Introduction
Atherosclerotic plaque injuries and intraplaque
inflammation are widely considered to serve a crucial role in the
development of acute coronary syndromes (1–3). It
has been demonstrated that vulnerable plaques of human coronary
arteries contain abundant macrophages and T lymphocytes (3). The authors of the present study
previously demonstrated that distinct accumulation of neutrophils
additionally occurs in culprit lesions of acute coronary syndromes
(4,5). Activated neutrophils can release
enzymes, including elastase and myeloperoxidase. Neutral
endopeptidase 24.11 (NEP), a membrane protein that regulates
inflammatory reactions, can also be identified in neutrophils. NEP
can hydrolyze a large number of peptides, including the natriuretic
peptide (NP) family (6). It has
already been reported (4) that
neutrophils were positive for NEP in ruptured plaques, while in
eroded plaques the majority of neutrophils lacked NEP
positivity.
Previous epidemiological studies have identified
that the level of NPs in the peripheral blood correlates positively
with the risk of coronary atherosclerosis and acute myocardial
infarction (AMI) (7,8). Two types of NP receptors (NPRs), the
biologically active receptor and the clearance receptor, have been
reported and the biologically active receptors are further
classified into two subtypes, NPR-A and NPR-B (9,10). A
previous study of human coronary atherosclerotic lesions
demonstrated that smooth muscle cells (SMCs) in early
atherosclerotic hypercellular lesions were positive for C-type NP
(CNP), whereas SMCs in advanced lesions were negative for CNP
(11). Furthermore, it has been
reported by examination of autopsy specimens, in addition to
atherectomy specimens, that the expression of NPRs was detected in
neointimal SMCs at the site of percutaneous coronary intervention
(PCI) (12).
Experimental studies have demonstrated that
neutrophils express the biologically active receptors of NPRs,
which limit neutrophil activation via the generation of
intracellular cyclic guanosine monophosphate (cGMP) (13). Although the effects of NPs on
neutrophils remain unclear, previous experimental studies reported
the priming of superoxide anions in neutrophils by atrial NP (ANP)
and by brain NP (14,15). In addition, Izumi et al
(16) demonstrated the
advantageous result of the blocking of NPR-A in models of
ischemia/reperfusion in mice.
Therefore, identification of the cellular
localization of NPR-A and -B is important for clarifying the
pathophysiological role of these receptors in plaque instability.
However, the localization of NPR-A and -B in the coronary culprit
lesions of AMI remains to be reported. The present study provides
information on the immunohistochemical localization that was used
to define the pattern of NPR-A and -B expression in ruptured and
eroded plaques from patients with AMI.
Materials and methods
Coronary tissue specimens
A total of 82 coronary artery segments were
collected at autopsy from 43 patients (13 segments from 13 patients
with AMI, 69 segments from 30 patients with non-cardiovascular
diseases). Each segment was obtained within 3 h following
mortality. The age range of these patients was between 11 and 74
years (non-cardiovascular disease 54±19 yr; AMI 50±17 years, mean ±
standard deviation). These two study groups were composed
predominantly of males [non-cardiovascular disease (males, 25;
females 5); AMI 77% (males, 10; females, 3)]. In the 69 segments
obtained from the patients with non-cardiovascular diseases, 20
segments contained normal coronary arteries with diffuse intimal
thickening (American Heart Association classification type I)
(17,18) and 49 segments contained
atherosclerotic lesions. These atherosclerotic lesions were
classified histologically according to the previously described
system (11) either as early
atherosclerotic lesions with hypercellularity (n=12) or as advanced
atherosclerotic lesions (n=37). The advanced atherosclerotic
lesions were further classified into two types; fibrolipid (type
Va; n=22) or fibrous (type Vc; n=15). The definition of these
various types of atherosclerotic lesions has been described
previously (4,11). The current study was approved by
the Osaka City General Hospital Ethical Committee (approval number
606; Osaka, Japan). Written informed consent from the families of
all the autopsy subjects was obtained.
A total of 13 segments, obtained from the lesions
responsible for mortality in the patients with AMI, were then
separated into either ruptured (n=7) or eroded (n=6) plaques,
according to the definition described previously (4). In the 13 patients with AMI, emergency
PCI was performed in 7 patients. The interval between the AMI onset
and mortality varied from 0 to 2 days (mean interval <1 day). No
significant differences were observed between patients with
ruptured or eroded plaques, with respect to age, sex, or risk
factors.
The coronary arteries were dissected from the
epicardial surface and a 2 mm slice from each segment was
snap-frozen and stored at −80°C. The snap-frozen specimens were
sectioned serially at 6 µm thickness and fixed with acetone. Every
first section was stained with hematoxylin-eosin; the other
sections were used for immunohistochemical investigation.
