Introduction
Primary open angle glaucoma (POAG) is a progressive
optic neuropathy often associated with an abnormal elevation of
intraocular pressure (IOP) and progressive death of retinal
ganglion cells (RGCs) (1,2). The major risk factor for the
elevation of IOP is the resistance of aqueous humor (AH) outflow
through the trabecular meshwork (TM) (3). On this basis, the preferable
treatment for POAG is essentially aimed at maintaining TM function,
lowering IOP and protecting RGCs.
Large population-based prevalence and incidence
studies (4–6) have identified a positive association
between diabetes and POAG. Sato and Roy reported that high glucose
levels in the AH of patients with diabetes may increase fibronectin
syntheses and accumulation in TM, and accelerate the depletion of
TM cells (7). Basic studies
(8–11) have also demonstrated that diabetes,
not only affects vascular tissues, but also compromises neuronal
and glial functions and metabolism in the retina, which ultimately
leads to the apoptotic death of retinal neurons including RGCs.
This impaired metabolism of neurons and glial by diabetes may
render RGCs susceptible to additional stresses including elevated
IOP (8). Although there have been
a number of explanations (12–15)
for this association, more evidence is required to confirm how
diabetes influences IOP and glaucoma. In view of this, a previous
study detected the change of the insulin receptors (InsRs) in the
high air-pressure cell model employed by this current study
(16). Insulin works by binding to
the cell surface receptor InsR. There are two main intracellular
pathways that are activated by InsRs: The InsR substrate
(IRS)-phosphatidylinositol 3-kinase (PI3K) pathway and the
Ras-mitogen-activated protein kinase (MAPK) pathway (17–19).
The σ-1 receptor (Sig-1R), which has been studied
thoroughly in the central nervous system (CNS), is also recognized
to be overabundant in the eye, including the lacrimal glands,
retina, iris-ciliary body, cornea and lens (20). Modulating Sig-1R activity can lower
IOP and protect RGCs (21–23). Sig-1R is a 26-kDa protein that is
categorized as a unique non-opioid receptor. It is located at the
endoplasmic reticulum (ER)-mitochondrion membrane (MAM) (24) and is a modulator of a variety of
receptors and ion channels, acting as an amplifier in signal
transduction cascades (25). It
functions by binding to various types of exogenous and endogenous
ligands, including (+)-pentazocin (PTZ), pregnenolone and
N'-[2-(3,4-dichlorophenyl)ethyl]-N,N,N'-trimethylethane-1,2-diamine
(26).
A previous study demonstrated that a Sig-1R agonist
decreased IOP and protected against retinal damage in a rat model
of chronic ocular hypertension glaucoma (27). Nevertheless, the mechanism of this
IOP-lowering effect in vitro remains to be elucidated. It is
assumed that this effect may be mediated by the increasing outflow
of the AH through TM. In the present study, human trabecular
meshwork cells (hTMCs) were cultured under different air pressures
and Sig-1R agonist (+)-PTZ and antagonist
N-(2-(3,4-dichlorophenyl)ethyl)-N-methyl-2-(dimethylamino)
ethylamine (BD-1063) were administered to the cells at high
pressure. It was identified that (+)-PTZ can protect hTMCs from
pressure-induced apoptosis and death and that the protection was
associated with the activation of InsR and its MAPK pathway. The
effects of Sig-1Rs on IOP lowering and RGCs protection may make it
an efficient therapy for POAG.
Materials and methods
Drugs
The (+)-PTZ (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in warm 0.1 N HCl and then diluted with pH
7.0 Dulbecco's modified Eagle's medium (DMEM); the BD-1063 (Tocris
Cookson Inc., Ellisville, MO, USA) was dissolved in distilled water
and then diluted with DMEM.
Cell culture
Cells from the immortalized hTMC line were kindly
provided as a gift by the State Key Laboratory of Ophthalmology,
Zhongshan Ophthalmic Center of Sun Yat-sen University (Guangzhou,
Guangdong, China). The cells were grown in culture flasks in
Dulbecco's modified Eagle's medium with 100 µ/ml
penicillin/streptomycin (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), 20 mM/l HEPES buffer and 10% fetal bovine serum
(GE Healthcare Life Sciences) in an atmosphere containing 5%
CO2 and 95% air at 37°C. The cells were passaged every 3
days using 0.125% trypsinogen and 0.02% EDTA buffer (Beyotime
Institute of Biotechnology, Haimen, China).
To study the effect of pressure, the cells were
cultured in the same conditions as above and the well-grown 50%
confluent cells placed in culture flasks or clusters into the
pressure equipment. The cells were subjected to 0, 20, 40, 60 and
80 mmHg air pressure respectively for 48 h. To study the effect of
(+)-PTZ and BD-1063, the cells were cultured under 80 mmHg pressure
for 44 h, after which the cells were treated with (+)-PTZ (20 µM),
BD-1063 (20 µM) administered 30 min prior to (+)-PTZ, or BD-1063
(20 µM) and then exposed to 80 mmHg again until the 48 h
time-point.
Pressure equipment
The pressure equipment used in this experiment was
designed by the authors and their colleagues and manufactured by a
teaching equipment company (Yuying Teaching Device Co., Harbin,
China). The equipment comprised an airtight box, a homeothermic
incubator (Boxun Medical Biological Instrument Corp., Shanghai,
China), a mixed-gas (5% CO2 and 95% air) cylinder
(Liming Gas Group Co. Ltd., Harbin, China) and rubber tubes. The
airtight box was made from plexiglass, which can withstand
pressures of 0–120 mmHg. A dial manometer was connected to the top
wall and two switch valves to the sidewall of the airtight box; the
upper valve was the gas outlet and the lower was the gas inlet,
which was connected to the cylinder by rubber tubes. The culture
flasks or clusters were placed in the airtight box, the door and
the gas outlet closed, then the gas inlet and the cylinder gate
opened. When the correct pressure was read from the manometer, the
gas inlet and the cylinder gate was closed, to maintain the
pressure. Then the homeothermic incubator was set to 37°C. The
atmosphere was renewed every hour to balance the pH value and the
gas concentration in the box.
Ethidium bromide/acridine orange
(EB/AO) dual-staining assay
An EB/AO dual-staining kit (Nanjing Keygen Biotech.
Co. Ltd., Nanjing, China) was used to assess apoptosis and death of
the cells which were cultured under the pressure of 0, 20, 40, 60
and 80 mmHg for 48 h respectively, 80 mmHg plus (+)-PTZ (20 µM) at
44 h, 80 mmHg plus BD-1063 (20 µM) 30 min prior to (+)-PTZ at 44 h
and 80 mmHg plus BD-1063 (20 µM) at 44 h. AO can permeate through
an unbroken cell membrane and exhibits green fluorescence, while EB
can only permeate through a broken cell membrane and exhibits
orange-red fluorescence. Normal cells stain green, late apoptotic
and dead cells (broken membranes) stain orange-red. Cells were
dissociated using 0.125% trypsinogen and washed twice with 1X PBS,
then incubated with a 5% EB and 5% AO mixture for 5 min at room
temperature. The cells were then placed onto slides and visualized
using a fluorescence microscope (Olympus BX41; Olympus Corporation,
Tokyo, Japan). The number of EB- and AO-stained cells were counted
and expressed as the ratio of orange-red cells to total cells for
late apoptosis and death rate and then averaged over three
different fields.
