Introduction
Mesenchymal stem cells (MSCs) represent a
heterogeneous population of fibroblast-like multipotent cells which
may differentiate into various mesodermal lineages and may be
isolated from a number of tissues, including bone marrow, umbilical
cord, amniotic membrane, or adipose tissue (1). Cardiac ischemia following acute
myocardial infarction leads to impaired cardiac function and is
associated with increased morbidity and mortality. MSCs
administration in cases of cardiac ischemia/reperfusion injury
(IRI) has been associated with a significant reduction of
myocardial cell death and an effective improvement in cardiac
function (2,3). However, a randomized control trial
(4) and meta-analyses (5,6) have
demonstrated that improvement of cardiac function resulting from
MSCs transplantation is limited. One major limiting factor in MSCs
transplantation therapy is the low survival rate of the
transplanted cells (7,8). This is thought to be due to local
inflammatory reactions, ischemia and hypoxia (9). Previous studies have focused on
improving the survival of MSCs through various methods, including
the use of genetically engineered MSCs, coupled with suitable
tissue engineering materials, or preconditioning with optimal
culture conditions (10–13).
microRNAs (miRs) are small (20–22 nucleotides long)
noncoding RNAs that suppress protein translation by binding to
target mRNAs, reducing their stability and/or inhibiting
translation. Growing evidence indicates that miRs are involved in
the regulation of cell survival, proliferation and migration,
through mediating the expression of their target genes (14). Several miRs, including miR-29,
miR-34a and miR-133, are involved in pathways modulating cellular
apoptosis (15–17). However, the exact role of miRs in
the survival of MSCs, in addition to the associated underlying
mechanisms, remains to be elucidated.
Accordingly, the present study was designed to
improve the survival of MSCs by cell culture. Specifically, it
identified the expression of miR-29a in human amnion-derived
(hA)MSCs cultured in different culture media. In addition, it also
identified novel target genes of miR-29a and explored the
underlying mechanisms associated with MSCs survival.
Materials and methods
Cell culture
Human amnion-derived (hA)MSCs were isolated from
amniotic membranes of healthy donors using enzymatic digestion as
previously described (18). All
donors (between May 2015 and January 2016) gave their informed
consent and the Ethics Committee of the First Affiliated Hospital
of China Medical University (Shenyang, China) approved the study
protocol. The hAMSCs were cultured in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences) and 100 U/ml penicillin and streptomycin
(Hyclone; GE Healthcare Life Sciences) at 37°C in an incubator with
5% CO2. For the endothelial cell culture conditions,
cells were cultured in endothelial growth medium (EGM-2) with 5%
FBS and endothelial cell growth supplement (ECGS; Sciencell
Research Laboratories, Inc., Carlsbad, CA, USA). All the subsequent
assays were performed with 7-day cultured hAMSCs.
HAMSCs immunophenotyping
The immunophenotype of hAMSCs cultured in either
hAMSCs culture conditions or EC culture conditions was analyzed
using flow cytometry. A total of 1×106 cells were
detached from culture dishes using trypsin solution (Hyclone; GE
Healthcare Life Sciences) and stained with 5 µl antibodies against
cluster of differentiation (CD)31 (cat. no 303105), CD34 (cat. no
343505), CD73 (cat. no 344003), CD90 (cat. no 32810) and CD105
(cat. no 323205) (all undiluted; BioLegend, Inc., San Diego, CA,
USA). Immunoglobulin G of the appropriate isotype was used as
negative control. Data from 10,000 viable cells were acquired. List
mode files were analyzed by FCS Express Software (version 3; BD
Biosciences, Franklin Lakes, NJ, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 1×106 hAMSCs
using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. RNA
concentration was determined by a NanoDrop ND-1000 (Nano-Drop
Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
For miR-29a detection, poly A tail was added to RNase-free DNase
digested total RNA using the poly A tailing kit (Ambion; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
SYBR RT-qPCR was used to assay miRNA expression with the specific
forward primers and the universal reverse primer complementary to
the anchor primer. U6 primer was used as an miRNA internal control.
For the mRNA analysis of myeloid cell leukemia (MCL)-1, cDNA was
synthesized using PrimeScript™ RT reagent (Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol. Reactions were
performed using the SYBR PrimeScript RT-PCR kit (Takara Bio, Inc.)
with an ABI 7500 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The PCR reactions used for the amplification of miRNAs
were conducted at 95°C for 30 sec, followed by 45 cycles of 95°C
for 5 sec and 60°C for 34 sec. As an internal control, β-actin
level was quantified in parallel with the target genes.
Normalization and fold-alterations were calculated using the
2−ΔΔCq method (19).
