Introduction
Apoptosis is involved in a series of cardiovascular
ailments, including diabetic cardiomyocytes. Previous studies have
identified a higher rate of apoptosis in diabetic hearts in
vivo and in vitro (1,2).
However, the underlying mechanisms of pathogenesis remain to be
elucidated.
In order to effectively pump blood, the heart relies
on gap junctions (GJ), which allow for the rapid propagation of the
electrical impulse to all cardiomyocytes (3). Apart from the transmission of
electrical signals, GJ also mediate the exchange of ions, nutrients
and small molecules between adjacent cells (4). GJ consist of a transmembrane protein,
termed connexin (Cx). Connexin 43 (Cx43) belongs to the Cx family
and is the primary connexin in ventricular cardiomyocytes (5). Previous studies revealed that the
abundance of Cx43 is altered during various cardiovascular
diseases, such as arrhythmia (6),
myocardial infarction (7), heart
failure (8) and hypertension
(9). Furthermore, previous studies
suggested that Cx43 expression was also altered in hyperglycemic
conditions (10–12). However, there is controversy on the
expression of cardiac Cx43 in diabetic rats or cardiomyocytes
exposed to hyperglycemic medium, which indicated that Cx43 may be
strongly related with the dysfunction of the diabetic rat heart.
Previous studies demonstrated that the autophagy/lysosome signaling
pathway may be involved in the regulation of Cx43 protein
degradation (8,13). Martins-Marques et al
(14) revealed that
ischemia-reperfusion induced degradation of cardiac Cx43 by
autophagy involved the recruitment of Beclin-1 and p62.
Autophagy (also termed macroautophagy) is an
intracellular bulk degradation process that involves the
elimination of damaged or unused proteins and organelles such as
the mitochondria (15). Autophagy
occurs on a regular basis in cells; however, dysfunction in its
regulation may contribute to the pathology of various conditions,
including ischemia-reperfusion (16), starvation (17), heart failure (8), hypertension (9) and diabetes (18,19),
suggesting that autophagy may have an important role in heart
disease. In addition, dysfunctional autophagy has been observed in
the diabetic heart and associated with an increase in cardiac
apoptosis, which was improved by the activation of autophagy
following metformin administration (2). This is in accordance with a previous
study which indicated that metformin could normalize cardiac
autophagy and attenuate high glucose-induced apoptosis (1). Previous studies on induced autophagy
reported that resveratrol treatment protected cardiac cells against
injury under various pathological conditions, including diabetes,
ischemia-reperfusion and myocardial infarction (20–22).
However, excessive or restricted autophagy may lead to cell death
in the heart. A previous study revealed that autophagy may have a
detrimental effect in an alcoholic cardiomyopathy mouse model
(23). Therefore, it remains to be
elucidated whether autophagy is beneficial or detrimental to the
heart. Previous studies determined that autophagic markers,
including microtubule associated protein light chain 3 (LC3),
Beclin-1 and p62 were required for cytoplasm-to-lysosome delivery
(24–26).
Resveratrol is a natural polyphenol contained in
wine, grapes and vegetables. This agent was investigated due to its
potential health benefits associated with its cardioprotective,
anti-inflammatory, antioxidant and antiplatelet properties
(27–29). Previous studies suggested that
resveratrol protected against apoptotic cell death and improved
cardiac function in ischemia-reperfusion injury or post-infarction
heart failure model via an autophagy-dependent pathway (21,22,27).
In addition, a previous study demonstrated that resveratrol may be
a potential therapeutic strategy for diabetic cardiomyopathy
through the autophagy signaling pathway (20). To the best of our knowledge, this
is the first study to investigate the effect of resveratrol on Cx43
expression and the role of autophagy in hyperglycemic conditions.
The present study aimed to evaluate the effect of resveratrol on
autophagy and Cx43 expression in H9c2 cardiac muscle cells exposed
to a high glucose medium.
Materials and methods
Materials
Resveratrol and chloroquine were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). The H9c2 cells
were obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Lactate dehydrogenase (LDH)
activity assay and MTT kits were purchased from Beyotime Institute
of Biotechnology (Shanghai, China). Primary antibodies against Cx43
(cat no. 3512), AMP-protein activated kinase (AMPK; cat no. 2532)
and phosphorylated (p)-AMPK (cat no. 2531S), mammalian target of
rapamycin (mTOR; cat no. 2972), p-mTOR (cat no. 2971), Beclin-1
(cat no. 3738), p62 (cat no. 5114), LC3-I/II (cat no. 2775), B-cell
lymphoma-2 (Bcl-2; cat no. 2870) and Bcl-2-associated X protein
(Bax; cat no. 2772) were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Cell culture and treatments
The cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified incubator with 5% CO2. Confluent cells
(80–90% confluence) were used for the subsequent experiments. The
H9c2 cells were exposed to medium containing 5.5 mM glucose
[control (Con) group], 25 mM glucose [high glucose (HG) group], or
25 mM glucose and resveratrol at concentration of 10 or 25 µM
(Res10 and Res25 group, respectively) for 24 h. Additionally, H9c2
cells were incubated with 25 µM resveratrol in the presence of
chloroquine (50 µM) in hyperglycemic conditions induced by 25 mM
glucose.
Cell viability assay
Cell viability was quantified with an MTT assay. The
H9c2 cells were seeded in 96-well plates at a density of
2.0×104 cells/well. Following 24 h incubation, MTT
solution (final concentration of 0.5 mg/ml) was added to each well
and incubated for 4 h at 37°C. Following the removal of the medium,
DMSO was added to dissolve the blue-colored formazan product.
Absorbance was measured with a microplate reader at 490 nm. Cell
survival rate was expressed as the absorbance (optical density) of
the various groups.
LDH release
Cell damage was assessed by the quantity of LDH
recorded. The LDH assay kit was used according to the
manufacturer's protocol. The cell medium was collected and then
mixed with LDH reaction buffer for 30 min at room temperature. The
reaction was stopped and the absorbance was measured at 490 nm
using a microplate reader. Cell damage was expressed as the
absorbance (optical density) of the various groups.
