Introduction
Vascular development is guided by two distinct
mechanisms: Vasculogenesis, during which a primary vascular plexus
is formed by angioblasts and hemopoietic cells, and angiogenesis,
during which new capillaries are generated from existing blood
vessels. Angiogenesis is tightly regulated by pro- and
antiangiogenic molecules (1,2) and
can be divided into the following steps: Endothelial cell
proliferation and migration, tube formation, vessel elongation and
maturation (3). Numerous factors
are involved in the regulation of angiogenic processes, including
the various vascular endothelial growth factor (VEGF) isoforms and
their receptors (VEGFRs) (4,5),
angiopoietins (Ang1 and Ang2) and their receptors (6,7),
fibroblast growth factors (8) and
hypoxia-inducible factors (HIFs) (9). Angiogenesis usually occurs under low
O2 conditions; in the human placenta, angiogenesis
progresses in a ~1.5–8% O2 environment (10). HIFs are hypoxia-responsive
transcription factors that act as O2 sensors in
mammalian cells; within the HIF family, HIF1α and HIF2α have been
implicated in early placental angiogenesis (11,12).
VEGFA and placental growth factor (PlGF) are members
of the VEGF family, and are critical for the regulation of
angiogenesis (13). The VEGFA gene
can undergo alternative splicing and produce several splice
variants, among which VEGF121/VEGF120, VEGF165/VEGF164 and
VEGF189/VEGF188 are the most notable and stable. The various VEGFA
isoforms with distinct biochemical properties may serve distinct
roles during the various stages of angiogenesis (14–16).
VEGFRs belong to the receptor tyrosine kinase family, and include
VEGFR1, encoded by the FLT1 gene, VEGFR2, encoded by the KDR gene,
and soluble fms-like tyrosine kinase-1 (sFlt1), an alternatively
spliced form of VEGFR1, all of which have been implicated in
angiogenesis (17).
Peroxisome proliferator-activated receptor (PPAR)-γ
belongs to the superfamily of nuclear receptors, and is a
ligand-activated transcription factor predominantly expressed in
adipose tissue and endothelial cells (18,19).
PPARγ has been implicated in placentation in mice, as
PPARγ−/− embryos exhibit severe impairments in placental
vascularization, leading to increased mortality (20,21).
In addition, a role for PPARγ has been suggested during the
differentiation of human labyrinthine trophoblasts, which may be
associated with HIF signaling (22). Furthermore, the synchronized
activation of G-protein coupled receptor 120 and PPARγ has been
demonstrated to enhance VEGF production in adipocytes (23). Therefore, it may be hypothesized
that PPARγ is implicated in porcine placental angiogenesis, and the
molecular mechanisms underlying its actions involve HIF-, VEGF- and
angiopoietin-mediated signaling.
Vascular endothelial cells (VECs) serve key roles in
numerous physiological and pathological processes, including
angiogenesis, blood pressure regulation, vascular permeability,
wound healing and tumor metastasis (24). PPARγ has previously been revealed
to be expressed in porcine placenta, mainly localized in VECs, thus
suggesting a role for PPARγ in placental vascularization (25). The present study aimed to further
investigate the roles of PPARγ in porcine placental vascularization
and explore the molecular mechanisms involved in its actions. In
the present study, VECs were isolated and incubated with PPARγ
ligands to investigate the angiogenic potential of PPARγ in
vitro. In addition, the mRNA expression levels of components of
HIF- VEGF- and angiopoietin-mediated signaling pathways were also
assessed.
Materials and methods
VEC isolation and identification
All studies were approved by the Animal Care and Use
Committee of Hunan Agricultural University (Hunan, China). VECs
were isolated from the umbilical vein of the delivered placenta of
primiparity Landrace pigs (n=16, 13 months old) as previously
described (26,27), with minor modifications. Briefly,
umbilical veins were collected form delivered placenta, ligated
with Serrefines (Zendainc instrument, Inc., Shanghai, China) and
filled with 0.1% (w/v) collagenase (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for digestion at 37°C 10 min. Digested cells
were collected by centrifugation at 560 × g for 5 min at room
temperature (RT), washed with PBS, and cultured in complete medium,
which contained RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.), at
37°C in a 5% CO2 atmosphere. Isolated VECs were cultured
for three passages before identification using immunofluorescence.
Cells were fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) at RT for 30 min, permeabilized with 0.1% Triton X-100
(Sigma-Aldrich; Merck KGaA) at RT for 10 min, blocked using 2%
bovine serum albumin (Sigma-Aldrich; Merck KgaA) at RT for 2 h and
incubated with rabbit anti-von Willebrand factor (vWF) (1:200; cat
no. PB0273 Wuhan Boster Biological Technology, Ltd., Wuhan, China)
and anti-cluster of differentiation (CD)31 (1:200; cat no. BA1346
Wuhan Boster Biological Technology, Ltd.) primary antibodies at 4°C
overnight. Cells were then incubated with Cyanine 3-labelled Goat
anti-rabbit secondary antibodies (1:100; cat no. BA1032 Wuhan
Boster Biological Technology, Ltd.) at RT for 1 h, and
counterstained with 0.4 µg/ml DAPI (Wuhan Boster Biological
Technology, Ltd.) at RT for 10 min. Cells were incubated with
rabbit immunoglobulin G (1:20; cat no. AR1010 Wuhan Boster
Biological Technology, Ltd.) in place of the primary antibody to
serve as the negative control. Stained cells were observed under a
fluorescence microscope (Olympus Corporation, Tokyo, Japan). The
positive rate of cells was determined using Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Cultures
between passages 3 and 6, with a positive rate of ~95% were used
for further experiments.
Cellular impedance assay
VEC proliferation was assessed using a cellular
impedance assay. VECs were seeded into 8-well E-plates (ACEA
Biosciences, Inc., San Diego, CA, USA) at a density of 7,500
cells/well and cultured overnight. Cells were cultured with
complete medium, supplemented with 0.1% dimethyl sulfoxide (DMSO)
(Sigma-Aldrich; Merck KGaA) as a control, the PPARγ agonist
rosiglitazone (10 µM; Sigma-Aldrich; Merck KGaA) or the PPARγ
antagonist T0070907 (15 µM; Sigma-Aldrich; Merck KGaA)
respectively, as previously described (28,29).
Cellular proliferation was dynamically monitored using the
iCELLigence™ real-time cell analysis (RTCA) system (ACEA
Biosciences, Inc.) at 37°C in a 5% CO2 atmosphere for
100 h. The cell index, which reflects the adhesion, proliferation
and viability of the cells through electrical impedance across
interdigitated microelectrodes integrated on the bottom of the
E-plates, was automatically calculated for each E-plate well using
RTCA software version 1.2 (Roche Diagnostics, Basel, Switzerland)
and graphs were generated in real-time using the iCELLigence™
system (30,31). Each treatment was performed in
duplicate and three independent experiments were conducted.
Scratch-wound assay
VECs were seeded into 6-well plates at a density of
2.5×105 cells/well. When the cells had reached 90%
confluence, cells were washed twice with PBS and serum-starved in
RPMI 1640 medium for 9 h. The confluent cell layer was scratched
with a 10 µl pipette tip, detached cells were removed by washing
with PBS, and cells were cultured in the presence of 10 µM
rosiglitazone, 15 µM T0070907 or 0.1% DMSO, respectively, in RPMI
1640 medium for 24 h. Photomicrographs of the scratch wounds were
obtained using an inverted phase-contrast microscope (Olympus
Corporation) equipped with a digital camera. The wound width was
determined using Image-Pro Plus software version 6.0.