All experiments were performed in triplicate and repeated three
times. The primers used for RT-qPCR were as follows: Forward,
5′-TAGCACCATCTGAAATCGGTTA-3′ and reverse,
5′-GCTGTCAACGATACGCTACGT-3′ for miR-29a; forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-TTCACGAATTTGCGTGTCAT-3′
for U6; forward, 5′-GAGGAGGACGAGTTGTACCG-3′ and reverse,
5′-CACAATCCTGCCCCAGTTTG-3′ for MCL-1 and forward,
5′-AGGATTCCTATGTGGGCGAC-3′ and reverse, 5′-ATAGCACAGCCTGGATAGCAA-3′
for β-actin.
miR-29a overexpression and
suppression
miR-29a mimic, negative control (nc), miR-29a
inhibitor and inhibitor nc were purchased from Suzhou GenePharma
Co. Ltd. (Suzhou, China) and the sequences were as follows:
5′-UAGCACCAUCUGAAAUCGGUUA-3′ for miR-29a mimic;
5′-UAACCGAUUUCAGAUGGUGCUA-3′ for miR-29a inhibitor;
5′-UUCUCCGAACGUGUCACGUTT-3′ for NC; and 5′-CAGUACUUUUGUGUAGUACAA-3′
for inhibitor NC. Small interfering (si)RNA targeting human MCL-1
and control siRNA were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
hAMSCs at 80% confluence were transfected with
miR-29a mimic, inhibitor, MCL-1 siRNA and their corresponding
controls (50 nM for each) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Following 48 h, cells were incubated with serum-free
DMEM with 300 µM cobalt (II) chloride for a further 48 h and
examined with Hoechst 33258 staining or flow cytometry.
Hoechst 33258 staining
Following cell incubation with serum-free DMEM with
300 µM cobalt (II) chloride for 48 h, a Hoechst 33258 staining kit
(Beyotime Institute of Biotechnology, Haimen, China) was used to
observe the apoptotic cells induced by mimic or inhibitor of
miR-29a, according to the manufacturer's protocol. Each assay was
performed at least three times.
Caspases activities assay
Caspase 3 and 7 activities were determined using
Apo-ONE® Homogeneous Caspase-3/7 Assay according to the
manufacturer's protocols (cat. no G7792; Promega Corporation,
Madison, WI, USA). The plate was read by a microplate reader at a
wavelength of 405 nm. Activities of caspases 3 and 7 were expressed
as the ratio of treated cells to corresponding controls.
Flow cytometry
Following 48 h incubation in serum-free DMEM with
300 µM cobalt (II) chloride, a FITC Annexin V Apoptosis Detection
kit (BD Biosciences) was used to double stain cells with
FITC-Annexin V and propidium iodide according to the manufacturer's
protocols. A total of 2.5×105 cells were seeded into
6-well plates for 48 h and removed using trypsin without EDTA,
stained cells were analyzed using a flow cytometer (BD
Biosciences). FCS Express Software (version 3; BD Biosciences) was
used to observe cell apoptosis. In the graphs, cells were
distinguished as dead, living, early apoptotic and late apoptotic
cells. The aggregate of early and late apoptotic cells was regarded
as an observation index to compare the experimental and negative
groups. Each experiment was performed at least three times.
Luciferase reporter assay
The wild-type (WT) or mutant (MUT) 3′-untranslated
region (UTR) segment of MCL-1 containing the putative miR-29a
binding site was amplified and inserted into the pLUC Luciferase
vector (Ambion; Thermo Fisher Scientific, Inc.). Site-directed
mutagenesis of the miR-29a binding site in the MCL-1 3′-UTR was
achieved with a commercially available kit (Beyotime Institute of
Biotechnology). All the plasmids were confirmed by DNA sequencing.
HEK293T cells (American Type Culture Collection, Manassas, VA, USA;
80% confluence) were co-transfected with 100 ng reporter constructs
and 50 nM miR-29a mimic, inhibitor, or control miRNA using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. At 24 h posttransfection,
cells were harvested and luciferase activity was assayed using the
Dual Luciferase Reporter Assay System (Promega Corporation). A
Renilla luciferase construct was used as the internal control. All
experiments were repeated in triplicate.
Western blot analysis
Total proteins from hAMSC were harvested in lysis
buffer (Beyotime Institute of Biotechnology) and quantified using
the bicinchoninic acid method. Protein lysates were separated using
10% SDS-acrylamide gels and transferred onto Protran nitrocellulose
membranes (Whatman; GE Healthcare Life Sciences, Little Chalfont,
UK). For immunodetection, membranes were incubated with antibodies
directed against MCL-1 (1:1,000; cat. no ab32087; Abcam), CD31
(1:1,000; cat. no ab28364; Abcam), CD105 (1:1,000; cat. no
ab107595; Abcam), or β-actin (1:10,000; cat. no ab8227; Abcam).
Signals from horseradish-peroxidase-conjugated secondary antibodies
(1:5,000; cat. no KC-RB-035; KangChen Bio-tech, Shanghai, China)
were generated by enhanced chemiluminescence solution (ECL; GE
Healthcare Life Sciences) and recorded on film. Quantification was
performed using ImageJ software (version 1.45S; National institutes
of Health, Bethesda, MD, USA). Experiments were repeated in
triplicate.
