Introduction
Micro (mi)RNAs are a class of single-stranded
regulatory RNAs composed of 20–24 non-coding nucleotides that
inhibit gene expression at the post-transcriptional level and
regulate cell function (1,2); ~30% of mRNAs are regulated by miRNAs.
Individual miRNAs are predicted to target dozens to hundreds of
genes simultaneously (3,4), and a single mRNA may contain multiple
miRNA binding sites often targeted by separate miRNAs, which may
increase regulation (5–7). miRNAs regulate gene expression by
underlying mechanisms that are primarily dependent on the degree of
miRNA sequence match to its cognate binding site within the target
mRNA (8). miRNAs serve important
roles in the regulation of numerous cellular and biological
processes, including growth, development, differentiation,
proliferation and apoptosis (9).
Aberrant expression of miRNA-21 has been observed in a number of
physiological and pathological processes; for example,
downregulation of endogenous miRNA-21 in glioblastoma may initiate
the caspase signaling pathway and increase apoptotic cell death
(10), and upregulation of
miRNA-21 in the cortex and hippocampus may promote repair following
traumatic brain injury (11,12).
miRNA-21 contributes to pathophysiological processes directly and
indirectly by regulating expression of target genes, including
those involved in cell apoptosis, programmed cell death 4 (PDCD4),
phosphatase and tensin homolog (PTEN), tissue inhibitor of
metalloproteinase 3 and B-cell lymphoma 2 (13). A previous study demonstrated that
miRNA-21 regulates cell apoptosis, proliferation and repair by
regulating gene expression in cancer and following central nervous
system (CNS) injury (14). Spinal
cord injury (SCI) is a serious injury resulting in varying degrees
of paraplegia. While numerous studies on SCI have centered on
post-traumatic neuron cell repair and anti-apoptotic measures
(15), the involvement of miRNA-21
in SCI pathophysiology remains to be investigated. The present
study examined the expression levels of miRNA-21 and its predicted
target genes PDCD4 and PTEN in rats following SCI. Additionally,
the effect of miRNA-21 on neurite outgrowth and regulation of PDCD4
expression levels was determined, and PDCD4 was demonstrated to be
a target gene of miRNA-21. These results may further the
understanding of the function of miRNA-21 in traumatic SCI.
Materials and methods
Spinal cord contusion injury
A total of 150 adult female Sprague-Dawley rats
(weight, 200–250 g) were purchased from the Shanghai Animal Center,
Chinese Academy of Sciences (Shanghai, China) and housed in a
specific pathogen free facility with a constant humidity and
temperature at 12 h:12 h light:dark cycle with free access to food
and water in the animal research facility in accordance with an
approved protocol. The study protocol was approved by the Ethics
committee at the Nanjing Medical University Affiliated Changzhou
No. 2 People's Hospital (Changzhou, China). Rats in the injured
group received SCI and were sacrificed (saline perfusion after 1%
pentobarbital sodium anesthesia) at 4 or 8 h, or 1, 3 or 7 days
following injury. Rats in the sham control group received
laminectomy only. SCI was performed as previously reported
(16). Briefly, rats were
anesthetized with 1% pentobarbital sodium injected
intraperitoneally (50 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and kept warm on a heating pad. Following laminectomy at
the T9 vertebra to expose the spinal cord (circular incision,
~2.5-mm in diameter), rats received moderate SCI at T9 using a New
York University weight-drop device to drop a 10 g rod onto the
dorsal surface of the cord from a height of 12.5 mm. Rats received
fluid infusion (crushed sterile food mixed with sterile distilled
water) and antibiotics (gentamicin, 50 mg/kg) for the first 3 days
post-operation to minimize infection. Bladders were manually
manipulated three times daily until normal voiding reflexes
returned.
Protein extraction
The 10 mm long spinal cord segment containing the
injury epicenter or a 10 mm long tissue at the same vertebral
position from an uninjured spinal cord was dissected. Tissues were
homogenized in 400 µl radioimmunoprecipitation assay (RIPA) buffer
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to
extract total protein. Ultrasonic cellular lysis was performed on
ice for ~5 min following the addition of RIPA buffer. The
homogenate was incubated on ice for 30 min and subsequently
centrifuged twice at 10,000 × g for 10 min at 4°C. Protein
concentrations were determined using a Bicinchoninic Acid Protein
Quantification kit (Sangon Biotech Co., Ltd., Shanghai, China), and
samples were stored at −80°C at a concentration of 5 µg/µl until
further use.
Western blotting
A total of 25 µg extracted proteins per animal
underwent 10% denaturing SDS-PAGE and were subsequently transferred
onto polyvinylidene difluoride membranes (0.08A, 3 h). The
membranes were blocked with 1% bovine serum albumin in TBS
containing Tween-20 (TBST) for 30 min at 30°C. Membranes were
incubated with a 1:1,000 dilution of PDCD4 (9535), PTEN (5384) and
β-actin (12620; all from Cell Signaling Technology, Inc., Danvers,
MA, USA) rabbit monoclonal primary antibodies at 4°C overnight.