Western blotting
Following the treatments, H9c2 cells
(2.0×106 cells/well) were harvested and lysed for 30 min
at 4°C with radioimmunoprecipitation assay lysis buffer (50 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 1% sodium deoxycholate)
supplemented with protease and phosphatase inhibitor cocktails
(Roche Diagnostics GmbH, Mannheim, Germany). The supernatant
fractions were collected by centrifugation at 10,000 × g for 20 min
at 4°C and protein concentration was determined using bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology). Equal amounts
of extracted protein samples (10 µg) were separated by 12% SDS-PAGE
and electrophoretically transferred to polyvinylidene difluoride
membranes. The membranes were blocked in 5% (w/v) non-fat milk for
2 h at room temperature. The membranes were then incubated with the
following rabbit polyclonal primary antibodies: Anti-Cx43 (1:1,000
dilution), anti-AMPK (1:1,000 dilution), anti-p-AMPK (1:1,000
dilution), anti-mTOR (1:1,000 dilution), anti-p-mTOR (1:1,000
dilution), anti-p62 (1:1,000 dilution), anti-Beclin-1 (1:1,000
dilution), anti-LC3 (1:1,000 dilution), anti-Bcl-2 (1:1,000
dilution), anti-Bax (1:1,000 dilution) and rabbit monoclonal
anti-GAPDH antibody (cat no. 2118; 1:10,000 dilution; Cell
Signaling Technology, Inc.) at 4°C overnight. Subsequently, they
were incubated for 2 h at room temperature with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (cat no.
A0208; 1:4,000 dilution; Beyotime Institute of Biotechnology).
Protein bands were visualized using enhanced chemiluminescence
regent (EMD Millipore, Billerica, MA, USA) and were captured using
the ImageQuant LAS 4000 (GE Healthcare Life Sciences, Little
Chalfont, UK) image reader. Blots were semi-quantified by
densitometric analysis using Gel-Pro32 Analyzer software version
4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation
of 3 independent experiments. One-way analysis of variance followed
by the Student-Newman-Keuls test was used to identify significant
differences between HG and resveratrol groups. Student's t-test was
performed to compare the differences between the Con and HG groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
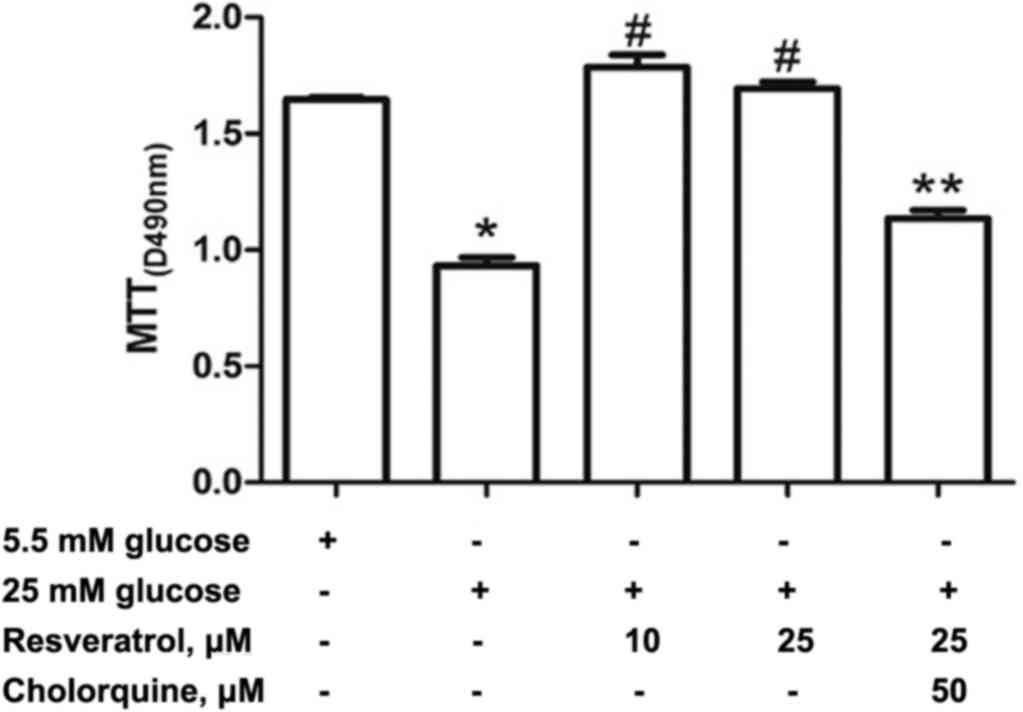
Effect of resveratrol treatment on
H9c2 cell viability
H9c2 cells were incubated with 5.5 or 25 mM glucose,
or 25 mM glucose and resveratrol (10, 25 µM) for 24 h. The
viability of H9c2 cells indicated by MTT assay was significantly
increased in resveratrol group compared with the HG group (Fig. 1). However, treatment with 50 µM
chloroquine and 25 µM resveratrol reduced cell survival compared
with the 25 µM resveratrol group (Fig.
1).
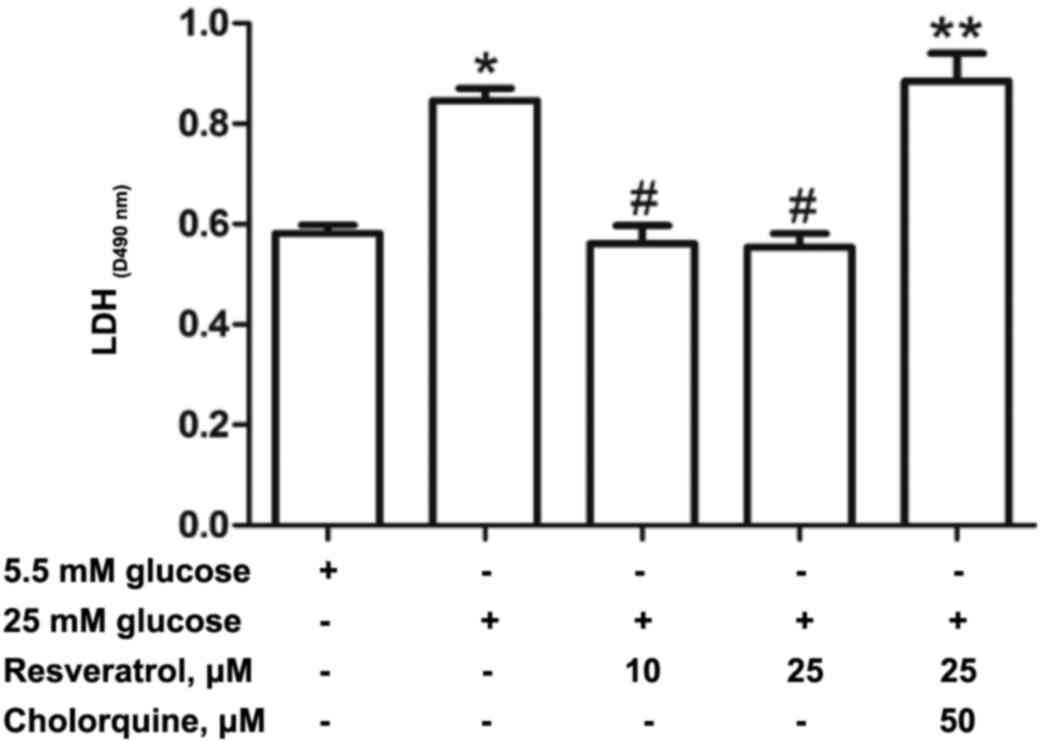
Effect of resveratrol treatment on
cell damage
Cell damage was indicated by LDH activity. The LDH
activity was significantly higher in the HG group compared with the
Con group (Fig. 2). However, H9c2
cells treated with resveratrol exhibited significantly reduced LDH
activity compared with the HG group. Treatment with 50 µM
chloroquine and 25 µM resveratrol significantly increased cell
damage as LDH release was increased compared with the Res10 and
Res25 groups.