Statistical analysis and
bioinformatics
TargetScan (www.targetscan.org), PicTar (pictar.mdc-berlin.de) and
miRanda (www.microrna.org) were used to predict
the target genes of miR-29a. Statistical analysis was performed
using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation and were
analyzed by a Student's t-test or one-way analysis of variance
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Phenotype characterization of hAMSCs
is variable depending on growth medium
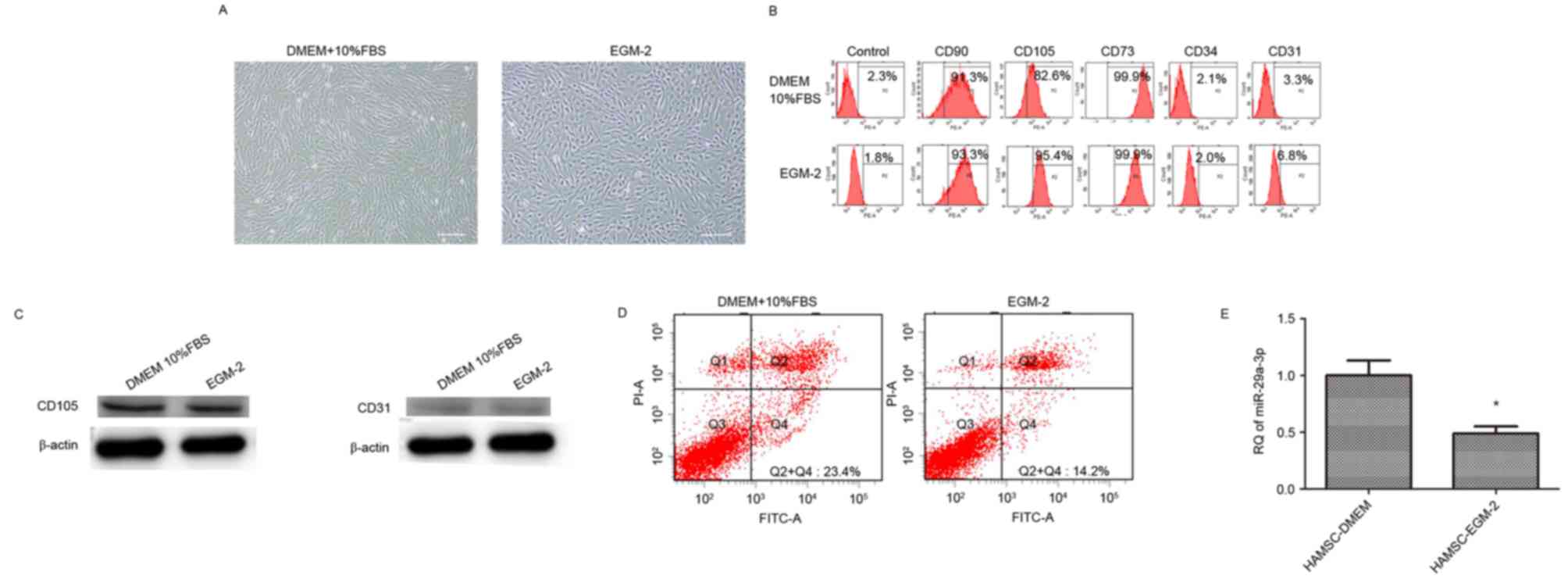
hAMSCs cultured in DMEM supplemented with 10% FBS
revealed a spindle fibroblast-like morphology, whereas those
cultured in EGM-2 exhibited a cobblestone-like morphology (Fig. 1A). Flow cytometry revealed the
expression of surface markers. hAMSCs cultured in the two types of
medium were positive for CD73, CD90 and CD105, but were negative
for CD31 and CD34 (Fig. 1B).
Western blotting revealed that hAMSCs cultured in the two types of
medium were positive for CD105 expression, and negative for CD31
expression (Fig. 1C).
EGM-2 culture decreases apoptosis of
hAMSCs and miR-29a expression
Flow cytometry results demonstrated that the
apoptosis of hAMSCs was significantly decreased in hAMSCs cultured
in EGM-2 (14.8±2.7%) compared with those cultured in DMEM
(21.5±3.1%; Fig. 1D). Fig. 1E indicated that miR-29a expression
was decreased in hAMSCs cultured in EGM-2 compared with hAMSCs
cultured in DMEM (~2-fold change).