Immunohistochemistry
Single staining
Table I presents
the source, specificity and working dilution of the antibodies
used. In the present study, monoclonal antibodies against human
NPR-A and NPR-B were employed; the specificity of these antibodies
has been reported previously (19). For the identification of NEP,
anti-common acute lymphocytic leukemia antigen (CALLA; CD10) was
used (20). The specificity of the
results obtained with NPR-A, NPR-B or NEP was checked by omitting
the primary antibodies and using non-immune mouse serum (DAKO;
Agilent Technologies, Inc., Santa Clara, CA, USA) as a negative
control. In the present immunohistochemical staining, a 3-step
staining procedure was used, with the streptavidin-biotin complex
method for color detection. Peroxidase activity was visualized by
incubation with 3-amino-9-ethyl-carbazole for 10 min at room
temperature, followed by faint counter-staining of the sections
with hematoxylin.
 | Table I.Antibodies. |
Table I.
Antibodies.
| Designation | Clone or cat.
no. | Type | Cell identified | Source | Working dilution | (Refs.) |
|---|
| NPR-A | A397 | MAb (IgG1) | – | Kitano et
al | 1:50 | (19) |
| NPR-B | B136 | MAb (IgG1) | – | Kitano et
al | 1:50 | (19) |
| NEP | CD10 | MAb (IgG1) | – | DAKO | 1:50 |
|
| α-Smooth muscle
actin |
1A4 | MAb (IgG2a) | Smooth muscle
cells | DAKO | 1:100 |
|
| CD68 | EBM11 | MAb (IgG1) | Macrophages | DAKO | 1:100 |
|
| Elastase | NP57 | MAb (IgG1) | Neutrophils, some
monocytes | DAKO | 1:200 |
|
| CD66b | 80H3 | MAb (IgG1) | Neutrophils | Beckman
Coulter | 1:50 |
|
| von Willebrand
factor | F8/86 | MAb (IgG1) | Endothelial
cells | DAKO | 1:50 |
Immunodouble staining
The simultaneous identification of SMCs and
macrophages was performed on the basis of two primary antibodies of
a different immunoglobulin G subclass (1A4 and CD68) (21). The enzymatic activity of
ß-galactosidase for 1A4 was visualized in turquoise (BioGenex kit,
BioGenex, Fremont, CA, USA) while that of alkaline phosphatase for
CD68 was visualized in red (New Fuchsin kit, DAKO; Agilent
Technologies, Inc.). To identify cell types that express NPR-A or
NPR-B, double immunostaining was performed between macrophages
(CD68) and each of NPR-A and NPR-B. In addition, double
immunostaining was also performed between neutrophils (CD66b) and
each of NPR-A, NPR-B and NEP using modifications of procedures
reported previously (21). In
these double immunostainings, alkaline phosphatase was visualized
with fast blue BB while peroxidase activity was identified by
3-amino-9-ethylcarbazole development.
Quantitative methods
The surface area containing NPR-A-positive cells and