Tube formation assay
BD Matrigel™ Basement Membrane Matrix (BD
Biosciences, Franklin Lakes, NJ, USA) was added into 96-well plates
(50 µl/well) and allowed to polymerize at 37°C for 30 min. VECs
were serum-starved overnight and seeded into 96-well plates
precoated with Matrigel at a density of 2×104
cells/well, in the presence of rosiglitazone (10 µM), T0070907 (15
µM) or 0.1% DMSO. Tube formation images were captured at 6 and 10 h
under an inverted microscope (Olympus Corporation) equipped with a
digital camera, and data were analyzed using Image-Pro Plus
software version 6.0. Differentiation of VECs into capillary-like
tubes was assessed by two independent investigators, via counting
the number of capillary branches under ×100 magnification in 3
random fields/well. The tube formation index was determined via
measuring the length of tubes (≥30 µm) in 3 random fields from each
well.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
VECs were seeded into 6-well plates at a density of
2.5×105 cells/well and cultured in complete medium at
37°C in 5% CO2 for 6 h. Cells were then treated with
serum free medium supplemented with rosiglitazone (10 µM), T0070907
(15 µM) or 0.1% DMSO at 37°C in a 5% CO2 for 24 h. When
the cells had reached >90% confluence, total RNA was extracted
from VECs with different treatments using Takara MiniBEST Universal
RNA Extraction kit (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol. The quantity and quality
of total RNA were determined using the NanoDrop 2000 UV–Vis
Spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). Total RNA (500 ng) was reverse transcribed into cDNA using
PrimeScript™ 1st strand cDNA synthesis kit (Takara Biotechnology
Co., Ltd.). qPCR results were calculated using absolute
quantification with the standard curve method. Fragments of the
indicated target genes (Table I)
were ligated into a pMD-T18 vector (Takara Biotechnology Co., Ltd.)
to create recombinant plasmids, which were amplified in E.
coli JM109 cells (Takara Biotechnology Co., Ltd.). qPCR was
performed using a SYBR® Premix Ex Taq™ kit (Takara
Biotechnology Co., Ltd.) on a StepOne™ Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) under the
following conditions: 95°C for 2 min, followed by 40 cycles of 95°C
for 5 sec at 60°C for 30 sec, followed by melt curve analysis. The
reaction volume was 20 µl, consisting of 10 µl SYBR Premix
DimerEraser, 0.4 µl ROX dye, 0.2 µl of each primer (20 µM), 2 µl
cDNA templates and water up to 20 µl. The standard curve was
obtained using 10-fold serially diluted plasmid samples as
templates, with R2 values >0.999. The specific
primers used for PCR are presented in Table I. The data were analyzed using the
comparative Cq method and gene expression was normalized to GAPDH
(32).
 | Table I.Primer sequences and standard curves
used in reverse transcription-quantitative polymerase chain
reaction. |
Table I.
Primer sequences and standard curves
used in reverse transcription-quantitative polymerase chain
reaction.
| Gene | Sequence | Length (bp) | Standard
equation | Reference
sequence |
|---|
| HIF1α | F:
5′-TTTTACTCATCCGTGCGACCAT-3′ | 276 |
y=−3.3524x+40.046 | EF070345.1 |
|
| R:
5′-GGTTCACAAATCAGCACCAAGC-3′ |
|
R2=0.9999 |
|
| HIF2α | F:
5′-TGGAGGGTTTCATTGCCGTAG-3′ | 287 |
y=−3.3795x+36.417 | EF375723.1 |
|
| R:
5′-CCAGGTGGCTGACTTGAGGTT-3′ |
|
R2=0.9995 |
|
| PPARγ | F:
5′-TTATGGAGCCCAAGTTTGAGTTT-3′ | 159 |
y=−3.2544x+38.805 | KM382175.1 |
|
| R:
5′-CTTGTAGCAGGTTGTCTTGAATG-3′ |
|
R2=0.999 |
|
| VEGF120 | F:
5′-AAGGCCAGCACATAGGAGAG-3′ | 101 |
y=−3.0243x+37.267 | KJ729036 |
|
| R:
5′-CCTCGGCTTGTCACATTTTT-3′ |
|
R2=0.9997 |
|
| VEGF164 | F:
5′-GAGGCAAGAAAATCCCTGTG-3′ | 150 |
y=−3.125x+36.848 |
|
|
| R:
5′-TCACATCTGCAAGTACGTTCG-3′ |
|
R2=0.9995 |
|
| VEGF188 | F:
5′-GGAGACCAGAAACCCCACGAAGT-3′ | 224 |
y=−3.2817x+37.074 |
|
|
| R:
5′-ATAATCTGCATGGCGATGTTGAA-3′ |
|
R2=0.9997 |
|
| PlGF | F:
5′-GCTGGTGGACATCGTGTCTGTG-3′ | 258 |
y=−3.3724x+36.761 | FJ177137.1 |
|
| R:
5′-CCGCACCTTTCTGGCTTCATCT-3′ |
|
R2=0.9993 |
|
| FLT1 | F:
5′-CACCCCGGAAATCTATCAGATC-3′ | 168 |
y=−3.0964x+37.958 | XM001925740.6 |
|
| R:
5′-GAGTACGTGAAGCCGCTGTTG-3′ |
|
R2=0.9994 |
|
| KDR | F:
5′-GATGCTCGCCTCCCTTTGA-3′ | 180 |
y=−3.1662x+37.922 | XM003128987.4 |
|
| R:
5′-AGTTCCTTCTTTCAGTCGCCTACA-3′ |
|
R2=0.9993 |
|
| sFlt1 | F:
5′-TGAGCACTGCAACAAAAAGG-3′ | 168 |
y=−3.3005x+38.014 | FJ263097.1 |
|
| R:
5′-CCGAGCCTGAAAGTTAGCAA-3′ |
|
R2=0.9993 |
|
| Ang-1 | F:
5′-GCCATAACCAGTCAGAGGCAGTA-3′ | 184 |
y=−3.22x+37.737 | AF233227.1 |
|
| R:
5′-AATCAGCACCATGTAAGATCAGG-3′ |
|
R2=0.9994 |
|
| Ang-2 | F:
5′-CCAGGTGTTAGTATCCAAGCAAA-3′ | 265 |
y=−3.3733x+39.653 | AF233228.1 |
|
| R:
5′-GTTAGGAAAGGTCAGCGTGTAGG-3′ |
|
R2=0.9995 |
|
| GAPDH | F:
5′-ACAGGGTGGTGGACCTCATG-3′ | 178 |
y=−3.3734x+38.727 | AK234838.1 |
|
| R:
5′-GGGTCTGGGATGGAAACTGG-3′ |
|
R2=0.999 |
|
Western blot analysis
VECs were seeded into 6-well plates at a density of
2.5×105 cells/well and cultured in complete medium at
37°C in 5% CO2 for 6 h. Cells were then treated with
serum free medium supplemented with rosiglitazone (10 µM), T0070907
(15 µM) or 0.1% DMSO at 37°C in a 5% CO2 for 24 h. When
the cells had reached >90% confluence, all treated cells were
lysed at 4°C for 30 min in radioimmunoprecipitation assay lysis
buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) containing
proteinase inhibitor cocktail (aprotinin and phenylmethanesulfonyl
fluoride). Protein concentration was determined using a
Bicinchoninic Acid assay with an Easy II Protein Quantitative kit
(Beijing Transgen Biotech Co., Ltd., Beijing, China). Equal amounts
of extracted protein samples (30 µg) were separated by 10% SDS-PAGE
and transferred onto nitrocellulose membranes. The membranes were
blocked with 2% bovine serum albumin at RT for 2 h and then
incubated with an anti-PPARγ antibody (1:1,000) (cat no. ab19481;
Abcam, Cambridge, UK) or anti-GAPDH antibody (1:2,000) (cat no.
