Introduction
Mitogen-activated protein-kinases (MAPKs), including
extracellular signal-regulated protein kinases (Erks), p38 and
c-Jun N-terminal kinases, are a group of well-described
serine/threonine-specific protein kinases typically expressed in
all cell types, and are essential components of the signal
transduction machinery that occupies a central position in
regulation of cell survival, motility, apoptosis, proliferation and
differentiation (1–4). However, different mitogen-activated
protein kinase (MAPK) family members are activated in response to
different extracellular stimuli and have different downstream
targets, thereby demonstrating distinct biological functions. It
has been reported that MAPKs serve crucial and complicated roles in
the chondrogenesis and osteogenesis of mesenchymal stem cells
(MSCs); however, the exact effects of Erk1/2 and p38 in
chondrogenesis and osteogenesis are contradictory in previous
studies (5–11). A previous study reported that Erk1⁄
2 serves a negative role, whereas p38 has a positive role in the
chondrogenesis of MSCs, including bone marrow stromal cells, dental
follicle stem cells and periodontal ligament stem cells (9). However, another other report
determined the opposite results (11). In addition, there are a number of
different results, promoting the osteogenesis or inhibiting the
osteogenesis, for the effect of Erk1/2 and p38 on the osteogenesis
of MSCs (5–8). These data suggested that the
biological effects of Erk1/2 and p38 on the
osteogenesis/chondrogenesis of MSCs may depend on cell type,
culture systems, developmental stage, culture time and stimulating
factors.
Dental pulp stem cells (DPSCs) may be easily
obtained from extracted third molars, deciduous teeth and other
healthy teeth. In addition, they are multipotent, meaning that they
are able to differentiate into several different cell types,
including neurons, odontoblasts, adipocytes, osteoblasts and
chondrocytes under specific stimuli (12,13).
These observations suggested that DPSCs may offer an alternative
cell source for bone and cartilage regeneration. Erk1/2 and p38
have been demonstrated to be important in the proliferation and
differentiation of DPSCs (14,15).
A previous study revealed that fibroblast growth factor 9 (FGF9)
was able to simultaneously promote the chondrogenesis of DPSCs by
activating the phosphorylation of Erk1/2 (16). However, the exact effect of Erk1/2
and p38 on the chondrogenesis and osteognesis of DPSC remains to be
completely elucidated. In the present study, the aim was to
investigate the function of Erk1/2 and p38 signals on the
chondrogenesis and osteognesis of DPSCs.
Materials and methods
Isolation and culture of DPSCs
DPSCs were obtained using direct cell outgrowth from
dental pulp tissue explants and cultured as previously reported
(16,17). Normal human third molars were
obtained from adults (age, 16–25 years) in the Department of Oral
and Maxillofacial Surgery, Stomatological Hospital, Shandong
University (Jinan, China). The teeth were cleaned, and the pulp
chamber was exposed using sterilized dental fissure burs.
Subsequently, the dental pulp tissues were dissected into fragments
(~0.5 mm), and were placed into a 6-cm dish containing Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) to
incubate at 37°C with 5% CO2 for 2–3 weeks. Following
reaching confluence, the DPSCs were continuously passaged, and
cells from the third passage (P3) were used for further
experiments. The cell morphology was observed under an inverted
phase contrast microscope (Nikon Corporation, Tokyo, Japan).
Colony-forming efficiency (CFE)
assays
The CFE of the P3 DPSCs was determined as previously
reported (16). A total of 1,000
mixed cells were seeded into a 10-cm culture dish, and cultured for
a further 14 days in DMEM. Subsequently the cells were fixed in
100% methanol for 20 min at room temperature, and stained with
Giemsa for 15 min at room temperature. Colonies containing >50
cells were counted, and the CFE was calculated by dividing the
total number of colonies by 1,000.
MTT assay
The vitality of the P3 DPSCs was determined as
previously reported (16).
Briefly, 104 DPSCs/well were plated in 96-well plates
containing 200 µl DMEM with 10% FBS. The proliferation of the DPSCs
was evaluated at different time points (1, 3, 5 and 7 days) via an
MTT assay.
