Introduction
Immune thrombocytopenia (ITP) is currently defined
as an autoimmune disease, characterized by a decreased platelet
count (due to autoantibodies mediating platelet destruction and
insufficient platelet production), which results in purpura and
increase of bleeding tendency (1).
However, the pathogenesis of ITP remains to be completely
elucidated. A previous study demonstrated that ITP was not always
associated with a decline in platelet number (2).
Platelets are essential for proper hemostasis and
thrombosis. Although platelets lack nucleus, they contain all other
necessary components to perform transcription and translation in a
signal-dependent manner (3–6).
Furthermore, researchers identified that platelets contain abundant
and diverse microRNAs (miRNAs/miRs), the key regulators in gene
expression alterations (7).
Extensive studies were performed to understand the transcriptome of
platelets using microarrays or an RNA deep sequencing approach
(8,9). miRNAs are present in platelets in
variable quantities, and are diverse in humans with specific
phenotypes and in different disease states (10,11).
Among them, hsa-miR-96 regulated the expression of
vesicle-associated membrane protein 8 (also known as endobrevin)
(12). However, miRNA targets in
ITP are unknown.
Increasing evidence has demonstrated that the
expression of aberrant miRNAs is associated with the pathogenesis
of ITP (13,14). However, the association between
miRNAs and the decrease in platelets in patients with ITP was
poorly investigated. In the present study differentially expressed
miRNAs were investigated in platelets from patients with ITP and
healthy control patients. Furthermore, the regulatory network of
miRNA-targets was established based on the information from the
differentially expressed miRNAs (hsa-miR-548a-5p,
hsa-miR-1185-2-3p, hsa-miR-30a-3p, hsa-miR-6867-5p, hsa-miR-765 and
hsa-miR-3125) identified. The present analyses may be important in
the understanding of the mechanisms of ITP, as well as future
therapy.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of Soochow University (Soochow, China) and written
informed consent was obtained from all the patients and healthy
donors involved.
Subjects
A total of 22 patients with ITP, and 8 age- and
sexual-matched healthy donators were recruited from the First
Affiliated Hospital of Soochow University (between March 1 and
December 31 2015; Table I). The
diagnosis of ITP was based on the criteria of the American Society
of Hematology (15) and
thrombocytopenia was defined as a platelet count of
<50×109 platelets/l. The patients with ITP had not
received glucocorticoids or immunosuppressive treatment. Patients
with the following complications were excluded: Diabetes,
hypertension, cardiovascular diseases, pregnancy, active infection
or autoimmune diseases other than ITP. Of the 22 ITP samples, 8
samples were studied by microarray together with 8 healthy samples,
the other 14 ITP patient samples were tested using the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
following performance of the microarray. Prior to the microarray,
the platelet concentrations from 8 patients with ITP were adjusted
to 100×109 platelets/l. A total of 1 ml each sample was
used for the ITP groups, for a total of 8 ml. The platelets for the
control groups were processed in the same way, for a total of 8
ml.
 | Table I.Clinical characteristics of the
patients with ITP and healthy controls. |
Table I.
Clinical characteristics of the
patients with ITP and healthy controls.
| Patient no. | Sex | Age, years | Bleeding
symptoms | PLT count,
PLTx109/l | Group | Test |
|---|
| 1 | F | 78 | EC, GH | 33 | ITP | Microarray |
| 2 | M | 45 | EC, EP | 42 | ITP | Microarray |
| 3 | M | 56 | PT | 40 | ITP | Microarray |
| 4 | F | 37 | PT, EC | 35 | ITP | Microarray |
| 5 | M | 53 | PT | 43 | ITP | Microarray |
| 6 | F | 52 | EC | 28 | ITP | Microarray |
| 7 | F | 21 | PT, GH | 28 | ITP | Microarray |
| 8 | F | 63 | EC, GH | 34 | ITP | Microarray |
| 9 | F | 22 | EC, EP | 42 | ITP | RT-qPCR |
| 10 | F | 33 | PT | 37 | ITP | RT-qPCR |
| 11 | M | 36 | PT, EC | 29 | ITP | RT-qPCR |
| 12 | F | 31 | PT | 43 | ITP | RT-qPCR |
| 13 | F | 40 | EC | 34 | ITP | RT-qPCR |
| 14 | F | 34 | PT, GH | 36 | ITP | RT-qPCR |
| 15 | M | 38 | PT | 38 | ITP | RT-qPCR |
| 16 | M | 29 | EC | 45 | ITP | RT-qPCR |
| 17 | F | 36 | EC, GH | 25 | ITP | RT-qPCR |
| 18 | F | 31 | EC, GH | 47 | ITP | RT-qPCR |
| 19 | F | 40 | EP | 41 | ITP | RT-qPCR |
| 20 | M | 63 | PT | 38 | ITP | RT-qPCR |
| 21 | F | 22 | PT | 42 | ITP | RT-qPCR |
| 22 | M | 33 | EC, GH | 41 | ITP | RT-qPCR |
| 23 | F | 36 | NA | 157 | Healthy
control | Microarray,
RT-qPCR |
| 24 | F | 31 | NA | 252 | Healthy
control | Microarray,
RT-qPCR |
| 25 | F | 40 | NA | 383 | Healthy
control | Microarray,
RT-qPCR |
| 26 | M | 34 | NA | 292 | Healthy
control | Microarray,
RT-qPCR |
| 27 | F | 38 | NA | 371 | Healthy
control | Microarray,
RT-qPCR |
| 28 | M | 29 | NA | 198 | Healthy
control | Microarray,
RT-qPCR |
| 29 | F | 36 | NA | 229 | Healthy
control | Microarray,
RT-qPCR |
| 30 | F | 31 | NA | 257 | Healthy
control | Microarray,
RT-qPCR |
Preparation of leukocyte-depleted
apheresis platelets (LDPs)
LDPs were prepared as previously reported (14). To deplete white blood cells (WBCs),
reticulocytes and red blood cells (RBCs), the platelets were
treated with pan-leukocyte [anti-cluster of differentiation
(CD)45+, anti-CD71+, and
anti-CD235+] immunomagnetic beads, according to the
manufacturer s instruction (Invitrogen, Carlsbad, CA, USA).
Following treatment, WBCs, RBCs and reticulocytes were not detected
by flow cytometry (16). The
leukocyte-depleted platelets from 8 ITP patients and 8 health
controls were pooled, respectively.