Following three washes with TBST (10 min/wash), the membranes were
incubated with a 1:5,000 dilution of a horseradish
peroxidase-conjugated goat anti-rabbit IgG (GW0103S; Beijing ConWin
Biotech, Co., Ltd., Beijing, China) for 1 h at 37°C. Following a
final wash, the membranes were developed using Western
Lightning® Enhanced Chemiluminescence (34080;
Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA), and data
were determined using Image Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Total RNA extraction
Total RNA was extracted from spinal cord tissue
using TRIzol® reagent (Sigma-Aldrich; Merck KGaA)
following the manufacturer's protocol. Briefly, 1 ml TRIzol was
used to homogenize the tissue in a 1.5 ml RNase-free tube, followed
by incubation at room temperature for 5–10 min. Chloroform (0.2 ml;
Sigma-Aldrich; Merck KGaA) was added and the tube was subsequently
vortexed for 15 sec and incubated at room temperature for 2–3 min.
Following this, the tissue was centrifuged at 10,000 × g for 15 min
at 4°C. The aqueous layer was transferred to a new RNase-free tube
and incubated with 0.5 ml isopropyl alcohol at room temperature for
10 min. The mixture was centrifuged at 10,000 × g for 10 min at 4°C
to precipitate RNA, and 75% ethyl alcohol was used to wash the RNA
pellet. Following this, the pellet was air-dried and resuspended in
diethyl pyrocarbonate H2O. RNA purity and concentration
were determined spectrophotometrically at 230, 260 and 280 nm.
Quantitative polymerase chain reaction
(qPCR)
The reverse transcription reaction was performed
using the Prime-Script RT reagent kit (RR420A; Takara Biotechnology
Co., Ltd., Dalian, China) and qPCR was performed using
SYBR® Premix Ex Taq™ kit (Tli RNaseH Plus; Takara
Biotechnology Co., Ltd.) to detect expression levels of miRNA-21 at
4 or 8 h, or 1, 3 or 7 days post-SCI. Stage 1 was 95°C for 30 sec
(1 cycle), stage 2 was 95°C for 5 sec and then 40 cycles at 60°C
for 30 sec. The full-length sequence of miRNA-21 was
5′-TAGCTTATCAGACTGATGTTGA-3′. The sequence of qPCR primer was
5′-GAGGTATTCGCACTGGATACG-3′ (Sangon Biotech Co., Ltd.). The U6 gene
served as an endogenous control (forward, 5′-CTCGCTTCGGCAGCACA-3′;
reverse, 5′-AACGCTTCACGAATTTGCGT-3′). Quantitation threshold (Cq)
values were determined using ABI Prism® 7900HT Fast
Real-Time PCR system software version SDS2.5 (Thermo Fisher
Scientific, Inc.). The relative quantity of miRNA-21 with respect
to U6 was calculated using the 2−ΔΔCq method (17).
Primary spinal cord neuron
culture
Spinal cord neurons of Sprague-Dawley rats at
postnatal days 3–5 were purchased from the Shanghai Animal Center,
Chinese Academy of Sciences (Shanghai, China) and were harvested
and cultured in vitro using a modified method (18). Rats were anesthetized with diethyl
ether (60297; Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) and total spinal cord tissues were isolated. The complete
spinal cord was placed in cold PBS
(−Ca+2/Mg+2); nerve roots and meninges were
dissected using fine forceps, and the cord was sectioned into 0.5
mm pieces. The tissue was transferred to a 50 ml tube and incubated
in 0.1% trypsin-EDTA at 37°C for 30 min in a water bath, with
agitation. The trypsin-EDTA was removed and replaced with 10 ml
Dulbecco's modified Eagle's medium/F-12 (DF12; 11330-057),
containing 10% fetal bovine serum (10099–141), 1% N-2 supplement
(17502–048), 2% B27 (17504–044) and 1% penicillin-streptomycin
(15070–063) all from Thermo Fisher Scientific, Inc. Following this,
the digested cells were gently and repeatedly pipetted using a 5 ml
pipette for 5 min, filtered through a 100 mesh filter, and single
cells in DF12 were plated onto coverslips coated with 200 µg/ml
poly-L-lysine and 3 µg/ml laminin. The DF12 was replaced with
Neurobasal medium (96% Neurobasal-A; 10888-022; Thermo Fisher
Scientific, Inc., 1% N-2 supplement, 2% B27 and 1%
penicillin-streptomycin) after 24 h.
Recombinant adeno-associated virus
(rAAV)-miRNA-21 transfection
To investigate the role of miRNA-21 on neurite
outgrowth and its target genes, primary spinal cord neurons were
transfected with an rAAV hU6-MCS-CMV-enhanced green fluorescent
protein (EGFP; hU6) vector (Sangon Biotech Co., Ltd.) containing
primary miRNA-21 (pri-miRNA-21), miRNA-21 inhibitor (in-miRNA-21)
or miRNA negative control (nc-miRNA) sequences. rAAV plasmid
production and purification were performed by GeneChem Co., Ltd
(Shanghai, China). Following the manufacturer's protocol,
1.0×1010 viral genomes of rAAV-pri-miRNA-21,
rAAV-in-miRNA-21 or rAAV-nc-miRNA, or PBS as a control, were added
to the cultured neurons 1 day post-plating.