Osteogenic induction of DPSCs
The osteogenic induction medium was composed of
DMEM, 10−8 M dexamethasone, 50 mg/ml L-ascorbic acid,
0.005 M KH2PO4, 50 mg/ml gentamycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 10% FBS. The
medium was replaced every 3 days for 3 weeks. The conditions used
in the different experimental groups were as follows: Control
group, DPSCs cultured in DMEM with 10% FBS; Dx group, DPSCs
cultured in the osteogenic induction medium dexamethasone (Dx);
Dx-PD98059 group, DPSCs cultured in Dx medium with 10 µM PD98059,
an inhibitor of Erk1/2; and Dx-SB203580 group, DPSCs cultured in Dx
medium with 10 µM SB203580, an inhibitor of p38. The inhibitors,
PD98059 (Merck KGaA) and SB203580 (Merck KGaA), were added to the
DMEM medium 2 h prior to the shift to osteogenic induction medium
(also including PD98059 and SB203580) for continuous culture.
DPSCs pellets cultured in vitro
In vitro chondrogenesis of DPSCs was
performed in pellet cultures using P3 DPSCs as previously described
(16,18). DPSCs were washed twice with PBS and
re-suspended in chondrogenic medium (Cyagen Biosciences, Santa
Clara, CA, USA) consisting of DMEM-F12 supplemented with 1%
insulin-transferrin-selenium (Sigma-Aldrich; Merck KGaA), 0.1 mM
L-ascorbate-2-phosphate, 0.4 mM proline (Sigma-Aldrich; Merck KGaA)
and 10 ng/ml transforming growth factor-β3 (TGFβ3; Sigma-Aldrich;
Merck KGaA). Subsequently, the cells were centrifuged in 15-ml
polypropylene tubes at 500 × g for 5 min at room temperature, and
maintained at 37°C under 5% CO2. The caps of the tubes
were loosened to allow for air exchange. The medium was changed
every 2 days. Following 6 weeks of culture, the pellets were
collected and processed for immunohistochemical analysis and
analysis of mRNA expression. All pellet groups for the different
experiments were prepared as follows: Control group, DPSCs cultured
in DMEM with 10% FBS; TGFβ3 group, DPSCs alone cultured in
chondrogenic medium with TGFβ3; TGFβ3-PD98059 group, DPSCs cultured
in chondrogenic medium with TGFβ3 and PD98059; and TGFβ3-SB203580
group, DPSCs cultured in chondrogenic medium with TGFβ3 and
SB203580.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from different cell/pellet
samples using the TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) as previously described (16,19).
The quality and quantity of the RNA was analyzed. Subsequently, the
total RNA was converted to cDNA using PrimeScript-RT reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). GAPDH was applied
as an internal control to normalize and quantify the results. The
gene-specific primers used for the amplification of Runt-related
transcription factor 2 (Runx2) were as follows: CGG AAT GCC TCT GCT
GTT ATG (forward) and GGT TCC CGA GGT CCA TCT ACT G (reverse), and
primers for GAPDH, alkaline phosphatase (ALP), type II collagen
(Col2), aggrecan (ACAN) and SRY-box 9 (Sox9) are described in
detail in previous reports (16).
The RT-qPCR reactions were performed using the SYBR-Green system
(Takara Biotechnology Co., Ltd., Dalian, China) in 96-well
microwell plates (total reaction volume, 20 µl) with a MyiQ
Single-Color Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The thermocycling parameters were as
follows: 95°C for 4 min, then 35 cycles of 95°C for 20 sec,
followed by 60°C for 15 sec, and 72°C for 20 sec. The amplification
efficiency of target genes was calculated relative to GAPDH (ΔCq=Cq
gene-Cq GAPDH). The relative mRNA expression levels of these target
genes were calculated compared with the expression of the controls
(2−ΔΔCq, ΔΔCq=ΔCq gene-ΔCq control) (20). All the experiments were performed
in triplicate.
ALP and alizarin red staining
Following culturing in vitro for 4 weeks, the
ALP staining of DPSCs was performed using an alkaline phosphatase
staining kit (Biyuntian, Haimen, China). Briefly, the culture dish
was washed twice with PBS, and then fixed in 4% paraformaldehyde
(PFA), followed by staining with alkaline dye for 30 min at room
temperature. Finally, DPSCs underwent thorough washing and
scanning. For alizarin red S staining, the DPSCs were fixed in 95%
ethanol following 28 days culturing, then stained with 1% alizarin
red S (Sigma-Aldrich; Merck KGaA) at room temperature for 20 min,
and thoroughly washed prior to scanning.