NPR-B-positive cells was quantified with the use of computer-aided
planimetry (WinROOF2015, Mitani Corporation, Fukui, Japan) and the
amount of NPR-A- or NPR-B-positive cells was estimated as a
percentage of the total surface area of the tissue section.
CD66b-positive neutrophil numbers and NEP-positive cell numbers
were calculated in the entire tissue sections and expressed as the
number of cells per mm2 of intimal tissue. NPR-A-,
NPR-B- or NEP-positive cells, or neutrophils within thrombi or
blood clots were excluded. In the morphometrical analysis,
quantification and calculation were performed by a single
investigator who was unaware of the histologic classification of
the patients. The results are expressed as mean ± standard
deviation. Statistical comparison between 2 groups was performed
using an unpaired Student's t-test or Mann-Whitney U test, as
appropriate. χ2 test or Fisher's exact test was used for
categorical variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunocytochemistry
Specimens obtained from patients with
non-cardiovascular diseases
In the 20 normal coronary arteries with diffuse
intimal thickening, macrophages were not detected. In the 12 early
atherosclerotic lesions with hypercellularity, 5 lesions consisted
predominantly of SMCs, while the other 7 lesions were characterized
by the presence of foci of macrophages. In the 15 advanced fibrous
plaques, scattered macrophages were identified in 5 lesions. In the
22 advanced fibrolipid plaques, abundant macrophages were observed
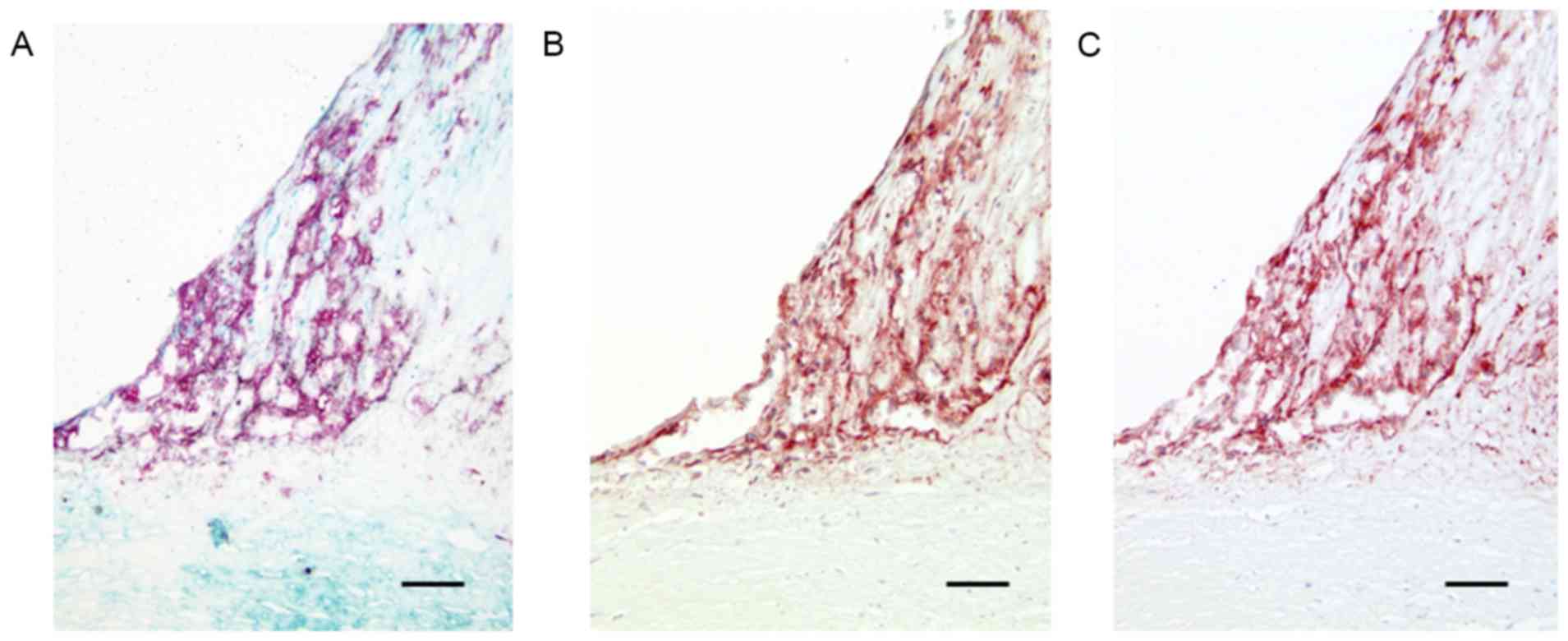
in all lesions (Fig. 1A).
Neutrophil infiltration was observed in 2 of the 22 advanced
fibrolipid plaques. In the normal coronary arteries with diffuse
intimal thickening, medial and intimal SMCs stained positive for
NPR-A, but negative for NPR-B. A similar staining pattern was
identified in medial SMCs of the early atherosclerotic lesions with
hypercellularity. However, SMCs within these early atherosclerotic
(hypercellular) lesions demonstrated distinct expression of NPR-A
with occasional positive staining for NPR-B. In advanced fibrous
and fibrolipid plaques, NPR-A expression was decreased markedly in
intimal and medial SMCs and SMCs within the plaque demonstrated
little or no expression of NPR-A (Fig.
1B). NPR-A positivity and NPR-B positivity were detected in
accumulated macrophages within the plaques (Fig. 1B and C). In normal coronary
arteries with diffuse intimal thickening, hypercellular lesions, or
advanced fibrous plaques, NEP positivity was not observed. However,
in the two advanced fibrolipid plaques with neutrophil
infiltration, NEP positivity was identified in these
neutrophils.