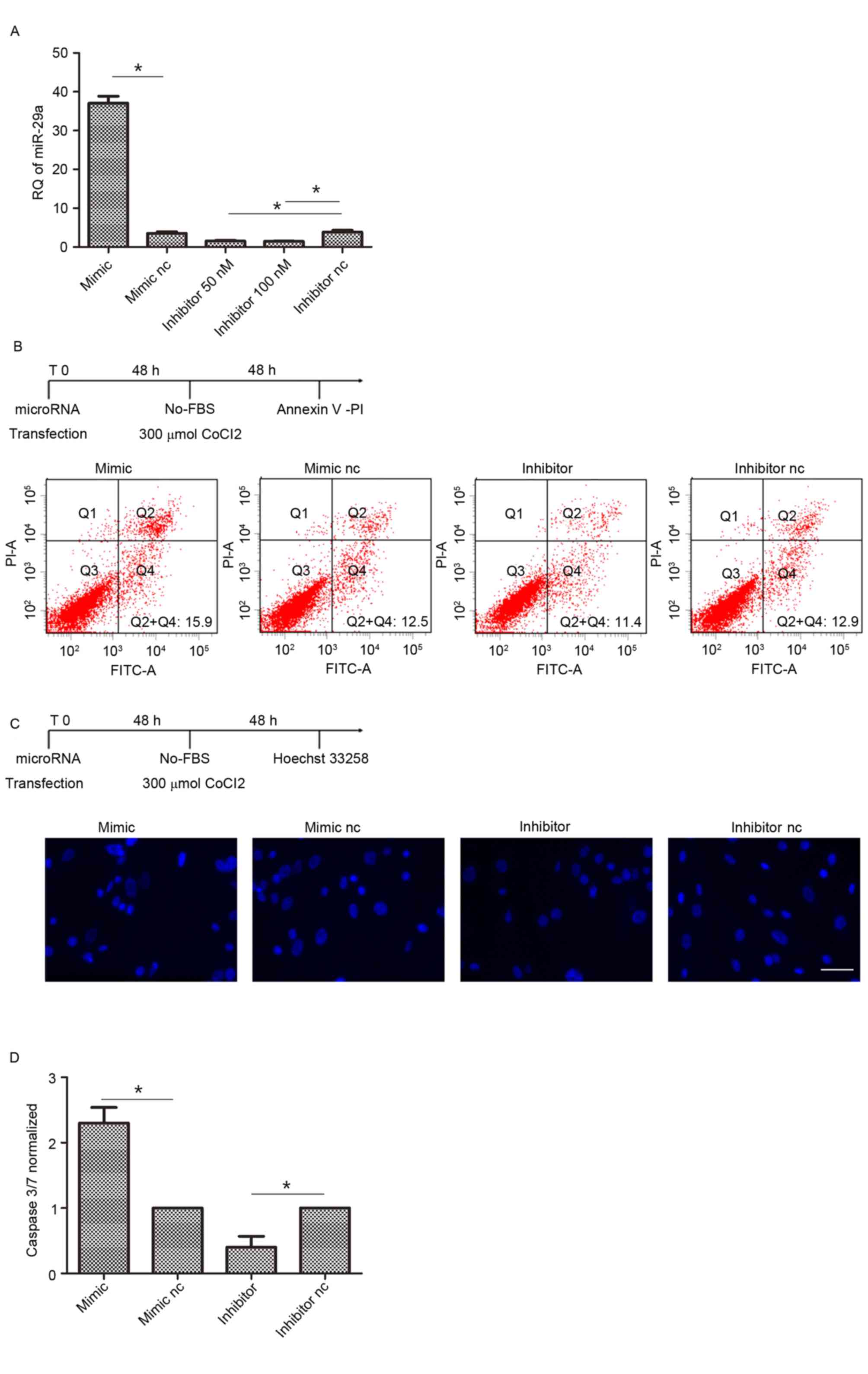
Suppression of miR-29a decreases
hAMSCs apoptosis
To evaluate the ability of miR-29a to control hAMSCs
apoptotic activity, these cells were transfected by miR-29a mimic,
inhibitor or their corresponding controls and incubated with
serum-free DMEM with 300 µM cobalt (II) chloride to undergo
apoptosis. hAMSCs at 80% confluence were transfected with miR-29a
inhibitor (50 and 100 nM) and 50 nM inhibitor successfully
suppressed the expression of miR-29a, so 50 nM concentrations were
selected for use in the present study (Fig. 2A). Following annexin-V/PI assays,
miR-29a-transfected hAMSCs presented an apoptotic cell rate of
18.4±2.3% compared with hAMSCs transfected by the scramble oligos
(12.7±1.3%). Meanwhile, knocking-out miR-29a by transfection with
inhibitor suppressed the apoptosis of hAMSCs (9.1±1.8%; Fig. 2B. The observations were confirmed
by Hoechst 33258 staining, demonstrating a suppression of apoptosis
in hAMSCs transfected with miR-29a inhibitor compared with the
negative control (Fig. 2C). These
observations were confirmed by caspase 3/7 activities measurements,
demonstrating an apoptosis suppression of hAMSCs transfected with
inhibitor (~2-fold) compared with the negative control (Fig. 2D).
MCL-1 is a target of miR-29a in
hAMSCs
The expression of experimentally validated targets
for miR-29a in hAMSCs were additionally examined. Potential targets
of miR-29a were identified based on bioinformatics prediction.
Through preliminary function screening, an anti-apoptotic protein,
MCL-1, was selected to further study its interaction with miR-29a
and its role in hAMSCs apoptosis. First, the basal level of MCL-1
mRNA in different culture mediums was observed by RT-qPCR. As
presented in Fig. 3A, a
significantly high level of MCL-1 mRNA was detected in the hAMSCs
cultured in EGM-2 compared with DMEM (P<0.05). Similar results
were obtained with western blotting analysis for MCL-1 protein
expression (Fig. 3B). To ascertain
if MCL-1 may be targeted by miR-29a, a gain-of-function experiment
was performed with miR-29a in hAMSCs. MiR-29a or negative control
was transiently transfected and endogenous MCL-1 expression was
detected by western blotting. Densitometric measurement of the
bands demonstrated that MCL-1 protein level was notably decreased
in miR-29a overexpressing cells, whereas it appeared to be
upregulated in miR-29a knockdown cells, compared with the control
(Fig. 3C).