Effect of resveratrol on Cx43 protein
expression level in hyperglycemia-cultured H9c2 cells
Cx43 expression was quantified using western blot
analysis (Fig. 3). Cx43 protein
expression was significantly increased in the HG compared with the
Con group (Fig. 3A and B).
Notably, treatment with resveratrol prevented the
hyperglycemia-induced increase in Cx43 protein expression levels
(Fig. 3B). No significant
difference was identified in terms of Cx43 expression between the
resveratrol-treated groups and the Con group.
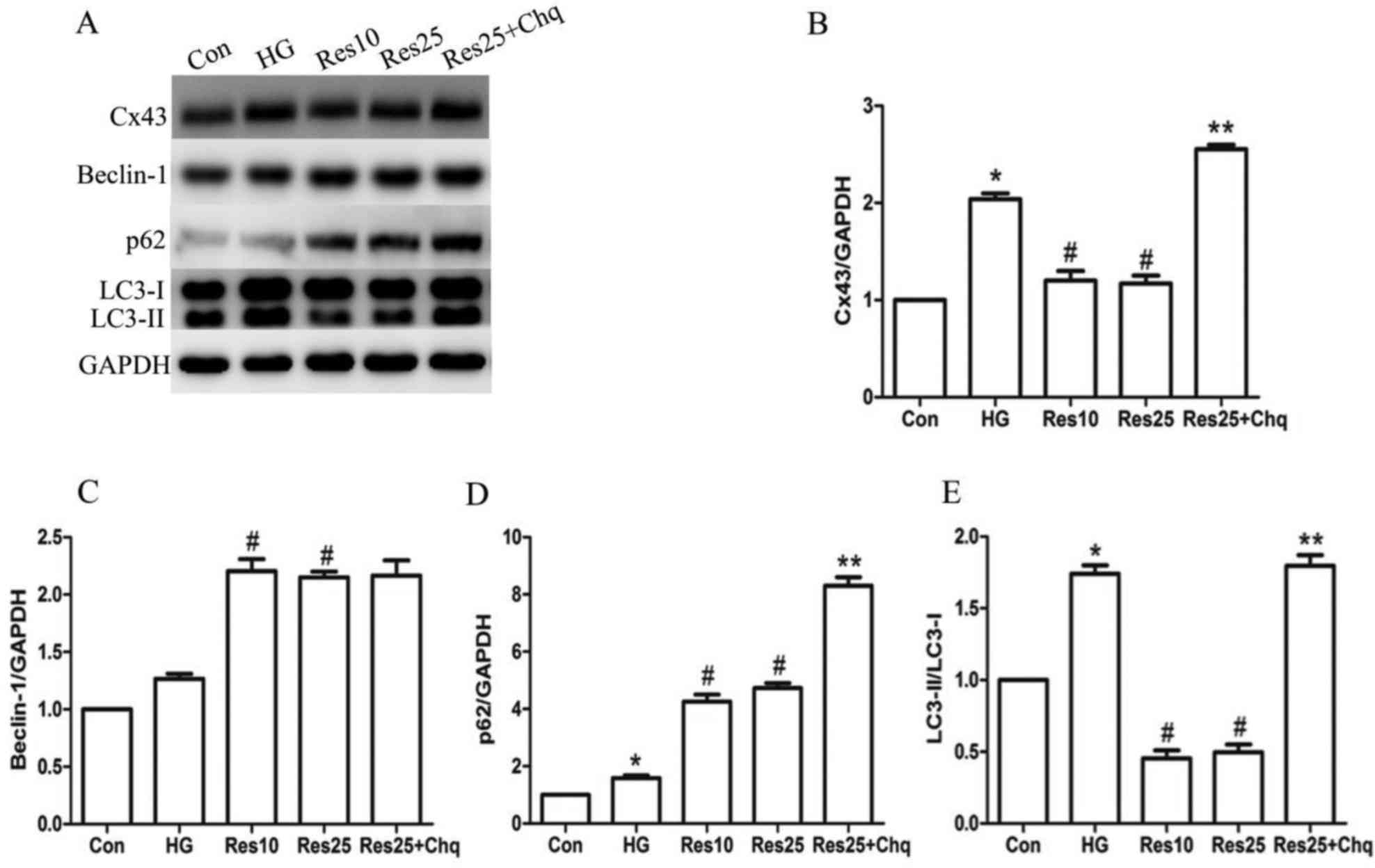 | Figure 3.Resveratrol treatment prevented Cx43
upregulation through autophagy pathway. (A) Western blotting was
performed in order to determine the expression of (B) Cx43, (C)
Beclin-1, (D) p62 and (E) LC3-II/LC3-I. The target protein density
was normalized to the control cells. Data are expressed as the mean
± standard deviation (n=3). *P<0.05 vs. Con,
#P<0.05 vs. HG, **P<0.05 vs. Res25. Cx43, connexin
43; LC3-I, -II, microtubule-associated protein 1 light chain 3-I,
-II; Con, control; HG, high glucose; Res10, resveratrol 10 µM;
Res25, resveratrol 25 µM; Chq, chloroquine. |
Effect of resveratrol on
autophagy-associated proteins in hyperglycemia-cultured H9c2
cells
Following 24 h incubation with 25 mM glucose, the
protein expression levels of autophagy-associated proteins were
upregulated. In addition, treatment with resveratrol in
hyperglycemia-cultured H9c2 cells induced autophagy. Autophagy is
evaluated by the protein expression levels of LC3-II and p62. The
p62 (Fig. 3D) expression level and
the LC3-II/LC3-I ratio (Fig. 3E)
were significantly increased in the HG group compared with the Con
group, indicating the onset of autophagy. It is of note that p62
protein expression was further increased in the Res treatment
groups compared with the HG group, whereas the LC3-II/LC3-I ratio
was reduced in the Res-treated groups compared with the HG group.