ab9484; Abcam) overnight at 4°C. Subsequently, membranes were
incubated with the HRP-conjugated Affinipure Goat Anti-Rabbit IgG
(1:5,000; cat no. SA00001-2, Wuhan Sanying Biotechnology, Wuhan,
China). Protein bands were visualized by enhanced chemiluminescence
using SuperSignal™ West Pico Chemiluminescent Substrate (Thermo
Fisher Scientific, Inc.) on a ChemiDoc™ XRS+ system (Bio-Rad
Laboratories, Inc.). PPARγ blots were normalized to GAPDH and
semi-quantified by densitometry using ImageJ software (v2.1.4.7;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
at least 3 independent experiments. The statistical significance of
the differences between groups was assessed using one-way analysis
of variance. Statistical analysis was performed using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Morphological and biochemical
characteristics of VECs
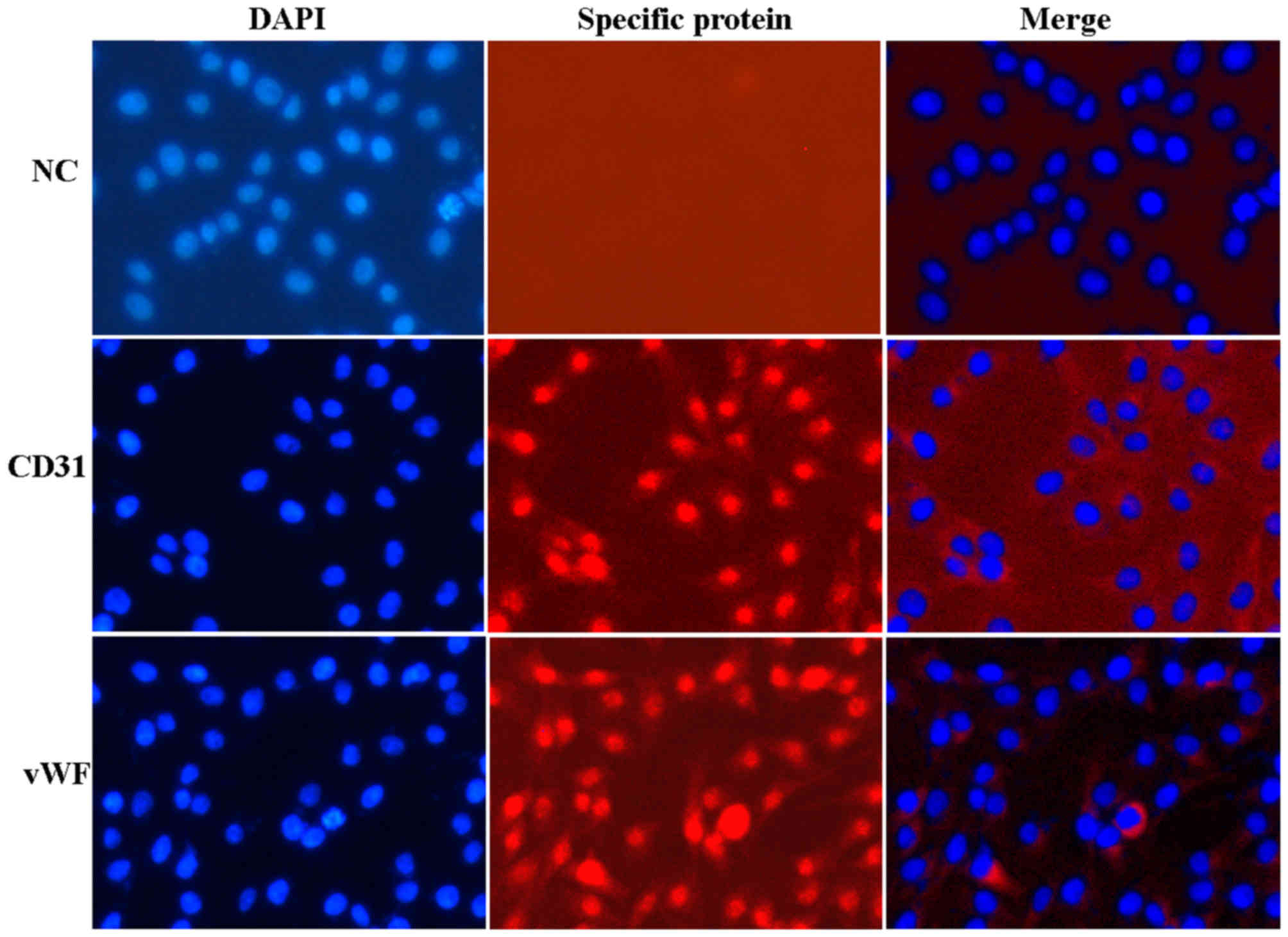
As presented in Fig.
1, isolated VECs grew as confluent monolayers with typical
cobblestone morphology, and had ovoid nuclei with 1 or 2 nucleoli.
The VEC markers vWF- and CD31 were positively stained in the nuclei
(Fig. 1). No specific staining was
detected in negative control cells.
