Introduction
Hypertension is a major health concern, since it can
increase the risk for acute vascular events, including myocardial
and cerebral infarction. According to the Chinese Hypertension
League 2009 report, the prevalence of hypertension in the Chinese
population is high, affecting one out of five individuals, i.e.
>2 billion (1). In the kidney,
dopamine receptors serve a pivotal role in blood pressure
regulation. Dysfunctional dopamine receptors may increase the
severity of essential hypertension. Dopamine is synthesized by the
dehydration of the amino acid tyrosine to L-dihydroxyphenylalanine
(L-DOPA), which is catalyzed by tyrosine hydroxylase, and the
subsequent decarboxylation of L-DOPA, which is catalyzed by
aromatic L-amino acid decarboxylase. The kidney does not express
tyrosine hydroxylase; however, it can absorb circulating L-DOPA
(2). Renal epithelial cells absorb
L-DOPA through the Na+-independent and pH-sensitive
L-type amino acid transporter type 2 (LAT2). L-DOPA uptake through
LAT2 increases with the elevation of blood pressure (3). The structure of LAT2 consists of two
polypeptides, the light-chain subunit solute carrier family 7,
member 8 (SLC7A8) and the heavy-chain subunit solute carrier family
3, member 2 (4). SLC7A8, which is
responsible for the uptake of L-DOPA, is a non-glycosylated
12-transmembrane-spanning membrane protein, and a member of the SLC
superfamily of amino acid transporters (5). It has previously been reported that
gene expression differs between spontaneously hypertensive rats
(SHR) and their normotensive controls, Wistar Kyoto (WKY) rats,
with 19 genes being markedly upregulated in SHR (6). Previous studies have demonstrated
that the production and secretion of dopamine is significantly
higher in SHR compared with in WKY rats (7,8).
Renal dopamine synthesis has been reported to increase in SHR,
possibly as a result of the deficiency in dopamine-mediated
natriuresis, which has previously been demonstrated in aged Fischer
344 rats (9).
The present study evaluated the expression of L-DOPA
transporters in SHR and WKY rats, and investigated the mechanism
underlying the increased dopamine synthesis and uptake in renal
epithelial cells of the proximal tubule.
Materials and methods
Animals
SHR and WKY rats were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). All rats were
allowed to acclimate for 1 week prior to experimentation. Male
13-week old rats (weight, 370–390 g) were chosen for the present
experiments (n=5 rats/group). All rats were housed in a specific
pathogen-free laboratory animal room under the following
conditions: Temperature, 18–29°C; relative humidity, 40–70%; 12 h
light/dark cycle. All rats received standard rat chow and water
ad libitum. All experimental procedures were approved by the
Animal Research Committee of Wenzhou Medical University (Wenzhou,
China).
Cell cultures
The rat renal epithelial cell line NRK-52E was
purchased from the Cell Resource Center of the Shanghai Institutes
for Biological Sciences of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 5% fetal bovine serum (Thermo Fisher Scientific,
Inc.), containing 1% penicillin and streptomycin. Cells were
maintained in an incubator at 37°C in a 5% CO2
atmosphere.
Blood pressure measurements
Pentobarbital (30 mg/kg weight) was used to
anesthetize the rats. Systolic blood pressure was measured in the
arteria caudalis using the MedLab Version 5.0 Bio-signal
collect-processing system (Nanjing MedEase Science and Technology
Co., Ltd., Nanjing, China). Measurements were repeated three times
for each rat and the average blood pressure was noted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
All rats were sacrificed by cervical dislocation,
and kidney tissues were collected and maintained in liquid
nitrogen. The kidney samples were ground, and total RNA was
extracted from a 1:1 mix of kidneys and second-order mesenteric
artery samples, as well as NRK-52E cells using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Total RNA (1 µg) was reverse transcribed
into cDNA using Revert Aid™ First Strand cDNA Synthesis kit (cat.
no. K1622; Thermo Fisher Scientific, Inc.) at 42°C for 60 min; cDNA
was stored at −70°C. qPCR analysis was performed on cDNA using SYBR
Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) in a 20 µl reaction volume consisting of 10 µl SYBR Green
Mix, 1 µl forward/reverse primers and 0.5–1 µg template; volume was
made up to 20 µl with water. The following primers were used for
RT-qPCR of targeted gene expression: SLC7A8, forward
5′-CTCCACTGGAAAAAAGGTAGCA-3′, reverse 5′-TGGTGAATGAAGCCACATCTG-3′;
and GAPDH, forward, 5′-TCCTGCACCACCAACTGCTTAG-3′ and reverse,
5′-AGTGGCAGTGATGGCATGGACT-3′. The amplification conditions were as
follows: Initial cycle at 50°C for 2 min and 95°C for 2 min,
followed by 40 cycles of denaturation at 95°C for 15 sec, and 40
cycles of annealing and extension at 59°C for 1 min. RT-qPCR was
performed using an ABI Prism 7900 Sequence Detector (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The PCR products were separated by 1.2%
agarose gel electrophoresis (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), stained with GoldView (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and observed using the Image
Master VDS-CL gel-imaging system (Amersham; GE Healthcare Life
Sciences, Tokyo, Japan). Relative expression levels of SLC7A8 mRNA
were calculated using the 2−∆∆Cq method (10); the results were normalized to GAPDH
mRNA expression.
Construction of rat SLC7A8 recombinant
adenoviral vector
cDNA from rats was amplified by PCR using the primer
specific for rat SLC7A8 (forward 5′-GCTGTCACTTTTAGAGCCTAGGAG-3′ and
reverse 5′-CAGGGATACAGGGCAGAAAGGATGA-3′). PCR was performed using
PrimeSTAR®HS DNA Polymerase (Takara Biotechnology Co.,
Ltd., Dalian, China) for SLC7A8. Amplification conditions were as
follows: Hot start for 5 min at 94°C, 35 cycles of denaturation
(98°C for 10 sec), annealing (57.8°C for 15 sec) and extension
(72°C for 2 min), and a final extension step at 72°C for 5 min. The
PCR products were gel purified using a PCR Clean-Up kit
(Sigma-Aldrich; Merck KGaA), according to the manufacturer's
protocol, and cloned into pGEM-T Easy vector using T4 DNA ligase
(Promega Corporation, Madison, WI, USA). The connection product
(pGEM-T Easy vector containing the PCR product) was subsequently
transformed into DH5α Escherichia coli cells (BioVector
Science Lab, Inc., Beijing, China) according to the manufacturer's
protocol, named T-SLC7A8. NotI restriction enzyme was used
to digest the adenovirus shuttle plasmid pShuttle-CMV-green
fluorescent protein (GFP) (Shanghai GenePharma Co., Ltd., Shanghai,
China) and T-SLC7A8, the SLC7A8 coding sequence, and the vector was
linked with T4 DNA ligase, resulting in formation of
pShuttle-SLC7A8. Linearized pShuttle-SLC7A8 was dephosphorylated
using calf intestinal alkaline phosphatase [New England Biolabs
(Beijing) Ltd., Beijing, China] and was transfected into Bj5183
competent cells (Shanghai Weidi Biotechnology Co., Ltd., Shanghai,
China), which contain the skeleton plasmid pAdEasy-1 and a
homologous recombination enzyme. Following recombination, the
correct plasmid, pAdxsi-GFP-SLC7A8, was identified and obtained.