Western blotting
The protein extraction of DPSCs was performed as
previously described (16,19). Protein concentrations were
determined using a BCA protein assay kit (cat. no. 23227; Pierce;
Thermo Fisher Scientific, Inc.), and 50 µg protein per lane were
separated using 10% SDS-PAGE, and transferred to a polyvinylidene
difluoride membrane with a semidry transfer apparatus (Bio-Rad
Laboratories, Inc.). Subsequently, the membranes were blocked with
5% milk for 2 h at room temperature, and incubated with primary
antibodies against Erk1/2 (cat. no. 4695), p-Erk1/2 (cat. no.
4376), p38 (cat. no. 8690) and p-p38 (cat. no. 4511) (all dilution
1:1,000 in PBS Tween-20 buffer; Cell Signaling Technology, Inc.,
Danvers, MA, USA) overnight at 4°C. Finally, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(cat. no. ab97051; Abcam, Cambridge, UK; dilution, 1:5,000 in PBS)
for 2 h at room temperature. Then the membranes were detected with
enhanced chemiluminescence reagents (EMD Millipore, Billerica, MA,
USA), and were exposed to X-ray film (Fujifilm, Tokyo, Japan).
Immunohistochemistry
DPSCs pellets were fixed with 4% PFA for 4 h,
embedded in paraffin, and cut into 5 µm-thick sections.
Immunohistochemistry staining of stromal cell antigen-1 (STRO-1)
and Col2 in the sections was performed as previously described
(16). Antigen retrieval was
performed by boiling samples in a sodium citrate solution for 15