Roles of PPARγ in VEC adhesion and
proliferation
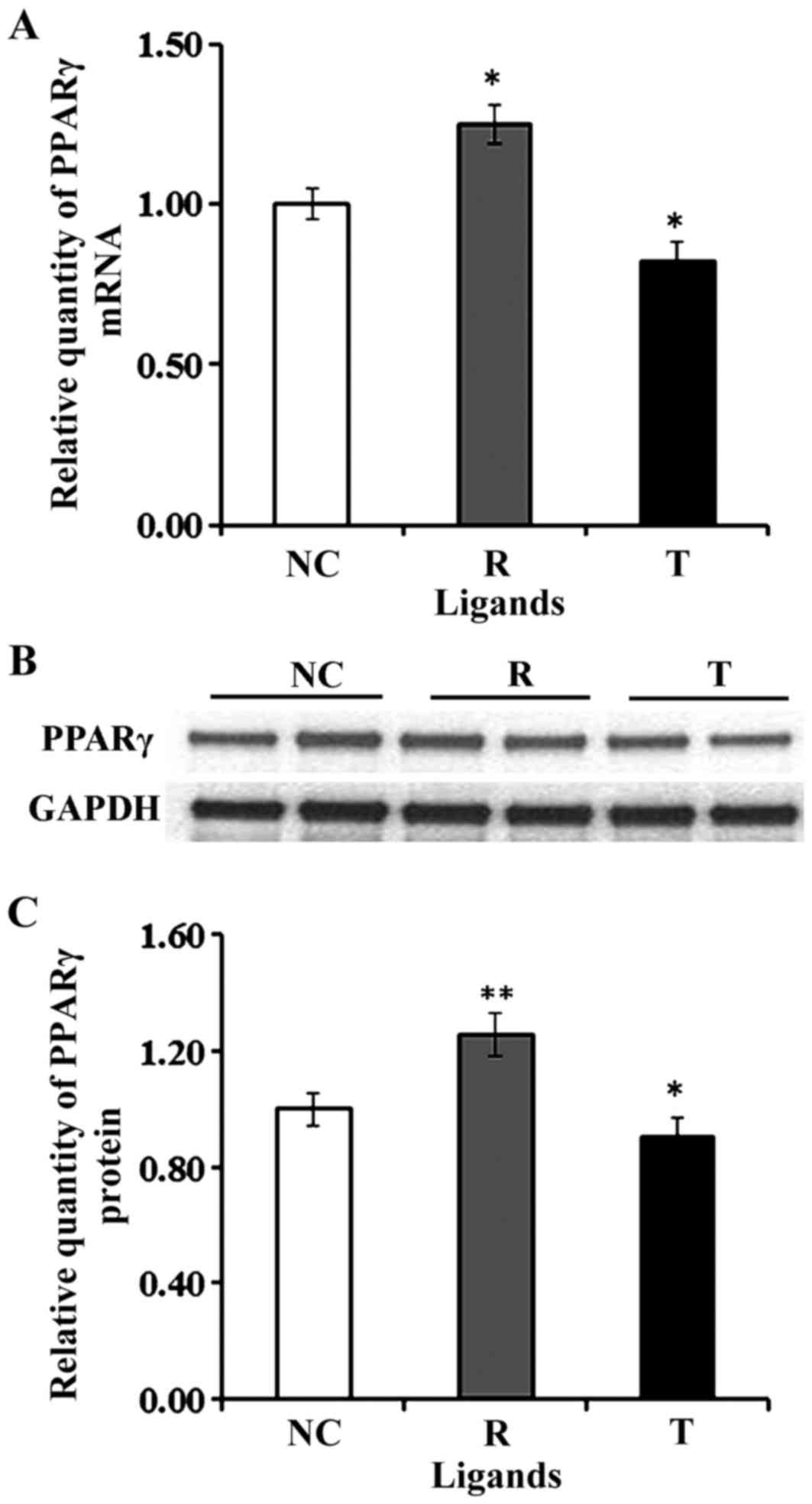
In the present study, the mRNA and protein
expression levels of PPARγ were revealed to be upregulated in VECs
following treatment with the PPARγ agonist rosiglitazone, whereas
they were downregulated following treatment with the antagonist
T007097 (Fig. 2). A cellular
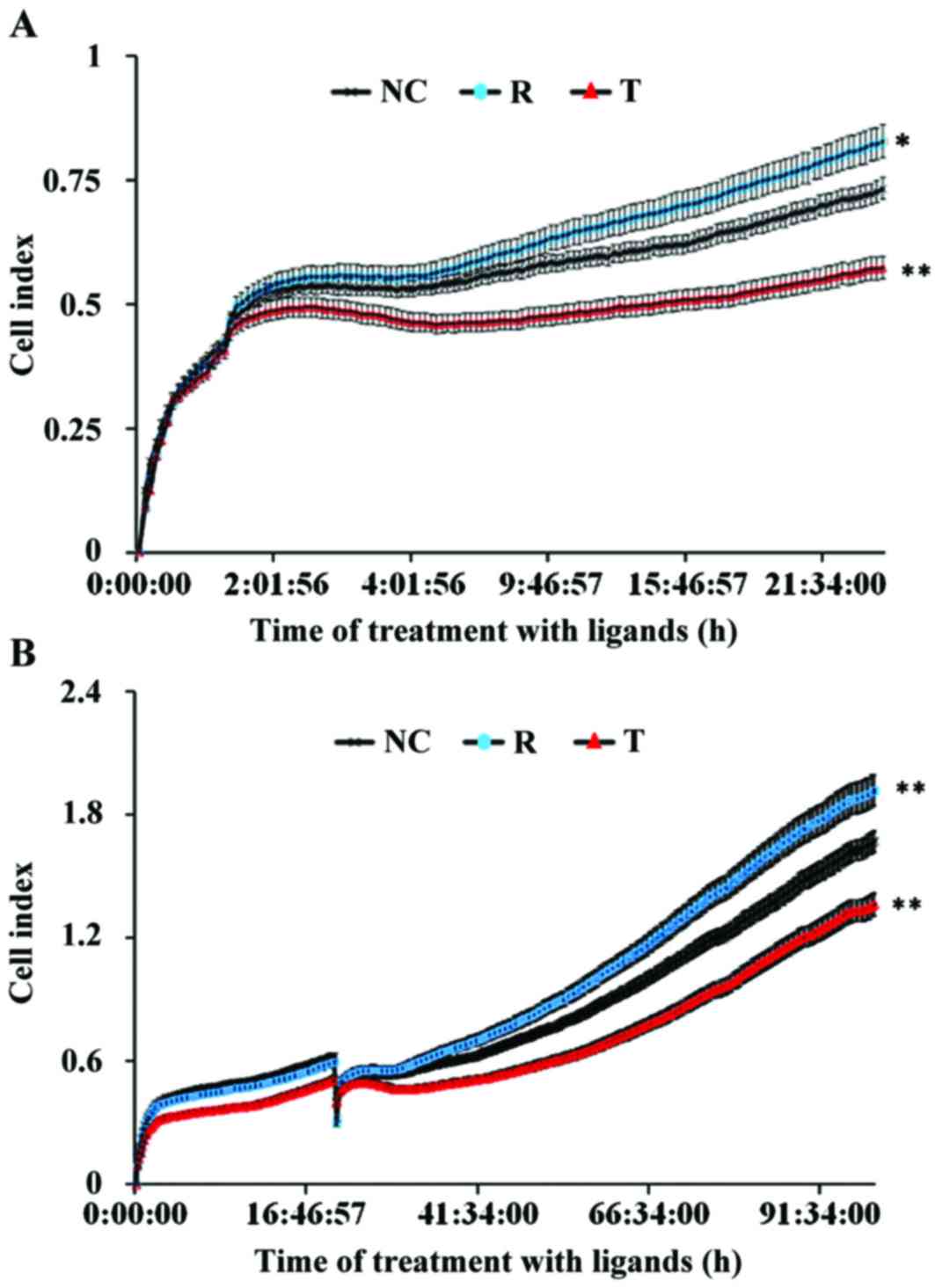
impedance assay demonstrated that following treatment with
rosiglitazone, the adhesive and proliferative capabilities of VECs
were enhanced, whereas treatment with T0070907 suppressed VEC
adhesion and proliferation (Fig.
3).