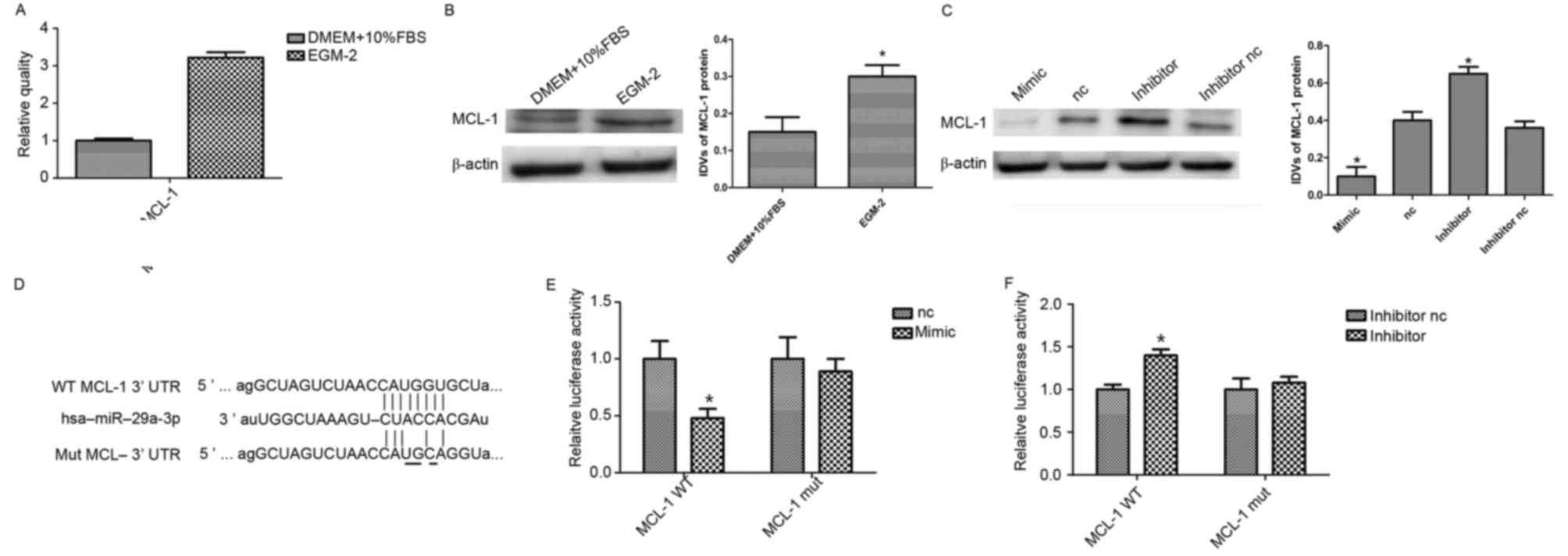 | Figure 3.miR-29a targets MCL-1 in hAMSCs. (A)
Reverse transcription-quantitative polymerase chain reaction
analysis of MCL-1 expression in hAMSCs cultured in DMEM and EGM-2.
(B) Western blot analysis of MCL-1 expression in hAMSCs cultured in
DMEM and EGM-2. EGM-2 increased MCL-1 expression. β-actin was used
as an endogenous control. Data are presented as the mean ± standard
deviation (n=3/group). (C) Western blot analysis of MCL-1
expression in miR-29a-3p mimic, inhibitor and their corresponding
controls. β-actin was used as an endogenous control. Data are
presented as the mean ± standard deviation (n=3/group). (D)
Alignment of miR-29a with MCL-1 at the 3′-UTR and the mutation site
of seed area. A luciferase reporter assay was performed to
determine whether MCL-1 is a target of miR-29a. A WT or MUT of
MCL-1 3′UTR was subcloned into the pLUC Luciferase vector. (E)
Vector plus miR-29a mimics or (F) miR-29a inhibitor were
co-transfected into hAMSCs. *P<0.05 vs. nc. miR, microRNA;
MCL-1, myeloid cell leukemia-1; hAMSCs, human amnion-derived
mesenchymal stem cells; EGM-2, endothelial growth medium; DMEM,
Dulbecco's modified Eagle's medium; nc, negative control; WT,
wildtype; MUT, mutated; IDV, integrated density value; FBS, fetal
bovine serum. |
To confirm if miR-29a binds directly to the 3′-UTR
of MCL-1 in HEK 293T cells, luciferase reporter vectors were
constructed containing the WT or MUT 3′-UTRMCL-1 (Fig. 3D). miR-29a effectively reduced
luciferase activity in 293T cells transfected with the WT 3′-UTR of
MCL-1. Conversely, miR-29a inhibitor significantly induced
luciferase activity in cells transfected with WT MCL-1 3′UTR,
whereas the MUT MCL-1 3′-UTR eliminated the suppression by miR-29a,
compared with the control group (Fig.