min, and pretreating with 0.01% Triton X-100 (Sigma-Aldrich; Merck
KGaA) for 20 min, then 3% H2O2 for 20 min and
3% BSA (Sigma-Aldrich; Merck KGaA) for 30 min. Subsequently, the
slides were incubated at 4°C overnight with the primary antibody
stromal cell antigen-1 (STRO-1; cat. no. sc-47733HRP; Santa Cruz
Biotechnology, Dallas, Texas, USA) or Col2 (cat. no. ab21291,
Abcam; both dilution, 1:100 in PBS; Abcam) in a humid chamber. Then
the samples were washed and incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. ab97051;
Abcam; dilution, 1:150 in PBS) at room temperature for 2 h. The
samples were stained with a diaminobenzidine staining kit (Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) for 3 min, and were
counterstained with hematoxylin.
Statistical analysis
The data are presented as the mean ± standard
deviation, and an independent samples t-test was chosen to compare
the data between two groups using SPSS software (version 18.0;
SPSS, Inc., Chicago, IL, USA). P<0.05 was used to indicate a
statistically significant difference.
Results
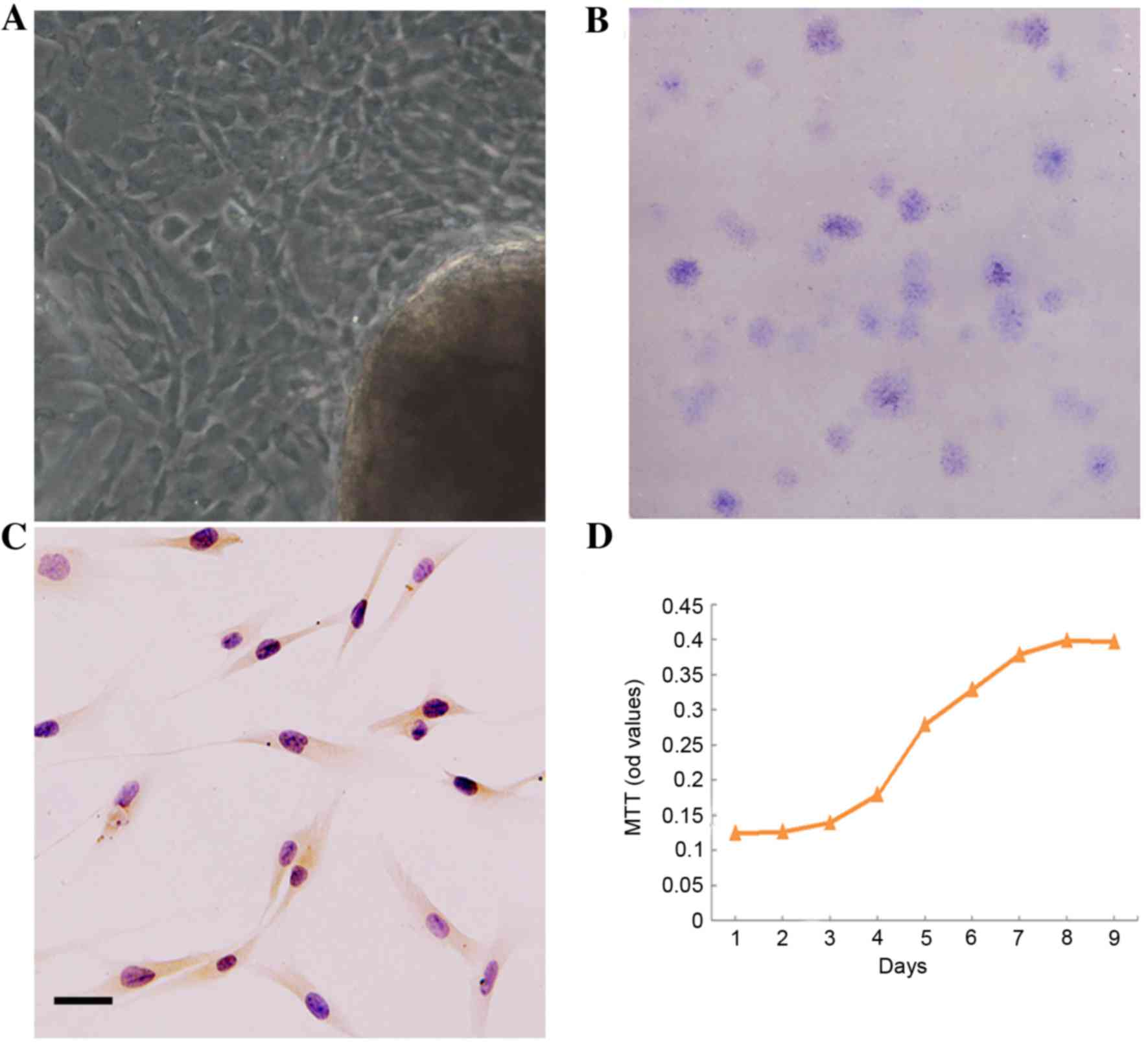
Features of DPSCs
The DPSCs outgrew from dental pulp tissue explants,
and exhibited a predominantly long and spindle-shaped morphology
(Fig. 1A). DPSCs are able to
readily form a colony from a single cell in vitro, and the
colony-forming efficiency (CFE) was 7% (Fig. 1B). In addition, DPSCs expressed