Roles of PPARγ in VEC migration
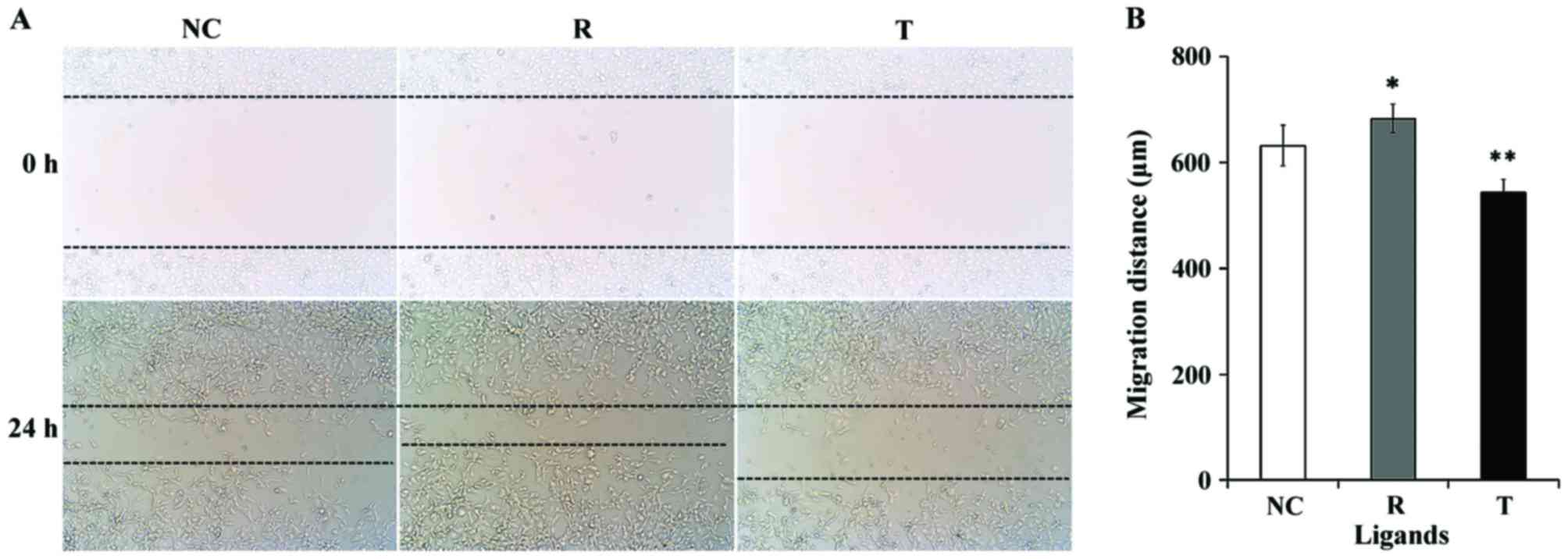
The migratory capabilities of VECs were investigated
using a wound healing assay, as previously described (33). As presented in Fig. 4, VEC migration was significantly
enhanced following treatment with rosiglitazone for 24 h compared
with control cells (P<0.05). Inhibition of PPARγ with T0070907
was revealed to decrease the migratory activity of VECs
(P<0.01).
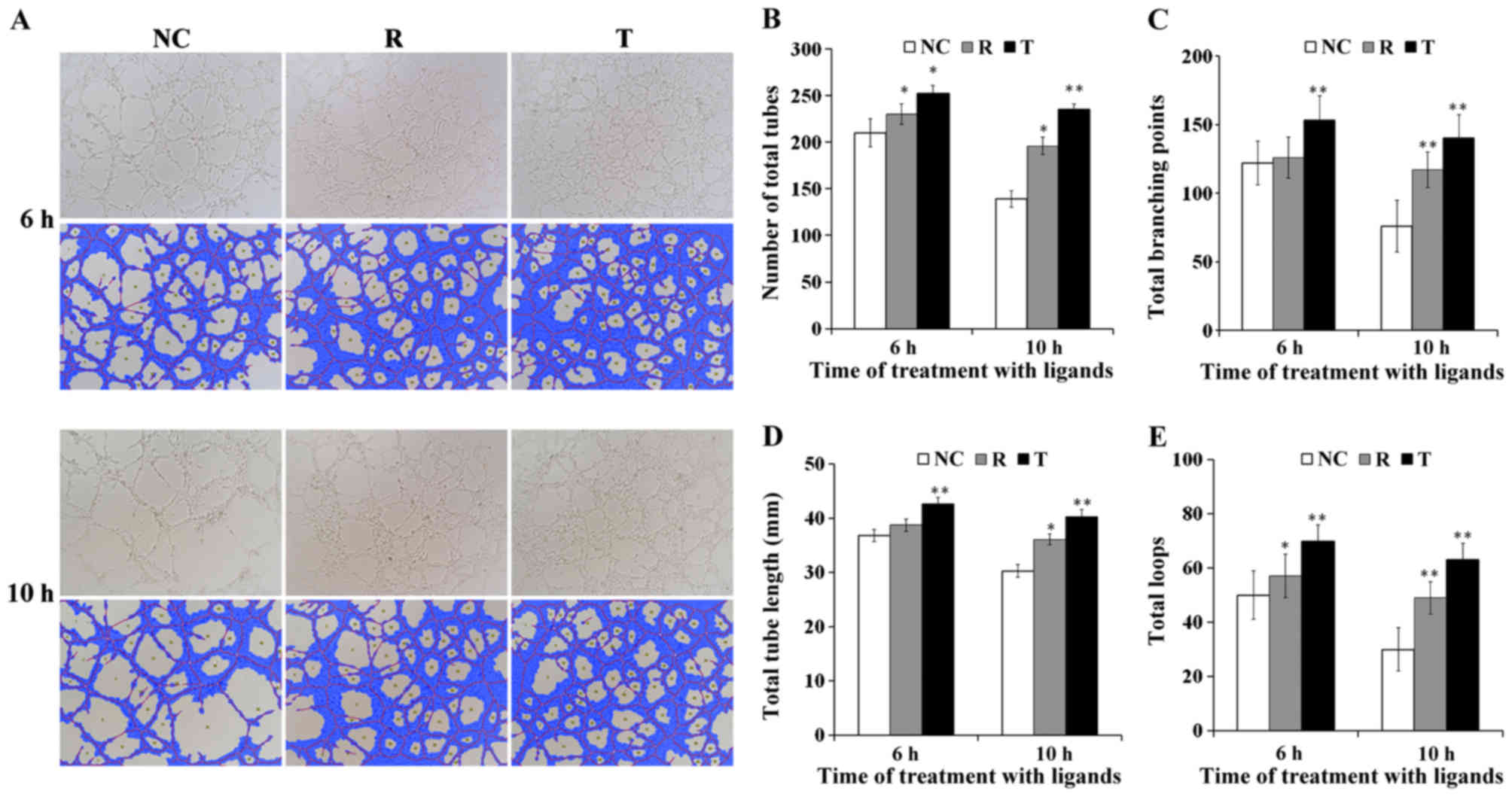
Roles of PPARγ in VEC capillary-like
tube formation
A tube-formation assay was performed to investigate
the roles of PPARγ on the angiogenic potential of VECs. As
demonstrated in Fig. 5A, following
6 h of culture on Matrigel-coated substrates, VECs exhibited
capillary-like tubular structures when observed under a
phase-contrast microscope. The quantitative parameters of
angiogenesis, including the number of total tubes, total tube
length, total branching points and total loops, were revealed to be
potentiated following treatment of VECs with rosiglitazone for 6
and 10 h compared with control cells (P<0.05; Fig. 5B-E). Notably, treatment with
T0070907 for 6 and 10 h also resulted in a significant increase in
tube, loop and branching point numbers, and in tube length compared
with the control group (P<0.01; Fig. 5B-E).
Angiogenic factor expression
The mRNA expression levels of several angiogenic
factors were investigated using RT-qPCR in VECs following treatment
with rosiglitazone and T0070907. As presented in Fig. 6A, the mRNA expression levels of
HIF1α and HIF2α were significantly upregulated following treatment
with rosiglitazone, whereas they were significantly downregulated
following treatment with T0070907.