pAdxsi-GFP-SLC7A8 was transfected into HEK293 cells (Cell Resource
Center of the Shanghai Institutes for Biological Sciences of the
Chinese Academy of Sciences, Shanghai, China) using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) for 24 h. GFP expression was
used to confirm successful transfection. After 7–10 days, cells
were collected and underwent freeze-thaw cycles at −80 and 37°C.
The viral supernatant was obtained by centrifugation (12,000 × g
for 5 min, 4°C) and viral titer was determined for successful
transduction.
Adenoviral vector transduction
(pAdxsi-GFP-SLC7A8)
The recombinant adenoviral vector
(pAdxsi-GFP-SLC7A8) was transduced into NRK-52E cells directly
using various viral titers without any transfection media, between
106 PFU/ml and 108 PFU/ml. Blank control (BC)
cells were untransduced; negative control (NC) cells were
transduced with the empty pAdxsi adenoviral vector. A total of 48 h
post-transduction, cells were digested by trypsin (0.05%) for 5 min
at room temperature. Subsequently, cells were scraped, centrifuged
(1,000 × g for 5 min at 4°C) and resuspended in PBS; transduction
efficiency was confirmed using flow cytometry (BD FACSCalibur; BD
Biosciences, San Jose, CA, USA). RNA and protein were then
extracted from the cells.
Western blot analysis
A total of 48 h post-transduction, total protein was
isolated from NRK-52E cells. Cells were homogenized in a
radioimmunoprecipitation assay lysis buffer with
phenylmethanesulfonyl fluoride; the buffer contained 50 mM Tris,
150 mM NaCl, 0.1 % sodium dodecyl sulfate and protease inhibitor.
Total protein concentration was determined using a bicinchoninic
acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Equal amounts of
extracted protein samples (50–80 µg) were separated by 10% SDS-PAGE
and transferred onto a polyvinylidene difluoride membrane. The
membrane was blocked in 5% skim milk and 0.5% Tween-20 for 2.5 h at
room temperature. Subsequently, blots were incubated with
anti-SLC7A8 primary antibody (1:1,000; cat. no. ARP43930_T100;
AVIVA Systems Biology, Co., San Diego, CA, USA) at 4°C overnight
and secondary antibody (1:5,000; cat. no. ab6721; Abcam, Cambridge,
UK) at 37°C for 2 h. GAPDH was used as a loading control (1:1,0000;
cat. no. ab181602; Abcam). The bands were visualized using the
Image Master VDS-CL gel-imaging system (Amersham; GE Healthcare
Life Sciences) and analyzed by Quantity One software version 4.6.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
L-DOPA uptake
After aspirating the culture medium, cell monolayers
were pre-incubated for 30 min in Hanks' medium (Thermo Fisher
Scientific, Inc.) at 37°C. In saturation experiments, cells were
incubated with 300 µl L-DOPA (1 µg/ml; (Toronto Research Chemicals,
Inc., North York, ON, Canada) for 6 min with increasing
concentrations of SLC7A8 vector (100, 300 and 1,000 ng/ml) in 1 ml
PBS. In time-course studies, cells were incubated with 1,000 ng/µl
SLC7A8 vector and 300 µl L-DOPA (1 µg/ml) in 1 ml PBS for 3, 6, 12,
30 and 60 min. The experiments were terminated by the rapid removal
of the uptake solution, followed by a rapid wash with cold PBS. The
cells were collected and permeabilized by repeated freeze-thaw
cycles. L-DOPA uptake was analyzed using reverse-phase high
performance liquid chromatography (HPLC; Agilent Technologies,
Santa Clara, CA, USA). Chromatographic conditions: Sample volume,
10 µl; Column YWG C18 4.6×250 mm, 10 µm C18; the mobile phase
consisted of 0.05 mol/l citric acid (Merck KGaA, Darmstadt,
Germany), 0.05 mol/l sodium acetate (Shanghai Shenggong Biology
Engineering Technology Service, Ltd., Shanghai, China), 5 mmol/l
ethylamine (Shanghai Shenggong Biology Engineering Technology
Service, Ltd.), 0.2 mmol/l EDTA (Merck KGaA), pH 3.6; flow rate,
0.5 ml/min; detector working potential, 0.7V; sensitivity, 5nA. The
standard curve was constructed using L-DOPA standards (Toronto
Research Chemicals, Inc.).
Statistical analysis
The statistical significance of the difference
between groups was assessed by one-way analysis of variance,
followed by a post hoc Cochran's Q-test, or a Kruskal-Wallis test
for non-parametric data, followed by a post hoc Nemenyi test for
multiple comparisons. Data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference. Analyses were performed using SPSS software
version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Gene expression arrays in SHR and WKY
rats
A previous study measured gene expression in SHR and
WKY rats by microarray analysis of 10,000 genes (6). A total of 38 genes of interest were
detected, including those coding for signal transducers, cell cycle
mediators, metabolic enzymes and transcription factors. In the
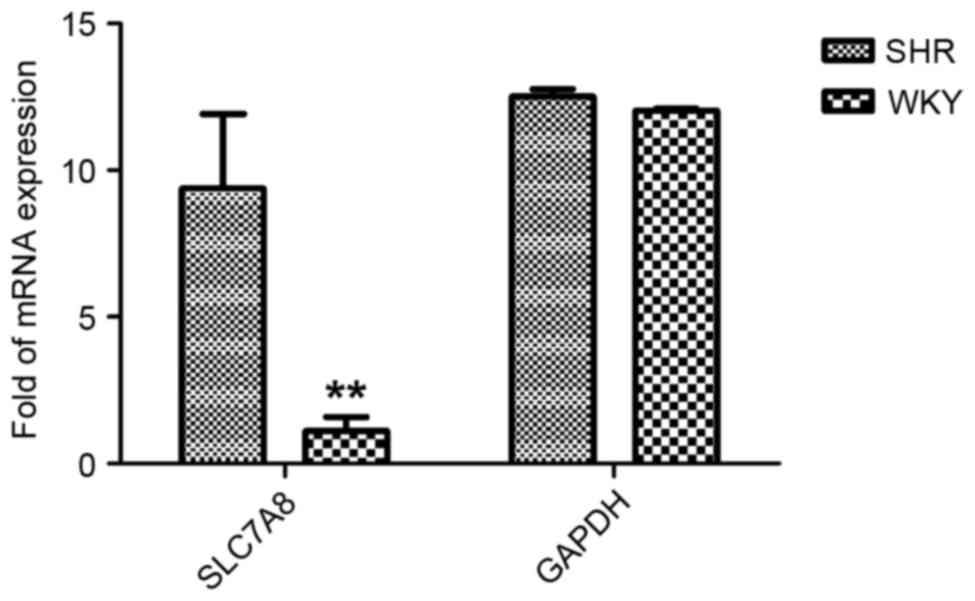
present study, as verified by RT-qPCR, the expression of SLC7A8 in
SHR kidneys and second-order mesenteric arteries was significantly
increased (P<0.01) compared with in the WKY group (Fig. 1).