RNA extraction
Total RNA was extracted using an miRNA isolation kit
(Beijing CoWin Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocol. The quantity and purity was determined
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and Agilent Bioanalyzer 2100 (Agilent
Technologies, Inc., Santa Clara, CA, USA), respectively.
miRNA microarray analysis
The Agilent Human miRNA (8×60K) Array (version 21.0;
design ID: 70156; Agilent Technologies, Inc.), which covers 2549
human miRNAs (based on miRBase release 21.0) (17), was used to detect miRNA expression
in platelets. Microarray experiments were performed by Shanghai
Biotechnology Company (www.ebioservice.com; Shanghai, China). Normalization
was performed using Gene Spring software (version 11.0; Agilent
Technologies, Inc.). Student's t-tests were used in the gene
screening. P<0.05 was considered to indicate a statistically
significant difference, and the fold change threshold values were
>3.0 and <0.33. Hierarchical clustering was performed to
generate miRNA and sample trees based on Pearson correlation using
MeV software (version 4.0; Multi Experiment Viewer; www.tm4.org/#/welcome).
miRNA RT-qPCR analysis
A total of 9 differentially expressed miRNAs
identified by microarray were selected for further validation using
RT-qPCR. For the reverse transcription of total RNA, the miRNA cDNA
kit (Beijing CoWin Biotech Co., Ltd.) was used, according to the
manufacturer's protocol. Total RNAs were initially treated with
Escherichia coli poly-A polymerase to generate a poly-A tail
at the 3′-end of each miRNA. Following polyadenylation, the miRNA
first strand cDNA was synthesized using the poly (T) adapter
(GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN) as primer, at 42°C
for 1 h. To measure the expression of mature miRNAs, the
miRNA-first strand cDNAs was analyzed using the miRNA Real-Time PCR
Assay kit (Beijing CoWin Biotech Co., Ltd.) and a StepOnePlus™
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primers for the RT-qPCR analysis are listed in Table II. Results were normalized to 5S
ribosomal RNA. The thermocycling conditions were 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
data were quantified using the 2−ΔΔCq method (18). The data were processed using
StepOne™ software (version 2.2.2; Applied Biosystems; Thermo Fisher
Scientific, Inc.).
 | Table II.List of primers used for the reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II.
List of primers used for the reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer (5′ to
3′) |
|---|
| 5S rRNA
forward |
TACGGCCATACCACCCTGAA |
| 5S rRNA
reverse |
TAACCAGGCCCGACCCTGCT |
| hsa-miR-338-5p |
AACAATATCCTGGTGCTGAGTG |
| hsa-miR-122-5p |
TGGAGTGTGACAATGGTGTTTG |
| hsa-miR-451b |
TAGCAAGAGAACCATTACCATT |
| hsa-miR-452-5p |
AACTGTTTGCAGAGGAAACTGA |
| hsa-miR-15a-3p |
CAGGCCATATTGTGCTGCCTCA |
|
hsa-miR-548a-5p |
AAAAGTAATTGCGAGTTTTACC |
| hsa-miR-30a-3p |
CTTTCAGTCGGATGTTTGCAGC |
| hsa-miR-765 |
TGGAGGAGAAGGAAGGTGATG |
| hsa-miR-765 |
TGGAGGAGAAGGAAGGTGATG |
| hsa-miR-224-3p |
AAAATGGTGCCCTAGTGACTACA |
|
hsa-miR-133a-3p |
TTTGGTCCCCTTCAACCAGCTG |
| hsa-miR-491-5p |
AGTGGGGAACCCTTCCATGAGG |
| hsa-miR-3125 |
TAGAGGAAGCTGTGGAGAGA |
|
hsa-miR-1185-2-3p |
ATATACAGGGGGAGACTCTCAT |
|
hsa-miR-6867-5p |
TGTGTGTGTAGAGGAAGAAGGGA |
| Universal reverse
primer |
GCGAGCACAGAATTAATACGACTC |
Prediction of target genes of
differentially expressed miRNAs
The target genes of the candidate miRNAs were
predicted using online tools contained within miRWalk software
(www.umm.uni-heidelberg.de/apps/zmf/mirwalk)
(19) and six bioinformatic
algorithms (DIANAmT, miRanda, miRDB, miRWalk, PicTar and
TargetScan).
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analyses
To further understand the biological function of
differentially expressed miRNAs, the Gene Ontology and KEGG
analyses were conducted using the Database for Annotation,
Visualization and Integrated Discovery online analysis tool
(20) and GENECODIS (21). Fisher's exact test and
χ2 tests were used to select the significant GO category
or KEGG pathway, and the false discovery rate (FDR) was calculated
to correct the p-value. FDR <0.01 and P<0.01 were considered
to be statistically significant.
Regulatory network construction
between miRNAs and their targets
The post-transcriptional regulatory network is
defined as a directed and bipartite graph in which expressions of
miRNAs and their targets are reversely correlated. A regulatory
network of miRNAs and their potential targets was presented using
Cytoscape software (22).
Results
Identification of the differentially
expressed miRNAs in patients with ITP
ITP is a severe disease that affects humans,
previous results have demonstrated that miRNAs may serve an
important role in ITP pathogenesis (23–25).
However, the role of platelet-derived miRNAs in ITP remains to be
examined. To determine miRNA alterations in ITP platelets compared
with the healthy controls, microarray analysis was performed. The
results demonstrated that 537 and 544 miRNAs were expressed in the
ITP and control samples, respectively. Among them, 115 miRNAs were
differentially expressed (fold-change of >3 or <0.33;
P<0.05). Of these, 57 miRNAs were upregulated while 58 miRNAs
were downregulated in the ITP samples compared with the healthy
samples.
