The culture medium was changed every
third day. A total of two weeks later, contaminated microglia and
oligodendrocytes were removed by shaking for 5 h, and shaking was
repeated again after 5 days to remove microglial cells that were
detached from the layer of astrocytes. Following successful removal
of microglia, astrocytes were collected using 0.125% trypsin. The
purity of astrocytes was ~95–98%, which was identified by analyzing
immunofluorescence using anti-glial fibrillary acidic protein
(astrocyte marker; Sigma-Aldrich; Merck Millipore). Further
analyses were performed on 22-day-old cultures.
Treatment of astrocytes
Astrocytes were randomly assigned to one of five
groups: Control group (no medium exposure), medium group
(serum-free DMEM exposure), FTY720 group (exposed to serum-free
DMEM supplemented with 0.6 µM FTY720; Novartis, Basel,
Switzerland), vitamin E group (exposed to serum-free DMEM
supplemented with vitamin E (10 µg/ml, Sigma-Aldrich; Merck
Millipore), and FTY720 + vitamin E group (exposed to serum-free
DMEM supplemented with FTY720 and vitamin E). Following culture for
24 h, the medium in all groups was removed and substituted for
glucose-free Earle's Balanced Salt solution (EBSS; Gibco; Thermo
Fisher Scientific, Inc.) that was pre-incubated with 95%
N2 and 5% CO2 for 0.5 h to remove the oxygen
in the medium. To induce an OGD condition, the cells were then
incubated for 12 h in an airtight box filled with 95% N2
and 5% CO2. Meanwhile, the cells in the control group
were treated with EBSS supplemented with 10 mmol/l glucose in
CO2 incubator.
Cell viability assay
The cell viability of astrocytes was determined by
an MTT assay. Non-treated cells were regarded as the control group.
Briefly, 0.5 mg/ml MTT reagent (Sigma-Aldrich; Merck Millipore) was
added to each well (1×104 cells/well) following 12 h of
exposure to OGD and incubated for 4 h at 37°C. Dimethylsulfoxide
(10%; 100 µl) was then added to dissolve the blue reaction product
formazan by shaking the plates at room temperature for 10 min. The
absorbance value at 570 nm was monitored by using a microplate
reader (SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA).
Each experiment was carried out in triplicate.
Lactate dehydrogenase (LDH) release
assay
LDH release was assessed in order to evaluate the
extent of cell injury using a LDH cytotoxicity detection kit (Roche
Applied Science, Penzberg, Germany) according to the manufacturer's
instructions. Following exposure to OGD for 12 h, the culture
supernatant was harvested and dead cells were removed by
centrifugation. The remaining supernatant was used to determine LDH
levels. Absorbance at 492 nm was read using a microplate reader.
Each experimental was done in triplicate.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
A TUNEL assay was performed to detect the staining
of astrocytes cells using a commercially available kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
instructions. A total of 12 h after exposure to OGD, the astrocytes
were collected, washed with PBS, and examined under a fluorescence
microscope.
Apoptosis assay
Cell apoptosis was detected by Annexin V-Cy5 and
propidium iodide (PI) staining (BioVision, Inc., Milpitas, CA, USA)
followed by fluorescence-activated cell sorting (FACS) analysis.
The cells (2×105/35 mm culture dish) were collected,
pelleted and re-suspended in Annexin V-binding buffer containing
Annexin V-Cy5 and PI, and incubated at room temperature for 5 min.
Thereafter, the cells were evaluated with a FACS Calibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The percentage
of total apoptotic cells was (early apoptotic + apoptotic)
calculated.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of secreted tumor necrosis factor
(TNF)-α, interleukin (IL)-6, and IL-1β in the culture supernatants
that were exposed to OGD for 12 h were evaluated by using a
commercially available ELISA kit (EMD Millipore, Billerica, MA,
USA) according to the manufacturer's instructions. Absorbance at
450 nm was read using a microplate reader.
Measurement of antioxidant levels
Total antioxidant capacity (TAC) levels were
assessed using Randox total antioxidant status kit (Randox
Laboratories, Ltd., Crumlin, Northern Ireland). Briefly, the
cultured astrocytes were collected following 12 h of exposure to
OGD, washed twice with PBS, and lysed with 10 mM phosphate buffer.
Following centrifugation at 300 × g for 10 min at 4°C, the
supernatant was collected to evaluate the levels of TAC.
Western blot analysis
Following 12 h of exposure to OGD, astrocyte
cultures were collected and washed with ice-cold PBS. The proteins
were extracted from the cells using RIPA lysis buffer (Cell
Signaling Technology, Inc., Danvers, MA, USA). The protein
concentrations were determined using a BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Equal amounts of the protein samples
(20 µg) were resolved on 10–12% SDS-PAGE, and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules,
CA, USA). The membranes were then blocked with 5% nonfat dry milk
in TBS with 0.1% Tween (TBST) for 1 h at room temperature. The
membranes were incubated overnight at 4°C with rabbit
anti-polyclonal intercellular adhesion molecule (ICAM)-1 (catalog
no. ab124760; 1:1,000), vascular cell adhesion molecule (VCAM)-1
(catalog no. ab215380; 1:1,000), chemokine (C-X-C motif) ligand
(CXCL)-10 (catalog no. ab7206; 1:1,000), heme oxygenase (HO)-1
(catalog no. ab68477; 1:1,000) or superoxide dismutase (SOD)-1
(catalog no. ab16831; 1:1,000) antibody. All antibodies were
obtained from Abcam (Cambridge, MA, USA). The membranes were then
incubated with horseradish peroxidase-conjugated secondary antibody
(catalog no. ab191866; 1:5,000; Abcam) for 2 h at room temperature.