SLC7A8 expression in NRK-52E
cells
A 1,819 bp cDNA fragment was isolated by RT-qPCR and
inserted into a pGEM-T Easy vector. The full SLC7A8 gene was
successfully cloned into a pShuttle-CMV-GFP plasmid and packaged
into a pAdxsi adenoviral vector. The successful insertion of the
target sequence was confirmed by enzyme digestion analysis and
sequencing (Fig. 2). The
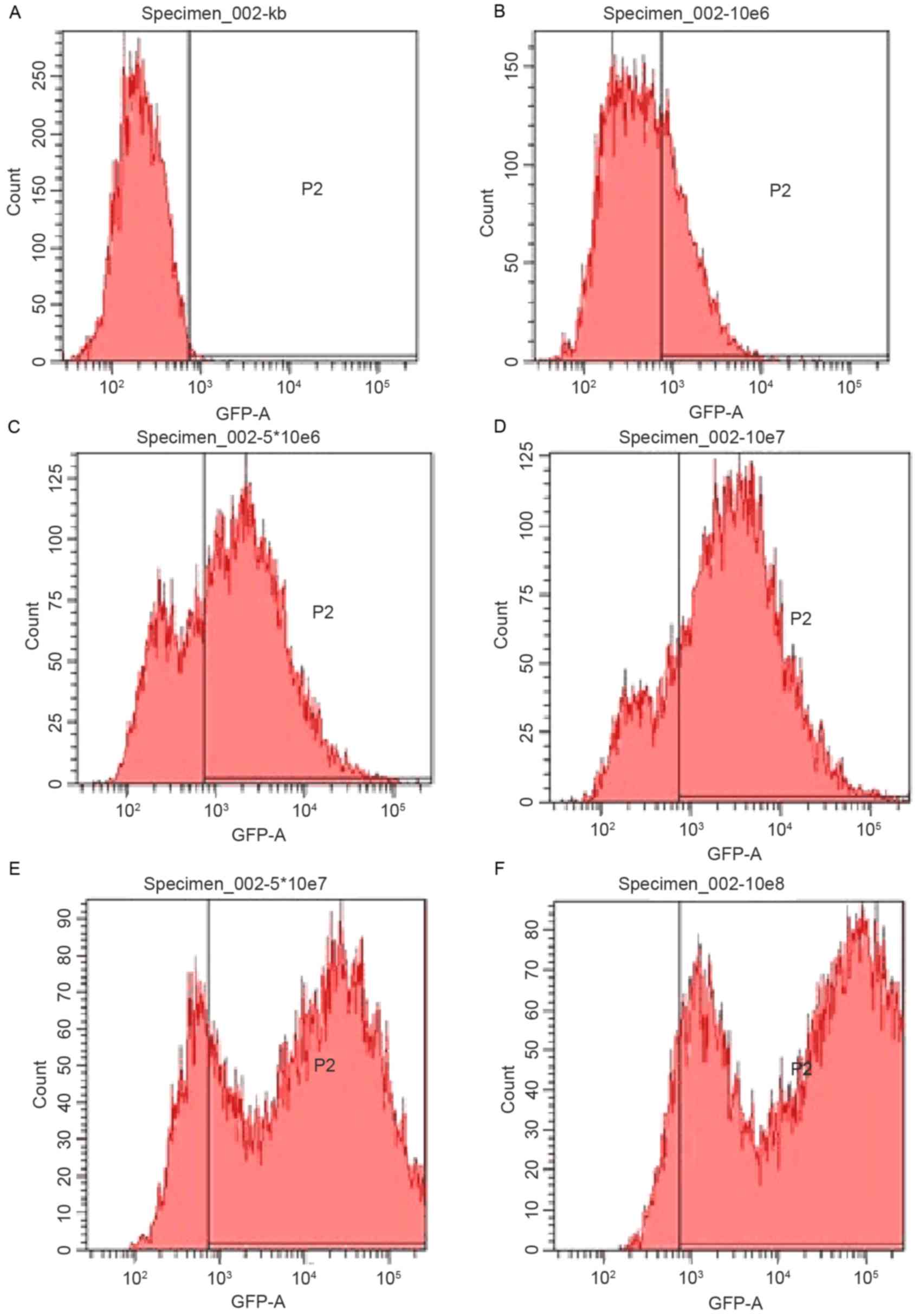
recombinant adenoviral vector (pAdxsi-GFP-SLC7A8) was transduced
into NRK-52E cells. Transduction efficiency was confirmed using
flow cytometry (Fig. 3).