Transmission electron microscopy of
hTMCs
Cells cultured under the pressure of 0 and 80 mmHg
were respectively dissociated by 0.125% trypsinogen, centrifuged at
1,800 × g for 5 min at 4°C and fixed with 2% glutaraldehyde in
phosphate buffer overnight at 4°C. Following post-fixation with 1%
OsO4 in cacodylate buffer for 1 h at 4°C, the pellet was
dehydrated in graded ethanol solutions and embedded in Epon.
Ultrathin sections (80 nm) of pellet were counterstained with
uranyl acetate and lead citrate and observed under a transmission
electron microscope (JEM1220; JEOL Ltd., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of Sig-1R and InsR in
experimental hTMCs
Experiments were performed to detect the mRNA
expression of Sig-1R and InsR in hTMCs which had been cultured
under the pressure of 0, 20, 40, 60 and 80 mmHg, and the effects of
agonist and antagonist on Sig-1R and InsR expression under the
pressure of 80 mmHg. Total RNA was isolated using the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). cDNA was reverse transcribed from 1 µg total RNA in 20 µl RT
reaction mix using the manufacturer's protocol (Promega
Corporation, Madison, WI, USA). Primers and Taqman probes (Shanghai
Chaoshi Biotechnology Co. Ltd., Shanghai, China) for human Sig-1R
(NM_005866.2) were 5′-AGCTCACCACCTACCTCTTTGG-3′ (forward primer),
5′-ACATGGGCTCCAGCAAGTG-3′ (reverse primer) and
5′-FAM-CCTTGACCAGCCAGGCCTGAAGG-BHQ1 (Probe); for human InsR
(NM_000208.2) were 5′-GCAGGAGCGTCATCAGCATA-3′ (forward primer),
5′-TCCACCCACTGTGAAGGAGAG-3′ (reverse primer) and
5′-FAM-TAAATGGATGTGCTGTAGTCCCAGTGCT-BHQ1 (Probe); for internal
control human β-actin (NM_001101.3) were
5′-CCCAGCACAATGAAGATCAAGATCAT-3′ (forward primer),
5′-ATCTGCTGGAAGGTGGACAGCGA-3′ (reverse primer) and
5′-FAM-TGACAAGTACTCCGTGTGGATCGGCG-BHQ1 (Probe); qPCR was performed
in an ABI Prism 7900 DNA Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Cycling variables were: 2 min at
50°C, 10 min at 95°C and then 40 cycles of 30 sec at 95°C and 30
sec at 55°C. Cycle threshold values of Sig-1R and InsR were
normalized to β-actin for each sample and calculated by comparison
of 2ΔΔCq (28).
Western blot analysis of Sig-1R, InsR
and phosphorylated and total (p/t) ERK in experimental hTMCs
Experiments were performed to detect the protein
expression of Sig-1R and InsR in hTMCs which had been cultured
under the pressure of 0, 20, 40, 60 and 80 mmHg, and the effects of
agonist and antagonist on Sig-1R, InsR and p/t ERK expression under
the pressure of 80 mmHg. Cells were lysed on ice in RIPA lysis
buffer (Beyotime Institute of Biotechnology) plus 1 protease and 1
phosphatase inhibitor cocktail tablets (Complete and PhosSTOP;
Roche Applied Science, Penzberg, Germany) per 10 ml lysis. Lysates
were centrifuged at 15,000 × g for 5 min at 4°C. The protein
content of each sample was determined by a spectrophotometer
(SmartSpec 3000; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equivalent amounts of protein from each sample was boiled with
protein loading buffer at 95°C for 5 min, loaded onto SDS-PAGE gels
for electrophoresis and then transferred onto nitrocellulose
membrane with a semi-dry transfer cell (Trans-Blot; Bio-Rad
Laboratories, Inc.). Membranes were blocked with 5% skimmed milk
for 1 h, incubated with anti-Sig-1R monoclonal antibody (cat. no.
sc-166392; 1:100), anti-InsR polyclonal antibody (cat. no. sc-710;
1:200), anti-pERK polyclonal antibody (cat. no. sc-16982R; 1:200),
anti-tERK monoclonal antibody (cat. no. sc-514302; 1:200; all Santa
Cruz Biotechnology Inc., Dallas, TX, USA) and anti-GAPDH polyclonal
antibody (cat. no. KC-5G4; 1:1,000; Kangcheng Biotechnology Co.,
Ltd., Shanghai, China) respectively at 4°C overnight, and GAPDH was
used as an internal control. The membranes were then incubated with
horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG
antibodies (cat. nos. ZB-2301 and ZB-2305; 1:5,000; Beijing
Zhongshan Goldenbridge Biotechnology; OriGene Technologies, Inc.,
Beijing, China) as the secondary antibodies for 1 h at 37°C.
Proteins were visualized using a electrochemiluminescence Detection
Reagent (cat. no. DQ111-01; Beijing Transgen Biotech Co., Ltd.,
Beijing, China), according to the manufacturer's instructions, and
captured with a scanner (Epson V30, Seiko Epson Corporation, Tokyo,
Japan). Data were quantified using Quantity One version 4.6.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and expressed as
the relative value of band density of Sig-1R/GAPDH, InsR/GAPDH and
p/t ERK1/2.
Statistical analysis
Statistical analyses were performed with the SAS
statistical package for Microsoft Windows version 9.1.3 (SAS
Institute, Cary, NC, USA). Data were expressed as mean ± standard
deviation for all the measurements. Analysis of variance was used
for comparison of experimental groups with control group in EB/AO
dual staining assay, RT-qPCR and western blot analysis. The q-test
was the post hoc statistical test. P<0.05 was considered to
indicate a statistically significant difference.
Results
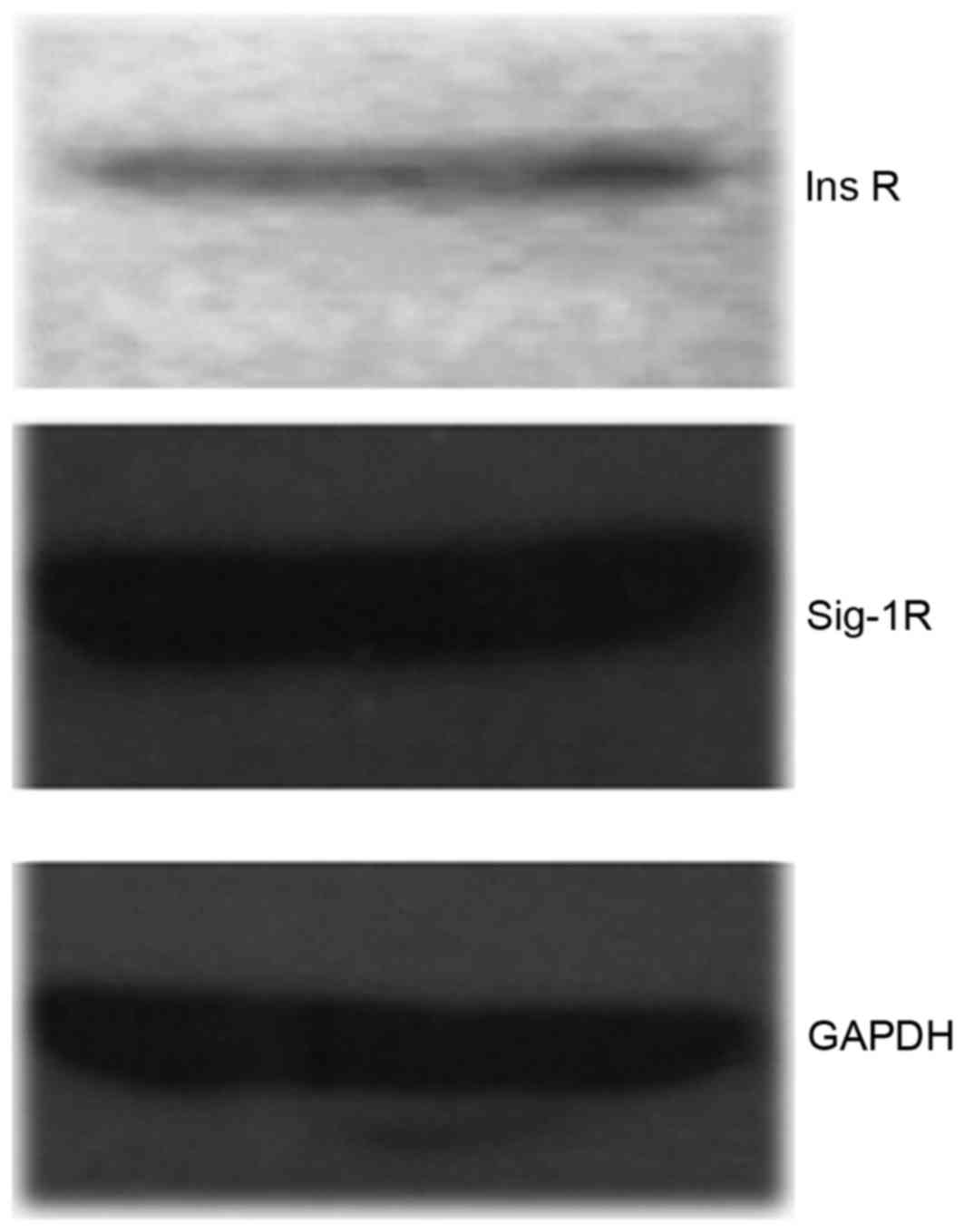
Expression of Sig-1R and InsR in
hTMCs
RT-qPCR and western blot analysis were used to
detect the expression of Sig-1R and InsR and demonstrated moderate
amounts of Sig-1R and InsR in hTMCs (Fig. 1).