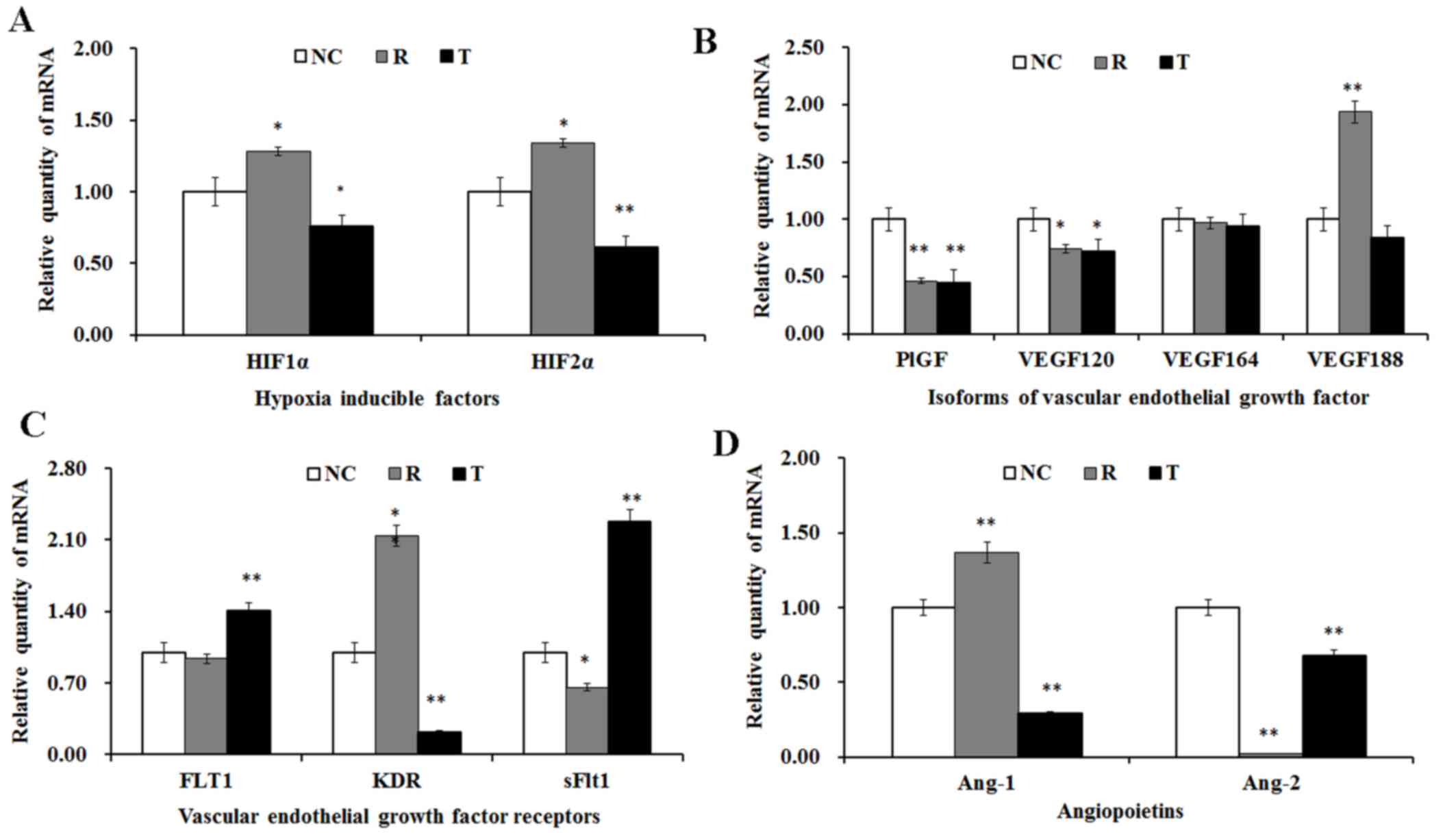 | Figure 6.Effects of rosiglitazone and T0070907
on mRNA expression of angiogenic factors in VECs. VECs were treated
with the PPARγ agonist rosiglitazone and the antagonist T0070907.
mRNA expression levels of (A) HIFs, (B) VEGF isoforms, (C) VEGFR
subtypes and (D) Ang subtypes were assessed using reverse
transcription-quantitative polymerase chain reaction following 24 h
of treatment. NC cells were treated with dimethyl sulfoxide. Data
are presented as the mean ± standard error of at least 3
independent experiments. *P<0.05, **P<0.01 vs. NC. VEC,
vascular endothelial cell; PPAR, peroxisome proliferator-activated
receptor; HIF, hypoxia-inducible factor; VEGF, vascular endothelial
growth factor; VEGFR, VEGF receptor; Ang, angiopoietin; NC,
negative control; R, rosiglitazone; T, T0070907; PlGF, placental
growth factor; FLT1, VEGFR subtype 1; KDR, VEGFR subtype 2; sFlt1,
soluble fms-like tyrosine kinase-1. |
Treatment with rosiglitazone and T0070907 resulted
in the significant downregulation of PlGF and VEGF120 mRNA
expression; however, the VEGF188 mRNA levels were significantly
upregulated following treatment with rosiglitazone, whereas they
remained unaltered following PPARγ inhibition (Fig. 6B). Furthermore, the modulation of
PPARγ activation did not appear to exert an effect on VEGF164 mRNA
expression levels (Fig. 6B).
PPARγ inhibition resulted in the significant
upregulation of FLT1 expression, whereas PPARγ activation had no
effect on FLT1 mRNA levels (Fig.
6C). Following treatment with rosiglitazone, the mRNA
expression levels of KDR were significantly increased, whereas
T0070907 was demonstrated to suppress KDR expression (Fig. 6C). Conversely, the mRNA expression
levels of sFlt1 were significantly downregulated in
rosiglitazone-treated VECs, and significantly upregulated following
T0070907 administration (Fig.
6C).
The mRNA expression levels of Ang-1 in VECs were
significantly enhanced following PPARγ activation, and
significantly suppressed in T0070907-treated cells (Fig. 6D). However, Ang-2 mRNA expression
was consistently decreased following PPARγ activation and blockade
(Fig. 6D).
Discussion
In a previous study, the PPARγ agonist troglitazone
exhibited species-specific effects in human and mouse endothelial
cells, as it was reported to increase the proliferation and
survival of mouse mammary fat pad microvascular endothelial cells,
whereas it did not affect human dermal microvascular endothelial
cells (34). In the present study,
the PPARγ agonist rosiglitazone was revealed to enhance the
adhesive, proliferative and migratory capabilities of porcine VECs;
conversely, the PPARγ antagonist T0070907 inhibited VEC adhesion,
proliferation and migration. These results suggested that PPARγ may
exert proangiogenic effects during porcine placental development.
In accordance with a previous study reporting dysregulation of
placental layers and vasculature defects in PPARγ−/−
mice (20), the present findings
suggested that PPARγ may promote placental vascularization in
porcine VECs, possibly via enhancing VEC differentiation,
proliferation and energy metabolism.
Currently, the role of PPARγ during angiogenesis
remains controversial. Previous studies have suggested that PPARγ
activation may inhibit angiogenesis, as demonstrated by the
inhibition of capillary-like tube formation in human retinal
pigment epithelial and bovine choroidal endothelial cells (35), and by the suppression of the
proliferative and migratory capabilities of human umbilical vein
endothelial cells (36,37). However, contradictory studies have
suggested proangiogenic effects for PPARγ, exerted through the
regulation of VEGF expression in myocardial (38) and pulmonary capillary cells
(39). These discrepancies may be
attributed to inter-species and cell type-specific differences, and
the different types and doses of PPARγ ligands that were used in
the various studies. Notably, in the present study, the
quantitative parameters of angiogenesis appeared to be invariably
enhanced following the activation and inhibition of PPARγ in VECs.