Furthermore, Beclin-1 protein expression levels were upregulated in
the HG group and in the Res treatment groups compared with the HG
group (Fig. 3C).
In the cells treated with resveratrol and
chloroquine, which is an inhibitor of autophagy, the expression of
p62 and the LC3-II/LC3-I ratio were further increased compared with
the resveratrol only treatment groups, suggesting that resveratrol
may increase autophagic turnover in H9c2 cells during hyperglycemic
conditions. However, Beclin-1 expression was unaffected by
chloroquine treatment (Fig.
3C).
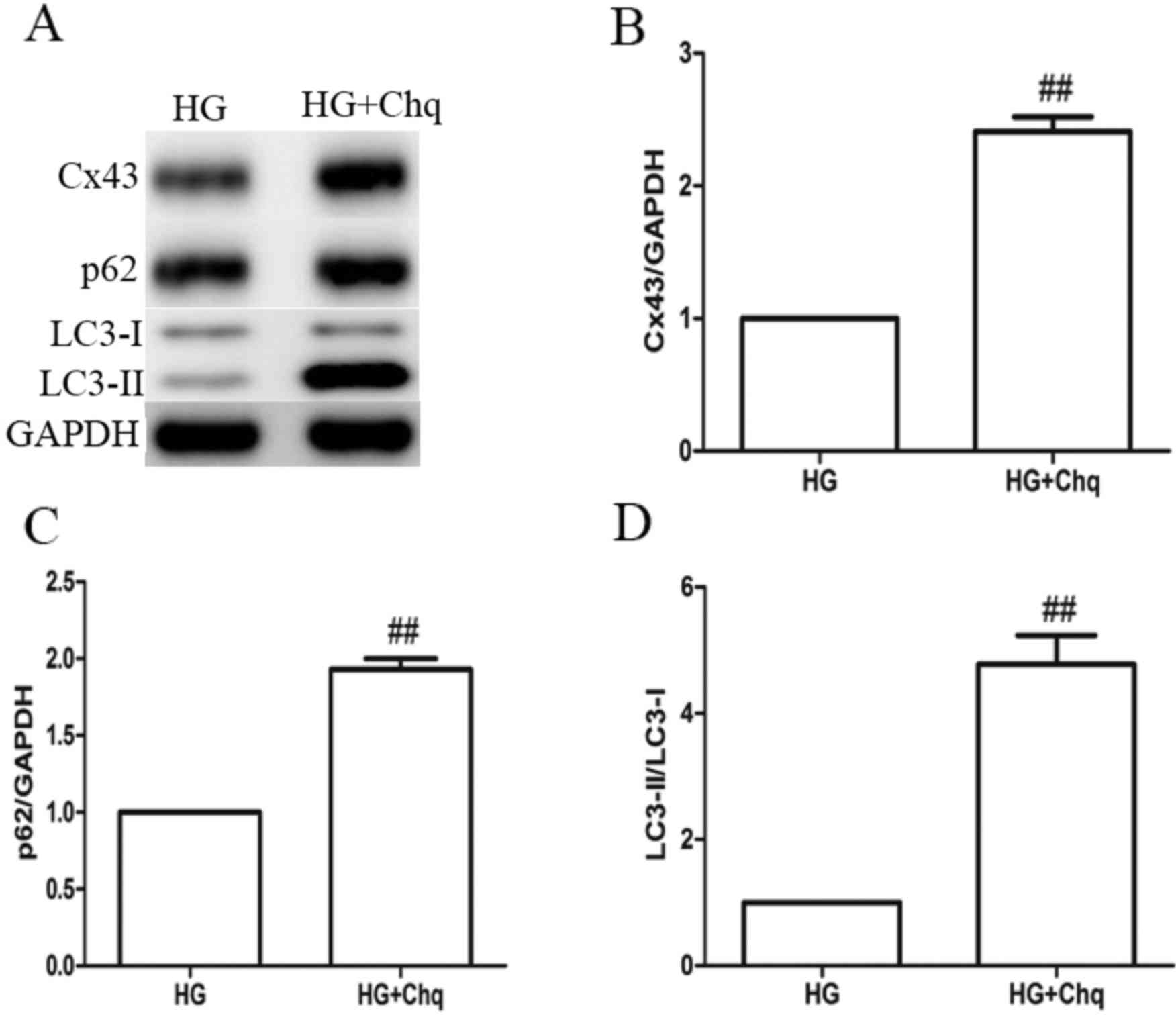
Effect of chloroquine on
resveratrol-induced Cx43 downregulation in hyperglycemia-cultured
H9c2 cells
Resveratrol treatment was observed to increase
autophagic flux in H9c2 cells under hyperglycemic conditions, along
with downregulation of Cx43 expression. When the cells were
incubated with 25 µM resveratrol and 50 µM chloroquine, Cx43
expression was increased compared with the 25 µM resveratrol alone
treatment group, which indicated that resveratrol may promote Cx43
downregulation through activation of the autophagy pathway
(Fig. 4A and B).
Effect of chloroquine on Cx43
expression and autophagy activity in hyperglycemia-cultured H9c2
cells
Cells were incubated with chloroquine (50 µM) in
hyperglycemic conditions and Cx43 expression was increased compared
with the HG only group. The p62 expression level and LC3-II/LC3-I
ratio were also increased compared with the HG only group,
suggesting that the inhibition of autophagy was prevented in the
chloroquine group (Fig. 4C and D).
Beclin-1 expression was unaffected by chloroquine treatment (data
not shown).