Pressure-induced abnormalities of
hTMCs
Pressure-induced changes in hTMCs were significant.
The EB/AO staining assay demonstrated that treatment with pressure
increased the number of EB-positive cells considerably, and the
rates of EB-positive cells to total cells of the former five groups
(cells cultured under 0, 20, 40, 60 or 80 mmHg of pressure for 48
h, respectively) were 2.63±1.25, 2.80±1.25, 24.50±2.78, 39.17±1.76
and 57.67±1.04 (P<0.0001 with the exception of the 0 and 20 mmHg
groups, Fig. 2Aa-e). (+)-PTZ
reduced the pressure-induced cell apoptosis and death (36.00±2.16,
P<0.0001; Fig. 2A-f) compared
with the 80 mmHg-treated cells without (+)-PTZ. To confirm that
cell protection was mediated by Sig-1R activation, the 80
mmHg-treated cells were exposed to BD-1063 prior to (+)-PTZ, which
apparently increased cell apoptosis and death (65.30±1.48,
P<0.0001; Fig. 2A-g) compared
with the (+)-PTZ group. These data suggested that BD-1063 was able
to block the protective effect of (+)-PTZ. The 80 mmHg-treated
cells that were exposed only to BD-1063 experienced almost total
apoptosis and death (99.07±1.07, P<0.0001; Fig. 2A-h) compared with the 80
mmHg-treated cells, which suggested that antagonism of Sig-1R can
cause pressure associated with cell death. In Fig. 2B, data are presented as the mean
percentage ± standard error of the mean in three fields of cells,
where each field contained 60–150 cells (P<0.0001 between each
two groups with the exception of the 0 and 20 mmHg groups).
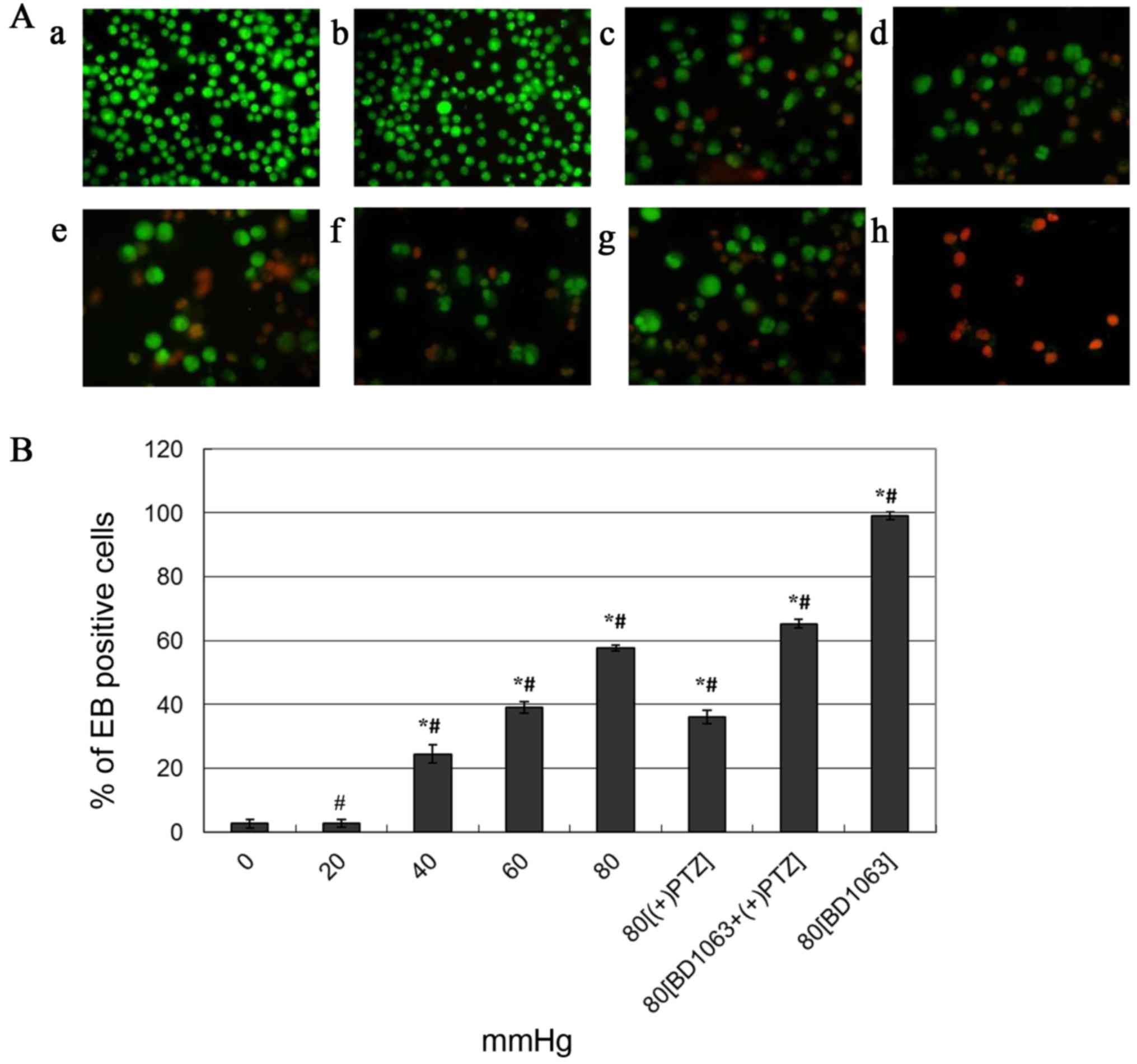 | Figure 2.Pressure-induced apoptosis and death
of hTMCs by EB/AO dual staining assay. Fluorescence images of cells
captured under the microscope (×200 magnification), normal cells
were stained green (AO positive), advanced apoptotic and dead cells
were stained orange-red (EB positive). (Aa-e) Pressure of 0, 20,
40, 60 and 80 mmHg, respectively, for 48 h. (A-f) 80 mmHg plus
(+)-PTZ (20 µM) at 44 h. (A-g) 80 mmHg plus BD-1063 (20 µM) 30 min
prior to (+)-PTZ at 44 h. (A-h) 80 mmHg plus BD-1063 (20 µM) at 44
h treated cells. (B) Summary of pressure-induced cell apoptosis and
death. The quantitative data collected from the fluorescence images
are expressed as the mean percentage ± standard error of the mean
of the ratio of apoptotic and dead cells to total cells of three
different fields of cells, where each field contained 6–150 cells.