Neurite outgrowth assay
Spinal cord neurons were transfected with
rAAV-pri-miRNA-21, rAAV-in-miRNA-21 or rAAV-nc-miRNA at day 1. At
day 5, neurons were fixed in 4% paraformaldehyde and stained with a
rabbit anti-β-III-tubulin antibody (ab11270; 1:1,000; Abcam,
Cambridge, UK). Neurites co-stained with goat anti-rabbit rhodamine
(R415; 1:100; Thermo Fisher Scientific, Inc.) and EGFP (PA1-18401;
Thermo Fisher Scientific, Inc.) were observed under a fluorescent
microscope (Leica Microsystems GmbH, Wetzlar, Germany) with
representative photomicrographs being captured throughout. The
longest neurite length for each of the first 100 neurons
encountered during scanning (regardless of size and number of
neurites) was measured using the Leica QW in software version 3
image analysis program (Leica Microsystems GmbH). Neurite outgrowth
analyses were performed in quadruplicate.
Luciferase reporter assay
Wild-type (wt; 5′-GAGAUGGAAUUUUAUGUAATT-3′) and
mutant (mt; 5′-UUACAUAAAAUUCCAUCUCCA-3′) PDCD4 3′-untranslated
regions (UTRs) were subcloned into the pRL-CMV miRNA expression
vector containing Renilla luciferase (Promega Corporation,
Madison, WI, USA) to generate wt-PDCD4 and mt-PDCD4. HEK293 human
embryonic kidney cells (Cell bank of Chinese Academy of Sciences,
Shanghai, China) were cultured in 24-well plates with Dulbecco's
modified Eagle's medium (11995065; Thermo Fisher Scientific, Inc.)
with 10% FBS for 24 h and subsequently transfected with 200 ng
wt-PDCD4, mt-PDCD4 or 100 ng empty pRL-CMV vector, and 10 µM
synthetic miRNA-21 mimic or miRNA NC (Sangon Biotech Co., Ltd.)
using Lipofectamine 2000™ (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Firefly and
Renilla luciferase activities were measured 48 h
post-transfection using the dual-luciferase reporter assay system.
The Renilla luciferase values were normalized to firefly
luciferase values.
Statistical analysis
Statistical analysis of the experimental data was
conducted using SPSS software version 17.0 (SPSS, Inc., Chicago,
IL, USA). All data are presented as the mean ± standard deviation.
One-way analysis of variance followed by Tukey's post hoc test was
used to analyze the differences in gene expression between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA-21, PDCD4 and PTEN expression
profiles following SCI
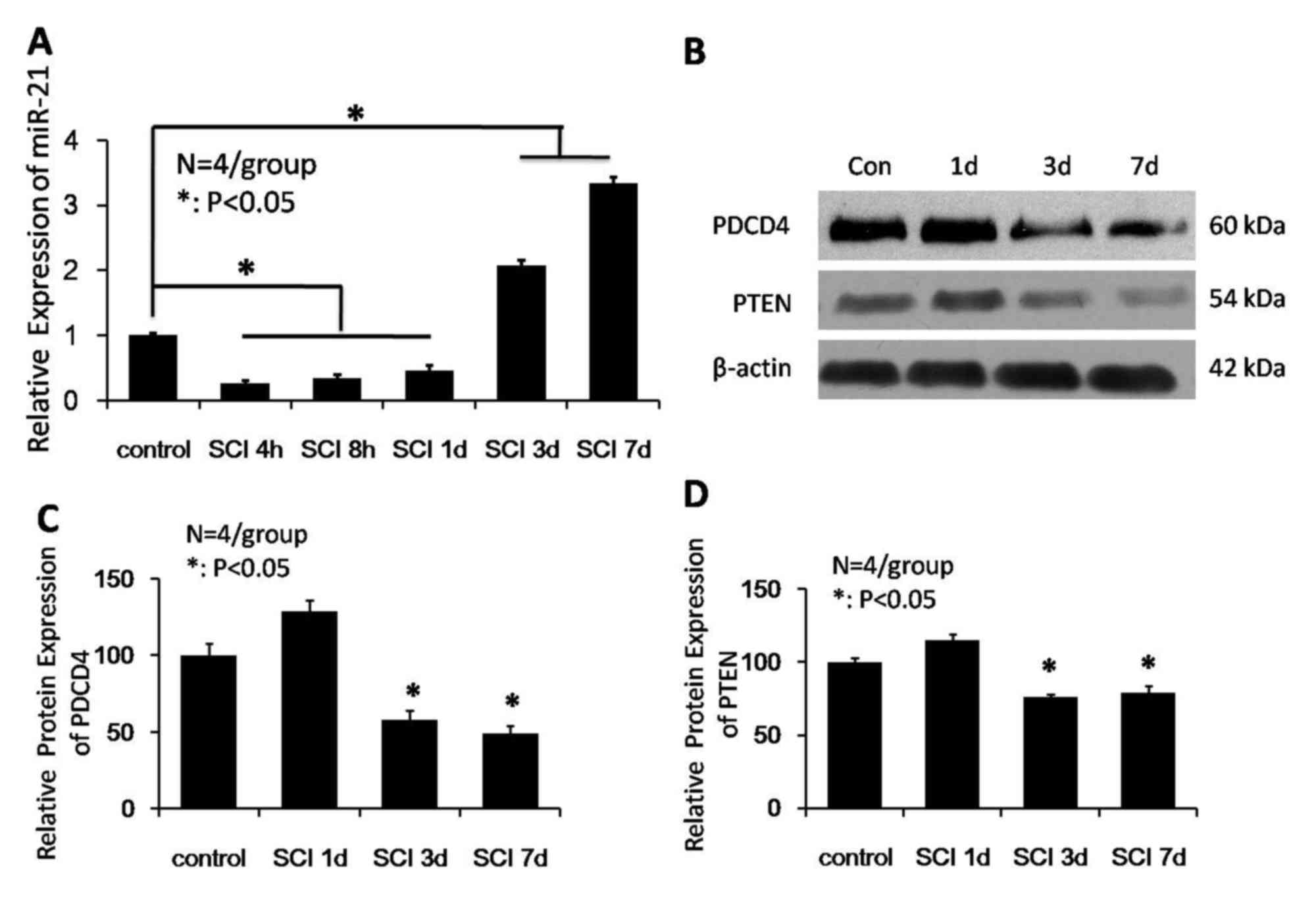
The present study investigated the expression levels