The positive bands were visualized with enhanced chemiluminescence
western blotting substrate (Pierce; Thermo Fisher Scientific, Inc.)
and autoradiography film. β-actin was used as an internal
control.
Statistical analysis
All data were expressed as the mean ± standard
deviation. The statistical significance of differences among
different groups was evaluated using SPSS software (version, 18.0;
SPSS Inc., Chicago, IL, USA) with one-way analysis of variance. The
Tukey-Kramer's post hoc test was performed for the multiple
comparisons. P<0.05 was used to indicate a statistically
significant difference.
Results
Combined effects of FTY720 and vitamin
E on cell viability on LDH release
To explore the combined effect of FTY720 and vitamin
E on cerebral ischemia, the cultured rat astrocytes were subjected
to medium, FTY720, vitamin E, or FTY720 combined with vitamin E. A
total of 24 h later, the cultured astrocytes were exposed to OGD
for 12 h to simulate cerebral ischemia. The combined effect of
FTY720 and vitamin E on cell viability and LDH release was
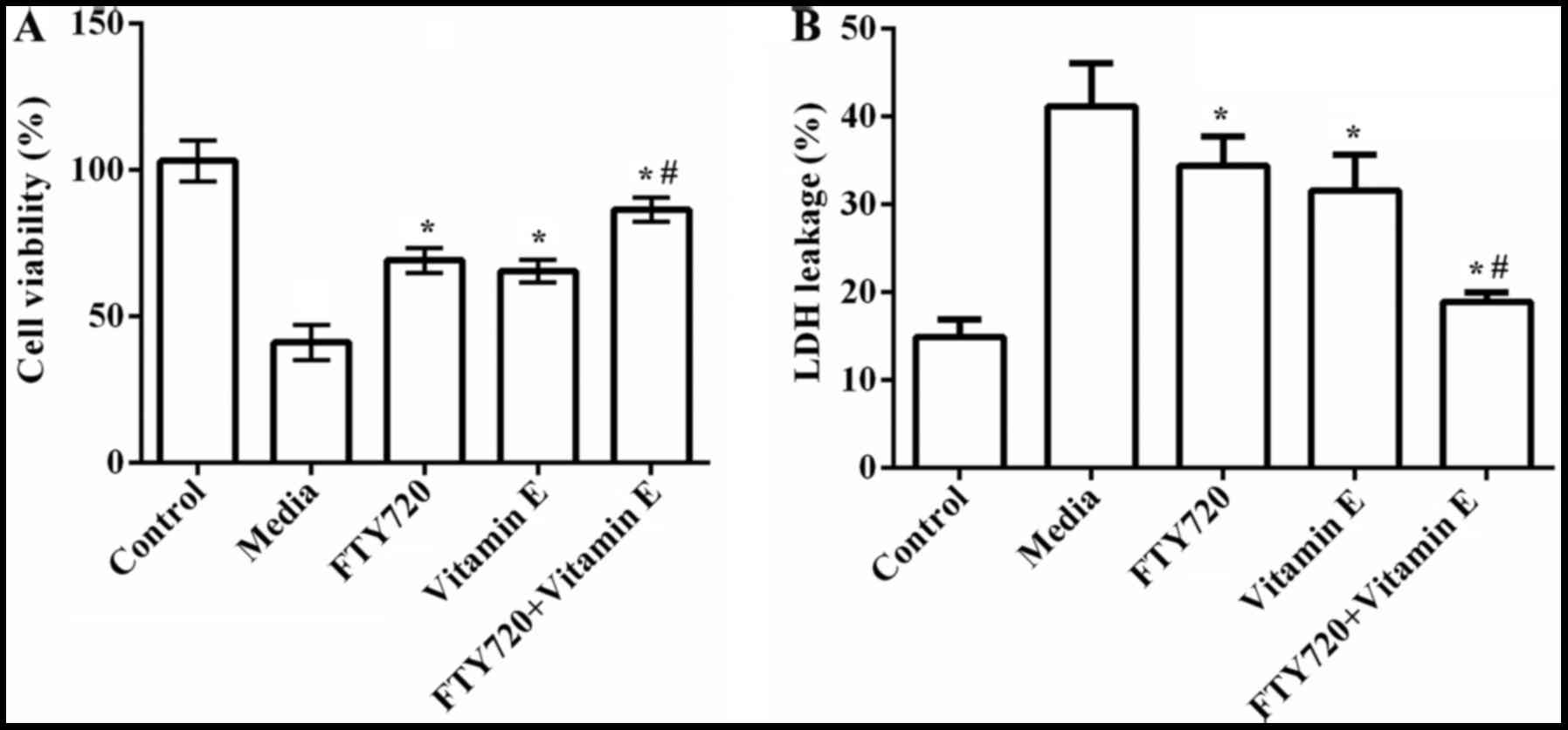
initially investigated. As indicated in Fig. 1A, the results demonstrated that the
cell viability was significantly decreased by exposure to OGD when
compared to the control group (P=0.032). However, the cell
viability was markedly increased by pre-treatment with FTY720 or
vitamin E compared to non-treatment (P=0.023 or P=0.036). No
significant differences were observed between the FTY720 group and
vitamin E group. In addition, the cell viability was markedly
higher in the FTY720 combined with vitamin E group than that in the
FTY720 group or vitamin E group (P=0.029 or P=0.018).