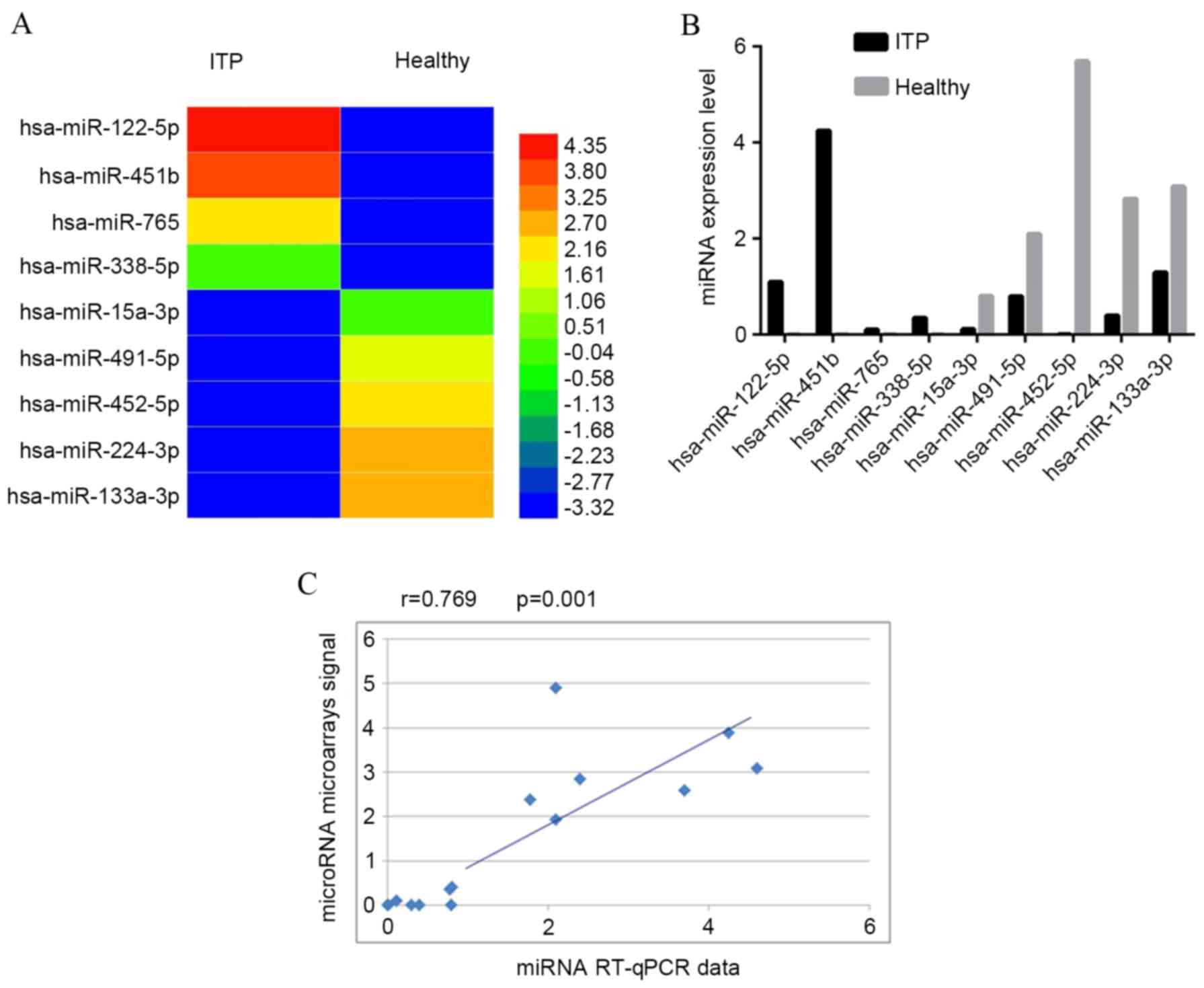
Of the differentially expressed miRNAs,
hsa-miR-338-5p, hsa-miR-765, hsa-miR-122-5p and hsa-miR-451b
(specifically expressed in ITP), and hsa-miR-133a-3p,
hsa-miR-224-3p, hsa-miR-452-5p, hsa-miR-491-5p and hsa-miR-15a-3p
(restricted to control group) were verified by RT-qPCR analysis.
The RT-qPCR results demonstrated similar patterns as observed in
the microarray data (Fig. 1). The
top 20 differentially expressed miRNAs are listed in Table III.
 | Table III.Top 20 differentially expressed
miRs. |
Table III.
Top 20 differentially expressed
miRs.
| A, Upregulated |
|---|
|
|---|
| miR | Fold change |
|---|
| hsa-miR-4730 | 305.53 |
| hsa-miR-122-5p | 297.44 |
|
hsa-miR-6716-3p | 273.18 |
| hsa-miR-575 | 230.61 |
|
hsa-miR-6867-5p | 219.03 |
|
hsa-miR-4800-5p | 208.65 |
|
hsa-miR-4778-5p | 180.35 |
|
hsa-miR-4716-3p | 179.61 |
|
hsa-miR-5581-5p | 164.55 |
|
hsa-miR-6767-5p | 159.14 |
| hsa-miR-451b | 147.17 |
|
hsa-miR-4653-3p | 131.93 |
|
hsa-miR-5088-5p | 120.57 |
|
hsa-miR-6751-3p | 115.94 |
|
hsa-miR-6512-5p | 105.84 |
|
hsa-miR-6873-3p | 104.97 |
| hsa-miR-5194 | 104.28 |
| hsa-miR-4634 | 96.54 |
| hsa-miR-3125 | 94.56 |
|
hsa-miR-3607-3p | 92.27 |
|
| B,
Downregulated |
|
| miR | Fold change |
|
| hsa-miR-299-3p | 0.01 |
|
hsa-miR-487a-3p | 0.01 |
| hsa-miR-370-3p | 0.01 |
| hsa-miR-136-3p | 0.01 |
|
hsa-miR-1307-5p | 0.01 |
|
hsa-miR-133a-3p | 0.01 |
|
hsa-miR-1185-2-3p | 0.01 |
| hsa-miR-3174 | 0.01 |
| hsa-miR-412-5p | 0.01 |
|
hsa-miR-3194-5p | 0.01 |
| hsa-miR-224-3p | 0.01 |
|
hsa-miR-548a-5p | 0.01 |
| hsa-miR-30a-3p | 0.02 |
|
hsa-miR-3617-5p | 0.02 |
| hsa-miR-542-3p | 0.02 |
| hsa-miR-9-3p | 0.02 |
| hsa-miR-452-5p | 0.02 |
|
hsa-miR-5187-5p | 0.02 |
| hsa-miR-766-5p | 0.02 |
| hsa-miR-100-5p | 0.02 |
Identification of the targets of
differentially expressed miRNAs
To identify the target genes of the differentially
expressed miRNAs in ITP, bioinformatic prediction was performed
using the miRWalk database. A total of 677 pairs of miRNA-target
genes were identified for the upregulated miRNAs and 1,274 pairs
for the downregulated miRNAs (data not shown).