of miRNA-21 for 1 week following SCI by qPCR. miRNA-21 expression
levels reduced 1 day following injury, and increased at days 3 and
7. As presented in Fig. 1A,
miRNA-21 expression levels at 4 and 8 h, and 1 day post-SCI were
significantly reduced compared with the control group (P<0.05).
miRNA-21 expression levels gradually increased at days 3 and 7
post-SCI, and were significantly increased compared with uninjured
spinal cords at the 2 time points (P<0.05). To elucidate the
role of miRNA-21 in SCI, the present study further analyzed the
expression levels of PDCD4 (the predicted target gene of miRNA-21)
at various time points following SCI to determine if there was a
correlation with altered miRNA-21 expression. Western blot analysis
(Fig. 1B) revealed that the
expression levels of PDCD4 significantly decreased 3 and 7 days
following injury compared with the uninjured group (P<0.05;
Fig. 1C). Additionally, PTEN
expression levels at days 3 and 7 days following injury were
reduced compared with control spinal cords (P<0.05; Fig. 1D). The western blot analysis
results suggested that miRNA-21-regulated PTEN activity may have an
impact on cell survival and apoptosis.
miRNA-21 promotes neurite outgrowth
and regulates PDCD4 expression levels
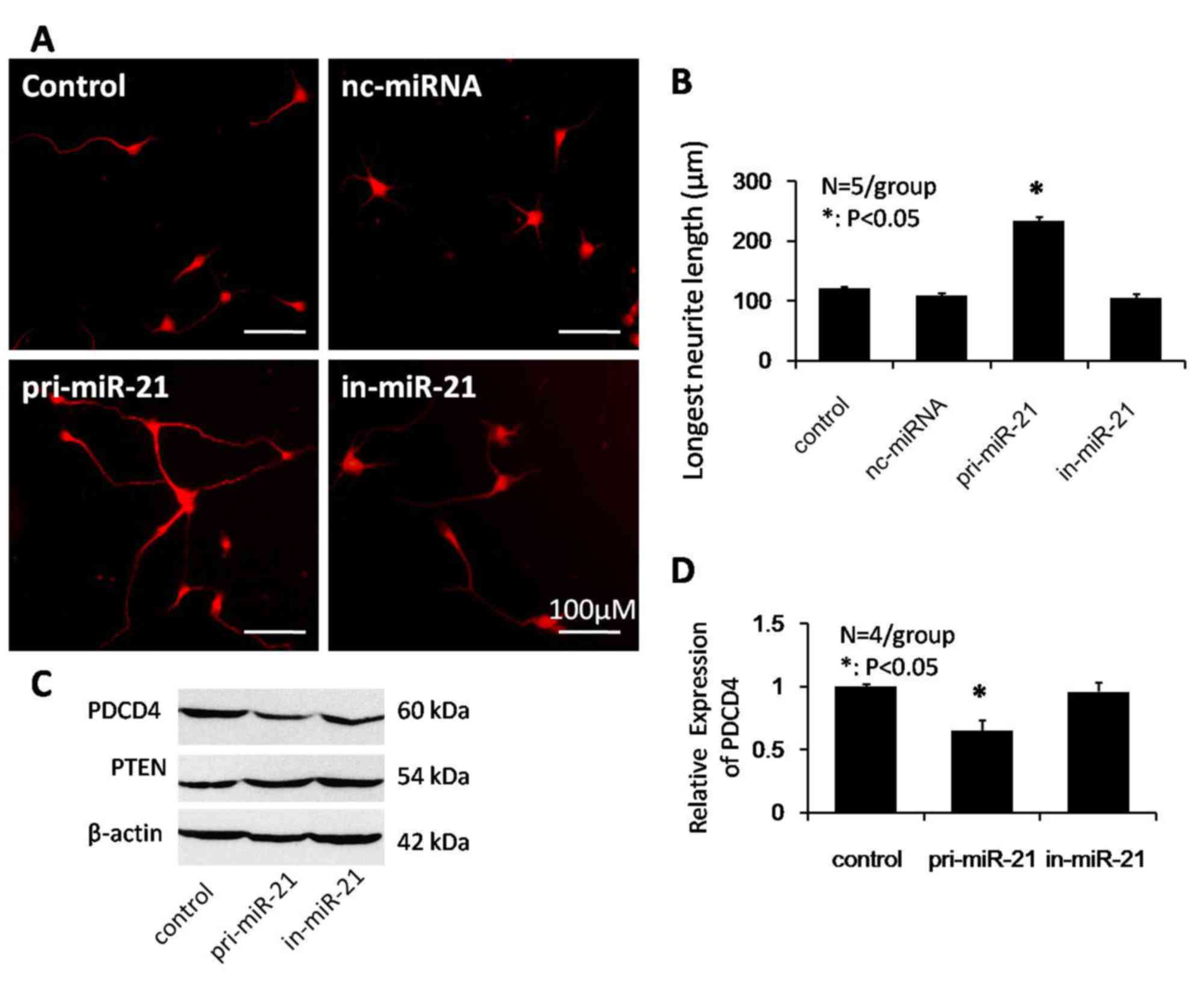
To examine the effect of miRNA-21 on neurite
outgrowth, the present study overexpressed miRNA-21 in monolayer
cultures of postnatal rat spinal cord neurons. Transfection
efficiency of miRNA-21 in dissected neurons was determined by EGFP
expression from the hU6-MCS-CMV-EGFP rAAV vector. In neuron
cultures transfected with in-miRNA-21, neurons produced short or no
neurites. By contrast, miRNA-21 overexpressing neurons demonstrated
a significant increase in neurite outgrowth compared with control
or nc-miRNA groups (Fig. 2A).
Quantification of the longest fiber outgrowth from transfected
neurons indicated that miRNA-21 significantly increased the mean
length of the longest neurite compared with the control and
nc-miRNA groups (234±7.1, 121±3.9 and 109±4.7 mm, respectively;
P<0.05; Fig. 2B). These data
demonstrated that overexpression of miRNA-21 promotes neurite
outgrowth in postnatal rat spinal cord neurons by increasing
neurite extension.
It was subsequently investigated whether miRNA-21
reduced PDCD4 and PTEN protein expression levels in postnatal
spinal cord neurons. As revealed by western blot analysis (Fig. 2C), compared with the control group,