Specimens obtained from patients with AMI
Ruptured and eroded plaques contained numerous
macrophages (Figs. 2A and B;
3A and B). Neutrophil infiltration
was distinctly identified in all lesions with plaque rupture or
erosion (Figs. 2C and 3C). With respect to the number of
neutrophils, no significant differences were observed between
ruptured and eroded plaques. Regarding the neutrophil number in the
culprit lesions, no significant differences were observed between
patients with AMI with PCI and those without PCI. NPR-A and NPR-B
were expressed in macrophages, in addition to in neutrophils in
ruptured and eroded plaques (Figs. 2D
and F; 3D and F). Double
immunostaining for neutrophils and NPR-A or NPR-B demonstrated that
the majority of NPR-A- or NPR-B-positive cells were neutrophils and
NPR-A- or NPR-B-positivity was also identified in occasional
macrophages (Figs. 2E and G;
3E and G). Regarding NEP
expression, neutrophils were positive for NEP in ruptured plaques,
while in eroded plaques the majority of the neutrophils were
negative for NEP (Figs. 2H and
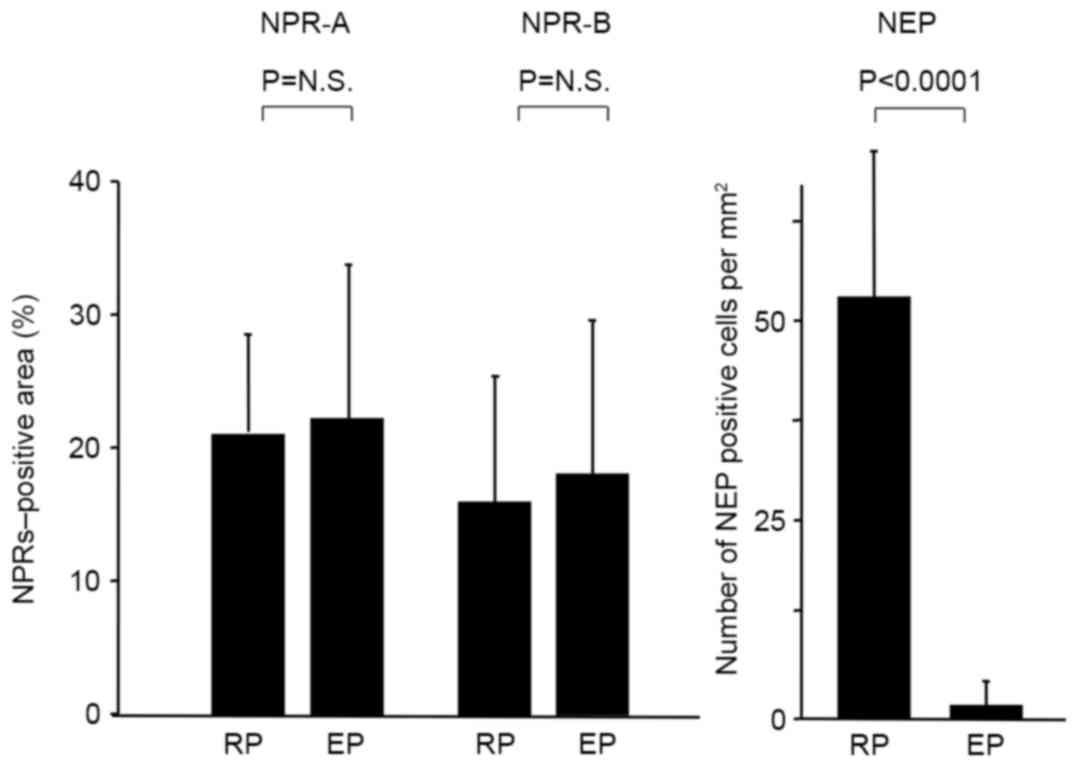
3H). Morphometric analysis
demonstrated that the percentage of NPR-A- and NPR-B- positive
cells did not differ between ruptured and eroded plaques, while the
number of NEP-positive cells in ruptured plaques was significantly
higher compared with eroded plaques (P<0.0001; Fig. 4). In ruptured and eroded plaques,
NPR-A expression in intimal and medial SMCs was decreased.
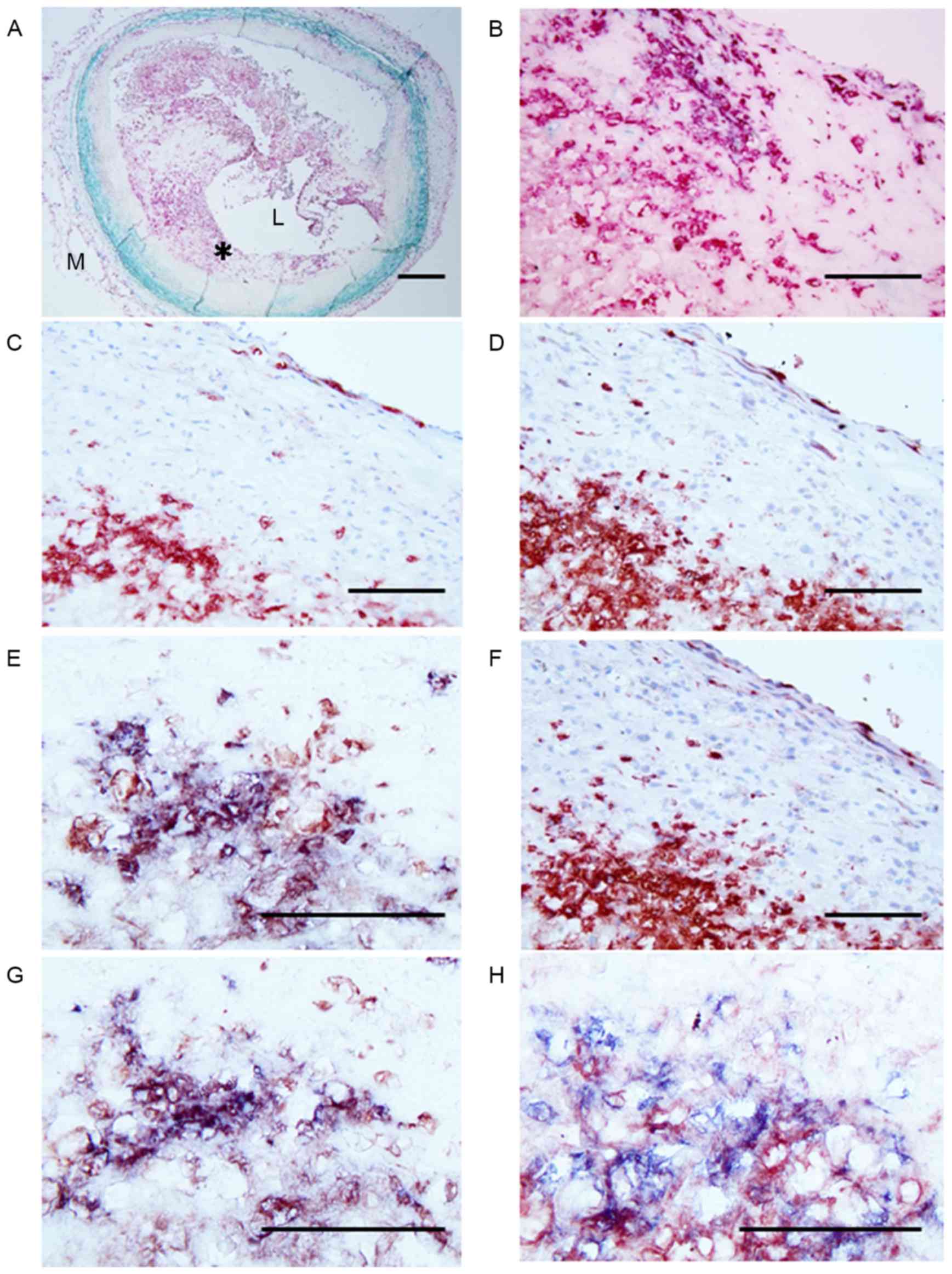 | Figure 2.Micrographs of the site of plaque
rupture obtained at autopsy in a patient with AMI. (A) Double
immunostaining (SMC, turquoise; macrophage, red) showing a
lipid-rich plaque containing numerous macrophages and a thin
fibrous cap with SMCs. The area indicated by the asterisk is
demonstrated at higher magnification in adjacent serial sections
labeled B-H. (B) Double immunostaining (SMC, turquoise; macrophage,
red) showing part of the lipid-rich plaque with numerous