Following this, the combined effect of FTY720 and
vitamin E on LDH release was investigated, which is another
indicator of cell toxicity (16).
As presented in Fig. 1B, LDH
leakage was significantly increased when the cells were exposed to
12 h OGD compared to the control group (P=0.034). Pre-treatment
with FTY720 (P=0.031) or vitamin E (P=0.019) significantly
attenuated OGD-induced LDH leakage, and while pre-treatment with
FTY720 combined with vitamin E represented the best protective
effect (P=0.013). These results indicated that pre-treatment with
FTY720 combined with vitamin E presented a synergistic protective
effect on OGD-induced cell viability and toxicity of
astrocytes.
Combined effects of FTY720 and vitamin
E on astrocyte death
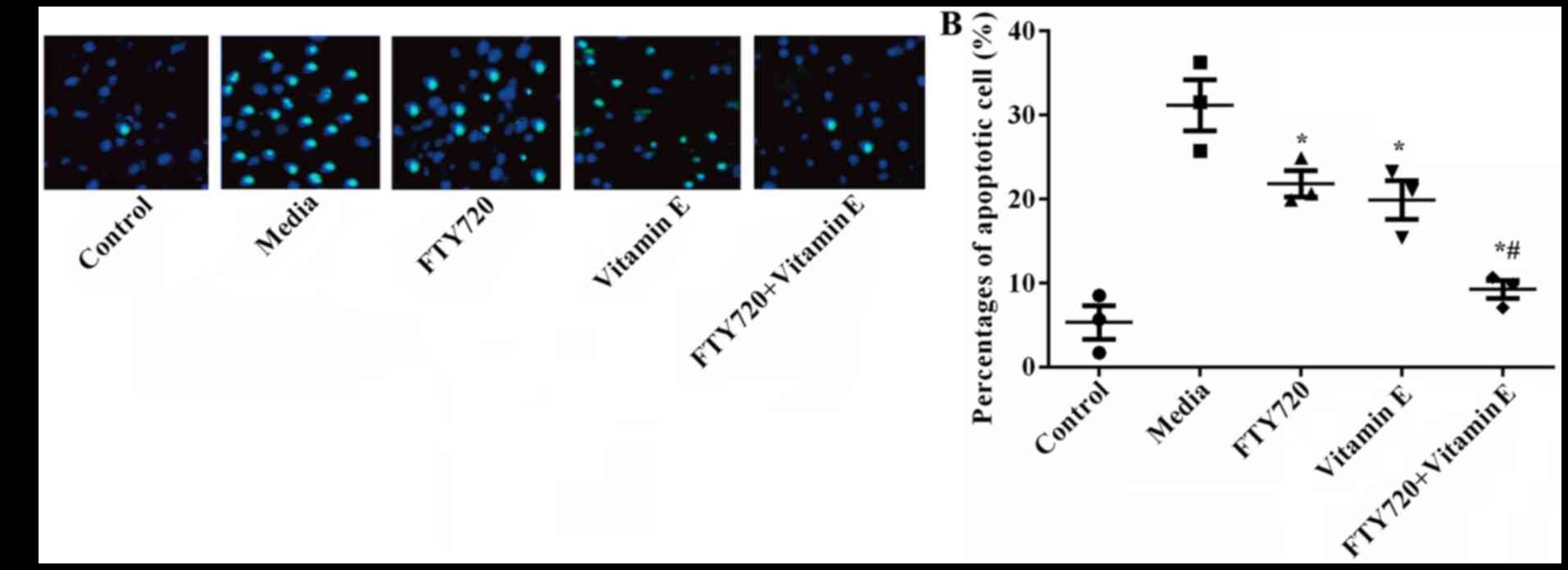
A total of 12 h following exposure to OGD, apoptotic
cell death was detected by TUNEL staining and an apoptosis assay.
As demonstrated in Fig. 2A and B,
there was a significantly higher number of TUNEL-positive cells in
media exposed to OGD when compared with the control group
(P=0.016). Pre-treatment with FTY720 or vitamin E significantly
reduced the number of TUNEL-positive cells (P=0.024 or P=0.017),
and pre-treatment with FTY720 combined with vitamin E presented the
fewest number of TUNEL-positive cells (P=0.012). The results
suggested that pre-treatment with FTY720 and vitamin E showed a
synergistic protective effect on OGD-induced astrocyte death.