no significant differences were observed in PTEN protein expression
levels, whereas there was a 35% decrease in PDCD4 expression levels
(P<0.05; Fig. 2D).
PDCD4 is the target gene of
miRNA-21
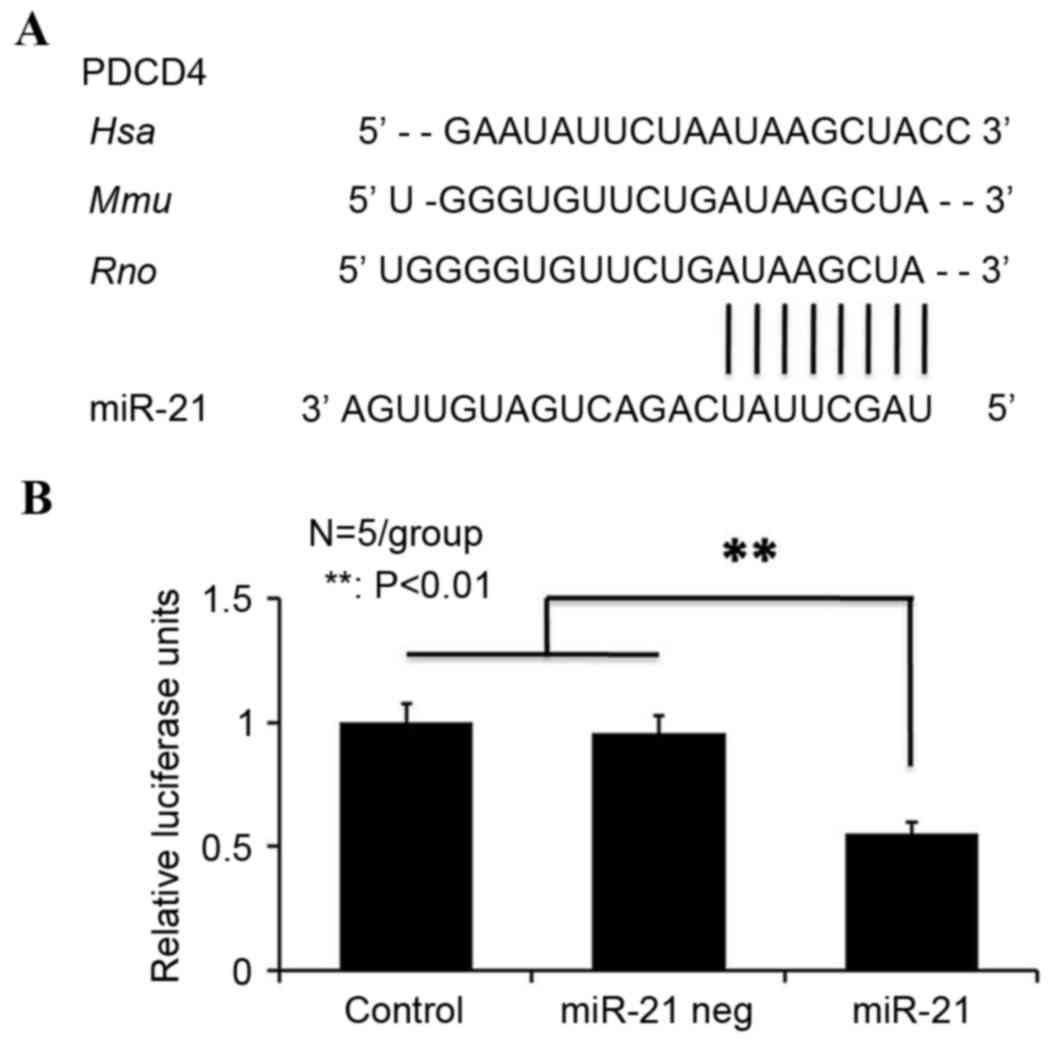
miRbase (www.mirbase.org) indicates that miRNA-21 has a
high-energy binding site at 281–288 bp in human, mouse and rat
PDCD4 3′-UTRs (Fig. 3A). To
determine whether miRNA-21 directly targeted PDCD4, the present
study cloned the 3′-UTR of rat PDCD4 downstream from the pRL-CMV
luciferase reporter. Cotransfection of wt-PDCD4, mt-PDCD4 or empty
pRL-CMV vectors along with an miRNA-21 mimic revealed that miRNA-21
decreased luciferase activity in wt-PDCD4-transfected cells by
45±5.1%, compared with mt-PDCD4 and negative control (miRNA-21 neg)
neurons (Fig. 3B). Taken together,
the western blot analysis and luciferase reporter assay results
indicated that miRNA-21 binds directly to the 3′-UTR of rat PDCD4
mRNA and downregulates PDCD4 protein expression levels in postnatal
spinal cord neurons.
Discussion
miRNAs are a class of endogenous noncoding small
RNAs that negatively regulate gene expression at the
post-transcriptional level by binding to the 3′-UTR of target
mRNAs, leading to their translational inhibition or degradation. In
humans, 20–30% of protein-coding genes are regulated by miRNAs
(5,19,20).
Following SCI, a primary mechanical injury causes considerable
alterations in gene expression, which leads to more serious
secondary damage, mediated by multiple injury processes (21,22).
Previous studies have suggested that miRNAs are expressed
specifically in the CNS and may regulate downstream protein
expression levels by redistributing and altering expression levels
in response to CNS injury, which may eventually affect repair and
regeneration in the CNS, and the pathogenesis of secondary injury
following SCI (23,24). Recent investigations have focused
on the role of miRNAs in SCI.
miRNA-21 has previously been identified in the
spinal cord (25–27). A previous study demonstrated that
overexpression of miRNA-21 in astrocytes caused a decrease in cell
size and glial fibrillary acidic protein expression levels,
suggesting a potential role for miRNA-21 in regulating astrogliosis
following SCI (28). Furthermore,
miRNA-21 has been suggested to be a potential therapeutic target
for manipulating gliosis and enhancing functional outcome by