macrophages. (C) Anti-neutrophil CD66b antibody positivity in large
numbers of neutrophils at the same site. (D) Anti-NPR-A antibody
positivity indicating NPR-A expression in infiltrated macrophages
and neutrophils. (E) Double immunostaining for neutrophils (blue)
and NPR-A (red) showing the presence of NPR-A-positive neutrophils.
(F) Anti-NPR-B antibody positivity indicating NPR-B expression in
the majority of neutrophils in the plaque. (G) Double
immunostaining for neutrophils (blue) and NRR-B (red) showing that
almost all the cells are double stained (purple) and therefore are
neutrophils. (H) Double immunostaining (neutrophil CD66b, blue;
NEP, red) indicated that almost all cells show double staining
(purple), indicating that the NEP-positive cells are neutrophils.
Scale bars: A, 500 µm; B-H, 100 µm. AMI, acute myocardial
infarction; SMC, smooth muscle cell; NPR, natriuretic peptide
receptor; L, lumen; M, media. |
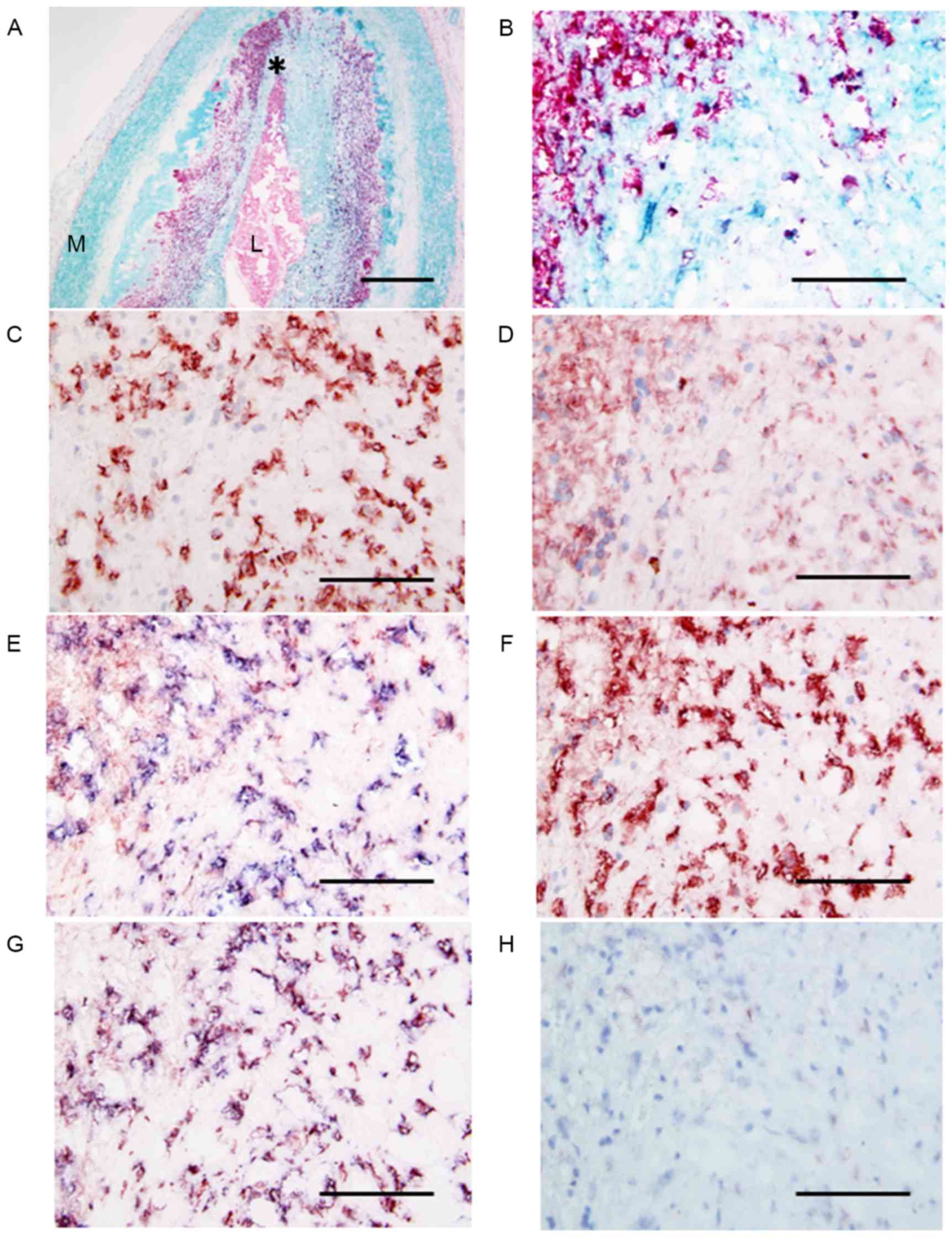 | Figure 3.Micrographs of the site of plaque
erosion obtained at autopsy in a patient with AMI. (A) Double
immunostaining (SMC, turquoise; macrophage, red) indicates abundant
macrophages within the plaque. L, lumen, M, media. The area
indicated by the asterisk is demonstrated at a higher magnification
in adjacent serial sections, labeled B-H. (B) Double immunostaining
(SMC, turquoise; macrophage, red) indicates large numbers of
macrophages. (C) Anti-neutrophil CD66b antibody positivity in large
numbers of neutrophils at the same site. (D) Anti-NPR-A antibody
positivity indicating NPR-A expression in infiltrated macrophages
and neutrophils. (E) Double immunostaining for neutrophils (blue)
and NPR-A (red) showing the presence of NPR-A-positive neutrophils.
(F) Anti-NPR-B antibody positivity indicating NPR-B expression in
the majority of neutrophils in the plaque. (G) Double
immunostaining for neutrophils (blue) and NRR-B (red) showing that
almost all the cells are double stained (purple) and therefore are