STRO-1, an early MSCs cell-surface molecule marker (Fig. 1C). At day 2, the P3 DPSCs began to
grow exponentially. The population doubling time (PDT) was 1.93
days in the logarithmic phase (Fig.
1D).
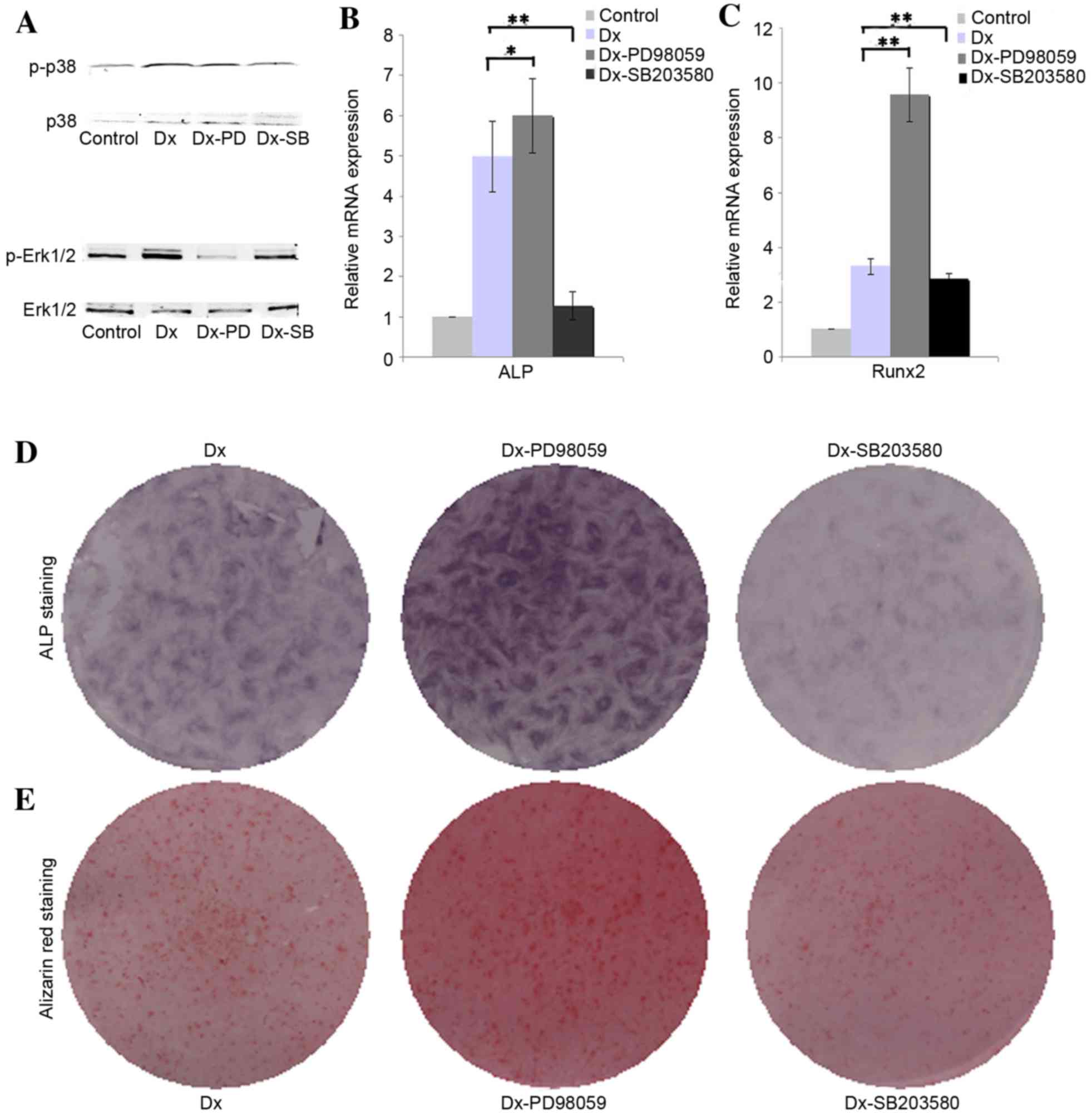
Effect of Erk1/2 and p38 on the
osteogenic differentiation of DPSCs
The results of western blotting demonstrated that
osteogenic induction medium is able to augment the phosphorylation
of ERK1/2 in DPSCs, whereas PD98059 effectively inhibited the
phosphorylation of ERK1/2. The presented western blotting results
indicated that osteogenic induction medium slightly inhibited the
phosphorylation of p38, and SB203580 were able to further inhibit
the phosphorylation of p38 (Fig.
2A). The RT-qPCR analysis results revealed that inhibition of
phosphorylation of Erk1/2 with PD98059 upregulated the expression
of ALP and Runx2 in DPSCs cultured in osteogenic medium when
compared with the Dx group (Fig.
2B, P<0.05; Fig. 2C,
P<0.01), whereas inhibition of phosphorylation of p38 with
SB203580 were able to downregulate the expression of ALP and Runx2
when compared with the Dx group (Fig.
2B and C, P<0.01). The ALP and alizarin red staining results
further confirmed that inhibition of phosphorylation of Erk1/2
promote the expression of ALP and ossification, whereas inhibition
of phosphorylation of p38 showed opposed effect (Fig. 2D and E).