*P<0.0001 vs. control (0 mmHg pressure); #P<0.0001
vs. each other group. hTMCs, human trabecular meshwork cells;
EB/AO, ethidium bromide/acridine orange; PTZ, pentazocin; BD-1063,
N-(2-(3,4-dichlorophenyl)ethyl)-N-methyl-2-(dimethylamino)
ethylamine. |
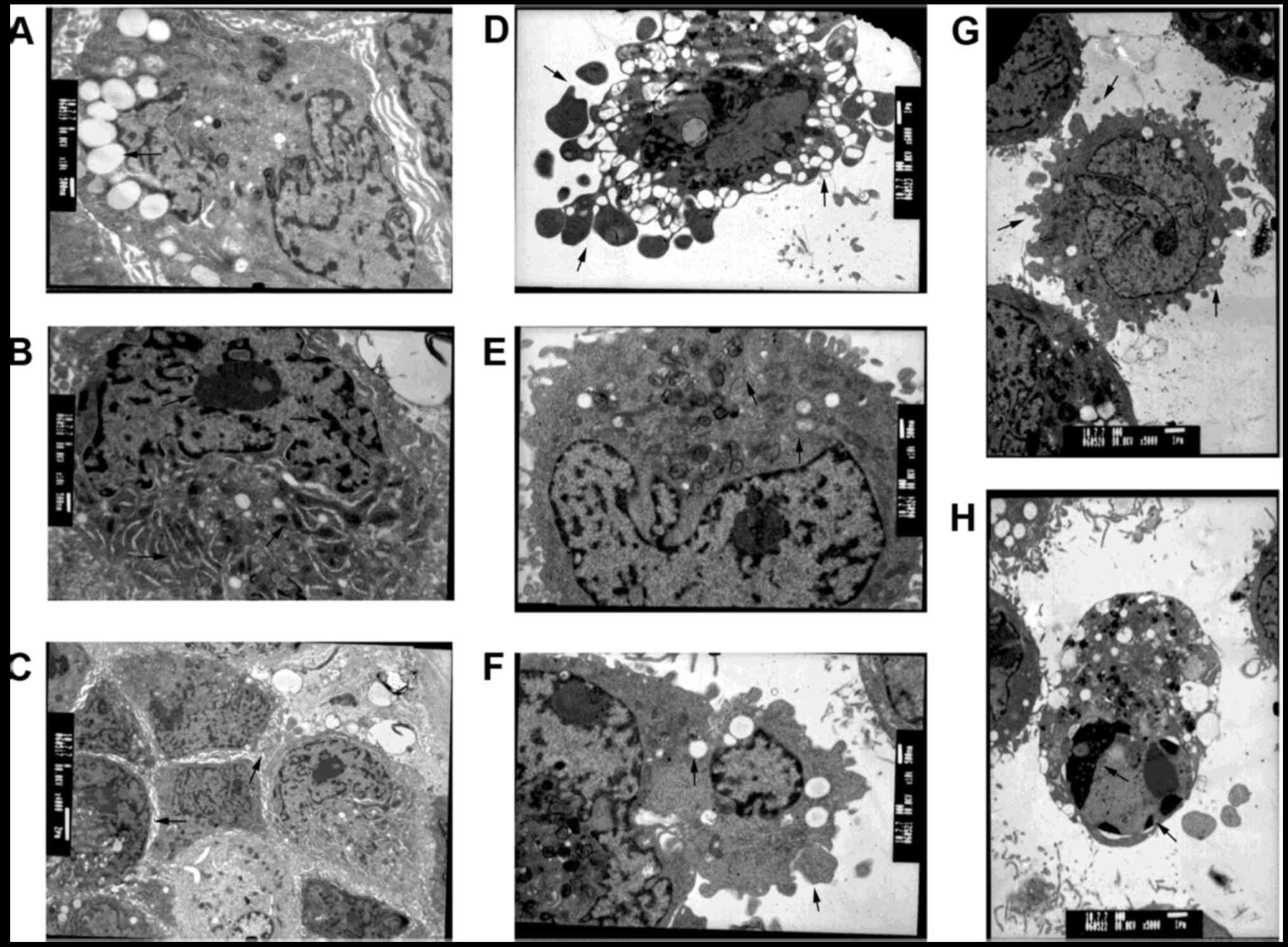
Pressure-induced morphologic changes of hTMCs were
observed by transmission electron microscopy. Normal hTMCs
exhibited multiple cell villous projections at cell surfaces,
abundant organelles (including mitochondria, rough ER and Golgi
bodies), numerous pinosomes, clear nucleoli and prominent spiked
bands of heterochromatin along the nuclear circumference (Fig. 3A-C). The ultra-microstructural
changes observed in the 80 mmHg-treated group were the formation of
apoptotic bodies, swelling and disappearance of mitochondrial
cristae, reduction of ER and pinosomes, aggregation of chromatins
and karyopyknosis (Fig. 3D-H). The
images indicated that high pressure-treated cells were undergoing
an apoptotic procedure.