Combined effects of FTY720 and vitamin
E on IL-1β, TNF-α, IL-6 and antioxidant release
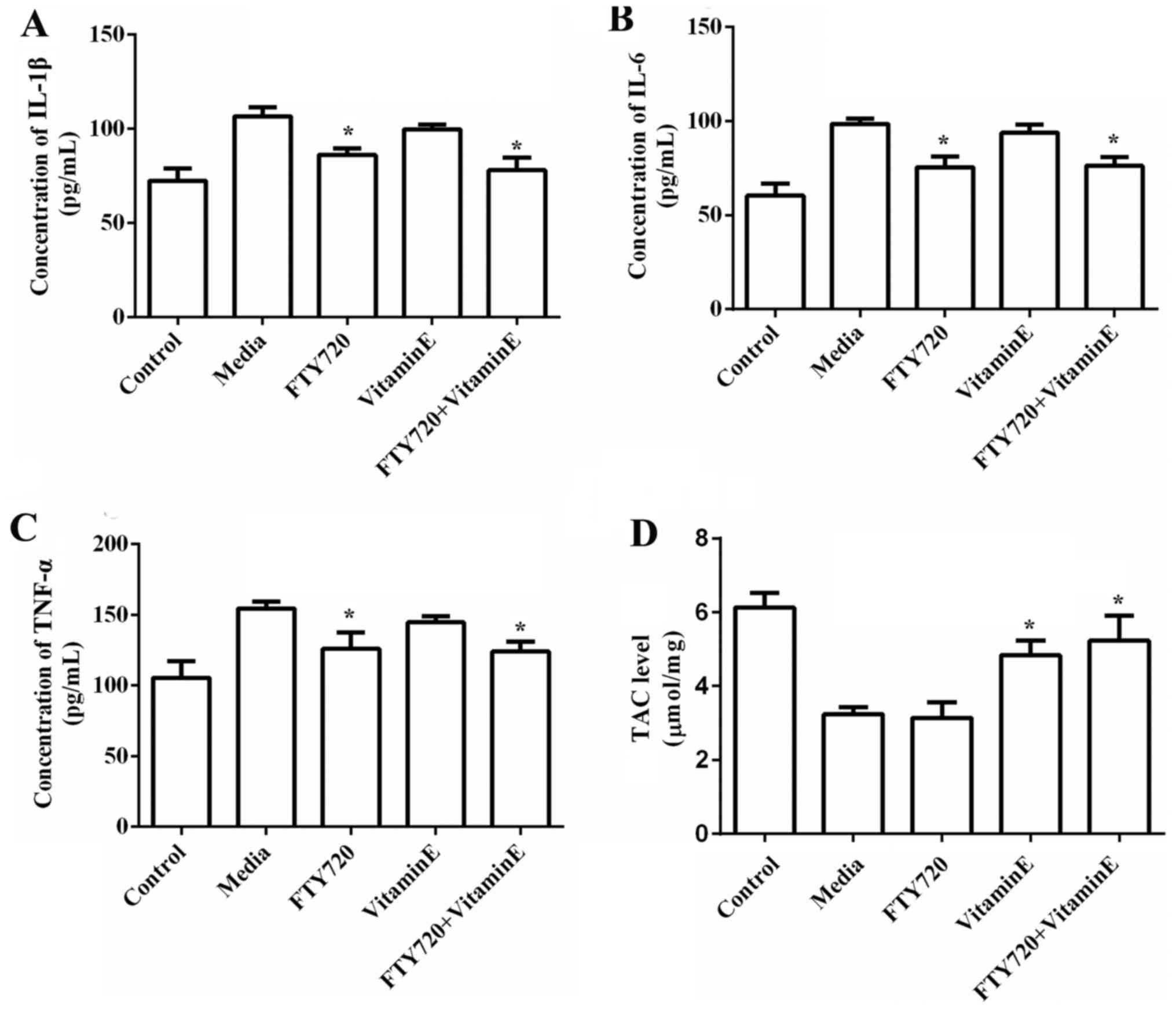
Exposure of astrocytes to OGD for 12 h lead to
increased IL-1β (P=0.034), TNF-α (P=0.029) and IL-6 release
(P=0.042), but significantly decreased TAC (P=0.038). Pre-treatment
with FTY720 markedly reduced the release of IL-1β (P=0.042), TNF-α
(P=0.038) and IL-6 (P=0.036), however had no effect on the release
of antioxidant level, confirming that FTY720 protects against
cerebral ischemia by reduction of the levels of pro-inflammatory
cytokines. However, pre-treatment with vitamin E markedly increased
the release of antioxidant level (P=0.022) but had no effect on the
release of IL-1β, TNF-α and IL-6, confirming that vitamin E
protects against cerebral ischemia by decrease of the levels of
antioxidants. The combination of FTY720 and vitamin E significantly
reduced the levels of IL-1β, TNF-α and IL-6, however simultaneously
elevated the levels of antioxidant, presenting a synergistic
protective effect (Fig. 3A-D).