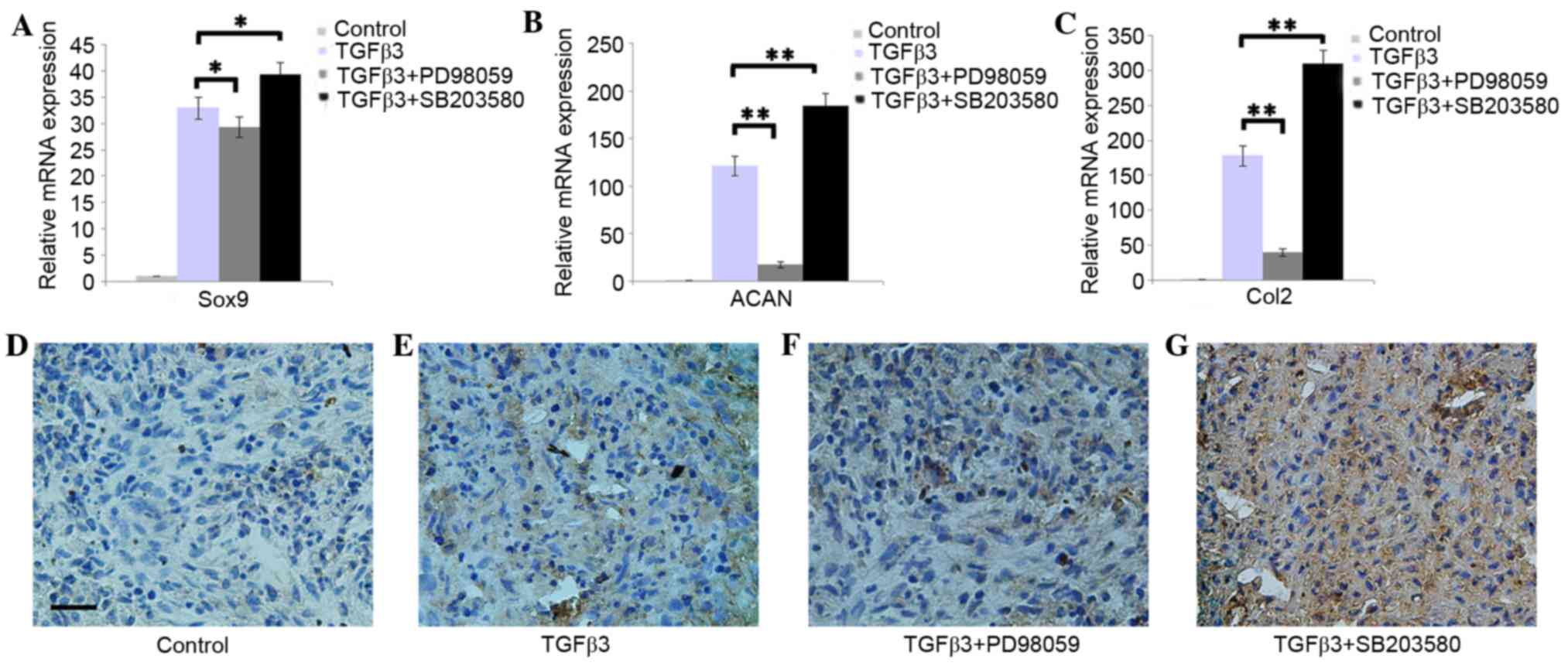
Effect of Erk1/2 and p38 on the
chondrogenic differentiation of DPSCs
The RT-qPCR results indicated that the addition of
PD98059 was able to downregulate the expression of Sox9, ACAN and
Col2, whereas SB203580 was able to upregulate the expression of
Sox9, ACAN and Col2 in DPSCs pellets cultured in chondrogenic
medium (Fig. 3A-C; P<0.05). The
immunohistochemistry results demonstrated that DPSCs pellets
cultured in chondrogenic medium with PD98059 presented weak Col2
staining. However, SB203580 was able to enhance the Col2 staining
(Fig. 3D-G).
Discussion
To the best of the authors' knowledge, the present
study is one of the first to investigate the effects of Erk1/2 and
p38, on the osteogenic and chondrogenic differentiation of DPSCs.
The results suggested that Erk1/2 and p38 exhibit an opposing
effect on the osteogenic and chondrogenic differentiation of DPSCs.