Considering the cytotoxicity and transduction efficiency, a viral
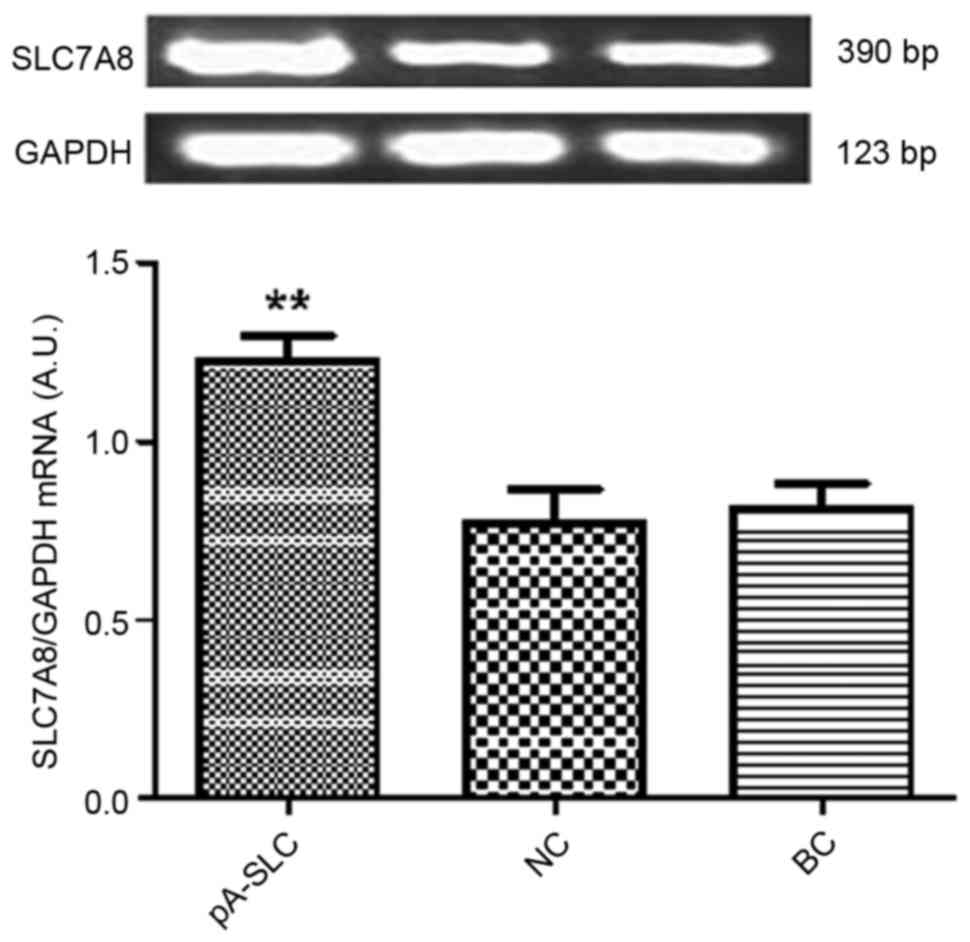
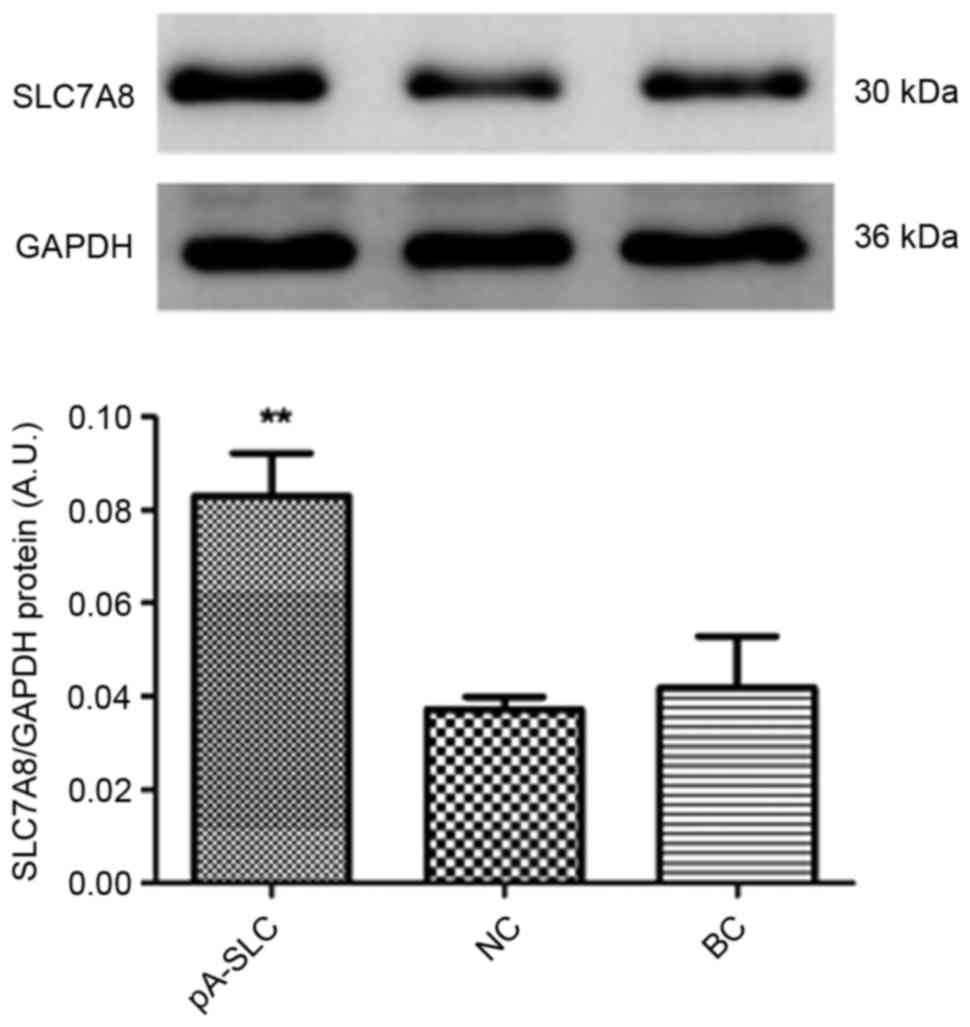
titer of 107 PFU/ml was chosen. The mRNA and protein
expression levels of SLC7A8 were significantly increased in
pAdxsi-GFP-SLC7A8-transduced NRK-52E cells (P<0.01) compared
with in the NC and BC cells (Figs.
4 and 5).