3E and F). These findings indicated that MCL-1 is the direct
target of miR-29a.
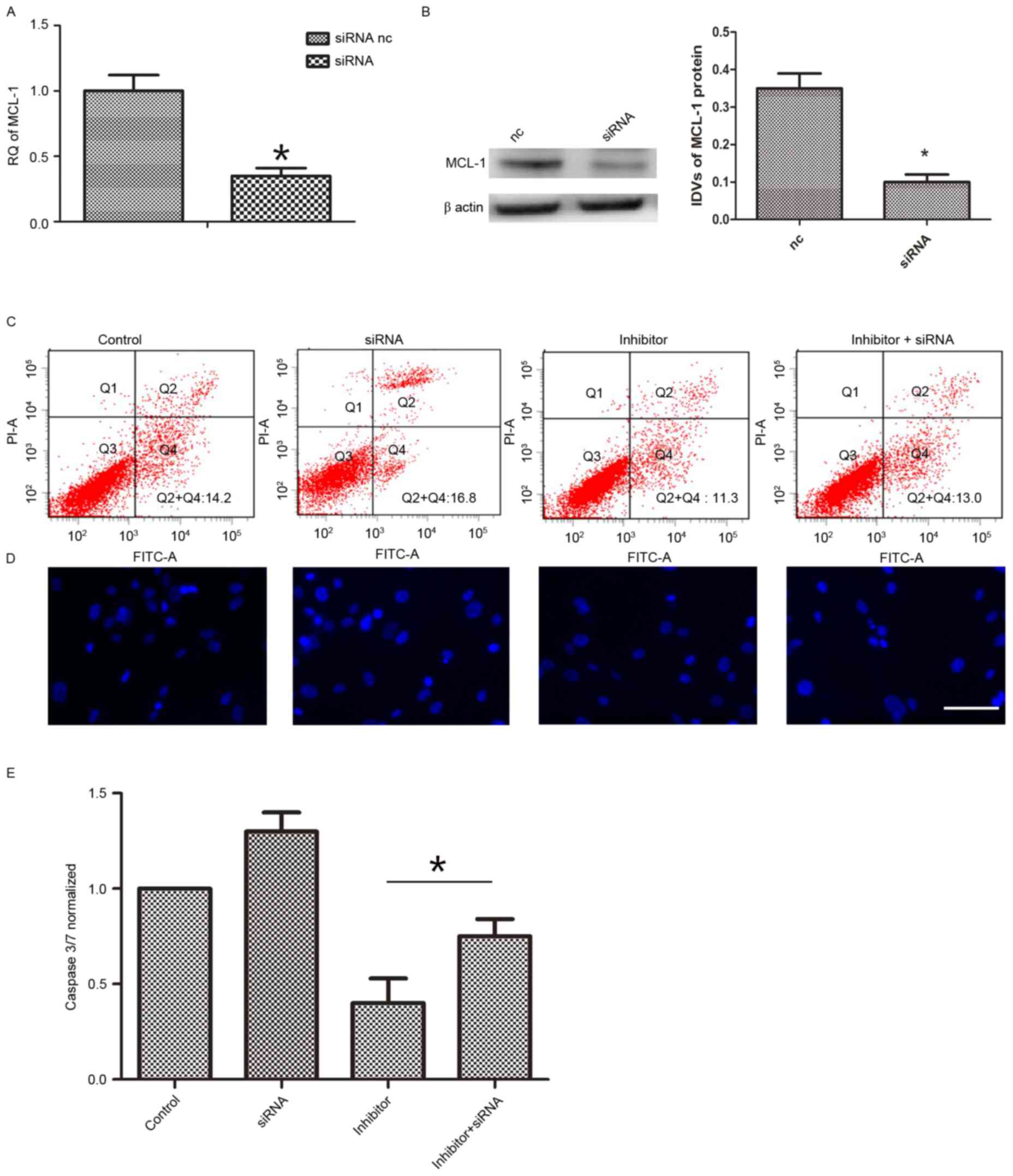
Furthermore, it was additionally determined whether
the effect of miR-29a on hAMSCs apoptosis was via MCL-1. The mRNA
levels of MCL-1 were knocked down (Fig. 4A and B). Following 48 h of
co-transfection with siRNA and miR-29a inhibitor, apoptosis
induction was conducted in each group. Then, FITC/PI assay, Caspase
3/7 activity assay and Hoechst 33258 staining assay were performed
to determine cell apoptosis. As demonstrated in Fig. 4C, MCL-1 inhibition (13.2±1.4%)
reversed the function of miR-29a inhibitor (9.7±1.6%) on cell
apoptosis. The observations were confirmed by Hoechst 33258
staining (Fig. 4D) and Caspase 3/7
activity assay (Fig. 4E)
demonstrating an apoptosis reduction with miR-29a inhibitor
compared with controls. Taking these findings together, it is
suggested that increased miR-29a results in increased levels of
apoptosis via targeting of MCL-1 (decreasing its levels).