Combined effects of FTY720 and vitamin
E on ICAM-1, VCAM-1, CXCL-10, HO-1 and SOD-1
Results of western blot analysis indicated that
exposure of astrocytes to OGD for 12 h markedly increased the
levels of ICAM-1, VCAM-1 and CXCL-10, but markedly decreased the
levels of HO-1 and SOD-1. Pre-treatment with FTY720 markedly
reduced the protein levels of ICAM-1, VCAM-1 and CXCL-10, however
appeared to have no effect on the levels of HO-1 and SOD-1.
Meanwhile, pre-treatment with vitamin E markedly increased the
levels of HO-1 and SOD-1, however, had no effect on the levels of
ICAM-1, VCAM-1 and CXCL-10. Additionally, pre-treatment with FTY720
combined with vitamin E statistically reduced all the levels of
ICAM-1, VCAM-1, CXCL-10, and simultaneously increased the levels of
HO-1 and SOD-1, revealing a synergistic protective effect of FTY720
and vitamin E on cerebral ischemia (Fig. 4).
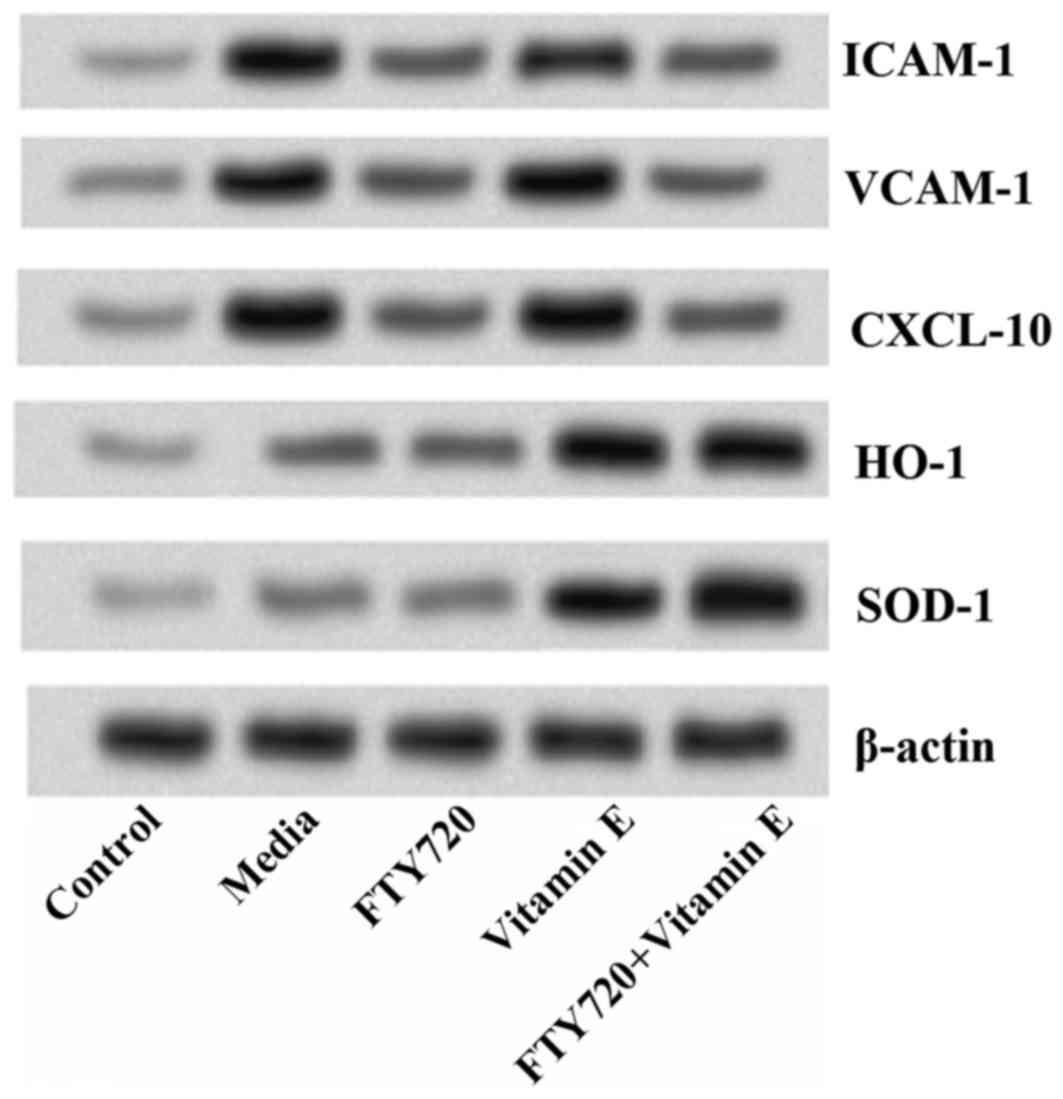 | Figure 4.Combined effects of FTY720 and vitamin
E on ICAM-1, VCAM-1, CXCL-10, HO-1 and SOD-1. Pre-treatment with
FTY720 significantly reduces the protein levels of ICAM-1, VCAM-1
and CXCL-10, while pre-treatment with vitamin E markedly increases
the levels of HO-1 and SOD-1. Additionally, pre-treatment with
FTY720 combined with vitamin E indicates a synergistic effect.
ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion
molecule; CXCL, chemokine (C-X-C motif) ligand; HO, heme oxygenase;
SOD, superoxide dismutase. |
Discussion
Not only do astrocytes serve significant roles in
regular neuronal function, but they are also involved in the
pathology of stroke (17,18). Following ischemic stroke,
astrocytes may exhibit the ability of proliferation and
differentiation (astrogliosis). Therefore, astrocytes were used in
the present study and treated with or without FTY720, vitamin E, or
combination of FTY720 and vitamin E. The results indicated that
combination of FTY720 and vitamin E exerted a synergistic
protective effect on astrocyte viability, toxicity and apoptosis
induced by OGD. In addition, it was identified that FTY720 markedly
reduced the levels of pro-inflammatory cytokines TNF-α, IL-1β and
IL-6, cell adherence molecules ICAM-1 and VCAM-1, and chemokine
CXCL-10, whereas vitamin E increased the levels of antioxidant
enzymes, HO-1 and SOD-1. The current results suggested that FTY720
combined with vitamin E possesses a synergistic neuroprotective
effects in the simulated cerebral ischemia in vitro.
FTY720 is a novel immunomodulatory drug approved by
the Food and Drug Administration to treat multiple sclerosis
(19). FTY720 has been previously
identified to reduce the trafficking of T cells, B cells, natural
killer cells, and other S1PR-bearing cells into the CNS, leading to
the reduction of relapses and brain volume loss (12,14).
FTY720 has emerged as a promising therapeutic modality, which has
demonstrated the beneficial effects on experimental models of
stroke (13,20–23).
The protective effect of FTY720 on stroke is predominantly involved
in induction of lymphocytopenia, and concomitant reduction of
microvascular thrombosis (23).
The interactions between lymphocytes and endothelial cells enhance
the dysfunction of microvascular, possibly leading to secondary
infarct growth. In addition to FTY720, vitamin E has long been
identified as a major lipid-soluble antioxidant protecting against
cerebral ischemia. Kraft et al (23) suggested that vitamin E prevents
cerebral ischemia neuronal apoptosis by lowering radical damage to
hippocampal neurons. However, rare studies are focused on the
combination effects of FTY720 and vitamin E on cerebral ischemia.
Therefore, combination effects were studied in our present study.
As indicated in our results, we observed that pre-treatment with
either FTY720 or vitamin E both significantly elevated the
astrocytes viability, and markedly decreased astrocytes
cytotoxicity (LDH release) and number of apoptotic cells,
demonstrating the protective roles of FTY720 or vitamin E in
cerebral ischemia. Our results were in line with previous studies
which showed FTY720 or vitamin E prevented against cerebral
ischemia. These effects, however, were obviously enhanced by
combination of FTY720 and vitamin E, indicating a synergistic
protective effect. The underlying mechanisms were then
investigated.
It has been well established that local and systemic
inflammatory responses involving neutrophils, lymphocytes,
macrophages and capillary endothelial cells are responsible for the
development of ischemic stroke (24–26).
Inflammatory molecules, such as adhesion molecules, chemokines and
cytokines are involved in the inflammatory process. ICAM-1 and
VCAM-1 are members of adhesion molecules, which are expressed on
endothelial cells during inflammation (27,28).
Furthermore, the inflammatory process is promoted by various
pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, produced
by several subtypes of T cells (29). Chemokines, including CXCL-10, are
secreted by the activated cells in ischemic regions to attract the
inflammatory leukocytes into the region of infarction (30). Elevation of these inflammatory
molecules clearly demonstrates detrimental effects on viable brain
tissue (31). Therefore,
administration of specific antagonists that reduce the release of
inflammatory molecules is beneficial for preventing the development
and deterioration of cerebral ischemia. In the current study,
pre-treatment with FTY720 statistically decreased the levels of
pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), adhesion
molecules (ICAM-1 and VCAM-1) and chemokines (CXCL-10). However,
these effects were not observed by pre-treatment with vitamin E,
suggesting that reduction of the expression of inflammatory
mediators caused by FTY720, but not by vitamin E, prevents against
cerebral ischemia.
On the other hand, one of the pathological
mechanisms of cerebral ischemia is oxidative/nitrative stress
(32). Oxidative/nitrosative
stress results in cellular macromolecular destruction and cell
death (33). HO is a microsomal
enzyme that degrades heme from heme-containing proteins, leading to
the production of carbon monoxide, iron and biliverdin (34). The characteristics of HO make it as
an crucial antioxidant enzyme in the nervous system (35). HO-1 is a rate-limiting enzyme in
heme catabolism, which has potent antioxidant and anti-apoptosis
effects (36). Recently, some
studies have suggested that overproduction of HO-1 is
neuroprotective against cerebral ischemia injury (37,38).