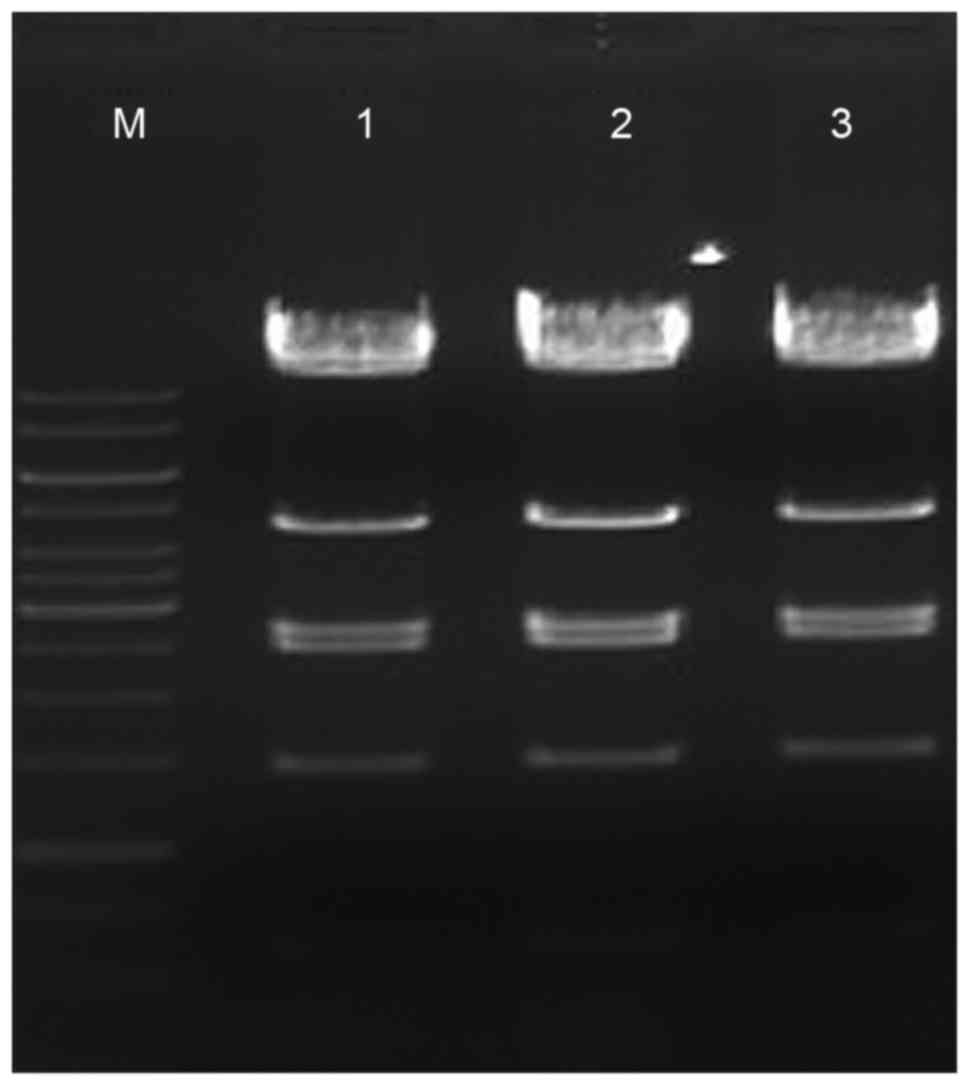 | Figure 2.pAdxsi-green fluorescent
protein-solute carrier family 7 member 8 sequencing results. Marker
(1 kb DNA ladder); from top to bottom: 10 kb, 8 kb, 6 kb, 5 kb, 4
kb, 3.5 kb, 3 kb, 2.5 kb, 2 kb, 1.5 kb, 1 kb, 750 bp, 500 bp, 250
bp; lanes 1–3, three positive clones; from top to bottom: 14.5 kb,
11.7 kb, 4.4 kb, 2.66 kb, 2.47 kb, 1.45 kb, 0.6 kb (7 specific
bands following enzyme digestion). |
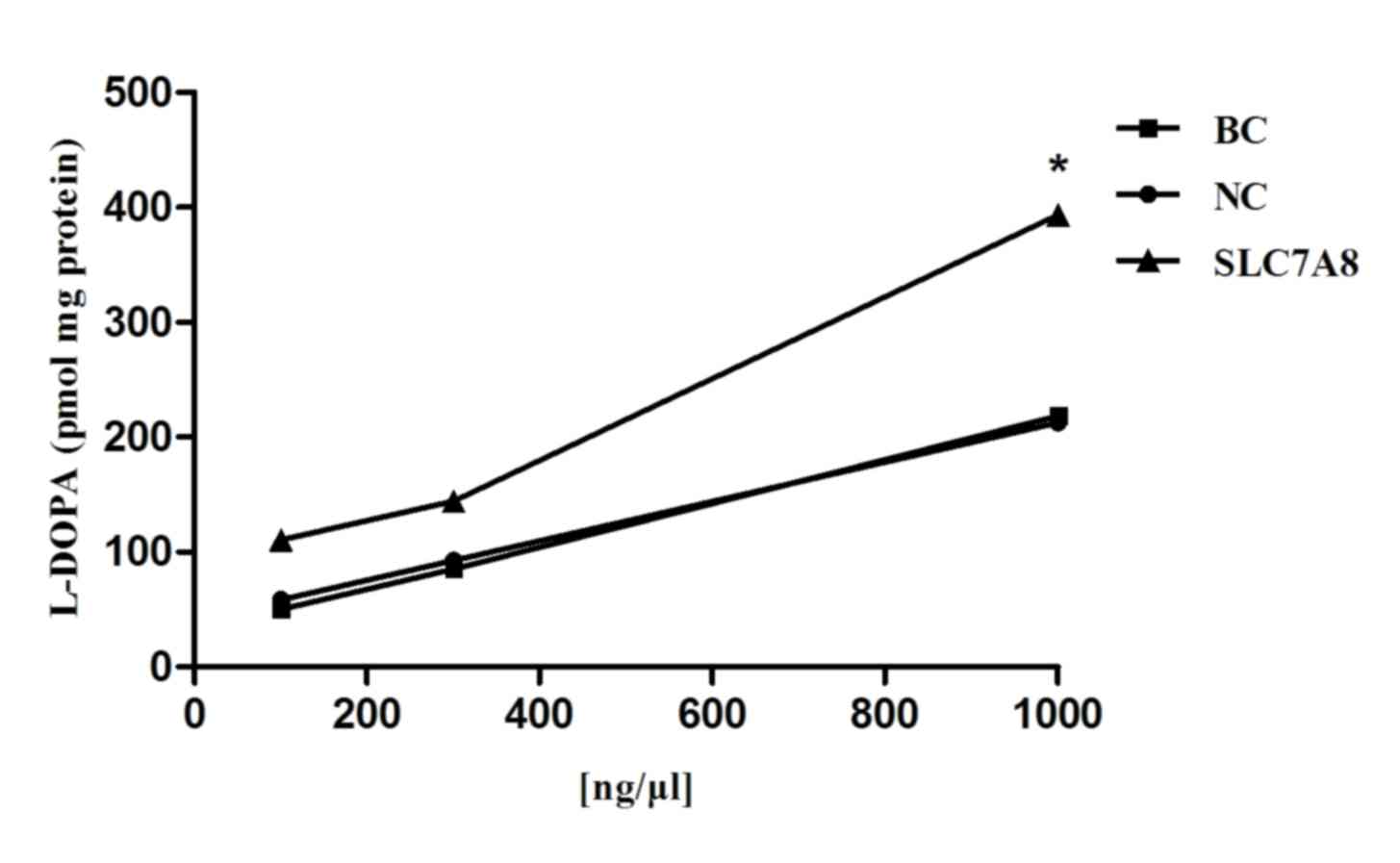
L-DOPA uptake in transduced NRK-52E
cells
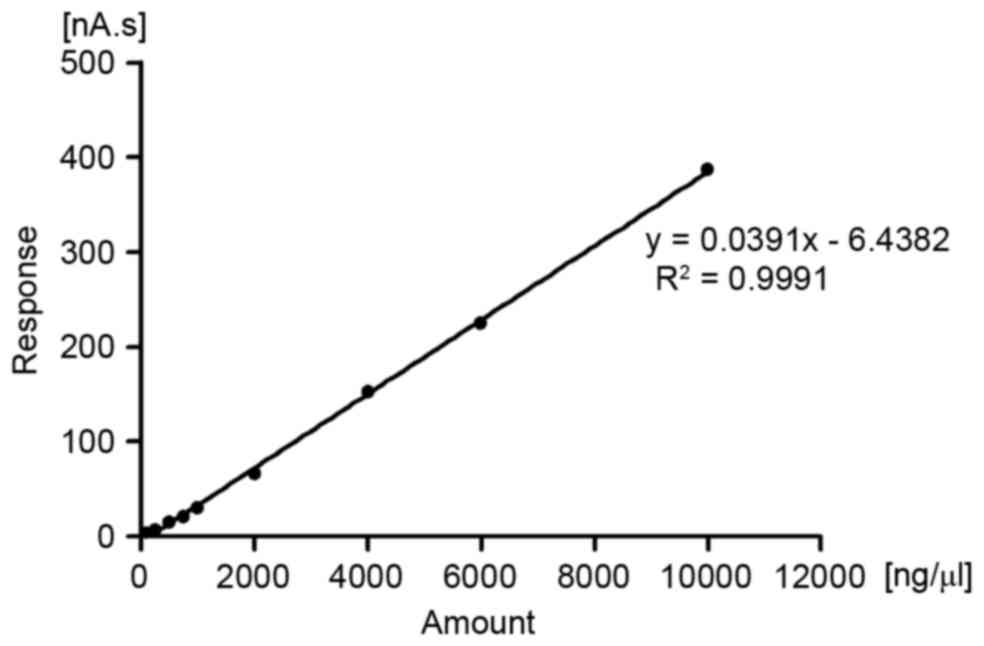
When analyzing samples under the same conditions and
using the standard curve (Fig. 6),
L-DOPA was absorbed by NRK-52E cells co-incubated with L-DOPA and
various doses of SLC7A8 for 6 min. The linear range is 100–10,000
ng/ml. The uptake of L-DOPA in NRK-52E cells transduced with the
SLC7A8 gene progressively increased with the dose of SLC7A8,
whereas no increase in uptake was apparent in the blank or negative
control cells (Fig. 7). The
results of the time-course studies revealed that the uptake of
L-DOPA in NRK-52E cells overexpressing the SLC7A8 gene increased
with incubation time, whereas L-DOPA uptake did not appear to be
increased in the blank or negative control cells (Fig. 8).
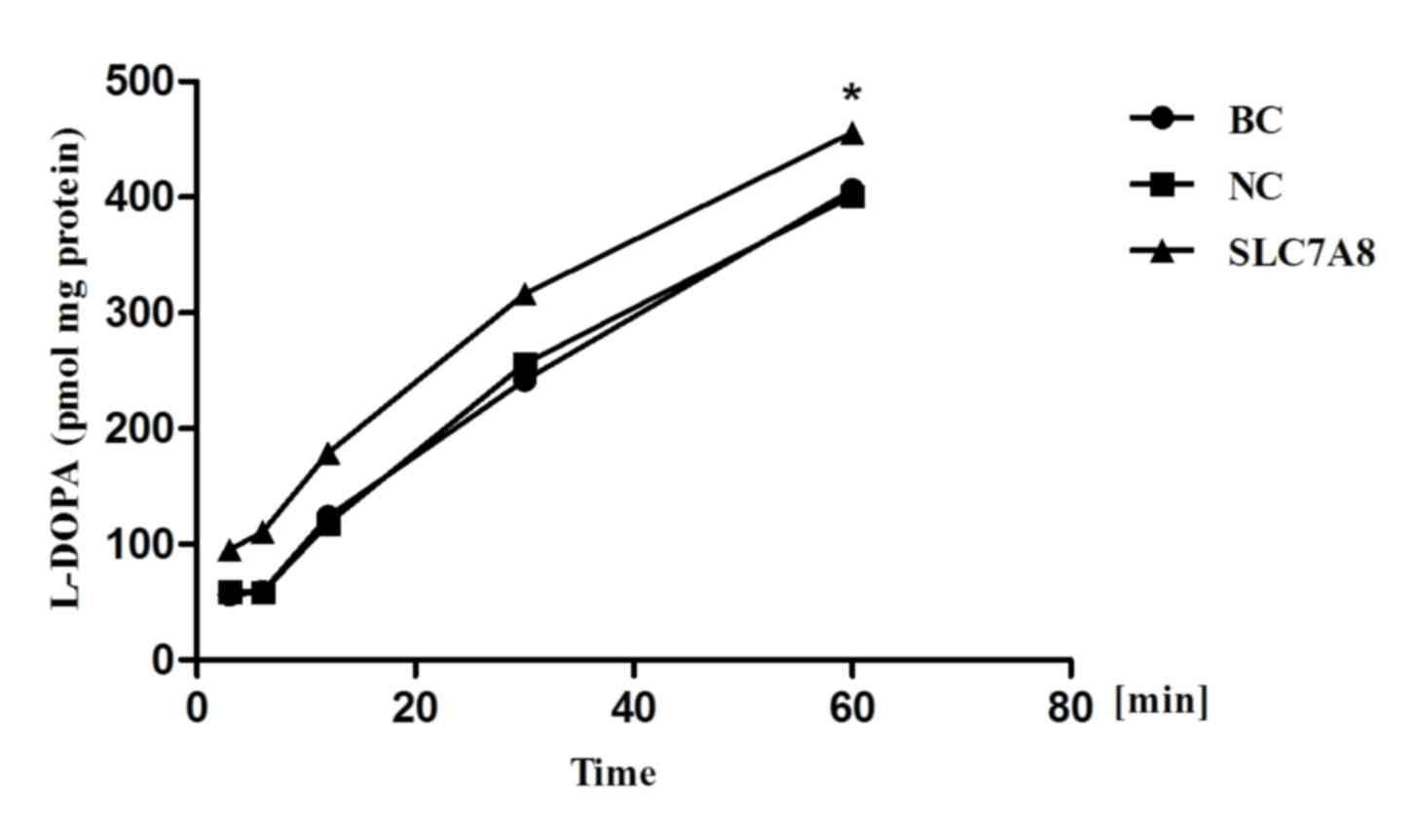 | Figure 8.Time-dependent uptake of L-DOPA. Cells
were incubated with 1,000 ng/µl SLC7A8 and 300 µl L-DOPA (1 µg/ml)
for 3, 6, 12, 30 and 60 min. Uptake was measured via high
performance liquid chromatography. Compared with BC or NC groups,
transduction with the recombinant adenoviral vector (pAdxsi-green
fluorescent protein-SLC7A8) resuted in significantly increased
L-DOPA uptake. *P<0.05 vs. NC and BC cells L-DOPA,
L-3,4-dihydroxyphenylalanine; BC, blank control; NC, negative
control; SLC7A8, solute carrier family 7 member 8. |
Discussion
Essential hypertension is a chronic disease in
humans. It has previously been reported that gene expression
differs between SHR and WKY rats, whereas 19 genes have been
identified as being markedly upregulated in SHR (6). Therefore, it may be hypothesized that
these genes are associated with essential hypertension. The
upregulated genes have been reported to participate in several
cellular processes, including Ca2+ homeostasis (11,12),
hydrogen peroxide metabolism (13), signal transduction, cell cycle
control, cellular proliferation and migration. They may also be
involved in pathophysiological processes, such as tissue fibrosis