These effects may be associated with the various VEGF isoforms and
their receptors: PlGF and VEGF120 mRNA expression levels were
downregulated following PPARγ activation and blockade; however,
PPARγ activation may promote tube formation through the
potentiation of VEGF188/KDR signaling. Conversely, PPARγ blockade
may enhance capillary-like tube formation via promoting
VEGF164/FLT1 and VEGF188/FLT1 signaling.
Angiogenesis is an adaptive response to hypoxia
in vivo and in vitro, and HIFs are the key mediators
responsible for the activation of several angiogenic factors,
including VEGFA (40). However,
the various HIF isoforms may be characterized by differential
expression and distinct functions (41). In the present study, the mRNA
expression levels of HIF1α and HIF2α were modulated by PPARγ
activation or inhibition, indicating that HIF and PPARγ were both
involved in the recruitment of growth factors and induction of
vascularization. Therefore, VEC adhesion, proliferation and
migration may be modified by the synergistic effect of HIF and
PPARγ.
Three stable VEGFA isoforms, namely VEGF120, VEGF164
and VEGF188, have been identified in the porcine peri-implantation
conceptus (14,42). VEGFA has been implicated in
angiogenesis; however, the various VEGFA isoforms are characterized
by distinct properties and expression patterns (43). In addition, the VEGFA isoforms
differ with regard to their binding affinity for the various VEGFR
subtypes (14,43). In the present study, three VEGF
isoforms, namely PlGF, VEGF120 and VEGF188, were revealed to be
modulated by PPARγ activation or inhibition; whereas VEGF164 did
not appear to be affected by PPARγ modulation. These results may
indicate that PPARγ mediates vascularization through the modulation
of VEGF120/VEGFRs, VEGF188/VEGFRs and PlGF/VEGFRs, similarly with
the situation observed during early pregnancy in the pig (42,44).
These results suggested that various VEGF isoforms and VEGFR
subtypes may be differentially implicated in the various stages of
the angiogenic process, and may differentially regulate
vascularization.
In present study, the mRNA expression levels of
Ang-1 and Ang-2 were assessed in VECs, as has previously been
reported in perivascular and endothelial tip cells (45). The balance between Ang-1 and Ang-2
is critical for vascular stability, and Ang-1/Ang-2 imbalance has
been associated with vascular disruption and the initiation of
angiogenesis in tumor tissues (46). In addition, aberrant angiogenesis
has been reported in Ang-1−/− mice (47). In the present study, PPARγ
modulation was demonstrated to exert distinct effects on Ang-1 and
Ang-2 mRNA expression, whereby PPARγ activation significantly
upregulated Ang-1 and downregulated Ang-2.
In conclusion, the present results suggested that
PPARγ may bind to a PPAR-responsive element in the VEGFA promoter
region (23), and promote the
translation of the VEGF188 isoform instead of VEGF120 or VEGF164,
thus promoting VEGFA/KDR and VEGFA/Flt1 interactions, and
increasing capillary density and the total number of capillary-like
tubes. Furthermore, PPARγ may interact with HIFs and thus activate
VEGF transcription. Therefore, the present findings suggested that
PPARγ may be implicated in angiogenesis, through the promotion of
endothelial cell adhesion, proliferation and migration, and through
enhancing the formation and the stability of capillary-like
tubules. However, further studies are required to elucidate the
detailed molecular mechanisms that underlie the involvement of
PPARγ in angiogenic processes.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 31172377, 31272630 and
31572591) and the Key Projects of Hunan Province Education Office
(grant no. 14A069).
Glossary
Abbreviations
Abbreviations:
|
CD31
|
cluster of differentiation 31
|
|
DMSO
|
dimethyl sulfoxide
|
|
FCS
|
fetal calf serum
|
|
HIF
|
hypoxia-inducible factor
|
|
PlGF
|
placental growth factor
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
sFlt1
|
soluble fms-like tyrosine kinase-1
|
|
T0070907
|
2-chloro-5-nitro-N-4-pyridinyl-benzamide
|
|
VEC
|
vascular endothelial cell
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
VEGF receptor
|
|
vWF
|
von Willebrand factor
|
References
|
1
|
Risau W and Flamme I: Vasculogenesis. Annu
Rev Cell Dev Biol. 11:73–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sato Y, Poynter G, Huss D, Filla MB,
Czirok A, Rongish BJ, Little CD, Fraser SE and Lansford R: Dynamic
analysis of vascular morphogenesis using transgenic quail embryos.
PLoS One. 5:e126742010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charnock-Jones DS, Clark DE, Licence D,
Day K, Wooding FB and Smith SK: Distribution of vascular
endothelial growth factor (VEGF) and its binding sites at the
maternal-fetal interface during gestation in pigs. Reproduction.
122:753–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ventureira MR, Sobarzo CMA, Naito M,
Zanuzzi C, Barbeito C and Cebral E: Early placental
angiogenesis-vascularization and VEGF/KDR receptor expression
during mouse organogenesis after perigestational alcohol
consumption. Placenta. 36:4912015. View Article : Google Scholar
|
|
6
|
Saharinen P and Alitalo K: The yin, the
yang, and the angiopoietin-1. J Clin Invest. 121:2157–2159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hwang B, Lee SH, Kim JS, Moon JS, Jeung
IC, Lee NG, Park J, Hong HJ, Cho YL, Park YJ, et al: Stimulation of
angiogenesis and survival of endothelial cells by human monoclonal
Tie2 receptor antibody. Biomaterials. 51:119–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang YZ, Li Y, Wang K, Dai CF, Huang SA,
Chen DB and Zheng J: Distinct roles of HIF1A in endothelial
adaptations to physiological and ambient oxygen. Mol Cell
Endocrinol. 391:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burton GJ, Charnock-Jones D and Jauniaux
E: Regulation of vascular growth and function in the human
placenta. Reproduction. 138:895–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soleymanlou N, Jurisica I, Nevo O, Letta
F, Zhang X, Zamudio S, Post M and Caniggia I: Molecular evidence of
placental hypoxia in preeclampsia. J Clin Endocrinol Metab.
90:4299–4308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koch S and Claesson-Welsh L: Signal
transduction by vascular endothelial growth factor receptors. Cold
Spring Harb Perspect Med. 2:a0065022012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vempati P, Popel AS and MacGabhann F:
Extracellular regulation of VEGF: Isoforms, proteolysis, and
vascular patterning. Cytokine Growth Factor Rev. 25:1–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grünewald FS, Prota AE, Giese A and
Ballmer-Hofer K: Structure-function analysis of VEGF receptor
activation and the role of coreceptors in angiogenic signaling.
Biochim Biophys Acta. 1804:567–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ng YS, Rohan R, Sunday ME, Demello DE and
D'amore PA: Differential expression of VEGF isoforms in mouse
during development and in the adult. Dev Dyn. 220:112–121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nieminen T, Toivanen PI, Rintanen N,
Heikuraa T, Jauhiainena S, Airennea KJ, Alitaloc K, Marjomäkib V
and Ylä-Herttuala S: The impact of the receptor binding profiles of
the vascular endothelial growth factors on their angiogenic
features. Biochim Biophys Acta. 1840:454–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Zhao B, Liu Y and Wang N:
Peroxisome proliferator-activated receptor gamma regulates the
expression of lipid phosphate phosphohydrolase 1 in human vascular
endothelial cells. PPAR Res. 2014:7401212014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kepez A, Oto A and Dagdelen S: Peroxisome
proliferator-activated receptor-gamma: Novel therapeutic target
linking adiposity, insulin resistance, and atherosclerosis.