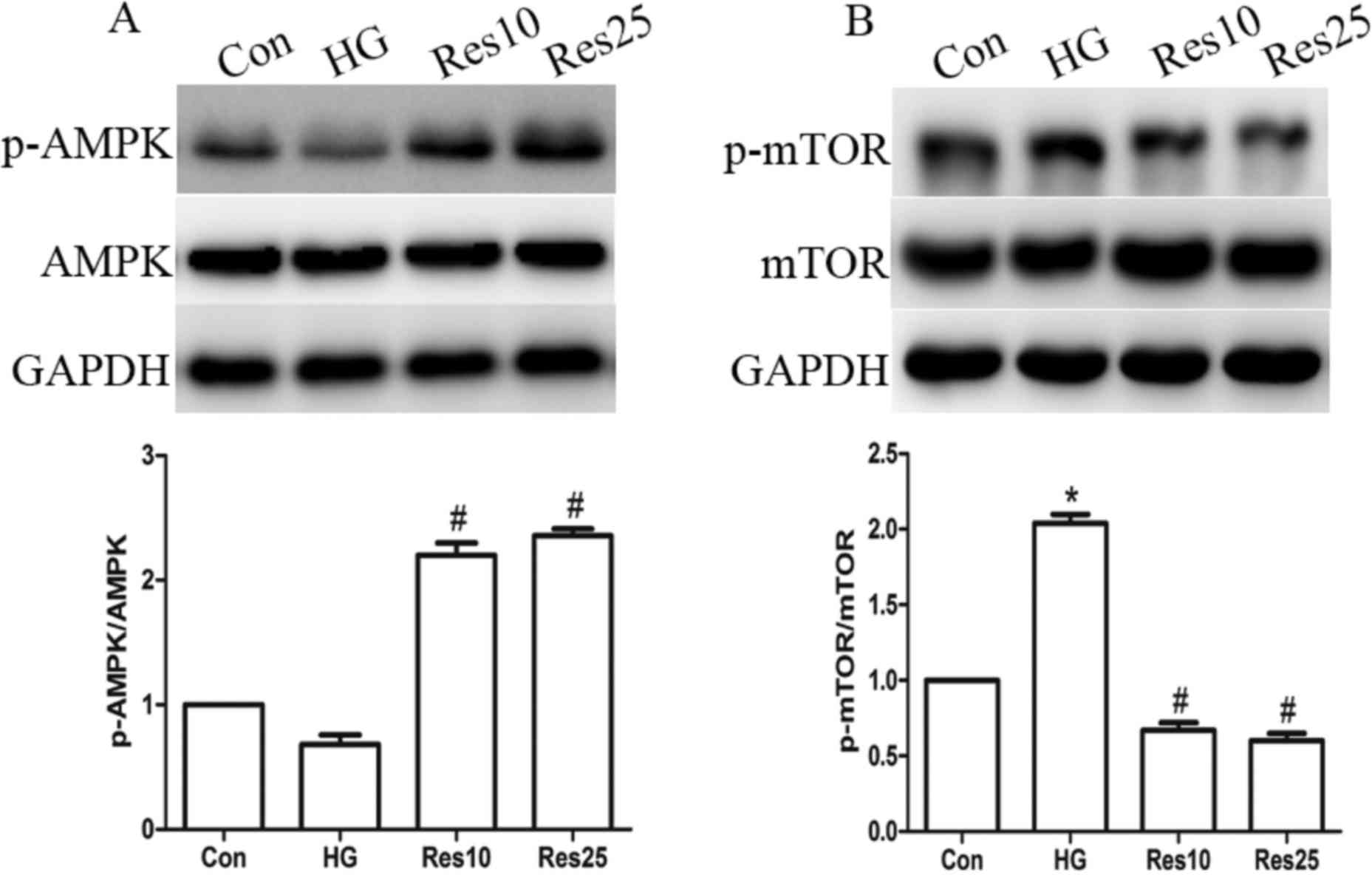
Resveratrol treatment upregulates AMPK
and downregulates mTOR expression levels
It has been previously established that AMPK may act
as an energy sensor, which is activated in response to a reduction
in ATP content (22). The present
study revealed a reduction in the phosphorylation level of AMPK in
the HG group, which represents the activated form. However,
resveratrol treatment increased the level of p-AMPK without
affecting the total AMPK (Fig.
5A). In addition, it was determined that the phosphorylation
and activation of mTOR (p-mTOR) was increased in the HG group.
However, resveratrol treatment increased the expression of p-mTOR
without affecting total mTOR expression (Fig. 5B).
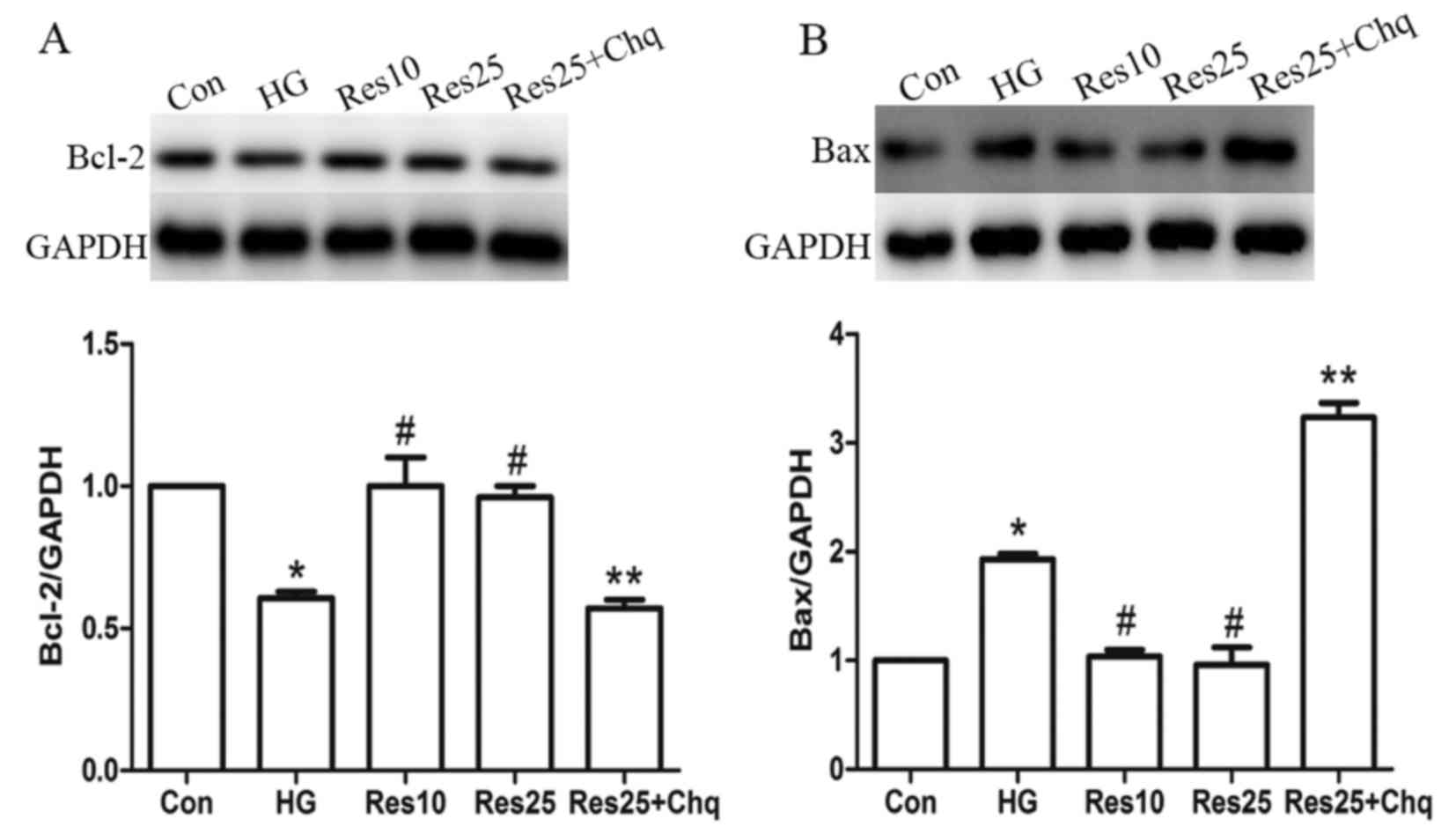
Resveratrol ameliorates cell apoptosis
depending on autophagic induction
Bcl-2 and Bax protein expression levels were used to
assess apoptosis. Resveratrol treatment increased Bcl-2 expression
and reduced Bax expression compared with the HG group. However,
when the autophagy flux was inhibited by chloroquine, the effect of
resveratrol was abolished as Bcl-2 expression was reduced and Bax
levels were increased compared with the Res25 group (Fig. 6).
Discussion
The primary findings of the present study included
the inhibitory effect of resveratrol on hyperglycemia-induced Cx43
upregulation in H9c2 cells combined with an increase in autophagic
flux and resveratrol treatment led to an increase in autophagic
flux along with increased cell survival and reduced cell damage in
H9c2 cells exposed to hyperglycemic conditions.
Gap junctions primarily consist of Cx43 in
ventricular cardiomyocytes, which have an important role in the
maintenance of normal heart functions, such as orderly electric and
metabolic coupling (3). Therefore,
changes in the quantity of Cx43 expression has been associated with
various heart diseases, such as myocardial infarction,
ischemia-reperfusion, arrhythmia, diabetes and heart failure
(7,8,10,12,30–32).
A previous study demonstrated that Cx43 expression was
significantly reduced in ischemic conditions, thus contributing to
the impairment of impulse propagation (33). Greener et al (33) demonstrated that transfection with
Cx43 could improve conduction velocity and reduce ventricular
arrhythmia susceptibility in pigs with myocardial infarction. This
was also observed by Rutledge et al (7), when mice were treated with c-Src
inhibitors following a myocardial infarction. However, the
alterations of Cx43 expression in diabetic hearts have remained
controversial. It was previously reported that Cx43 expression was
markedly reduced in various tissues under hyperglycemic conditions
(34,35). However, Howarth et al
(12) reported that total Cx43
expression was increased in hearts isolated from 12 weeks-old
diabetic rats, and prolonged QT interval and QRS complex were
observed. Therefore, it is of note, that increased cardiac Cx43
expression may not be consistently beneficial for the heart under
certain circumstances. These findings were supported by Kanno et