GO and KEGG analyses of target genes
of downregulated miRNAs in ITP
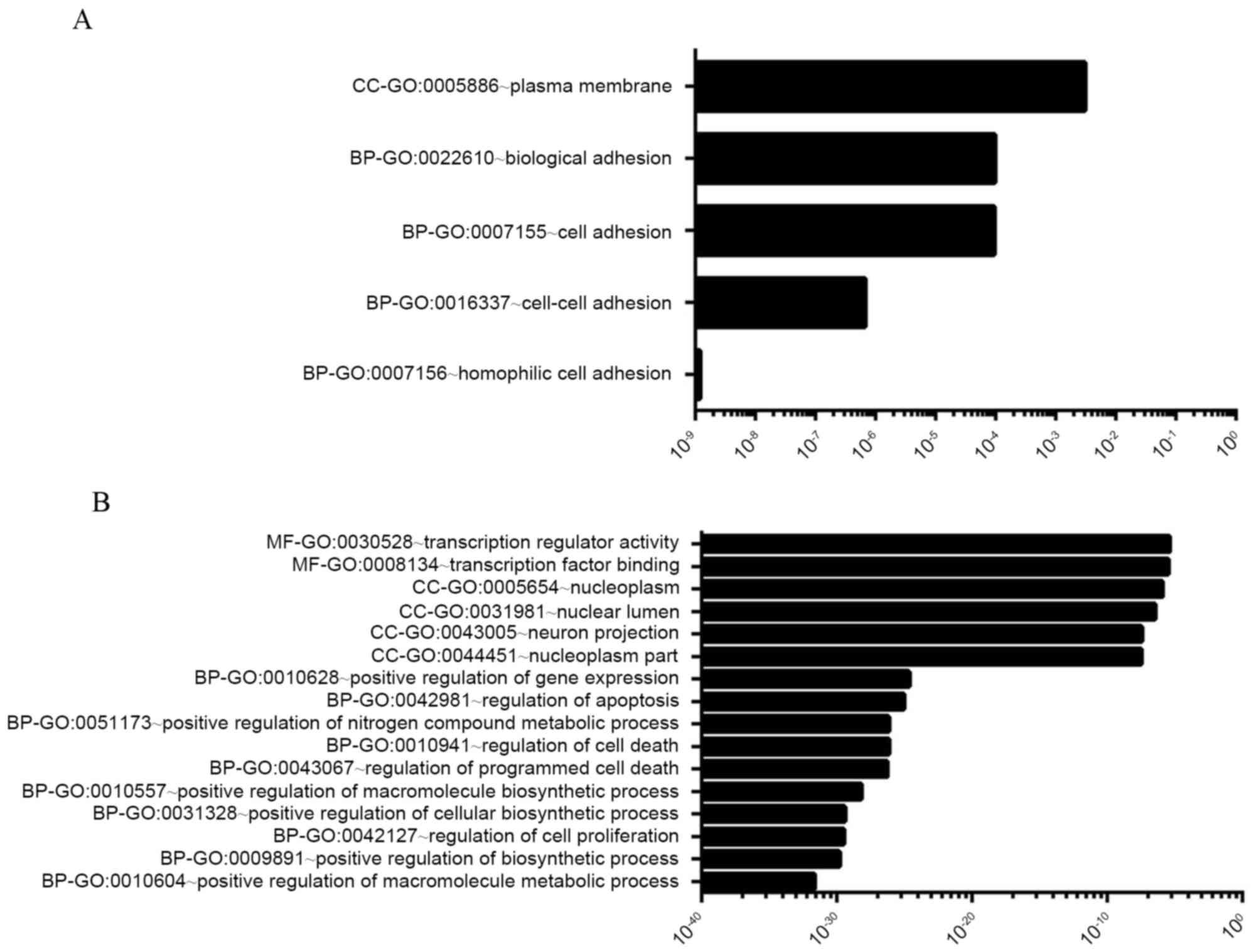
GO functional and KEGG pathway analyses were
performed for the 1,274 target genes of the downregulated ITP
miRNAs. GO terms were assigned to the potential targets. The GO
terms associated with the targets were categorized (FDR<0.01;
P<0.01) into 16 classes. In order to better understand the
function of the involved genes, these GO terms were divided into
three groups including biological processes (BP; 10 classes),
molecular function (MF; 2 classes) and cellular components (CC; 4
classes). In the BP group, the major regulatory pathways included
GO:0010604-positive regulation of macromolecular metabolic
processes, GO:0009891-positive regulation of biosynthetic
processes, GO:0042127-regulation of cellular proliferation,
GO:0031328-positive regulation of cellular biosynthetic processes,
GO:0010557-positive regulation of macromolecular biosynthetic
processes, GO:0043067-regulation of programmed cell death,
GO:0010941-regulation of cell death, GO:0051173-positive regulation
of nitrogen compound metabolic processes, GO:0042981-regulation of
apoptosis and GO:0010628-positive regulation of gene expression
(Fig. 2A). The most predicted
results were involved with apoptosis and cell death.
To better understand the function of potential
targets, signaling pathways were analyzed using the KEGG database
(24 signaling pathways). The pathways associated with the
downregulated miRNAs in ITP (P<0.01) included the Wnt signaling
pathway (KEGG pathway, hsa04,310; P=2.24×10−5), the
global cancer pathway map (KEGG pathway, hsa05,200;
P=1.93×10−4), a small cell lung cancer-associated
pathway (KEGG pathway, hsa05, 222; P=1.20×10−3), the
mechanistic target of rapamycin signaling pathway (KEGG pathway,
hsa04, 150; P=3.11×10−3), a pancreatic cancer-associated
pathway (KEGG pathway, hsa05212; P=3.14×10−3).
GO and KEGG analyses of target genes
of upregulated miRNAs in ITP
GO enrichment and KEGG pathway analyses were
performed for the 677 target genes of the upregulated ITP miRNAs.
The GO terms associated with the targets were categorized into 5
classes (FDR<0.20; P<0.01), including BP (4 classes;
GO:0007156-homophilic cell adhesion, GO:0016337-cell-cell adhesion,
GO:0007155-cell adhesion, GO:0022610-biological adhesion) and CC (1
class; CC-GO:0005886-plasma membrane) (Fig. 2B).
To better understand the function of potential
targets, signaling pathways were analyzed using the KEGG database
(7 signaling pathways; Fig. 3).
The pathway associated with the upregulated miRNAs in ITP
(P<0.05) was the cell adhesion molecules (CAMs) pathway, it was
significantly enriched in the analysis (hsa04, 514;
P=1.47×10−2).
Regulatory network of miRNAs and their
target genes
In order to investigate the association among the
miRNAs of interest, the miRNA target gene regulatory network in ITP
was created using Cytoscape software. The upregulated miRNAs,
downregulated miRNAs and their targets formed a regulatory network.