For osteogenic differentiation, previous studies indicated that p38
and Erk1/2 involved in bone morphogenetic factor-9-induced
osteogenic differentiation of rat dental follicle stem cells, and
inhibition of p38 is able to inhibit the osteogenic
differentiation, whereas inhibition of Erk1/2 would promote the
osteogenic differentiation (6). Li
et al (7) reported that
cyclic tensile stress may enhance the osteogenic differentiation of
periodontal ligament cells via the Erk1/2 signaling pathway, and
inhibition of Erk1/2 may inhibit osteogenic differentiation. Wang
et al (21) reported that
hypoxia inhibits the osteogenic differentiation of rat bone
mesenchymal stem cells, potentially through Erk1/2, but not the p38
signaling pathway. In addition, there are a number of additional
reports presenting contradictory results concerning the effects of
Erk1/2 and p38 on the osteogenic differentiation of MSCs under
different conditions (22–24). These previous findings implied that
different chemical and physical stimuli for osteogenic
differentiation of MSCs may result in different biochemical
responses through different underlying mechanisms, and that
different cell types, culture systems, developmental stages and
culture times may contribute to the effect (6,7,21–24).
In the present study, inhibition of p38 may inhibit the osteogenic
differentiation of DPSCs, whereas inhibition of Erk1/2 demonstrated
the opposite effect. Although the results were similar with certain
previous studies, these findings seem to contradict several other
reports. However, the presented results may further confirm a more
intricate set of events underlying the differentiation of MSCs.
For chondrogenesis, there are a number of different
effects of Erk1/2 and p38 on the chondrogenic differentiation of
MSCs. Bobick et al (11)
reported that knockdown of ERK1/2 pathway members MEK1 and ERK1
decreased expression of all chondrogenic markers in human bone
marrow-derived multipotent progenitor cells cultured in
chondrogenic medium with TGF-β3. An additional study suggests that
Erk1/2 and p38 serve opposing roles in mediating transcription of
cartilage-specific genes in rat bone marrow mesenchymal stem cells
cultured in chondrogenic medium with TGF-β1, and inhibition of
Erk1/2 may promote chondrogenesis (9). Kim and Im (10) reported that inhibition of Erk1/2
was able to suppress hypertrophy and promote chondrogenesis of bone
marrow-derived MSCs and adipose tissue-derived MSCs cultured in
chondrogenic medium with bone morphogenetic protein 7 and TGF-β2,
whereas inhibition of p38 had little effect. The results of the
present study suggest that inhibition of Erk1/2 may inhibit the
chondrogenic differentiation of DPSCs, whereas the effect of
inhibiting p38 demonstrated an opposing effect. These findings seem
to contradict previous reports, suggesting that inhibition of
Erk1/2 simultaneously promotes the chondrogenesis and hypertrophy
of DPSCs co-cultured with costal chondrocytes in chondrogenic
medium with TGF-β3 and FGF9 (16).
The present study further demonstrates the complexity of the effect
of Erk1/2 and p38 on the differentiation of MSCs.
It is well understood that modifying the signaling
pathway during the differentiation process of MSCs may influence
the ultimate commitment of the MSCs, and many differentiating
stimuli were applied to direct the MSCs to differentiate into the
expected cells. However, how these pathways affect the
differentiation program of MSCs and how manipulation of these
pathways may obtain more efficient differentiation protocols
remains unclear. MAPKs have been reported to be one of the most
important signaling pathways implicated in chondrogenic and
osteogenic differentiation of MSCs (4). However, as discussed above, the exact
mechanisms of how Erk1/2 and p38 are involved in these processes
remain unclear, and many factors may influence the results.
Therefore, future studies should consider the influence of
different chemical and physical stimuli, cell types, culture
methods, the times of adding the inhibitor and the dosage of
inhibitor, on the effect of Erk1/2 and p38 on the differentiation
of MSCs. In addition, the potential difference between the in
vivo and in vitro effects of Erk1/2 and p38 on the
differentiation of MSCs should be taken into consideration. The aim
is that these results will help to better understand the underlying
mechanisms that control this process, in the search for novel,
effective tissue regeneration and stem cell-based regenerative
therapies.
References
|
1
|
Gehart H, Kumpf S, Ittner A and Ricci R:
MAPK signalling in cellular metabolism: Stress or wellness? EMBO
Rep. 11:834–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garrington TP and Johnson GL: Organization
and regulation of mitogen-activated protein kinase signaling
pathways. Curr Opin Cell Biol. 11:211–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peter AT and Dhanasekaran N: Apoptosis of
granulosa cells: A review on the role of MAPK-signalling modules.
Reprod Domest Anim. 38:209–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stanton LA, Underhill TM and Beier F: MAP
kinases in chondrocyte differentiation. Dev Biol. 263:165–175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanton LA and Beier F: Inhibition of p38
MAPK signaling in chondrocyte cultures results in enhanced
osteogenic differentiation of perichondral cells. Exp Cell Res.