BioDrugs. 20:121–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nadra K, Quignodon L, Sardella C, Joye E,
Mucciolo A, Chrast R and Desvergne B: PPARgamma in placental
angiogenesis. Endocrinology. 151:4969–4981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meher A, Sundrani D and Joshi S: Maternal
nutrition influences angiogenesis in the placenta through
peroxisome proliferator activated receptors: A novel hypothesis.
Mol Reprod Dev. 82:726–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tache V, Ciric A, Moretto-Zita M, Li Y,
Peng J, Maltepe E, Milstone DS and Parast MM: Hypoxia and
trophoblast differentiation: A key role for PPARγ. Stem Cells Dev.
22:2815–2824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hasan AU, Ohmori K, Konishi K, Igarashi J,
Hashimoto T, Kamitori K, Yamaguchi F, Tsukamoto I, Uyama T,
Ishihara Y, et al: Eicosapentaenoic acid upregulates VEGF-A through
both GPR120 and PPARγ mediated pathways in 3T3-L1 adipocytes. Mol
Cell Endocrinol. 406:10–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cines DB, Pollak ES, Buck CA, Loscalzo J,
Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS,
et al: Endothelial cells in physiology and in the pathophysiology
of vascular disorders. Blood. 91:3527–3561. 1998.PubMed/NCBI
|
|
25
|
Zhang J, Xu J, Yang Q, Yuan A, Yang L and
Xue L: Peroxisome proliferator-activated receptor gamma (PPARγ)
expression in pig placenta. Mol Med Rep MMR-8037-156015 in
press.
|
|
26
|
Hong HX, Zhang YM, Xu H, Su ZY and Sun P:
Immortalization of swine umbilical vein endothelial cells with
human telomerase reverse transcriptase. Mol Cells. 24:358–363.
2007.PubMed/NCBI
|
|
27
|
Chrusciel M, Bodek G, Kirtiklis L, Lewczuk
B, Hyder CL, Blitek A, Kaczmarek MM, Ziecik AJ and Andronowska A:
Immortalization of swine umbilical vein endothelial cells (SUVECs)
with the simian virus 40 large-T antigen. Mol Reprod Dev.
78:597–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Young PW, Buckle DR, Cantello BC, Chapman
H, Clapham JC, Coyle PJ, Haigh D, Hindley RM, Holder JC, Kallender
H, et al: Identification of high-affinity binding sites for the
insulin sensitizer rosiglitazone (BRL-49653) in rodent and human
adipocytes using a radioiodinated ligand for peroxisomal
proliferator-activated receptor gamma. J Pharmacol Exp Ther.
284:751–759. 1998.PubMed/NCBI
|
|
29
|
Zaytseva YY, Wallis NK, Southard RC and
Kilgore MW: The PPARgamma antagonist T0070907 suppresses breast
cancer cell proliferation and motility via both PPARgamma-dependent
and -independent mechanisms. Anticancer Res. 31:813–823.
2011.PubMed/NCBI
|
|
30
|
Dolkart O, Liron T, Chechik O, Somjen D,
Brosh T, Maman and Gabet Y: Statins enhance rotator cuff healing by
stimulating the COX2/PGE2/EP4 pathway: An in vivo and in vitro
study. Am J Sports Med. 42:2869–2876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koval OA, Sakaeva GR, Fomin AS, Nushtaeva
AA, Semenov DV, Kuligina EV, Gulyaeva LF, Gerasimov AV and Richter
VA: Sensitivity of endometrial cancer cells from primary human
tumor samples to new potential anticancer peptide lactaptin. J
Cancer Res Ther. 11:345–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jonkman JE, Cathcart JA, Xu F, Bartolini
ME, Amon JE, Stevens KM and Colarusso P: An introduction to the
wound healing assay using live-cell microscopy. Cell Adh Migr.
8:440–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kakiuchi-Kiyota S, Vetro JA, Suzuki S,
Varney ML, Han HY, Nascimento M, Pennington KL, Arnold LL, Singh RK
and Cohen SM: Effects of the PPARgamma agonist troglitazone on
endothelial cells in vivo and in vitro: Differences between human
and mouse. Toxicol Appl Pharmacol. 237:83–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murata T, He S, Hangai M, Ishibashi T, Xi
XP, Kim S, Hsueh WA, Ryan SJ, Law RE and Hinton DR: Peroxisome
proliferator-activated receptor-gamma ligands inhibit choroidal
neovascularization. Invest Ophthalmol Vis Sci. 41:2309–2317.
2000.PubMed/NCBI
|
|
36
|
Park BC, Thapa D, Lee JS, Park SY and Kim
JA: Troglitazone inhibits vascular endothelial growth
factor-induced angiogenic signaling via suppression of reactive
oxygen species production and extracellular signal-regulated kinase
phosphorylation in endothelial cells. J Pharmacol Sci. 111:1–12.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KY, Ahn JH and Cheon HG:
Anti-angiogenic action of PPARγ ligand in human umbilical vein
endothelial cells is mediated by PTEN upregulation and VEGFR-2
downregulation. Mol Cell Biochem. 358:375–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Wei T, Jiang X, Li Z, Cui H, Pan
J, Zhuang W, Sun T, Liu Z, Zhang Z and Dong H: PEDF and 34-mer
inhibit angiogenesis in the heart by inducing tip cells apoptosis
via up-regulating PPAR-γ to increase surface FasL. Apoptosis.
21:60–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Biscetti F, Gaetani E, Flex A, Aprahamian
T, Hopkins T, Straface G, Pecorini G, Stigliano E, Smith RC,
Angelini F, et al: Selective activation of peroxisome
proliferator-activated receptor (PPAR)alpha and PPARgamma induces
neoangiogenesis through a vascular endothelial growth
factor-dependent mechanism. Diabetes. 57:1394–1404. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ndubuizu OI, Tsipis CP, Li A and LaManna
JC: Hypoxia-inducible factor-1 (HIF-1)-independent microvascular
angiogenesis in the aged rat brain. Brain Res. 1366:101–109. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arreola A, Cowey CL, Coloff JL, Rathmell
JC and Rathmell WK: HIF1α and HIF2α exert distinct nutrient
preferences in renal cells. PLoS One. 9:e987052014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaczmarek MM, Kiewisz J, Schams D and
Ziecik AJ: Expression of VEGF-receptor system in conceptus during
peri-implantation period and endometrial and luteal expression of
soluble VEGFR-1 in the pig. Theriogenology. 71:1298–1306. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanthou C, Dachs GU, Lefley DV, Steele AJ,
Coralli-Foxon C, Harris S, Greco O, Dos Santos SA, Reyes-Aldasoro
CC, English WR and Tozer GM: Tumour cells expressing single VEGF
isoforms display distinct growth, survival and migration
characteristics. PLoS One. 9:e1040152014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coultas L, Chawengsaksophak K and Rossant
J: Endothelial cells and VEGF in vascular development. Nature.
438:937–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Holash J, Maisonpierre P, Compton D,
Boland P, Alexander CR, Zagzag D, Yancopoulos GD and Wiegand SJ:
Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeansson M, Gawlik A, Anderson G, Li C,
Kerjaschki D, Henkelman M and Quaggin SE: Angiopoietin-1 is
essential in mouse vasculature during development and in response
to injury. J Clin Invest. 121:2278–2289. 2011. View Article : Google Scholar : PubMed/NCBI
|