Discussion
The therapeutic potential of MSCs for cardiac repair
is limited, partly due to their low survival rate within the
ischemic myocardial microenvironment into which they are introduced
(8,9). Hypoxia and serum deprivation,
imitating the ischemic engraftment environment, induce the
apoptosis of MSCs (20). To
improve cardiovascular cell therapy, continuous efforts have been
made to improve their function. The present study demonstrated that
hAMSCs cultured in EGM-2 conferred a protective effect against
serum-free and hypoxic conditions. In the specific culture
condition, hAMSCs expressed a lower level of miR-29a compared with
the normal conditions. This downregulation contributed in part to
the overexpression of MCL-1, the primary anti-apoptotic B-cell
lymphoma 2 (Bcl-2) family member and thus, to the hAMSCs
survival.
It was demonstrated that hAMSCs cultured in EGM-2
conferred a protective effect against serum-free and hypoxic
conditions, making them suitable for cell therapy. In endothelial
culture conditions, hAMSCs exhibited a cobblestone-like morphology,
yet expressed similar levels of surface makers compared with hAMSCs
cultured in DMEM. The present study is consistent with previous
studies demonstrating that EGM-2 mediated the morphological
alterations of hAMSCs. However, hAMSCs did not express the mature
endothelial cell markers, von Willebrand factor and vascular
endothelial-cadherin, and hAMSCs resisted undergoing a complete
differentiation into mature endothelial cells (21,22).
Therefore, hAMSCs cultured in EGM-2 medium still possess stem cell
characteristics and are unable to differentiate into endothelial
cells in the EGM-2 medium. Using RT-qPCR, a significantly lower
expression of miR-29a in EGM-2 was observed compared with cells
cultured in DMEM. The present study is the first, to the authors'
knowledge, to demonstrate the difference in expression of miR-29a
in hAMSCs cultured with EGM-2. Therefore miR-29a was selected for
the current study. Notably, miR-29a-inhibitor mediated protective
effects on the apoptosis of hAMSCs induced by serum starvation and
hypoxia. Results from the current study demonstrated that
endogenous MCL-1 protein expression was markedly decreased in
hAMSCs transfected with miR-29a.
It was demonstrated that MCL-1 is regulated at the
posttranscriptional level by miR-29a in hAMSCs, thus suggesting a
survival advantage in the silencing of miR-29a. MCL-1, in absence
of Bcl-2, promotes cell survival by inhibiting cell death (23,24).
It is known that miR-29 acts directly at the MCL-1 3′UTR. Mott
et al (25) were the first
to demonstrate in a cholangiocarcinoma cell model that MCL-1 may
additionally be regulated at the posttranscriptional level by
miR-29b. The results of the present study are in accordance with
those reported by Mott et al (25), Garzon et al (26) and Xiong et al (27), they identified that miR-29a,
another member of the miR-29 family, contributed to the
downregulation of MCL-1 in hAMSCs. It was observed in the present
study that targeted knockdown of MCL-1 by specific siRNA evidently
inhibited the protective effect of miR-29a-inhibitor on hypoxia-
and serum deprivation-induced apoptosis of hAMSCs.
However, there are studies suggesting that loss of
miR-29 results in cell death. In diabetic nephropathy, increasing
miR-29a action may protect against diabetic podocytopathy (28). In addition, in vivo evidence
demonstrated the neuronal cell death of brain-specific knockdown of
miR-29 (29). It therefore remains
to be elucidated whether the apoptotic action of miR-29a is
dependent on cell types or different culture conditions.
In conclusion, the present study demonstrated that
hAMSCs experienced decreased apoptosis when cultured in EGM-2,
partly through low expression of miR-29a, and this may provide a
novel tool to improve stem cell therapy in the future.
Acknowledgements
The present study was supported by the Science and
Technology Department of Liaoning Province (grant no
2013020200-206).
References
|
1
|
Parolini O, Alviano F, Bagnara GP, Bilic
G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M,
Mao N, et al: Concise review: Isolation and characterization of
cells from human term placenta: Outcome of the first international
workshop on placenta derived stem cells. Stem cells. 26:300–311.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bollini S, Pozzobon M, Nobles M, Riegler
J, Dong X, Piccoli M, Chiavegato A, Price AN, Ghionzoli M, Cheung
KK, et al: In vitro and in vivo cardiomyogenic differentiation of
amniotic fluid stem cells. Stem Cell Rev. 7:364–380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SW, Zhang HZ, Kim CE, Kim JM and Kim
MH: Amniotic mesenchymal stem cells with robust chemotactic
properties are effective in the treatment of a myocardial
infarction model. Int J Cardiol. 168:1062–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katritsis DG, Sotiropoulou PA, Karvouni E,
Karabinos I, Korovesis S, Perez SA, Voridis EM and Papamichail M:
Transcoronary transplantation of autologous mesenchymal stem cells
and endothelial progenitors into infarcted human myocardium.