neutrophils. (H) Double immunostaining (neutrophil CD66b, blue;
NEP, red) shows that all cells stain blue, indicating that these
neutrophils are negative for NEP. Scale bars: A, 500 µm; B-H, 100
µm. AMI, acute myocardial infarction; SMC, smooth muscle cell; NPR,
natriuretic peptide receptor. |
Discussion
Plaque rupture or erosion has been demonstrated to
be the most important mechanism that underlies the sudden onset of
acute coronary syndromes. Several pathophysiological mechanisms may
serve a significant role in the process of plaque disruption,
including inflammation, rheological factors, circumferential wall
stress and vasoconstriction.
The current study, based on frozen sections, is the
first, to the best of the authors' knowledge, to demonstrate cells
positive for NPR-A and NPR-B in ruptured and eroded plaques. A
previous study (22) demonstrated
the presence of mRNA and its translation products for NPs and their
receptors in human coronary atherosclerotic plaques. However, the
involvement of NPR-A and NPR-B in the development of the various
types of coronary atherosclerotic lesions, including ruptured and
eroded plaques, remains to be established. The findings of the
present immunohistochemical study indicated that cell types
involved in NPR-A and NPR-B expression levels in various stages of
coronary atherosclerotic lesions are different. In early
atherosclerotic lesions with hypercellularity, SMCs are
predominantly involved, while in advanced atherosclerotic lesions
NPR-A and NPR-B are mainly expressed in accumulated macrophages. In
ruptured and eroded plaques the great majority of NPR-A- or
NPR-B-positive cells are neutrophils. Previous experimental studies
have demonstrated that NPR-A and NPR-B are expressed in macrophages
(23), in bone marrow-derived
stromal cells (24) and in
neutrophils (13). These
experimental data and the results of the present study suggest that
NPR-A and NPR-B contribute to the progression of plaque instability
in human coronary atherosclerotic lesions.