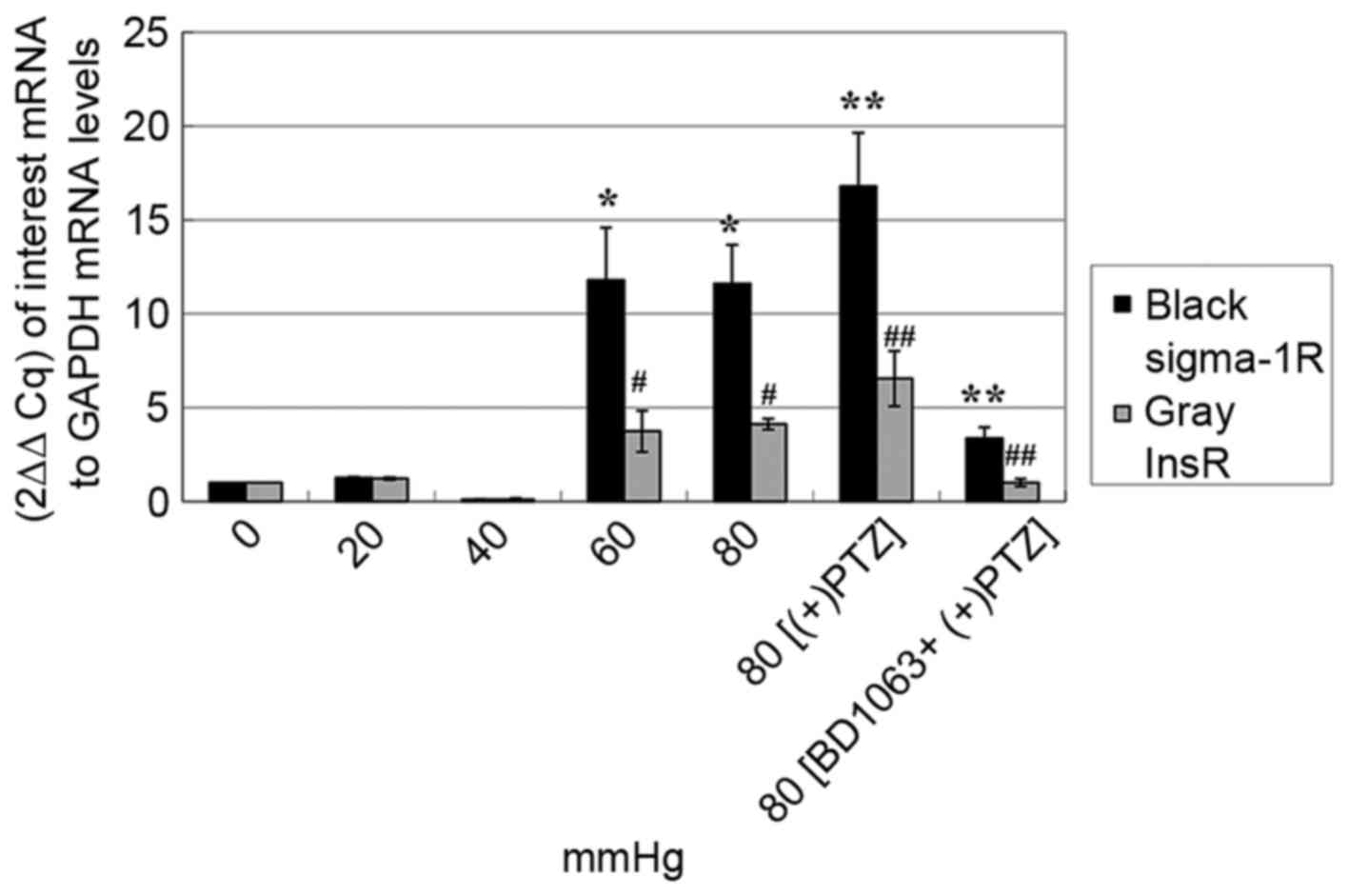
Pressure-induced change of Sig-1R and
InsR mRNA expression in hTMCs
RT-qPCR analysis of Sig-1R mRNA expression of seven
groups of cells [0, 20, 40, 60 and 80 mmHg for 48 h, respectively,
80 mmHg plus (+)-PTZ (20 µM) at the 44 h, 80 mmHg plus BD-1063 (20
µM) 30 min prior to (+)-PTZ at the 44 h] demonstrated that the
amount of Sig-1R under pressures of 60 and 80 mmHg were 11- to
12-fold higher compared with the 0, 20 and 40 mmHg groups; (+)-PTZ
upregulated the Sig-1R mRNA levels of 80 mmHg group, while the
upregulation was reversed by BD-1063 (P<0.0001; Fig. 4). The results suggested that
short-term exposure to high pressure can cause upregulation of
Sig-1R mRNA expression in vitro.
The expression of InsR mRNA in the seven groups of
cells was consistent with that of Sig-1R; the amount of InsR under
pressures of 60 and 80 mmHg were 3-to 4-fold higher compared with
the 0, 20 and 40 mmHg groups. Furthermore, the change in InsR mRNA
levels coincided with those of Sig-1R when administering
(+)-PTZ/BD-1063 (P<0.0001; Fig.
4).
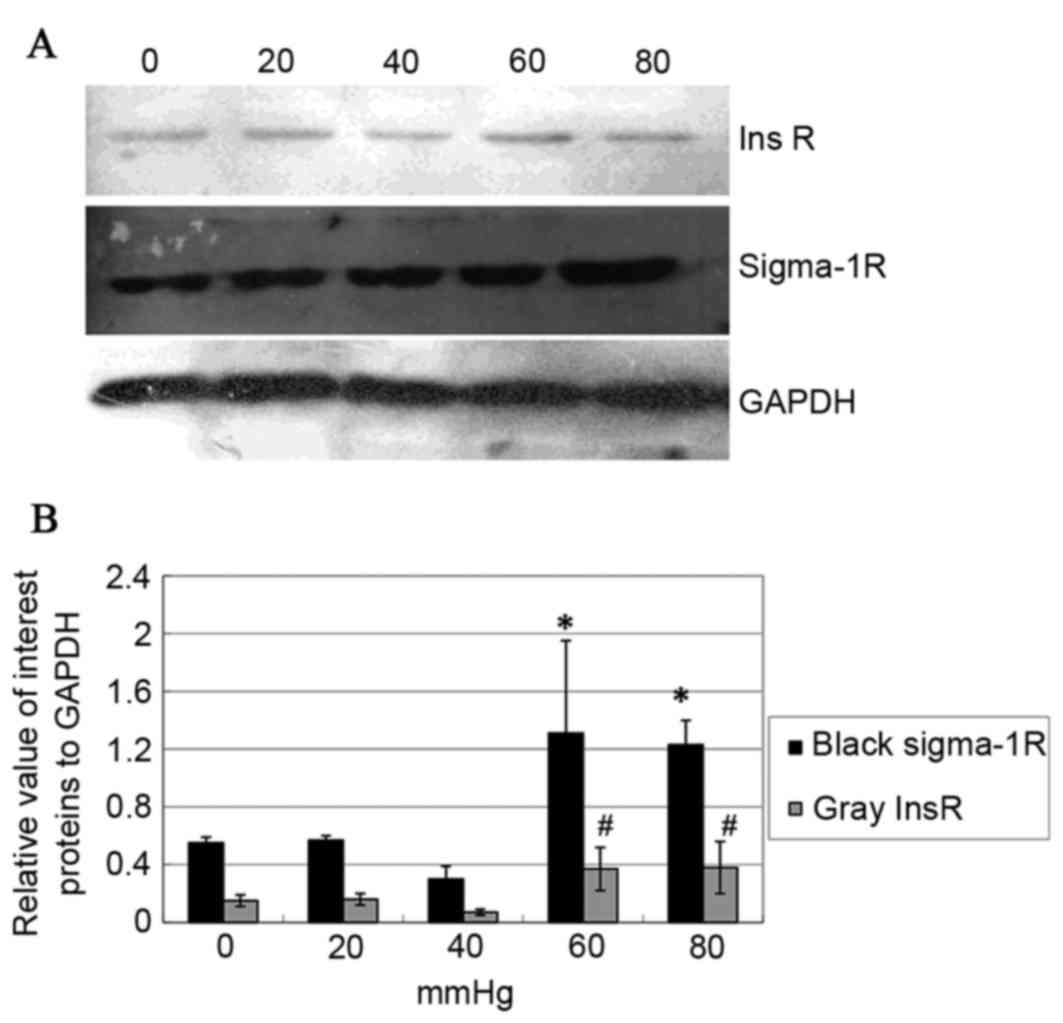
Pressure-induced change of Sig-1R,
InsR and p/t ERK protein expression in hTMCs
Western blot analysis of Sig-1R and InsR protein
(the seven groups of cells as with RT-qPCR analysis above) were
consistent with the result of RT-qPCR (P<0.05; Fig. 5A). This indicated that high
pressure can also cause the upregulations of Sig-1R and InsR
protein expression in vitro. To explore the association
between Sig-1R and InsR signaling, the phosphorylation of ERK (an
important downstream protein of the InsR-MAPK signal pathway) was
analyzed by western blotting. The data demonstrated that (+)-PTZ
caused an increase of Sig-1R itself, InsR and pERK protein
expression (P<0.05; Fig. 6;
tERK was unchanged). To confirm whether this effect was mediated by
Sig-1R, BD-1063 was used 30 min prior to (+)-PTZ and the results
demonstrated that the effect was attenuated by BD-1063 (P<0.05;
Fig. 6). It was notable that
Sig-1R ligands modulated InsR and pERK expression, which indicated
that Sig-1R served its functions partly by activating InsR and its
MAPK signal pathway.