regulating astrocytic hypertrophy and glial scar progression
following SCI (25). One study
reported that in a rat SCI model, exercise elevated expression
levels of miRNA-21 and decreased expression levels of
miRNA-199a-3p, which correlated with significant alterations in
mRNA expression levels of their target genes PTEN and mammalian
target of rapamycin, respectively (29). Given the potential role for
miRNA-21 in SCI, the present study investigated miRNA-21 expression
levels at 4 and 8 h, and 1, 3 and 7 days following injury, and
identified that its expression levels were significantly decreased
during the early phase of injury: 4 and 8 h and 1 day post-SCI. It
has previously been reported that acute SCI may result in extensive
hemorrhage around the central canal and regional gray matter, with
progressive neural degeneration and necrosis during the first 8 h
post-injury, which triggers apoptotic cell death to limit the
release of protease and toxic molecules (30). Secondary damage caused by tissue
edema, hemorrhage, microcirculation disturbance and release of
oxygen free radicals following acute neuron necrosis resulted in
the migration of hypertrophic reactive astrocytes and microglial
cells adjacent to the substantia nigra into the lesion sites, where
they served important roles in the reparative process. A previous
study indicated that overexpression of miRNA-21 may protect against
ischemic neuronal death; therefore, miRNA-21 may be a potential
therapeutic molecule for the treatment of stroke (31). Axotomy-induced miRNA-21 has
previously been reported to promote axon growth in adult dorsal
root ganglion neurons (32). The
results of the present study suggested that downregulation of
miRNA-21 during the early phase of injury may contribute to the
clearance of damaged debris from the lesion and facilitate the
repair and regeneration of neurons and neural precursor cells
bordering the lesion. The present study additionally demonstrated
that miRNA-21 expression levels were significantly upregulated 3
days post-SCI, with peak expression levels at day 7. As astrocytic
hypertrophy in the glial injury response may impede communication
between proximal and distal nerve fibers in the lesion (33), these observations suggested that
upregulation of miRNA-21 at a later phase of injury may contribute
to the reparative process by inhibiting the migration of
hypertrophic astrocytes and apoptosis of neural cells.