In the present study, no significant difference was
observed in the expression of NPR-A and NPR-B in neutrophils
between ruptured and eroded plaques. However, the number of
NEP-positive neutrophils was markedly higher in ruptured plaques
compared with eroded plaques, which is consistent with the results
of our previous study (4). NEP can
hydrolyze the NP family (6). NEP
on the surface of leukocytes degrades the chemotactic peptide
N-formyl-methionyl-leusyl-phenylaline and leukocyte adhesion, and
chemotaxis is increased by inhibition of NEP (6,20).
Indeed, NEP-negative neutrophils exhibit a greater chemotactic
reaction to the activated complement compared with NEP-positive
neutrophils (25,26). It has also been demonstrated that
NEP expression occurs on fully mature neutrophils (27,28).
Perchansky et al (29)
reported that NEP is detected only on fully mature and segmented
neutrophils, while the majority of newly generated neutrophils
exhibit a low expression of NEP due to the immaturity of the
neutrophil membrane. Martens et al (30) further demonstrated that NEP
expression in neutrophils was significantly decreased in patients
with septic shock, which may be associated with an increase of
immature neutrophils. These data are of interest, due to the fact
that the results of the present study indicate that eroded plaques
predominantly contain NEP-negative neutrophils. The biological
significance of this phenomenon in human coronary atherosclerotic
lesions remains to be elucidated. It may be hypothesized that the
differences in NEP expression in neutrophils between ruptured and
eroded plaques reflect differences in chemotaxis and, therefore,
differences in the underlying inflammatory processes. In addition,
the results of the present study indicated that the majority of the
neutrophils in eroded plaques were negative for NEP, indicating
that a rapid outburst of neutrophils had taken place, including
that observed in the early stages of infection. In this context, it
is conceivable that the underlying pathogenetic mechanism in plaque
erosion is different from that in plaque rupture.
The roles of NPR-A and NPR-B expressed by
neutrophils in ruptured and eroded plaques remain unclear. A
previous study (13) demonstrated
that neutrophils express NPR-A, the active receptor for NPs, and
that ANP limits neutrophil activation via a cGMP-dependent
mechanism. In addition, Mtairag et al (31) reported that ANP potentiation by NEP
inhibition further limited neutrophil activation and
neutrophil-vascular cell interactions. However, in those studies,
the mechanism of the effect of ANP on neutrophils was not
investigated in vivo. In the present study, strong
expression of NPR-A and NPR-B was identified in neutrophils in
ruptured and eroded plaques. However, ruptured plaques had a
significantly higher number of NEP-positive neutrophils compared
with eroded plaques. These observations suggested that the
inhibitory effect of NPs on neutrophil activation can be suppressed
by neutrophil NEP in ruptured plaques, while NPs can limit
neutrophil activation via NPR-A and NPR-B more markedly in eroded
plaques. Therefore, it can be hypothesized that the enhanced
expression of NPR-A and NPR-B on NEP-negative neutrophils in eroded
plaques may regulate inflammatory process and vascular activity in
these lesions.
Atherosclerosis is a complex phenomenon associated
with interaction of numerous factors. The NP system and its
receptors are not the only factors involved, therefore, other
factors must be taken into consideration to determine the
functional significance of this system. Nevertheless, the present
study provided data to implicate NPR-A and NPR-B in the changes in
vasomotor activity and inflammatory cell infiltration that occur in
plaque instability.
In conclusion, distinct expression of NPR-A and
NPR-B in culprit lesions underlying AMI strongly suggests that NPs
serve a role in regulating plaque instability in humans.