(14,15). In the present study, the SLC7A8
gene, coding for the LAT2 dopamine uptake transporter, was revealed
to be significantly upregulated in kidneys and second-order
mesenteric arteries of SHR rats. The SLC7 gene family comprises two
subfamilies, the cationic amino acid transporters, and the
glycoprotein-associated amino acid transporters, also called light
chain (16–18). SLC7A8 is a member of the SLC7
family and belongs to the light chain subfamily. SLC7A8 encodes two
proteins, containing 531 and 535 amino acids, which are 92%
identical.
A previous study by Pinho et al evaluated the
uptake of L-DOPA in isolated renal proximal tubules of SHR and WKY
rats, and the expression of LAT1 and LAT2 in the renal cortex and
intestinal mucosa was also investigated (19). Expression of LAT2 in the SHR renal
cortex was increased compared with in WKY tissue, as detected by
northern blotting. Tubular uptake of L-DOPA via LAT2 was revealed
to be the rate-limiting step of renal dopamine synthesis, whereas
uptake was increased in SHR compared with in WKY rats (19). It has previously been hypothesized
that the overexpression of LAT2 in SHR renal tissue may contribute
to the enhanced renal uptake of L-DOPA, which is organ-specific and
precedes the onset of hypertension (20). The results of the present study
indicated that overexpression of the SLC7A8 gene can increase the
uptake of L-DOPA in renal cells. L-DOPA uptake progressively
increased with increasing dose of SLC7A8 and incubation time. The
present results are consistent with previous studies, as they
demonstrated that by increasing the expression of SLC7A8 in tubular
epithelial cells a corresponding increase in L-DOPA uptake could be
achieved. L-type amino acid transporters are responsible for
transporting neutral amino acids with high affinity (Km
in the µM range), independent of Na+ concentration in
the extracellular medium, whereas they also exhibit a particularly
high capacity for trans-stimulation (21). NRK-52E cells can absorb L-DOPA
through the pH-dependent LAT2. This is further supported by the
present results, demonstrating that L-DOPA uptake was markedly
enhanced in NRK-52E cells transduced with the an adenoviral vector
containing the SLC7A8 gene in order to upregulate the expression of
the transporter. Therefore, it may be hypothesized that SLC7A8
serves an important role in the transportation and uptake of L-DOPA
by renal cells.
The renal proximal tubule is the main site of L-DOPA