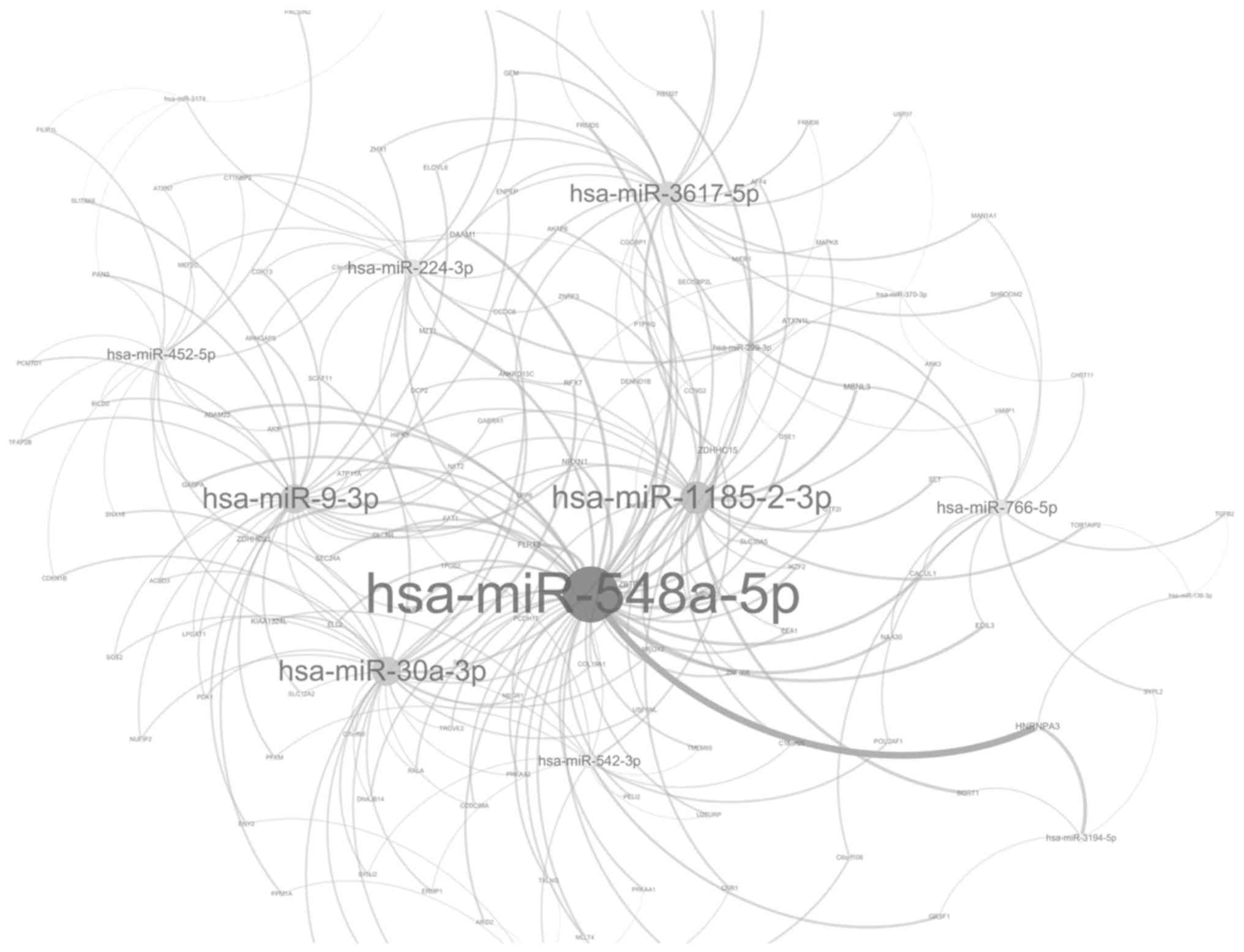
Among the downregulated miRNAs, hsa-miR-548a-5p exhibited the
maximum number of target genes (50 genes; Fig. 3). Among the upregulated miRNAs,
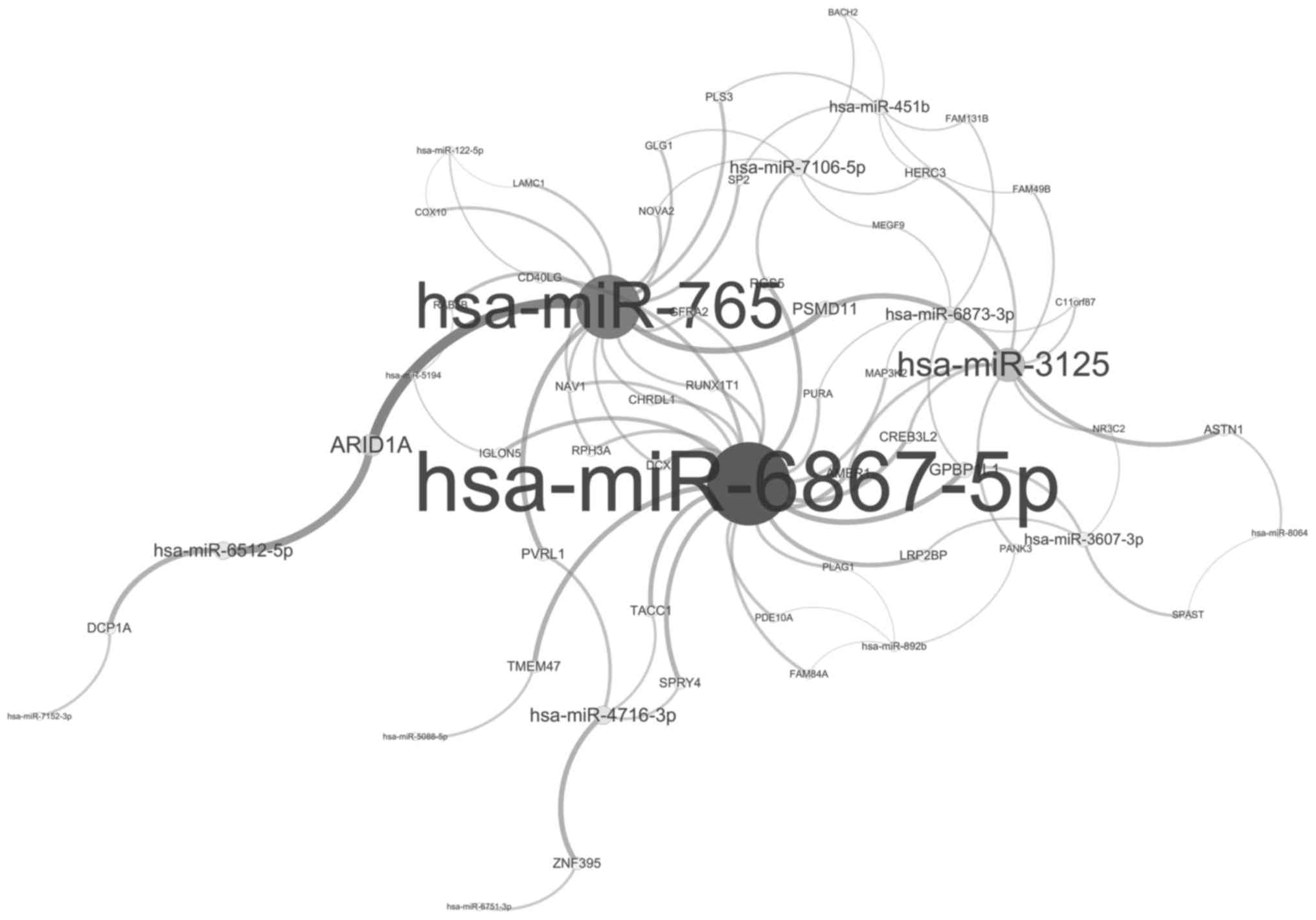
hsa-miR-6867-5p possessed 24 regulatory target genes, while
hsa-miR-765 and hsa-miR-3125 targeted 18 and 9 genes, respectively
(Fig. 4).