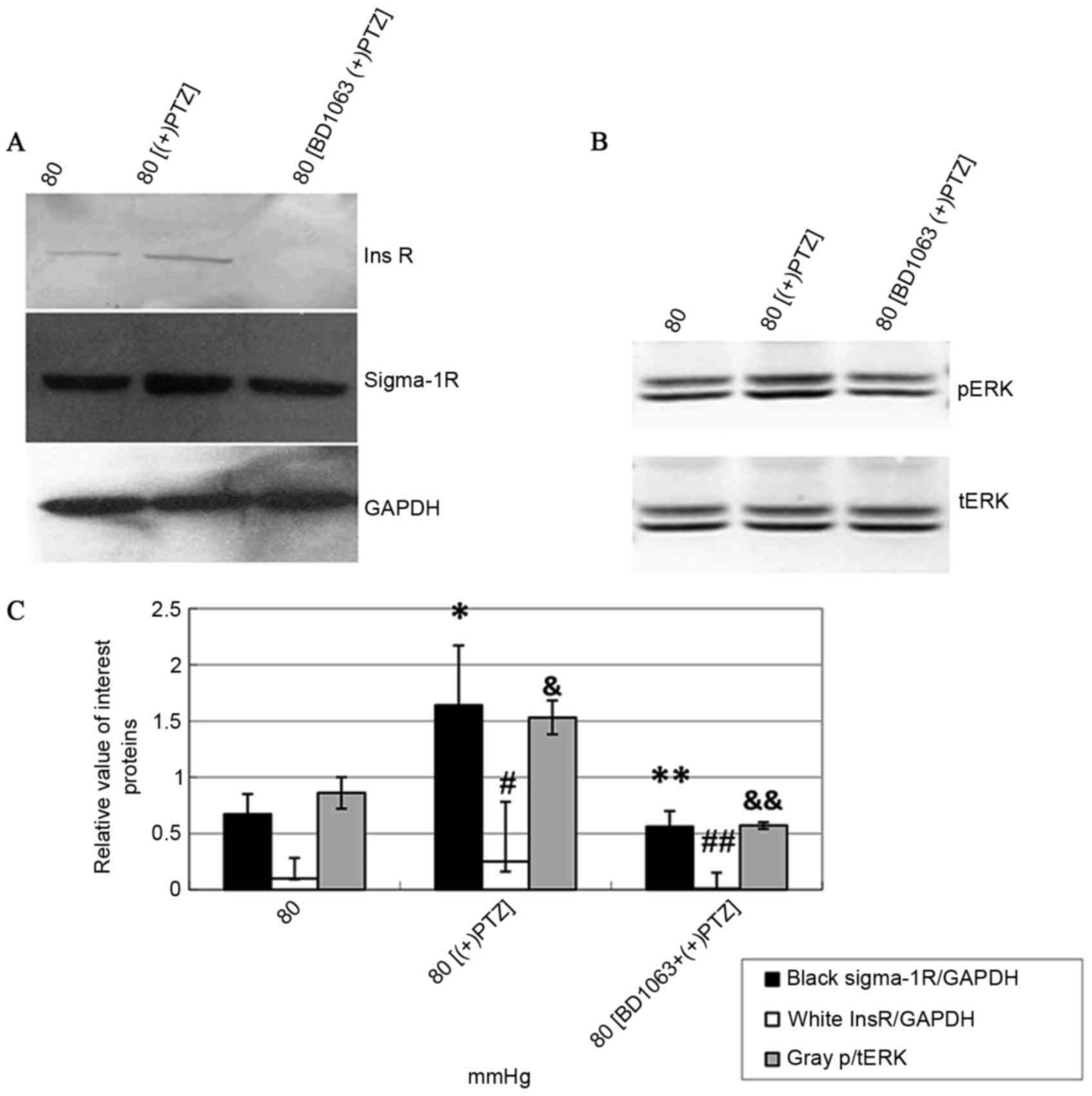 | Figure 6.Expression of Sig-1R, InsR and
p/tERK1/2 protein in hTMCs treated with (+)PTZ and BD-1063. (A)
Western blotting: The molecular sizes of the Sig-1R, InsR and GAPDH
bands of 80 mmHg, 80 mmHg + (+)PTZ, and 80 mmHg + BD-1063 + (+)PTZ
groups are indicated. (B) Western blotting: The molecular sizes of
the pERK1/2 and tERK1/2 bands of 80 mmHg, 80 mmHg + (+)PTZ, and 80
mmHg + BD-1063 + (+)PTZ groups are indicated. (C) Data from
densitometric scans of blots from (A and B); Sig-1R and InsR
proteins were increased simultaneously when (+)PTZ was
administered, and this effect was attenuated by BD-1063. Data are
presented as the mean ± standard deviation. *P=0.0002 and
#P=0.0039 vs. controls (80 mmHg); **P=0.0002 and
##P=0.0039 vs. 80 mmHg + (+)PTZ group. p/tERK1/2 was
increased when (+)PTZ was administered and such effect was also
attenuated by BD-1063. &P=0.0137 vs. control (0
mmHg); &&P=0.0137 vs. 80 mmHg + (+)PTZ group.
Data are presented as the mean ± standard deviation. Sig-1R, σ-1
receptor; InsR, insulin receptor; p/t, phosphorylated and total;
ERK, extracellular signal-regulated kinase; hTMCs, human trabecular
meshwork cells; PTZ, pentazocin; BD-1063,
N-(2-(3,4-dichlorophenyl)ethyl)-N-methyl-2-(dimethylamino)
ethylamine Sig-1R, σ-1 receptor; InsR, insulin receptor. |
Discussion
A previous study demonstrated that Sig-1R agonist
can decrease IOP and protect against retinal damage in a rat model
of chronic ocular hypertension glaucoma (26). Numerous researchers (29–31)
have performed experiments to study the anti-apoptotic mechanism of
Sig-1R agonist on RGCs, however, the mechanism of IOP lowering
remains to be fully elucidated. The present study identified that
the Sig-1R agonist (+)-PTZ protects hTMCs from pressure-induced
apoptosis and death by activating InsR and its MAPK pathway.
Elevated IOP is the main risk factor for the
development and progression of glaucomatous damage (32,33).
It is hypothesized that elevated IOP results from increased aqueous
outflow resistance and is due to several morphologic changes in the
TM, including loss of TMCs (34,35).
As a result, it was considered useful to observe what TMCs
experience under the high air-pressure directly. In the present
study, hTMCs were cultured under high pressure (80 mmHg) and it was
observed that the cells underwent an apoptotic process, with 50%
cell death at 48 h. A loss of the hTMCs, followed by substitution
with extracellular matrix (ECM), may contribute to an increased
resistance to AH outflow (36,37).
Morphologic changes were also observed in hTMCs cultured under high
pressure. Among the abnormalities, the reduction of pinosomes was
notable and possibly meant reduced phagocytic activity in
glaucomatous TMCs, which would induce increased deposition of ECM
material in the outflow pathway (38). Previous studies also suggested that
these structural changes in the TM and a decrease in TMCs are also
triggered by apoptosis (35,39,40).