decarboxylation and dopamine synthesis, indicating that the
activity of LAT2 may limit the synthesis of renal dopamine
(22). The
Na+-independent transport systems of L-DOPA include
system L (LAT1 and LAT2) and system b0,+ (23). LAT1 is primarily localized in brain
capillary endothelial cells (24).
The transporter system b0,+ is a
Na+-independent transporter for neutral and basic amino
acids that also recognizes the di-amino acid cysteine (25). LAT2 is a Na+-independent
transporter with a broad specificity for small and large neutral
amino acids that is stimulated by acid pH. The expression of LAT1
and LAT2 in SHR cells has been reported to differ significantly
compared with in cells from WKY rats (20). LAT2 gene silencing markedly reduced
the inward and outward transfer of [14C]-L-DOPA,
suggesting a major role of LAT2 in renal L-DOPA handling (26).
Following its synthesis in renal epithelial cells,
dopamine can exert natriuretic and diuretic effects via activation
of D1-like receptors located at various regions in the
nephron. In proximal tubules, dopamine can increase Na+
excretion via inhibiting the main Na+ transport
mechanisms at the apical membranes of the tubular cells, i.e. the
Na+/K+/ATPase and the
Na+/H+ exchanger (27). In SHR, dopamine D1-like
receptor-mediated natriuretic and diuretic responses are decreased
compared with in normotensive WKY rats (28).
Two limitations exist in the present study. Firstly,
although the present results demonstrated that overexpression of
the SLC7A8 gene can promote the uptake of L-DOPA in renal tubular
epithelial cells, it remains to be elucidated whether increased
SLC7A8 expression can promote dopamine synthesis. In addition, the
lack of in vivo evidence supporting that SLC7A8
overexpression can promote the renal uptake of L-DOPA and
subsequent blood pressure elevation further limits the impact of
the present study. Further studies are required, including in
vivo experiments, to elucidate the role of SLC7A8 in renal
dopamine synthesis and its implication in blood pressure
regulation.
In conclusion, the results of the present study
indicated that SLC7A8 may serve a role in the onset and progression
of essential hypertension. Further studies, investigating the
expression of SLC7A8 in vivo, and its association with the
dopaminergic system, are required to elucidate its role in the
regulation of blood pressure under various physiological and
pathophysiological conditions, including essential
hypertension.