Catheter Cardiovasc Interv. 65:321–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeevanantham V, Butler M, Saad A,
Abdel-Latif A, Zuba-Surma EK and Dawn B: Adult bone marrow cell
therapy improves survival and induces long-term improvement in
cardiac parameters: A systematic review and meta-analysis.
Circulation. 126:551–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hristov M, Heussen N, Schober A and Weber
C: Intracoronary infusion of autologous bone marrow cells and left
ventricular function after acute myocardial infarction: A
meta-analysis. J Cell Mol Med. 10:727–733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hua P, Liu JY, Tao J and Yang SR:
Application and progress of combined mesenchymal stem cell
transplantation in the treatment of ischemic cardiomyopathy. Biomed
Res Int. 2015:5685022015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geng YJ: Molecular mechanisms for
cardiovascular stem cell apoptosis and growth in the hearts with
atherosclerotic coronary disease and ischemic heart failure. Ann N
Y Acad Sci. 1010:687–697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamedi-Asl P, Halabian R, Bahmani P,
Mohammadipour M, Mohammadzadeh M, Roushandeh AM,
Jahanian-Najafabadi A, Kuwahara Y and Roudkenar MH:
Adenovirus-mediated expression of the HO-1 protein within MSCs
decreased cytotoxicity and inhibited apoptosis induced by oxidative
stresses. Cell Stress Chaperones. 17:181–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang YL, Tang Y, Zhang YC, Qian K, Shen L
and Phillips MI: Improved graft mesenchymal stem cell survival in
ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J
Am Coll Cardiol. 46:1339–1350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He J, Wang C, Sun Y, Lu B, Cui J, Dong N,
Zhang M, Liu Y and Yu B: Exendin-4 protects bone marrow-derived
mesenchymal stem cells against oxygen/glucose and serum
deprivation-induced apoptosis through the activation of the
cAMP/PKA signaling pathway and the attenuation of ER stress. Int J
Mol Med. 37:889–900. 2016.PubMed/NCBI
|
|
13
|
Jin J, Jeong SI, Shin YM, Lim KS, Shin Hs,
Lee YM, Koh HC and Kim KS: Transplantation of mesenchymal stem
cells within a poly (lactide-co-epsilon-caprolactone) scaffold
improves cardiac function in a rat myocardial infarction model. Eur
J Heart Fail. 11:147–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye Y, Hu Z, Lin Y, Zhang C and Perez-Polo
JR: Downregulation of microRNA-29 by antisense inhibitors and a
PPAR-gamma agonist protects against myocardial
ischaemia-reperfusion injury. Cardiovasc Res. 87:535–544. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang F, Cui J, Liu X, Lv B, Liu X, Xie Z
and Yu B: Roles of microRNA-34a targeting SIRT1 in mesenchymal stem
cells. Stem Cell Res Ther. 6:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H,
Xiao J, Shan H, Wang Z and Yang B: The muscle-specific microRNAs
miR-1 and miR-133 produce opposing effects on apoptosis by
targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci.
120:3045–3052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Wang Z, Wang R, Zhao F, Shi P,
Jiang Y and Pang X: Direct comparison of the potency of human
mesenchymal stem cells derived from amnion tissue, bone marrow and
adipose tissue at inducing dermal fibroblast responses to cutaneous
wounds. Int J Mol Med. 31:407–415. 2013.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem cells. 24:416–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
König J, Huppertz B, Desoye G, Parolini O,
Fröhlich JD, Weiss G, Dohr G, Sedlmayr P and Lang I: Amnion-derived
mesenchymal stromal cells show angiogenic properties but resist
differentiation into mature endothelial cells. Stem Cells Dev.
21:1309–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
König J, Weiss G, Rossi D, Wankhammer K,
Reinisch A, Kinzer M, Huppertz B, Pfeiffer D, Parolini O and Lang
I: Placental mesenchymal stromal cells derived from blood vessels
or avascular tissues: What is the better choice to support
endothelial cell function? Stem Cells Dev. 24:115–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Opferman JT, Letai A, Beard C, Sorcinelli
MD, Ong CC and Korsmeyer SJ: Development and maintenance of B and T
lymphocytes requires antiapoptotic MCL-1. Nature. 426:671–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maurer U, Charvet C, Wagman AS, Dejardin E
and Green DR: Glycogen synthase kinase-3 regulates mitochondrial
outer membrane permeabilization and apoptosis by destabilization of
MCL-1. Mol Cell. 21:749–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: Mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garzon R, Heaphy CE, Havelange V, Fabbri
M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA,
et al: MicroRNA 29b functions in acute myeloid leukemia. Blood.
114:5331–5341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenicity and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
28
|
Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY,
Chuang PC, Huang YT, Wang SY, Wu SL, Chen YS, et al: MicroRNA-29a
promotion of nephrin acetylation ameliorates hyperglycemia-induced
podocyte dysfunction. J Am Soc Nephrol. 25:1698–1709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roshan R, Shridhar S, Sarangdhar MA, Banik
A, Chawla M, Garg M, Singh VP and Pillai B: Brain-specific
knockdown of miR-29 results in neuronal cell death and ataxia in
mice. RNA. 20:1287–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|