In addition, SOD-1 is a well-known antioxidant enzyme, responsible
for detoxifying intracellular reactive oxygen species (ROS),
thereby protecting cells from oxidative damage. It has been
reported that overexpression of SOD-1 in transgenic rats or mice
protects neurons from death after focal cerebral ischemia (39). Zhang et al (15) found that vitamin E protects against
cerebral ischemia by inducing the expression of subunit of HIF-1
and its target genes, including HO-1. Administration of vitamin E
slowed the onset and the progression of amyotrophic lateral
sclerosis in SOD-1 transgenic mice (40). Similarly, in the present study,
astrocytes pretreated with vitamin E presented higher levels of
HO-1 and SOD-1; however, these effects were not observed by
pre-treatment with FTY720, indicating an antioxidant effect of
vitamin E but not FTY720.
In conclusion, the current study suggested that
pre-treatment with FTY720 combined with vitamin E reveals a
synergistic effect on cerebral ischemia. This provides novel
therapeutic strategies for cerebral ischemia. However, animal
experiments should be performed to confirm the results.
References
|
1
|
Tang Q, Han R, Xiao H, Shen J, Luo Q and
Li J: Neuroprotective effects of tanshinone IIA and/or
tetramethylpyrazine in cerebral ischemic injury in vivo and in
vitro. Brain Res. 1488:81–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suethanapornkul S, Kuptniratsaikul PS,
Kuptniratsaikul V, Uthensut P, Dajpratha P and Wongwisethkarn J:
Post stroke shoulder subluxation and shoulder pain: A cohort
multicenter study. J Med Assoc Thai. 91:1885–1892. 2008.PubMed/NCBI
|
|
3
|
WRITING GROUP MEMBERS, . Lloyd-Jones D,
Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB,
Ford E, Furie K, et al: Heart disease and stroke statistics-2010
update: A report from the American Heart Association. Circulation.
121:e46–e215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urban PP, Wolf T, Uebele M, Marx JJ, Vogt
T, Stoeter P, Bauermann T, Weibrich C, Vucurevic GD, Schneider A
and Wissel J: Occurence and clinical predictors of spasticity after
ischemic stroke. Stroke. 41:2016–2020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raichle ME: The pathophysiology of brain
ischemia. Ann Neurol. 13:2–10. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lees KR, Bluhmki E, von Kummer R, Brott
TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA,
et al: Time to treatment with intravenous alteplase and outcome in
stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and
EPITHET trials. Lancet. 375:1695–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brinkmann V, Davis MD, Heise CE, Albert R,
Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et
al: The immune modulator FTY720 targets sphingosine 1-phosphate
receptors. J Biol Chem. 277:21453–21457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yagi H, Kamba R, Chiba K, Soga H, Yaguchi
K, Nakamura M and Itoh T: Immunosuppressant FTY720 inhibits
thymocyte emigration. Eur J Immunol. 30:1435–1444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinkmann V, Pinschewer DD, Feng L and
Chen S: FTY720: Altered lymphocyte traffic results in allograft
protection. Transplantation. 72:764–769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung CG, Kim HJ, Miron VE, Cook S, Kennedy
TE, Foster CA, Antel JP and Soliven B: Functional consequences of
S1P receptor modulation in rat oligodendroglial lineage cells.
Glia. 55:1656–1667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cannon RE, Peart JC, Hawkins BT, Campos CR
and Miller DS: Targeting blood-brain barrier sphingolipid signaling
reduces basal P-glycoprotein activity and improves drug delivery to
the brain. Proc Natl Acad Sci USA. 109:15930–15935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen JA and Chun J: Mechanisms of
fingolimod's efficacy and adverse effects in multiple sclerosis.
Ann Neurol. 69:759–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasegawa Y, Suzuki H, Sozen T, Rolland W
and Zhang JH: Activation of sphingosine 1-phosphate receptor-1 by
FTY720 is neuroprotective after ischemic stroke in rats. Stroke.
41:368–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei Y, Yemisci M, Kim HH, Yung LM, Shin
HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V, et al:
Fingolimod provides long-term protection in rodent models of
cerebral ischemia. Ann Neurol. 69:119–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Tanaka J, Yang L, Yang L,
Sakanaka M, Hata R, Maeda N and Mitsuda N: Protective effect of
vitamin E against focal brain ischemia and neuronal death through
induction of target genes of hypoxia-inducible factor-1.
Neuroscience. 126:433–440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Broussas M, Broyer L and Goetsch L:
Evaluation of antibody-dependent cell cytotoxicity using lactate
dehydrogenase (LDH) measurement. Methods Mol Biol. 988:305–317.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barreto G, White RE, Ouyang Y, Xu L and
Giffard RG: Astrocytes: Targets for neuroprotection in stroke. Cent
Nerv Syst Agents Med Chem. 11:164–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sofroniew MV and Vinters HV: Astrocytes:
Biology and pathology. Acta Neuropathol. 119:7–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kappos L, Radue EW, O'Connor P, Polman C,
Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M,
Zhang-Auberson L, et al: A placebo-controlled trial of oral
fingolimod in relapsing multiple sclerosis. N Engl J Med.