To date, the genes targeted by miRNA-21 that
contribute to cell survival and apoptosis remain elusive. A
previous study demonstrated that miRNA-21 expression levels were
significantly upregulated in the spinal cord following passive
exercise in rats (34), indicating
that miRNA-21 may function as an anti-apoptotic factor by
inhibiting the expression levels of the pro-apoptotic factors PDCD4
and PTEN. In breast cancer cells, PDCD4 was revealed to be
regulated and targeted by miRNA-21 (35). To further examine the role of
endogenous miRNA-21 in SCI, the present study investigated the
expression levels of the miRNA-21 targets PDCD4 and PTEN following
injury. miRNA-21 has previously been demonstrated to serve a
protective role in limiting secondary cell death following SCI,
which may have been the result of its regulation of pro-apoptotic
genes (26); this was consistent
with the results of the present study. A negative correlation would
indicate that miRNA-21 may block glial scar progression by
regulating apoptotic factors and thus improve post-injury repair.
The present study demonstrated that PDCD4 expression levels
decreased 3 and 7 days post-injury. Similarly, PTEN expression
levels were markedly downregulated at days 3 and 7 post-SCI. Taken
together, these results suggested that the increased expression
levels of miRNA-21 resulted in reduced expression levels of the
target genes. Additionally, the present study demonstrated that
miRNA-21 promoted spinal cord neurite outgrowth at postnatal days
3–5 in rats and inhibited protein expression of PDCD4 in cultured
neurons in vitro. Luciferase reporter assays confirmed that
PDCD4 is a target gene of mi-R21. These results thus suggested that
miRNA-21 may repress glial scar progression and improve spinal cord
repair by inhibiting the expression of the pro-apoptotic PDCD4 gene
in the secondary phase of SCI.
Following SCI, a diverse set of cytokines is
involved in the complex processes of pathogenesis and repair.
Alterations in miRNAs following SCI may influence the biological
repair process, including cell apoptosis, inflammation, glial scar
formation and nerve fiber regeneration. The present study observed
that increased miRNA-21 expression levels negatively regulate PDCD4
gene expression, thus inhibiting factors detrimental to nerve
regeneration and repair and creating a more conducive environment
for repair around the lesion sites. SCI commonly occurs among
children and young adults and has a high rate of morbidity and
mortality (36). Studies
investigating the role of miRNA-21 on pathophysiological processes
following SCI remain limited. The results of the present study
facilitate a greater understanding of the role of miRNA-21 and the
mechanisms underlying regeneration following SCI, which may be of
significant value for the clinical treatment of nervous system
injury.
Acknowledgements
The authors would like to thank Dr Hai-bin Xia
(Institute of Biological Science, Shaanxi Normal University, Xi'an,
China) for his individual support and help with this study. The
present study was supported by the H-level Medical Talents Training
Project (grant no. 2016CZBJ033).
References
|
1
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griffiths-Jones S, Saini HK, van Dongen S
and Enright AJ: miRBase: Tools for microRNA genomics. Nucleic Acids
Res. 36:(Database issue). D154–D158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J,
Xing R, Sun Z and Zheng X: miR-16 family induces cell cycle arrest
by regulating multiple cell cycle genes. Nucleic Acids Res.
36:5391–5404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fazekas D, Koltai M, Türei D, Módos D,
Pálfy M, Dúl Z, Zsákai L, Szalay-Bekő M, Lenti K, Farkas IJ, et al:
SignaLink 2-a signaling pathway resource with multi-layered
regulatory networks. BMC Syst Biol. 7:72013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuzin A, Kundu M, Brody T and Odenwald WF:
The Drosophila nerfin-1 mRNA requires multiple microRNAs to
regulate its spatial and temporal translation dynamics in the
developing nervous system. Dev Biol. 310:35–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stark A, Brennecke J, Bushati N, Russell
RB and Cohen SM: Animal MicroRNAs confer robustness to gene
expression and have a significant impact on 3′UTR evolution. Cell.