al (36).
In the present study, the expression of Cx43
increased significantly in H9c2 cells in hyperglycemic conditions,
whereas resveratrol protected H9c2 cells from the increase in Cx43
induced by hyperglycemia. Therefore, it is possible that reduced
Cx43 expression following resveratrol treatment may have a
beneficial effect under the experimental conditions used in the
present study.
Previous studies determined that Cx43 expression
levels may be regulated by the autophagy signaling pathway
(3,8,14).
Autophagy may provide a source of energy when nutrients are scarce
and may also be involved in protein quality control through
discards defective cytoplasmic components. Previous studies have
demonstrated autophagy-mediated the degradation of Cx43 in
vivo and in vitro (14). The present study revealed that
resveratrol treatment may lead to degradation of Cx43 in H9c2 cells
through activation of the autophagy signaling pathway. Therefore,
multiple autophagy-associated proteins including Beclin-1, p62 and
LC3 were investigated. The current study determined that the levels
of Beclin-1 and p62 were markedly increased, whereas the
LC3-II/LC3-I ratio was significantly reduced in the resveratrol
treatment group. The accumulation of p62 may indicate either an
increase in autophagosome formation or a reduction in autophagic
clearance (22). Therefore, to
examine autophagic flux in vitro, the present study used
chloroquine in combination with resveratrol. It has been previously
established that chloroquine may inhibit lysosome fusion with
autophagosomes and elevate lysosomal pH, thus preventing the final
digestion step and inhibiting lysosomal activity, and is widely
used as an autophagic inhibitor (22). Therefore, the LC3-II/LC3-I ratio
and p62 expression were increased in H9c2 cells treated with
chloroquine and resveratrol, and Cx43 expression was also
upregulated. However, Beclin-1 protein expression was not affected
by the chloroquine and resveratrol treatment. These findings
suggested that resveratrol treatment increased autophagic flux as
opposed to reducing it, and the downregulation of Cx43 expression
by resveratrol treatment occurred via the activation of the
autophagy pathway. It is of note that the present study also
observed that the LC3-II/LC3-I ratio and p62 expression were
increased following chloroquine treatment under hyperglycemic
conditions. Furthermore, a hyperglycemia-induced increase in Cx43
expression was observed in the HG group, which may occur through an
alternative process, such as increased synthesis, whereas the net
effect of the HG treatment led to increased Cx43 expression.
However, further investigation is required to determine the
underlying mechanism.
In the present study, the expression levels of p62
were increased and the LC3-II/LC3-I ratio was reduced in the
resveratrol treatment groups, which was inconsistent with previous
studies (21,22). Previous studies demonstrated that
resveratrol increased autophagic flux, as indicated by increased
LC3-II/LC3-I ratio and reduced p62 expression (21,22).