362:387–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shichita T, Sugiyama Y, Ooboshi H,
Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ,
et al: Pivotal role of cerebral interleukin-17-producing
gammadeltaT cells in the delayed phase of ischemic brain injury.
Nat Med. 15:946–950. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunkhorst R, Kanaan N, Koch A, Ferreirós
N, Mirceska A, Zeiner P, Mittelbronn M, Derouiche A, Steinmetz H,
Foerch C, et al: FTY720 treatment in the convalescence period
improves functional recovery and reduces reactive astrogliosis in
photothrombotic stroke. PLoS One. 8:e701242013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Czech B, Pfeilschifter W, Mazaheri-Omrani
N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A and
Pfeilschifter J: The immunomodulatory sphingosine 1-phosphate
analog FTY720 reduces lesion size and improves neurological outcome
in a mouse model of cerebral ischemia. Biochem Biophys Res Commun.
389:251–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kraft P, Göb E, Schuhmann MK, Göbel K,
Deppermann C, Thielmann I, Herrmann AM, Lorenz K, Brede M, Stoll G,
et al: FTY720 ameliorates acute ischemic stroke in mice by reducing
thrombo-inflammation but not by direct neuroprotection. Stroke.
44:3202–3210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Upadhyay UM and Tamargo RJ:
Inflammation in stroke and focal cerebral ischemia. Surg Neurol.
66:232–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Simoni MG, Milia P, Barba M, De Luigi
A, Parnetti L and Gallai V: The inflammatory response in cerebral
ischemia: Focus on cytokines in stroke patients. Clin Exp
Hypertens. 24:535–542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsuo Y, Onodera H, Shiga Y, Shozuhara H,
Ninomiya M, Kihara T, Tamatani T, Miyasaka M and Kogure K: Role of
cell adhesion molecules in brain injury after transient middle
cerebral artery occlusion in the rat. Brain Res. 656:344–352. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connolly ES Jr, Winfree CJ, Springer TA,
Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC
and Pinsky DJ: Cerebral protection in homozygous null ICAM-1 mice
after middle cerebral artery occlusion. Role of neutrophil adhesion
in the pathogenesis of stroke. J Clin Invest. 97:209–216. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito K, Suyama K, Nishida K, Sei Y and
Basile AS: Early increases in TNF-alpha, IL-6 and IL-1 beta levels
following transient cerebral ischemia in gerbil brain. Neurosci
Lett. 206:149–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Ellison JA, Siren AL, Lysko PG,
Yue TL, Barone FC, Shatzman A and Feuerstein GZ: Prolonged
expression of interferon-inducible protein-10 in ischemic cortex
after permanent occlusion of the middle cerebral artery in rat. J
Neurochem. 71:1194–1204. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Merrill JE and Benveniste EN: Cytokines in
inflammatory brain lesions: Helpful and harmful. Trends Neurosci.
19:331–338. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lyden P and Wahlgren NG: Mechanisms of
action of neuroprotectants in stroke. J Stroke Cerebrovasc Dis.
9:9–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dalle-Donne I, Scaloni A, Giustarini D,
Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R and Milzani A:
Proteins as biomarkers of oxidative/nitrosative stress in diseases:
The contribution of redox proteomics. Mass Spectrom Rev. 24:55–99.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elbirt KK and Bonkovsky HL: Heme
oxygenase: Recent advances in understanding its regulation and
role. Proc Assoc Am Physicians. 111:438–447. 1999.PubMed/NCBI
|
|
35
|
Schipper HM: Heme oxygenase expression in
human central nervous system disorders. Free Radic Biol Med.
37:1995–2011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu ML, Ho YC, Lin CY and Yet SF: Heme
oxygenase-1 in inflammation and cardiovascular disease. Am J
Cardiovasc Dis. 1:150–158. 2011.PubMed/NCBI
|
|
37
|
Chao XD, Ma YH, Luo P, Cao L, Lau WB, Zhao
BC, Han F, Liu W, Ning WD, Su N, et al: Up-regulation of heme
oxygenase-1 attenuates brain damage after cerebral ischemia via
simultaneous inhibition of superoxide production and preservation
of NO bioavailability. Exp Neurol. 239:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Panahian N, Yoshiura M and Maines MD:
Overexpression of heme oxygenase-1 is neuroprotective in a model of
permanent middle cerebral artery occlusion in transgenic mice. J
Neurochem. 72:1187–1203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chan PH, Kawase M, Murakami K, Chen SF, Li
Y, Calagui B, Reola L, Carlson E and Epstein CJ: Overexpression of
SOD1 in transgenic rats protects vulnerable neurons against
ischemic damage after global cerebral ischemia and reperfusion. J
Neurosci. 18:8292–8299. 1998.PubMed/NCBI
|
|
40
|
Gurney ME, Cutting FB, Zhai P, Doble A,
Taylor CP, Andrus PK and Hall ED: Benefit of vitamin E, riluzole,
and gabapentin in a transgenic model of familial amyotrophic
lateral sclerosis. Ann Neurol. 39:147–157. 1996. View Article : Google Scholar : PubMed/NCBI
|