Discussion
The functions of platelets, including activation,
adhesion and aggregation, are crucial for coagulation physiology
and the maintenance of hemostatic balance. Platelet dysfunction is
associated with various pathologies, including atherosclerosis,
occlusive or thrombotic hemorrhagic disorders (26). The roles of miRNAs in platelets
have been given increasing attention due to their importance in ITP
pathogenesis. To date, the studies have investigated the roles of
miRNAs in biological processes in platelets. Girardot et al
(27) demonstrated that
hsa-miR-28, as well as other miRNAs, targets receptor of
thrombopoietin (MPL) and MPL repression is potentially involved in
the regulation of platelet count. Nagalla et al (28) reported that hsa-miR-107 targets
clock circadian regulator, which may regulate circadian platelet
reactivity. The authors previously demonstrated that hsa-miR-326
served a crucial role in platelet apoptosis during storage
(29).
It is well-known that platelets have mRNA and mRNA
splicing machinery, and are able to translate mRNA into proteins
(3,30). Platelets also contain miRNA
processing machinery, including endoribonuclease dicer (DICER1),
TAR RNA-binding protein 2 and protein argonaute-2, which is
involved in the processing of pre-miRNA into mature miRNA (7). Microarray-based studies demonstrated
that ≤32% of human mRNAs were expressed in platelets (31,32).
Several studies have focused on the analysis of the platelet
transcriptome (6,31–34)
and deduced a certain correlation between mRNA and coupled proteins
(31,34). The mRNA in platelets originated
from megakaryocytes and seem to be affected by aging and platelet
activation (8,35). Zhang et al (36) observed that 6 marker proteins with
significant differences in the platelet lysates of patients with
primary ITP patients compared with the secondary ITP and healthy
controls.
Patients with ITP exhibit a decreased platelet count
accompanied with dysfunction, including increased apoptosis and the
reduction of adhesion function (37–39).
However, the underlying mechanism of ITP pathogenesis remains
unclear. In the present study, the expression of platelet miRNAs
was analyzed by microarray. The miRNAs expressed in the platelets
of patients with ITP and healthy controls were compared, and there
were 115 differentially expressed miRNAs between the two groups. To
confirm the reliability of the microarray results, 9 differentially
expressed miRNAs were further verified using RT-qPCR. The results
of the RT-qPCR data were consistent with the microarray data
obtained (Fig. 1). Among a total
of 115 differentially expressed miRNAs, 57 miRNAs were upregulated
while 58 miRNAs were downregulated in ITP. The observations also
suggested that human platelets contain different types of miRNAs,
and these were stable and reproducible (Table IV). The data was consisted with
the report by Osman and Falker (40).
 | Table IV.Comparison between the top 20
differentially expressed platelet miRNAs in the present study and
the report by Osman and Falker (36). |
Table IV.
Comparison between the top 20
differentially expressed platelet miRNAs in the present study and
the report by Osman and Falker (36).
| ITP (miRbase
21) | Healthy control
(miRbase 21) | Characterization of
human platelet miRNA by reverse transcription-quantitative
polymerase chain reaction analysis (miRbase 14) |
|---|
| hsa-miR-7975 | hsa-miR-7975 | hsa-miR-223-3p |
| hsa-miR-7977 | hsa-miR-7977 | hsa-miR-92 |
| hsa-miR-5100 | hsa-miR-223-3p | hsa-miR-16-5p |
| hsa-miR-223-3p | hsa-miR-21-5p | hsa-miR-126-3p |
| hsa-miR-1260a |
hsa-miR-103a-3p | hsa-miR-142-3p |
| hsa-miR-21-5p | hsa-let-7a-5p | hsa-miR-26a-5p |
| hsa-miR-16-5p | hsa-miR-16-5p | hsa-miR-565 |
| hsa-miR-451a | hsa-let-7f-5p | hsa-miR-484 |
| hsa-miR-6090 | hsa-miR-26a-5p | hsa-miR-486 |
| hsa-let-7a-5p | hsa-let-7b-5p | hsa-miR-222 |
|
hsa-miR-103a-3p | hsa-miR-107 | hsa-miR-451a |
| hsa-let-7f-5p | hsa-miR-5100 | hsa-miR-191 |
| hsa-miR-4286 | hsa-miR-24-3p | hsa-miR-24-3p |
| hsa-miR-6089 |
hsa-miR-130a-3p | hsa-miR-650 |
|
hsa-miR-1273g-3p | hsa-miR-126-3p | hsa-miR-93 |
| hsa-miR-126-3p | hsa-miR-23a-3p | hsa-miR-20a-5p |
| hsa-miR-107 | hsa-let-7d-5p | hsa-miR-19b-3p |
| hsa-let-7b-5p | hsa-miR-19b-3p | hsa-miR-26b |
| hsa-miR-7641 | hsa-miR-20a-5p | hsa-miR-17 |
| hsa-miR-23a-3p | hsa-miR-15b-5p | hsa-miR-106b |
To better understand the function of miRNAs,
bioinformatic analysis was performed, including GO and KEGG pathway
analysis. The results revealed that 21 GO terms and 6 signaling
pathways were associated with the potential targets (P<0.01).