Guidelines for monitoring autophagy suggest that if the degradation
process of LC3-II due to lysosomal activity is rapid, it would it
would lead to a decrease in LC3-II/LC3-I ratio (26). In addition, changes to LC3 may be
rapid, whereas clearance of substrates of autophagy may require
more time. Furthermore, p62 upregulation has also been observed in
conditions where there is an increase in autophagic flux (37,38).
Therefore, combined with increased Beclin-1 protein expression and
the findings from the chloroquine treatment, the present study
determined that induction of autophagic flux by resveratrol
treatment is possible under the given set of experimental
conditions.
In order to investigate the mechanisms of
resveratrol-induced autophagic flux, the present study examined
mTOR expression in hyperglycemic conditions. A previous study
revealed that inhibition of mTOR by dephosphorylation may induce
autophagy (21). It has been
revealed that inhibition of mTOR with rapamycin induced autophagy
in H9c2 cells (21). In the
present study, resveratrol treatment inhibited mTOR
dephosphorylation at serine 2448, along with an increase autophagic
flux. These findings were consistent with a previous study
(21), where resveratrol increased
autophagy activity via inhibition of the phosphorylation of mTOR
(Ser2448). However, high glucose treatment in the present study
increased the activation of mTOR by enhancing the phosphorylation
level at Ser2448, the autophagic flux was also increased. It is
possible that hyperglycemia-induced autophagy is independent from
inhibition of mTOR under hyperglycemic conditions. These findings
suggested that resveratrol treatment increased the activation of
autophagy primarily through preventing the phosphorylation of mTOR
under the experimental conditions of the present study.
AMPK may act as a regulator of energy balance, and
has several cellular functions including autophagy in the
cardiovascular system (21,22).
A previous study revealed that AMPK triggered autophagy through
negatively regulating mTOR activity in H9c2 cells treated with
resveratrol (21). The present
study observed reduced AMPK expression in H9c2 cells under
hyperglycemic conditions, whereas resveratrol treatment increased
AMPK activation indicated by the increased phosphorylation level at
Threonine 172. Therefore, it is possible that resveratrol-activated
AMPK stimulated autophagic activity in H9c2 cells by modulating the
mTOR pathway.
It has been previously reported that autophagy may
regulate cell survival and cell death. He et al (1) demonstrated that induction of
autophagy protected cells against apoptosis in vivo and
in vitro. However, inhibition of autophagy may suppress this
effect. Matsui et al (16)
revealed that inhibition of autophagy in mice exposed to
ischemia/reperfusion leads to reduced apoptotic cell death. The
present study determined that resveratrol promoted cell survival,
and Bcl-2 expression was increased whereas Bax expression was
reduced. In addition, MTT assay and LDH release experiments also
confirmed these observations. This effect was inhibited by
chloroquine treatment, suggesting that autophagy is beneficial
under hypoglycemic conditions.
Resveratrol was not observed to have a
dose-dependent effect on the parameters examined in the current
study. Gurusamy et al (21)
demonstrated that 0.1 µM resveratrol was sufficient to induce
autophagy in H9c2 cells. Therefore, the two concentrations of
resveratrol (10 and 25 µM) used in the present study may have had a
similar effect on H9c2 cells.
In conclusion, the findings of the present study
revealed that resveratrol treatment led to downregulation of Cx43
expression and contributed to cell survival in H9c2 cells via
regulation of autophagic flux. This is supported by the finding
that inhibition of autophagic flux protected against
hyperglycemia-induced Cx43 upregulation and apoptosis. The findings
of the current study demonstrated that the AMPK/mTOR-mediated
autophagy pathway may be involved in this condition. Further
investigation is required to elucidate the underlying mechanism
behind the resveratrol-induced autophagy in hyperglycemic
conditions.
There are several limitations to the present study.
Although chloroquine is a widely-used inhibitor of autophagy, this
pharmacological approach lacks specificity. Therefore, a genetic
approach may be more effective. Electron microscopy is the most
accurate method for measuring autophagy; however, the present study
was only able to perform western blotting for autophagy-associated
proteins. Future investigations should aim to quantify the
autolysosome via electron microscopy to clarify the effect of
resveratrol on autophagy. Autophagy is a dynamic process;
therefore, it will be useful for the effects of the treatments to
be quantified at multiple time points. The findings of the present
study should be replicated in other cell types and animal models,
which may further elucidate the underlying mechanisms.
Acknowledgements
The present study was supported by the Shanghai
Committee of Science and Technology, China (grant no.
13ZR1431500).
Glossary
Abbreviations
Abbreviations:
|
Chq
|
chloroquine
|
|
HG
|
high glucose
|
|
Cx43
|
connexin43
|
|
Res
|
resveratrol
|
|
AMPK
|
AMP-activated protein kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
LDH
|
lactate dehydrogenase
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium
bromide
|
References
|
1
|
He C, Zhu H, Li H, Zou MH and Xie Z:
Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances
cardiac autophagy and protects against cardiomyocyte apoptosis in
diabetes. Diabetes. 62:1270–1281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie Z, Lau K, Eby B, Lozano P, He C,
Pennington B, Li H, Rathi S, Dong Y, Tian R, et al: Improvement of
cardiac functions by chronic metformin treatment is associated with
enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes.
60:1770–1778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martins-Marques T, Catarino S, Marques C,
Pereira P and Girão H: To beat or not to beat: Degradation of Cx43
imposes the heart rhythm. Biochem Soc Trans. 43:476–481. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rohr S: Role of gap junctions in the
propagation of the cardiac action potential. Cardiovasc Res.
62:309–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vozzi C, Dupont E, Coppen SR, Yeh HI and
Severs NJ: Chamber-related differences in connexin expression in
the human heart. J Mol Cell Cardiol. 31:991–1003. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou P, Zhang SM, Wang QL, Wu Q, Chen M
and Pei JM: Anti-arrhythmic effect of verapamil is accompanied by
preservation of cx43 protein in rat heart. PLoS One. 8:e715672013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rutledge CA, Ng FS, Sulkin MS, Greener ID,
Sergeyenko AM, Liu H, Gemel J, Beyer EC, Sovari AA, Efimov IR and
Dudley SC: c-Src kinase inhibition reduces arrhythmia inducibility
and connexin43 dysregulation after myocardial infarction. J Am Coll
Cardiol. 63:928–934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hesketh GG, Shah MH, Halperin VL, Cooke
CA, Akar FG, Yen TE, Kass DA, Machamer CE, Van Eyk JE and Tomaselli
GF: Ultrastructure and regulation of lateralized connexin43 in the
failing heart. Circ Res. 106:1153–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Zhao G, Hu X, Wang M, Li H, Ye Y,
Du Q, Yao J, Bao Z, Hong W, et al: Aliskiren-attenuated myocardium
apoptosis via regulation of autophagy and connexin-43 in aged
spontaneously hypertensive rats. J Cell Mol Med. 18:1247–1256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin H, Ogawa K, Imanaga I and Tribulova N:
Remodeling of connexin 43 in the diabetic rat heart. Mol Cell
Biochem. 290:69–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howarth FC, Nowotny N, Zilahi E, El Haj MA
and Lei M: Altered expression of gap junction connexin proteins may
partly underlie heart rhythm disturbances in the
streptozotocin-induced diabetic rat heart. Mol Cell Biochem.