123:1133–1146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP and Chen CZ: Micromanagers of
gene expression: The potentially widespread influence of metazoan
microRNAs. Nat Rev Genet. 5:396–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei P, Li Y, Chen X, Yang S and Zhang J:
Microarray based analysis of microRNA expression in rat cerebral
cortex after traumatic brain injury. Brain Res. 1284:191–201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Redell JB, Zhao J and Dash PK: Altered
expression of miRNA-21 and its targets in the hippocampus after
traumatic brain injury. J Neurosci Res. 89:212–221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Talotta F, Cimmino A, Matarazzo MR,
Casalino L, De Vita G, D'Esposito M, Di Lauro R and Verde P: An
autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1
activity in RAS transformation. Oncogene. 28:73–84. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Põlajeva J, Swartling FJ, Jiang Y, Singh
U, Pietras K, Uhrbom L, Westermark B and Roswall P: miRNA-21 is
developmentally regulated in mouse brain and is co-expressed with
SOX2 in glioma. BMC Cancer. 12:3782012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donnelly DJ and Popovich PG: Inflammation
and its role in neuroprotection, axonal regeneration and functional
recovery after spinal cord injury. Exp Neurol. 209:378–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dou F, Huang L, Yu P, Zhu H, Wang X, Zou
J, Lu P and Xu XM: Temporospatial expression and cellular
localization of oligodendrocyte myelin glycoprotein (OMgp) after
traumatic spinal cord injury in adult rats. J Neurotrauma.
26:2299–2311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Montoya-Gacharna JV, Sutachan JJ, Chan WS,
Sideris A, Blanck TJ and Recio-Pinto E: Preparation of adult spinal
cord motor neuron cultures under serum-free conditions. Methods Mol
Biol. 846:103–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abe M and Bonini NM: MicroRNAs and
neurodegeneration: Role and impact. Trends Cell Biol. 23:30–36.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krichevsky AM: MicroRNA profiling: From
dark matter to white matter, or identifying new players in
neurobiology. ScientificWorldJournal. 7:155–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baptiste DC and Fehlings MG: Update on the
treatment of spinal cord injury. Prog Brain Res. 161:217–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zai LJ, Yoo S and Wrathall JR: Increased
growth factor expression and cell proliferation after contusive
spinal cord injury. Brain Res. 1052:147–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bak M, Silahtaroglu A, Møller M,
Christensen M, Rath MF, Skryabin B, Tommerup N and Kauppinen S:
MicroRNA expression in the adult mouse central nervous system. RNA.
14:432–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Medina PP and Slack FJ: Inhibiting
microRNA function in vivo. Nat Methods. 6:37–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhalala OG, Pan L, Sahni V, McGuire TL,
Gruner K, Tourtellotte WG and Kessler JA: microRNA-21 regulates
astrocytic response following spinal cord injury. J Neurosci.
32:17935–17947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu JZ, Huang JH, Zeng L, Wang G, Cao M and
Lu HB: Anti-apoptotic effect of microRNA-21 after contusion spinal
cord injury in rats. J Neurotrauma. 30:1349–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu NK, Wang XF, Lu QB and Xu XM: Altered
microRNA expression following traumatic spinal cord injury. Exp
Neurol. 219:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahni V, Mukhopadhyay A, Tysseling V,
Hebert A, Birch D, Mcguire TL, Stupp SI and Kessler JA: BMPR1a and
BMPR1b signaling exert opposing effects on gliosis after spinal
cord injury. J Neurosci. 30:1839–1855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu G, Detloff MR, Miller KN, Santi L and
Houlé JD: Exercise modulates microRNAs that affect the PTEN/mTOR
pathway in rats after spinal cord injury. Exp Neurol. 233:447–456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu XZ, Xu XM, Hu R, Du C, Zhang SX,
McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, et al: Neuronal
and glial apoptosis after traumatic spinal cord injury. J Neurosci.
17:5395–5406. 1997.PubMed/NCBI
|
|
31
|
Buller B, Liu X, Wang X, Zhang RL, Zhang
L, Hozeska-Solgot A, Chopp M and Zhang ZG: MicroRNA-21 protects
neurons from ischemic death. FEBS J. 277:4299–4307. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strickland IT, Richards L, Holmes FE,
Wynick D, Uney JB and Wong LF: Axotomy-induced miR-21 promotes axon
growth in adult dorsal root ganglion neurons. PLoS One.
6:e234232011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu NK, Zhang YP, Titsworth WL, Jiang X,
Han S, Lu PH, Shields CB and Xu XM: A novel role of phospholipase
A2 in mediating spinal cord secondary injury. Ann Neurol.
59:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu G, Keeler BE, Zhukareva V and Houlé
JD: Cycling exercise affects the expression of apoptosis-associated
microRNAs after spinal cord injury in rats. Exp Neurol.
226:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boltshauser E: Spnal cord injury in the
child and young adult. Neuropediatrics. 2015.(Epub ahead of
print).
|