Networks of 16 GO terms and 5 pathways of interest were built
between downregulated miRNAs and their target genes. The results
demonstrated that downregulated miRNAs may be involved in platelet
apoptosis and cell death. Among these downregulated miRNAs,
hsa-miR-548a-5p was the most important modulator and was able to
modulate 50 target genes. The targets of hsa-miR-548a-5p, including
DICER1, histone acetyltransferase p300, low-density lipoprotein
receptor related protein 1B, ADAM metallopeptidase domain 8
(ADAM8), serine carboxypeptidase 1, topoisomerase (DNA) II α and
erb-b2 receptor tyrosine kinase 2, were involved in apoptosis.
Zhang et al (41) reported
that ADAM8 potentially served a role in the pathogenesis of
non-small cell lung cancer by resisting cisplatin-mediated
apoptosis. Excluding hsa-miR-548a-5p, the other downregulated
miRNAs were also predicted to serve important roles in cellular
apoptosis. miR-9-3p serves a role in tumor suppression by targeting
tafazzin in hepatocellular carcinoma cells. The results of the
present study indicated in GO terms that these downregulated miRNAs
in ITP may promote platelet apoptosis.
Networks of five GO terms and one pathway of
interest were built between upregulated miRNAs and their target
genes. The results demonstrated that upregulated miRNAs may be
involved in platelet adhesion. Among these upregulated miRNAs,
hsa-miR-6867-5p was the most important modulator and was able to
modulate 24 target genes. The targets of hsa-miR-6867-5p, including
cyclin D1, CD40 ligand, integrin subunit α11 and PLAG1 zinc finger,
were involved in cellular adhesion.
Following GO analysis, the KEGG database was
employed to analyze the pathways involved in the predicted miRNA
target genes. KEGG analysis demonstrated that these signaling
pathways were associated with the CAMs pathway. In the present
study, the CAMs pathway was the most associated pathway.
hsa-miR-6867-5p, hsa-miR-122-5p and hsa-miR-892b may comodulate the
CAMs pathway. The results suggested that several miRNAs
coparticipate in the same pathways and serve important roles in the
cell adhesion of platelets. Previous studies demonstrated that the
CAMs pathway was implicated in the pathogenesis of ITP (42,43).
The present research implied that miRNAs may serve an important
role in the platelet apoptosis of ITP. Further studies are required
to provide more information in understanding the underlying
mechanism of ITP pathogenesis.
In conclusion, 115 differentially expressed miRNAs
in the platelets from patients with ITP and healthy controls were
identified. The predication of these differentially expressed
miRNAs and their target genes provided information on the
understanding of ITP pathogenesis. A number of the miRNA-regulated
molecular networks and biological processes identified in the
present study are associated with platelet apoptosis and adhesion.
The molecular pathways presented in the present study constituted a
comprehensive resource for future investigations into the role of
specific miRNAs in ITP pathogenesis.
Acknowledgements
The present study was supported by grants from the
Project of Ningbo Medical Science and Technology Plans (grant no.
2016A17), the Ningbo City Natural Science Foundation (grant no.
2,015A610308) and Zhejiang Provincial Natural Science Foundation
(grant no. LY16H200005).
References
|
1
|
Neunert CE: Current management of immune
thrombocytopenia. Hematology Am Soc Hematol Educ Program.
2013:276–282. 2013.PubMed/NCBI
|
|
2
|
Varga-Szabo D, Pleines I and Nieswandt B:
Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc
Biol. 28:403–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Denis MM, Tolley ND, Bunting M, Schwertz
H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda
KJ, et al: Escaping the nuclear confines: Signal-dependent pre-mRNA
splicing in anucleate platelets. Cell. 122:379–391. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dittrich M, Birschmann I, Pfrang J,
Herterich S, Smolenski A, Walter U and Dandekar T: Analysis of SAGE
data in human platelets: Features of the transcriptome in an
anucleate cell. Thromb Haemost. 95:643–651. 2006.PubMed/NCBI
|
|
5
|
Schwertz H, Tolley ND, Foulks JM, Denis
MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss
LW, et al: Signal-dependent splicing of tissue factor pre-mRNA
modulates the thrombogenicity of human platelets. J Exp Med.
203:2433–2440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rowley JW, Oler AJ, Tolley ND, Hunter BN,
Low EN, Nix DA, Yost CC, Zimmerman GA and Weyrich AS: Genome-wide
RNA-seq analysis of human and mouse platelet transcriptomes. Blood.
118:e101–e111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Landry P, Plante I, Ouellet DL, Perron MP,
Rousseau G and Provost P: Existence of a microRNA pathway in
anucleate platelets. Nat Struct Mol Biol. 16:961–966. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bray PF, McKenzie SE, Edelstein LC,
Nagalla S, Delgrosso K, Ertel A, Kupper J, Jing Y, Londin E, Loher
P, et al: The complex transcriptional landscape of the anucleate
human platelet. BMC Genomics. 14:12013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Londin ER, Hatzimichael E, Loher P,
Edelstein L, Shaw C, Delgrosso K, Fortina P, Bray PF, McKenzie SE
and Rigoutsos I: The human platelet: Strong transcriptome
correlations among individuals associate weakly with the platelet
proteome. Biol Direct. 9:32014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freedman JE, Larson MG, Tanriverdi K,
O'Donnell CJ, Morin K, Hakanson AS, Vasan RS, Johnson AD, Iafrati
MD and Benjamin EJ: Relation of platelet and leukocyte inflammatory
transcripts to body mass index in the Framingham heart study.
Circulation. 122:119–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lood C, Amisten S, Gullstrand B, Jönsen A,
Allhorn M, Truedsson L, Sturfelt G, Erlinge D and Bengtsson AA:
Platelet transcriptional profile and protein expression in patients
with systemic lupus erythematosus: Up-regulation of the type I
interferon system is strongly associated with vascular disease.