305:145–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Howarth FC, Chandler NJ, Kharche S, Tellez
JO, Greener ID, Yamanushi TT, Billeter R, Boyett MR, Zhang H and
Dobrzynski H: Effects of streptozotocin-induced diabetes on
connexin43 mRNA and protein expression in ventricular muscle. Mol
Cell Biochem. 319:105–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fong JT, Kells RM, Gumpert AM, Marzillier
JY, Davidson MW and Falk MM: Internalized gap junctions are
degraded by autophagy. Autophagy. 8:794–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martins-Marques T, Catarino S, Zuzarte M,
Marques C, Matafome P, Pereira P and Girão H: Ischaemia-induced
autophagy leads to degradation of gap junction protein connexin43
in cardiomyocytes. Biochem J. 467:231–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei H, Liu L and Chen Q: Selective removal
of mitochondria via mitophagy: Distinct pathways for different
mitochondrial stresses. Biochim Biophys Acta. 1853:2784–2790. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lichtenstein A, Minogue PJ, Beyer EC and
Berthoud VM: Autophagy: A pathway that contributes to connexin
degradation. J Cell Sci. 124:910–920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanamori H, Takemura G, Goto K, Tsujimoto
A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M,
et al: Autophagic adaptations in diabetic cardiomyopathy differ
between type 1 and type 2 diabetes. Autophagy. 11:1146–1160. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Palanivel R, Rai E, Park M, Gabor
TV, Scheid MP, Xu A and Sweeney G: Adiponectin stimulates autophagy
and reduces oxidative stress to enhance insulin sensitivity during
high-fat diet feeding in mice. Diabetes. 64:36–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang B, Yang Q, Sun YY, Xing YF, Wang YB,
Lu XT, Bai WW, Liu XQ and Zhao YX: Resveratrol-enhanced autophagic
flux ameliorates myocardial oxidative stress injury in diabetic
mice. J Cell Mol Med. 18:1599–1611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanamori H, Takemura G, Goto K, Tsujimoto
A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Morishita K,
Kawasaki M, et al: Resveratrol reverses remodeling in hearts with
large, old myocardial infarctions through enhanced
autophagy-activating AMP kinase pathway. Am J Pathol. 182:701–713.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo R and Ren J: Deficiency in AMPK
attenuates ethanol-induced cardiac contractile dysfunction through
inhibition of autophagosome formation. Cardiovasc Res. 94:480–491.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena A Acevedo, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Liu YY, Liu XY, Jia SW, Zhao J,
Cui D and Wang L: Resveratrol protects neurons and the myocardium
by reducing oxidative stress and ameliorating mitochondria damage
in a cerebral ischemia rat model. Cell Physiol Biochem. 34:854–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vilar S, Quezada E, Santana L, Uriarte E,
Yánez M, Fraiz N, Alcaide C, Cano E and Orallo F: Design,
synthesis, and vasorelaxant and platelet antiaggregatory activities
of coumarin-resveratrol hybrids. Bioorg Med Chem Lett. 16:257–261.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu PL, Chong IW, Lee YC, Tsai JR, Wang
HM, Hsieh CC, Kuo HF, Liu WL, Chen YH and Chen HL:
Anti-inflammatory effects of resveratrol on
Hypoxia/Reoxygenation-induced alveolar epithelial cell dysfunction.
J Agric Food Chem. 63:9480–9487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghaly HA, Boyle PM, Vigmond EJ, Shimoni Y
and Nygren A: Simulations of reduced conduction reserve in the
diabetic rat heart: Response to uncoupling and reduced
excitability. Ann Biomed Eng. 38:1415–1425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okruhlicova L, Tribulova N, Misejkova M,
Kucka M, Stetka R, Slezak J and Manoach M: Gap junction remodelling
is involved in the susceptibility of diabetic rats to
hypokalemia-induced ventricular fibrillation. Acta Histochem.
104:387–391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santos-Almeida FM, Girão H, da Silva CA,
Salgado HC and Fazan R Jr: Cholinergic stimulation with
pyridostigmine protects myocardial infarcted rats against
ischemic-induced arrhythmias and preserves connexin43 protein. Am J
Physiol Heart Circ Physiol. 308:H101–H107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Greener ID, Sasano T, Wan X, Igarashi T,
Strom M, Rosenbaum DS and Donahue JK: Connexin43 gene transfer
reduces ventricular tachycardia susceptibility after myocardial
infarction. J Am Coll Cardiol. 60:1103–1110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernandes R, Girão H and Pereira P: High
glucose down-regulates intercellular communication in retinal
endothelial cells by enhancing degradation of connexin 43 by a
proteasome-dependent mechanism. J Biol Chem. 279:27219–27224. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu L, Zhao Y, Fan Y, Wang M, Xu S and Fu
G: Epigallocatechin-3 gallate, a green tea catechin, attenuated the
downregulation of the cardiac gap junction induced by high glucose
in neonatal rat cardiomyocytes. Cell physiol Biochem. 26:403–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanno S, Kovacs A, Yamada KA and Saffitz
JE: Connexin43 as a determinant of myocardial infarct size
following coronary occlusion in mice. J Am Coll Cardiol.
41:681–686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colosetti P, Puissant A, Robert G, Luciano
F, Jacquel A, Gounon P, Cassuto JP and Auberger P: Autophagy is an
important event for megakaryocytic differentiation of the chronic
myelogenous leukemia K562 cell line. Autophagy. 5:1092–1098. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng Q, Su H, Ranek MJ and Wang X:
Autophagy and p62 in cardiac proteinopathy. Circ Res. 109:296–308.
2011. View Article : Google Scholar : PubMed/NCBI
|