Acknowledgements
The present study was supported by the Ministry of
Health of the People's Republic of China Science Foundation (grant
no. WKJ-ZJ-1420), and the Hangzhou Science and Technology
Development Project (grant no. 20150633B05).
References
|
1
|
Sun H, Yang ZQ, Liu SY, Yu L, Huang K, Lin
KQ, Chu JY and Huang XQ: Correlation between natriuretic peptide
receptor C (NPR3) gene polymorphisms and hypertension in the Dai
people of China. Genet Mol Res. 14:8786–8795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinto V, Pinho MJ and Soares-da-Silva P:
Renal amino acid transport systems and essential hypertension.
FASEB J. 27:2927–2938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moura E, Silva E, Serrão MP, Afonso J,
Kozmus CE and Vieira-Coelho MA: α2C-Adrenoceptors modulate L-DOPA
uptake in opossum kidney cells and in the mouse kidney. Am J
Physiol Renal Physiol. 303:F928–F938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Camargo SM, Vuille-dit-Bille RN, Mariotta
L, Ramadan T, Huggel K, Singer D, Götze O and Verrey F: The
molecular mechanism of intestinal levodopa absorption and its
possible implications for the treatment of Parkinson's disease. J
Pharmacol Exp Ther. 351:114–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
del Amo EM, Urtti A and Yliperttula M:
Pharmacokinetic role of L-type amino acid transporters LAT1 and
LAT2. Eur J Pharm Sci. 35:161–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Wang B, Yang D, Shi X, Hong J,
Wang S, Dai X, Zhou X and Geng YJ: Reduced expression of FXYD
domain containing ion transport regulator 5 in association with
hypertension. Int J Mol Med. 29:231–238. 2012.PubMed/NCBI
|
|
7
|
Chen K, Deng K, Wang X, Wang Z, Zheng S,
Ren H, He D, Han Y, Asico LD, Jose PA and Zeng C: Activation of D4
dopamine receptor decreases angiotensin II type 1 receptor
expression in rat renal proximal tubule cells. Hypertension.
65:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Igreja B, Pires NM, Bonifácio MJ, Loureiro
AI, Fernandes-Lopes C, Wright LC and Soares-da-Silva P: Blood
pressure-decreasing effect of etamicastat alone and in combination
with antihypertensive drugs in the spontaneously hypertensive rat.
Hypertens Res. 38:30–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vieira-Coelho MA, Serrão P, Hussain T,
Lokhandwala MF and Soares-da-Silva P: Salt intake and intestinal
dopaminergic activity in adult and old Fischer 344 rats. Life Sci.
69:1957–1968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan TT, Li XF, Sun YM, Li YB and Su Y:
Role of the calpain on the development of diabetes mellitus and its
chronic complications. Biomed Pharmacother. 74:187–190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siklos M, BenAissa M and Thatcher GR:
Cysteine proteases as therapeutic targets: Does selectivity matter?
A systematic review of calpain and cathepsin inhibitors. Acta Pharm
Sin B. 5:506–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawada N, Kristensen DB, Asahina K,
Nakatani K, Minamiyama Y, Seki S and Yoshizato K: Characterization
of a stellate cell activation-associated protein (STAP) with
peroxidase activity found in rat hepatic stellate cells. J Biol
Chem. 276:25318–25323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ostendorf T, Boor P, van Roeyen CR and
Floege J: Platelet-derived growth factors (PDGFs) in glomerular and
tubulointerstitial fibrosis. Kidney Int Suppl (2011). 4:65–69.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leask A: Getting to the heart of the
matter: New insights into cardiac fibrosis. Circ Res.
116:1269–1276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fotiadis D, Kanai Y and Palacin M: The
SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med.
34:139–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schweikhard ES and Ziegler CM: Amino acid
secondary transporters: Toward a common transport mechanism. Curr
Top Membr. 70:1–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Closs EI, Boissel JP, Habermeier A and
Rotmann A: Structure and function of cationic amino acid
transporters (CATs). J Membr Biol. 213:67–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pinho MJ, Gomes P, Serrão MP, Bonifácio MJ
and Soares-da-Silva P: Organ-specific overexpression of renal LAT2
and enhanced tubular L-DOPA uptake precede the onset of
hypertension. Hypertension. 42:613–618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinho MJ, Serrão MP, Gomes P, Hopfer U,
Jose PA and Soares-da-Silva P: Over-expression of renal LAT1 and
LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal
tubular cells. Kidney Int. 66:216–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soares-da-Silva P and Serrão MP: High- and
low-affinity transport of L-leucine and L-DOPA by the hetero amino
acid exchangers LAT1 and LAT2 in LLC-PK1 renal cells. Am J Physiol
Renal Physiol. 287:F252–F261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sizova D, Velazquez H, Sampaio-Maia B,
Quelhas-Santos J, Pestana M and Desir GV: Renalase regulates renal
dopamine and phosphate metabolism. Am J Physiol Renal Physiol.
305:F839–F844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishiia H, Sasaki Y, Goshima Y, Kanai Y,
Endou H, Ayusawa D, Ono H, Miyamae T and Misu Y: Involvement of
rBAT in Na(+)-dependent and -independent transport of the
neurotransmitter candidate L-DOPA in Xenopus laevis oocytes
injected with rabbit small intestinal epithelium poly A(+) RNA.
Biochim Biophys Acta. 1466:61–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kageyama T, Nakamura M, Matsuo A, Yamasaki
Y, Takakura Y, Hashida M, Kanai Y, Naito M, Tsuruo T, Minato N and
Shimohama S: The 4F2hc/LAT1 complex transports L-DOPA across the
blood-brain barrier. Brain Res. 879:115–121. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gomes P and Soares-da-Silva P:
Na+-independent transporters, LAT-2 and b0,+,exchange L-DOPA with
neutral and basic amino acids in two clonal renal cell lines. J
Membr Biol. 186:63–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soares-Da-Silva P, Serrão MP, Pinho MJ and
Bonifácio MJ: Cloning and gene silencing of LAT2, the
L-3,4-dihydroxyphenylalanine (L-DOPA) transporter, in pig renal
LLC-PK1 epithelial cells. FASEB J. 18:1489–1498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pinho MJ, Serrão MP and Soares-da-Silva P:
High-salt intake and the renal expression of amino acid
transporters in spontaneously hypertensive rats. Am J Physiol Renal
Physiol. 292:F1452–F1463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Asico LD, Zheng S, Villar VA, He
D, Zhou L, Zeng C and Jose PA: Gastrin and D1 dopamine receptor
interact to induce natriuresis and diuresis. Hypertension.
62:927–933. 2013. View Article : Google Scholar : PubMed/NCBI
|