313:146–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Yang X, He Y, Ye G, Li X, Zhang X,
Zhou L and Deng F: Bone morphogenetic protein-9 induces osteogenic
differentiation of rat dental follicle stem cells in P38 and ERK1/2
MAPK dependent manner. Int J Med Sci. 9:862–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Han M, Li S, Wang L and Xu Y: Cyclic
tensile stress during physiological occlusal force enhances
osteogenic differentiation of human periodontal ligament cells via
ERK1/2-Elk1 MAPK pathway. DNA Cell Biol. 32:488–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelaez D, Arita N and Cheung HS:
Extracellular signal-regulated kinase (ERK) dictates osteogenic
and/or chondrogenic lineage commitment of mesenchymal stem cells
under dynamic compression. Biochem Biophys Res Commun.
417:1286–1291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ and Im GI: The effects of ERK1/2
inhibitor on the chondrogenesis of bone marrow- and adipose
tissue-derived multipotent mesenchymal stromal cells. Tissue Eng
Part A. 16:851–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bobick BE, Matsche AI, Chen FH and Tuan
RS: The ERK5 and ERK1/2 signaling pathways play opposing regulatory
roles during chondrogenesis of adult human bone marrow-derived
multipotent progenitor cells. J Cell Physiol. 224:178–186.
2010.PubMed/NCBI
|
|
12
|
Zhang W, Walboomers XF, Van Kuppevelt TH,
Daamen WF, Van Damme PA, Bian Z and Jansen JA: In vivo evaluation
of human dental pulp stem cells differentiated towards multiple
lineages. J Tissue Eng Regen Med. 2:117–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grottkau BE, Purudappa PP and Lin YF:
Multilineage differentiation of dental pulp stem cells from green
fluorescent protein transgenic mice. Int J Oral Sci. 2:21–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang FM, Hu T and Zhou X: p38
mitogen-activated protein kinase and alkaline phosphatase in human
dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:114–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Liu S, Zhou Y, Tan J, Che H, Ning
F, Zhang X, Xun W, Huo N, Tang L, et al: Natural mineralized
scaffolds promote the dentinogenic potential of dental pulp stem
cells via the mitogen-activated protein kinase signaling pathway.
Tissue Eng Part A. 18:677–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai J, Wang J, Lu J, Zou D, Sun H, Dong Y,
Yu H, Zhang L, Yang T, Zhang X, et al: The effect of co-culturing
costal chondrocytes and dental pulp stem cells combined with
exogenous FGF9 protein on chondrogenesis and ossification in
engineered cartilage. Biomaterials. 33:7699–7711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnstone B, Hering TM, Caplan AI,
Goldberg VM and Yoo JU: In vitro chondrogenesis of bone
marrow-derived mesenchymal progenitor cells. Exp Cell Res.
238:265–272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai J, Kuang Y, Fang B, Gong H, Lu S, Mou
Z, Sun H, Dong Y, Lu J, Zhang W, et al: The effect of
overexpression of Dlx2 on the migration, proliferation and
osteogenic differentiation of cranial neural crest stem cells.
Biomaterials. 34:1898–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Li J, Wang Y, Lei L, Jiang C, An
S, Zhan Y, Cheng Q, Zhao Z, Wang J and Jiang L: Effects of hypoxia
on osteogenic differentiation of rat bone marrow mesenchymal stem
cells. Mol Cell Biochem. 362:25–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Yang Y, Yang P, Gu Y, Zhao Z, Tan L,
Zhao L, Tang T and Li Y: The osteogenic differentiation of PDLSCs
is mediated through MEK/ERK and p38 MAPK signalling under hypoxia.
Arch Oral Biol. 58:1357–1368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang P, Wu Y, Dai Q, Fang B and Jiang L:
p38-MAPK signaling pathway is not involved in osteogenic
differentiation during early response of mesenchymal stem cells to
continuous mechanical strain. Mol Cell Biochem. 378:19–28. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi C, Liu D, Fong CC, Zhang J and Yang M:
Gold nanoparticles promote osteogenic differentiation of
mesenchymal stem cells through p38 MAPK pathway. ACS Nano.
4:6439–6448. 2010. View Article : Google Scholar : PubMed/NCBI
|