Blood. 116:1951–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gatsiou A, Boeckel JN, Randriamboavonjy V
and Stellos K: MicroRNAs in platelet biogenesis and function:
Implications in vascular homeostasis and inflammation. Curr Vasc
Pharmacol. 10:524–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burenbatu, Borjigin M, Eerdunduleng, Huo
W, Gong C, Hasengaowa, Zhang G, Longmei, Li M, Zhang X, et al:
Profiling of miRNA expression in immune thrombocytopenia patients
before and after Qishunbaolier (QSBLE) treatment. Biomed
Pharmacother. 75:196–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian BH, Ye X, Zhang L, Sun Y, Zhang JR,
Gu ML, Qin Q, Chen J and Deng AM: Increased miR-155 expression in
peripheral blood mononuclear cells of primary immune
thrombocytopenia patients was correlated with serum cytokine
profiles. Acta Haematol. 133:257–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodeghiero F, Stasi R, Gernsheimer T,
Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH,
Cooper N, et al: Standardization of terminology, definitions and
outcome criteria in immune thrombocytopenic purpura of adults and
children: Report from an international working group. Blood.
113:2386–2393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu S, Deng G, Qian D, Xie Z, Sun H, Huang
D and Li Q: Detection of apoptosis-associated microRNA in human
apheresis platelets during storage by quantitative real-time
polymerase chain reaction analysis. Blood Transfus. 12:541–547.
2014.PubMed/NCBI
|
|
17
|
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42:(Database issue). D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao X, Sherman BT, da Huang W, Stephens
R, Baseler MW, Lane HC and Lempicki RA: DAVID-WS: A stateful web
service to facilitate gene/protein list analysis. Bioinformatics.
28:1805–1806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabas-Madrid D, Nogales-Cadenas R and
Pascual-Montano A: GeneCodis3: A non-redundant and modular
enrichment analysis tool for functional genomics. Nucleic Acids
Res. 40:(Web Server issue). W478–W483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Zhao H, Xue F, Zhang X, Zhang D, Ge
J, Yang Y, Xuan M, Fu R and Yang R: Reduced expression of miR409-3p
in primary immune thrombocytopenia. Br J Haematol. 161:128–135.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Y, Qu W, Wang YH, Liu H, Li LJ, Wang
HQ, Fu R, Liu H, Wu YH, Guan J, et al: The role of miR-155 in
pathogenesis of immune thrombocytopenia. Zhonghua Yi Xue Za Zhi.
96:1103–1107. 2016.(In Chinese). PubMed/NCBI
|
|
25
|
Bay A, Coskun E, Oztuzcu S, Ergun S,
Yilmaz F and Aktekin E: Plasma microRNA profiling of pediatric
patients with immune thrombocytopenic purpura. Blood Coagul
Fibrinolysis. 25:379–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kottke-Marchant K: Importance of platelets
and platelet response in acute coronary syndromes. Cleve Clin J
Med. 76:(Suppl 1). S2–S7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Girardot M, Pecquet C, Boukour S, Knoops
L, Ferrant A, Vainchenker W, Giraudier S and Constantinescu SN:
miR-28 is a thrombopoietin receptor targeting microRNA detected in
a fraction of myeloproliferative neoplasm patient platelets. Blood.
116:437–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagalla S, Shaw C, Kong X, Kondkar AA,
Edelstein LC, Ma L, Chen J, McKnight GS, López JA, Yang L, et al:
Platelet microRNA-mRNA coexpression profiles correlate with
platelet reactivity. Blood. 117:5189–5197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu S, Huang H, Deng G, Xie Z, Ye Y, Guo R,
Cai X, Hong J, Qian D, Zhou X, et al: miR-326 targets antiapoptotic
Bcl-xL and mediates apoptosis in human platelets. PLoS One.
10:e01227842015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weyrich AS, Schwertz H, Kraiss LW and
Zimmerman GA: Protein synthesis by platelets: Historical and new
perspectives. J Thromb Haemost. 7:241–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McRedmond JP, Park SD, Reilly DF,
Coppinger JA, Maguire PB, Shields DC and Fitzgerald DJ: Integration
of proteomics and genomics in platelets: A profile of platelet
proteins and platelet-specific genes. Mol Cell Proteomics.
3:133–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gnatenko DV, Perrotta PL and Bahou WF:
Proteomic approaches to dissect platelet function: Half the story.
Blood. 108:3983–3991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colombo G, Gertow K, Marenzi G, Brambilla
M, De Metrio M, Tremoli E and Camera M: Gene expression profiling
reveals multiple differences in platelets from patients with stable
angina or non-ST elevation acute coronary syndrome. Thromb Res.
128:161–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rowley JW and Weyrich AS: Coordinate
expression of transcripts and proteins in platelets. Blood.
121:5255–5256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harrison P and Goodall AH: ‘Message in the
platelet’-more than just vestigial mRNA. Platelets. 19:395–404.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang HW, Zhou P, Wang KZ, Liu JB, Huang
YS, Tu YT, Deng ZH, Zhu XD and Hang YL: Platelet proteomics in
diagnostic differentiation of primary immune thrombocytopenia using
SELDI-TOF-MS. Clin Chim Acta. 455:75–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiao J, Liu Y, Li D, Wu Y, Li X, Yao Y,
Niu M, Fu C, Li H, Ma P, et al: Imbalanced expression of Bcl-xL and
Bax in platelets treated with plasma from immune thrombocytopenia.
Immunol Res. 64:604–609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mitchell WB, Pinheiro MP, Boulad N, Kaplan
D, Edison MN, Psaila B, Karpoff M, White MJ, Josefsson EC, Kile BT
and Bussel JB: Effect of thrombopoietin receptor agonists on the
apoptotic profile of platelets in patients with chronic immune
thrombocytopenia. Am J Hematol. 89:E228–E234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Winkler J, Kroiss S, Rand ML, Azzouzi I,
Bang KW Annie, Speer O and Schmugge M: Platelet apoptosis in
paediatric immune thrombocytopenia is ameliorated by intravenous
immunoglobulin. Br J Haematol. 156:508–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Osman A and Fälker K: Characterization of
human platelet microRNA by quantitative PCR coupled with an
annotation network for predicted target genes. Platelets.
22:433–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang W, Wan M, Ma L, Liu X and He J:
Protective effects of ADAM8 against cisplatin-mediated apoptosis in
non-small-cell lung cancer. Cell Biol Int. 37:47–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kroll H, Sun QH and Santoso S: Platelet
endothelial cell adhesion molecule-1 (PECAM-1) is a target
glycoprotein in drug-induced thrombocytopenia. Blood. 96:1409–1414.
2000.PubMed/NCBI
|
|
43
|
Ulger Z, Aksu S, Aksoy DY, Koksal D,
Haznedaroglu IC and Kirazli S: The adhesion molecules of L-selectin
and ICAM-1 in thrombocytosis and thrombocytopenia. Platelets.
21:49–52. 2010. View Article : Google Scholar : PubMed/NCBI
|