These hypotheses, together with the findings of the present study,
indicate that TMCs serve a key role in maintaining the balance of
drainage pathways, that air-pressure over 40 mmHg can cause a
decrease in hTMCs and a disequilibrium between the cells and ECM
and that such disequilibrium can concomitantly cause increased IOP.
As a result, finding a way to protect hTMCs from apoptosis and
death is important.
Sig-1R has been studied thoroughly in CNS, where it
acts as a neuroprotector. In the eye, Sig-1Rs are located in the
lacrimal glands, retina, iris-ciliary body, cornea and lens, and
the effects of modulating their activities include lowering IOP and
protecting RGCs against stress (15–17).
Bucolo et al (22)
demonstrated that topical agonists [(+)-PTZ and flunarizine])
caused a significant dose-related reduction of IOP in ocular
normotensive and hypertensive albino rabbits. A previous study also
demonstrated that intraperitoneal injection of a Sig-1R agonist
(pregnenolone) reduced IOP in a chronic ocular hypertension rat
model (27). It was hypothesized
that this may be caused by the increasing AH outflow through the
other quadrants of TM with normal episcleral vein pressure. Thus,
it was required to perform in vitro experiments to explain
the phenomenon. The present study demonstrated that air-pressure
over 40 mmHg may contribute to the apoptosis and death of hTMCs.
The Sig-1R agonist (+)-PTZ can prevent the process of apoptosis and
maintain the quantity and structure of these cells, which is
beneficial for the homeostasis of TM. A short time exposure to high
air-pressure of hTMCs caused an increase of Sig-1Rs, which may be
beneficial to the survival of the cells. This result corresponds to
the hypothesis that, besides ligands, Sig-1Rs can also response for
cellular stress, including deprivation of glucose, deplenishment of
calcium in ER and oxidation (24);
high pressure being a type of stress.
For years, an increasing number of epidemiological
studies demonstrated that patients with diabetes exhibit an
increased risk of developing POAG (15,41–44).
A possible reason for the impairment of InsRs in patients with
diabetes inducing InsR signaling dysfunction, may result in
decreased phosphorylation of protein kinase B and ERK1/2. This
decrease may cause apoptosis and death of hTMCs, unbalanced
homeostasis of TM and an increase in the resistance of AH outflows.
The present study also observed that the expression of InsR was
highly consistent with that of Sig-1R. Sig-1R agonist (+)-PTZ
upregulated the expression of Sig-1R and InsR at 80 mmHg and that
can be attenuated by the antagonist BD-1063. To evaluate whether
the upregulation of InsR triggered downstream cascade, the
phosphorylation of ERK1/2, a protein of InsR-MAPK signaling
pathway, was also detected. The phosphorylation of ERK1/2 increased
with the upregulation of InsR when (+)-PTZ was administered to 80
mmHg-cultured cells, and this effect can be attenuated by BD-1063.
These results led to the hypothesis that the anti-apoptotic
mechanism of Sig-1R on pressure-induced damage of hTMCs is
associated with the MAPK signal pathway. The current consensus is
that the acute metabolic effects of insulin require activation of
the IRS-PI3K pathway, while stimulation of cell growth and
proliferation requires the Ras-MAPK cascade (45).
Previous studies have demonstrated that the Sig-1R
is mainly located at the MAM (46–48).
Activation and cellular stress promote redistribution of the
receptors from MAM to other subcellular locations, including the
periphery of endoplasmic membranes, the vicinity of the cell
membrane or nuclear envelopes. This relocation possibly increases
the number or types of proteins with which the Sig-1R can interact.
The results from the current study may provide a new protein, InsR,
interacting with the Sig-1R, a hypothesis which requires further
elucidation.
In conclusion, the present study demonstrates that
high pressure can induce hTMCs apoptosis and death, which is one of
the causes of the progression of POAG. The finding that the Sig-1R
agonist (+)-PTZ protects hTMCs from pressure-induced apoptosis and
death by activating InsR and the MAPK signal pathway, together with
the retina protecting effect of the receptor, may make the Sig-1R
agonist a superior therapeutic for POAG in the near future.
Acknowledgements
The present study was supported by the Youth
Scientific Funds of Heilongjiang Province of China (grant no.
QC2014C089), the Doctoral Foundation of Ministry of Education of
China (grant no. 20112307110015) and the Sub-project of National
973 Foundation (grant no. 2100CB707502).
References
|
1
|
Quigley HA, Tielsch JM, Katz J and Sommer
A: Rate of progression in open-angle glaucoma estimated from
cross-sectional prevalence of visual field damage. Am J Ophthalmol.
122:355–363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harper MM, Adamson L, Blits B, Bunge MB,
Grozdanic SD and Sakaguchi DS: Brain-derived neurotrophic factor
released from engineered mesenchymal stem cells attenuates
glutamate and hydrogen peroxide-mediated death of
staurosporine-differentiated RGC-5 cells. Exp Eye Res. 89:538–548.
2006. View Article : Google Scholar
|
|
3
|
Boland MV and Quigley HA: Risk factors and
open-angle glaucoma: Classification and application. J Glaucoma.
16:406–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cruz-lñigo Y, Izquierdo NJ, García O and
Pérez R: Open-angle glaucoma in patients with diabetic retinopathy
at the Puerto Rico Medical Center. Bol Asoc Med P R. 104:10–13.
2012.
|
|
5
|
Chopra V, Varma R, Francis BA, Wu J,
Torres M and Azen SP: Los Angeles Latino Eye Study Group: Type 2
diabetes mellitus and the risk of open-angle glaucoma the Los
Angeles Latino Eye Study. Ophthalmology. 115:227–232.e1. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitchell P, Smith W, Chey T and Henley P:
Open-angle glaucoma and diabetes: The Blue Mountains Eye Study,
Australia. Ophthalmology. 104:712–718. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato T and Roy S: Effect of high glucose
on fibronectin expression and cell proliferation in trabecular
meshwork cells. Invest Ophthalmol Vis Sci. 43:170–175.
2002.PubMed/NCBI
|
|
8
|
Nakamura M, Kanamori A and Negi A:
Diabetes mellitus as a risk factor for glaucomatous optic
neuropathy. Ophthalmologica. 219:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Q, Xu Y, Xie P, Cheng H, Song Q, Su
T, Yuan S and Liu Q: Retinal neurodegeneration in db/db mice at the
early period of diabetes. J Ophthalmol. 2015:7574122015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Mao D, Chen X, Zhao L, Tian Q, Liu
C and Zhou BL: Decrease in retinal neuronal cells in
streptozotocin-induced diabetic mice. Mol Vis. 18:1411–1420.
2012.PubMed/NCBI
|
|
11
|
de Moraes G and Layton CJ: Therapeutic
targeting of diabetic retinal neuropathy as a strategy in
preventing diabetic retinopathy. Clin Exp Ophthalmol. 44:838–852.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soto I, Howell GR, John CW, Kief JL, Libby
RT and John SW: DBA/2J mice are susceptible to diabetic nephropathy
and diabetic exacerbation of IOP elevation. PLoS One.
9:e1072912014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong VH, Bui BV and Vingrys AJ: Clinical
and experimental links between diabetes and glaucoma. Clin Exp
Optom. 94:4–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faiq MA, Dada R, Saluja D and Dada T:
Glaucoma-diabetes of the brain: A radical hypothesis about its
nature and pathogenesis. Med Hypotheses. 82:535–546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen L, Walter S, Melles RB, Glymour MM
and Jorgenson E: Diabetes pathology and risk of primary open-angle
glaucoma: Evaluating causal mechanisms by using genetic
information. Am J Epidemiol. 183:147–155. 2016.PubMed/NCBI
|
|
16
|
Nakae J, Kido Y and Accili D: Distinct and
overlapping functions of insulin and IGF-I receptors. Endocr Rev.
22:818–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baumann CA and Saltiel AR: Spatial
compartmentalization of signal transduction in insulin action.
Bioessays. 23:215–222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnston AM, Pirola L and Van Obberghen E:
Molecular mechanisms of insulin receptor substrate protein-mediated
modulation of insulin signalling. FEBS Lett. 546:32–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ola MS, Moore P, El-Sherbeny A, Roon P,
Agarwal N, Sarthy VP, Casellas P, Ganapathy V and Smith SB:
Expression pattern of sigma receptor 1 mRNA and protein in
mammalian retina. Brain Res Mol Brain Res. 95:86–95. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campana G, Bucolo C, Murari G and
Spampinato S: Ocular hypotensive action of topical flunarizine in
the rabbit: Role of sigma 1 recognition sites. J Pharmacol Exp
Ther. 303:1086–1094. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bucolo C, Campana G, Di Toro R,
Cacciaguerra S and Spampinato S: Sigma1 recognition sites in rabbit
iris-ciliary body: Topical sigma1-site agonists lower intraocular
pressure. J Pharmacol Exp Ther. 289:1362–1369. 1999.PubMed/NCBI
|
|
23
|
Martin PM, Ola MS, Agarwal N, Ganapathy V
and Smith SB: The sigma receptor ligand (+)-pentazocine prevents
apoptotic retinal ganglion cell death induced in vitro by
homocysteine and glutamate. Brain Res Mol Brain Res. 123:66–75.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayashi T and Su TP: Sigma-1 receptor
chaperones at the ER-mitochondrion interface regulate Ca(2+)
signaling and cell survival. Cell. 131:596–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su TP and Hayashi T: Understanding the
molecular mechanism of sigma-1 receptors: Towards a hypothesis that
sigma-1 receptors are intracellular amplifiers for signal
transduction. Curr Med Chem. 10:2073–2080. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cobos EJ, Entrena JM, Nieto FR, Cendán CM
and Del Pozo E: Pharmacology and therapeutic potential of sigma(1)
receptor ligands. Curr Neuropharmacol. 6:344–366. 2009. View Article : Google Scholar
|
|
27
|
Sun X, Cheng F, Meng B, Yang B, Song W and
Yuan H: Pregnenolone sulfate decreases intraocular pressure and
changes expression of sigma receptor in a model of chronic ocular
hypertension. Mol Biol Rep. 39:6607–6614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Mysona BA, Qureshi A, Kim L,
Fields T, Gonsalvez GB, Smith SB and Bollinger KE: (+)-Pentazocine
reduces NMDA-induced murine retinal ganglion cell death through a
σ1-dependent mechanism. Invest Ophthalmol Vis Sci. 57:453–461.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mueller BH II, Park Y, Ma HY, Dibas A,
Ellis DZ, Clark AF and Yorio T: Sigma-1 receptor stimulation
protects retinal ganglion cells from ischemia-like insult through
the activation of extracellular-signal-regulated kinases 1/2. Exp
Eye Res. 128:156–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mueller BH II, Park Y, Daudt DR III, Ma
HY, Akopova I, Stankowska DL, Clark AF and Yorio T: Sigma-1
receptor stimulation attenuates calcium influx through activated
L-type voltage gated calcium channels in purified retinal ganglion
cells. Exp Eye Res. 107:21–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kass MA, Heuer DK, Higginbotham EJ,
Johnson CA, Keltner JL, Miller JP, Parrish RK II, Wilson MR and
Gordon MO: The ocular hypertension treatment study: A randomized
trial determines that topical ocular hypotensive medication delays
or prevents the onset of primary open-angle glaucoma. Arch
Ophthalmol. 120:701–713, 829–830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heijl A, Leske MC, Bengtsson B, Hyman L
and Hussein M: Early Manifest Glaucoma Trial Group: Reduction of
intraocular pressure and glaucoma progression: Results from the
Early Manifest Glaucoma Trial. Arch Ophthalmol. 120:1268–1279.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alvarado J, Murphy C and Juster R:
Trabecular meshwork cellularity in primary open-angle glaucoma and
nonglaucomatous normals. Ophthalmology. 91:564–579. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rohen JW, Lütjen-Drecoll E, Flugel C,
Meyer M and Grierson I: Ultrastructure of the trabecular meshwork
in untreated cases of primary open-angle glaucoma (POAG). Exp Eye
Res. 56:683–692. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lutjen-Drecoll E, Rittig M, Rauterberg J,
Jander R and Mollenhauer J: Immunomicroscopical study of type VI
collagen in the trabecular meshwork of normal and glaucomatous
eyes. Exp Eye Res. 48:139–147. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tripathi RC: Pathologic anatomy of the
outflow pathway of aqueous humor in chronic simple glaucoma. Exp
Eye Res. 25:(Suppl). S403–S407. 1977. View Article : Google Scholar
|
|
38
|
Zhang X, Ognibene CM, Clark AF and Yorio
T: Dexamethasone inhibition of trabecular meshwork cell
phagocytosis and its modulation by glucocorticoid receptor beta.
Exp Eye Res. 84:275–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao Y, Wei H, Pfaffl M, Da B and Li Z:
Apoptosis of human trabecular meshwork cells induced by
transforming growth factor-beta2 in vitro. J Huazhong Univ Sci
Technolog Med Sci. 24:87–89, 94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ziangirova GG and Antonova OV: Lipid
peroxidation in the pathogenesis of primary open-angle glaucoma.
Vestn Oftalmol. 119:54–55. 2003.(In Russian). PubMed/NCBI
|
|
41
|
Zhao D, Cho J, Kim MH, Friedman DS and
Guallar E: Diabetes, fasting glucose, and the risk of glaucoma: A
metaanalysis. Ophthalmology. 122:72–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ko F, Boland MV, Gupta P, Gadkaree SK,
Vitale S, Guallar E, Zhao D and Friedman DS: Diabetes, triglyceride
levels, and other risk factors for glaucoma in the National Health
and Nutrition Examination Survey 2005–2008. Invest Ophthalmol Vis
Sci. 57:2152–2157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mitchell P, Smith W, Chey T and Healey PR:
Open-angle glaucoma and diabetes: The Blue Mountains eye study,
Australia. Ophthalmology. 104:712–718. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klein BE, Klein R and Jensen SC:
Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye
Study. Ophthalmology. 101:1173–1177. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie L, Chen H, Overbeek PA and Reneker LW:
Elevated insulin signaling disrupts the growth and differentiation
pattern of the mouse lens. Mol Vis. 13:397–407. 2007.PubMed/NCBI
|
|
46
|
Zamanillo D, Romero L, Merlos M and Vela
JM: Sigma 1 receptor: A new therapeutic target for pain. Eur J
Pharmacol. 716:78–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mori T, Hayashi T, Hayashi E and Su TP:
Sigma-1 receptor chaperone at the ER-mitochondrion interface
mediates the mitochondrion-ER-nucleus signaling for cellular
survival. PLoS One. 8:e769412013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Su TP, Hayashi T, Maurice T, Buch S and
Ruoho AE: The sigma-1 receptor chaperone as an inter-organelle
signaling modulator. Trends Pharmacol Sci. 31:557–566. 2010.
View Article : Google Scholar : PubMed/NCBI
|