Glossary
Abbreviations
Abbreviations:
|
SMC
|
smooth muscle cell
|
|
AMI
|
acute myocardial infarction
|
|
NPR-A
|
natriuretic peptide receptor-A
|
|
NPR-B
|
natriuretic peptide receptor-B
|
|
NEP
|
neutral endopeptidase
|
|
NP
|
natriuretic peptides
|
|
CNP
|
C-type natriuretic peptide
|
|
PCI
|
percutaneous coronary intervention
|
|
cGMP
|
cyclic guanosine monophosphate
|
References
|
1
|
Davies MJ and Thomas AC: Plaque
fissuring-the cause of acute myocardial infarction, sudden ischemic
mortality, and crescendo angina. Br Heart J. 53:363–373. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fuster V, Badimon L, Badimon JJ and
Chesebro JH: The pathogenesis of coronary artery disease and the
acute coronary syndromes. N Engl J Med. 326:242–250. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Wal AC, Becker AE, van der Loos CM
and Das PK: Site of intimal rupture or erosion of thrombosed
coronary atherosclerotic plaques is characterized by an
inflammatory process irrespective of the dominant plaque
morphology. Circulation. 89:36–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naruko T, Ueda M, Haze K, van der Wal AC,
van der Loos CM, Itoh A, Komatsu R, Ikura Y, Ogami M, Shimada Y, et
al: Neutrophil infiltration of culprit lesions in acute coronary
syndromes. Circulation. 106:2894–2900. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kayo S, Ohsawa M, Ehara S, Naruko T, Ikura
Y, Hai E, Yoshimi N, Shirai N, Tsukamoto Y, Itabe H, et al:
Oxidized low-density lipoprotein levels circulating in plasma and
deposited in the tissues: Comparison between Helicobacter
pylori-associated gastritis and acute myocardial infarction. Am
Heart J. 148:818–825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connelly JC, Skidgel RA, Schulz WW,
Johnson AR and Erdos EG: Neutral endopeptidase 24.11 in human
neutrophils: Cleavage of chemotactic peptide. Proc Natl Acad Sci
USA. 82:8737–8741. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galvani M, Ferrini D and Ottani F:
Natriuretic peptides for risk stratification of patients with acute
coronary syndromes. Eur J Heart Fail. 6:327–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki S, Yoshimura M, Nakayama M, Mizuno
Y, Harada E, Ito T, Nakamura S, Abe K, Yamamuro M, Sakamoto T, et
al: Plasma level of B-type natriuretic peptide as a prognostic
marker after acute myocardial infarction: A long-term follow-up
analysis. Circulation. 110:1387–1391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang MS, Lowe DG, Lewis M, Hellmiss R,
Chen E and Goeddel DV: Differential activation by atrial and brain
natriuretic peptides of two different receptor guanylate cyclases.
Nature. 341:68–72. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maack T, Suzuki M, Almeida FA, Nussenzveig
D, Scarborough RM, McEnroe GA and Lewicki JA: Physiological role of
silent receptors of atrial natriuretic factor. Science.
238:675–678. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naruko T, Ueda M, van der Wal AC, van der
Loos CM, Itoh H, Nakao K and Becker AE: C-type natriuretic peptide
in human coronary atherosclerotic lesions. Circulation.
94:3103–3108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naruko T, Itoh A, Haze K, Ehara S,
Fukushima H, Sugama Y, Shirai N, Ikura Y, Ohsawa M and Ueda M:
C-Type natriuretic peptide and natriuretic peptide receptors are
expressed by smooth muscle cells in the neointima after
percutaneous coronary intervention. Atherosclerosis. 181:241–250.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumura T, Kugiyama K, Sugiyama S,
Ohgushi M, Amanaka K, Suzuki M and Yasue H: Neutral endopeptidase
24.11 in neutrophils modulates protective effects of natriuretic
peptides against neutrophils-induced endothelial cytotoxity. J Clin
Invest. 97:2192–2203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiedermann CJ, Niedermühlbichler M,
Braunsteiner H and Widermann CJ: Priming of polymorphonuclear
neutrophils by atrial natriuretic peptide in vitro. J Clin Invest.
89:1580–1586. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garlichs CD, Zhang H, Schmeisser A and
Daniel WG: Priming of superoxide anion in polymorphonuclear
neutrophils by brain natriuretic peptide. Life Sci. 65:1027–1033.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Izumi T, Saito Y, Kishimoto I, Harada M,
Kuwahara K, Hamanaka I, Takahashi N, Kawakami R, Li Y, Takemura G,
et al: Blockade of the natriuretic peptide receptor guanylyl
cyclase-A inhibits NF-kappaB activation and alleviates myocardial
ischemia/reperfusion injury. J Clin Invest. 108:203–213. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stary HC, Chandler AB, Dinsmore RE, Fuster
V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD and
Wissler RW: A definition of advanced types of atherosclerotic
lesions and a histological classification of atherosclerosis. A
report from the Committee on Vascular Lesions of the Council on
Arteriosclerosis, American Heart Association. Arterioscler Thromb
Vasc Biol. 15:1512–1531. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stary HC, Chandler AB, Glagov S, Guyton
JR, Insull W Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD
and Wissler RW: A definition of initial, fatty streak, and
intermediate lesions of atherosclerosis. A report from the
committee on vascular lesions of the council on arteriosclerosis,
American Heart Association. Arterioscler Thromb. 14:840–856. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitano K, Fukuda Y, Nagahira K, Nasu T,
Izumi R, Kawashima K and Nakanishi T: Production and
characterization of monoclonal antibodies against human natriuretic
peptide receptor-A or -B. Immunol Lett. 47:215–222. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Letarte M, Vera S, Tran R, Addis JB,
Onizuka RJ, Quackenbush EJ, Jongeneel CV and McInnes RR: Common
acute lymphocytic leukemia antigen is identical to neutral
endopeptidase. J Exp Med. 168:1247–1253. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Loos CM, Becker AE and van den
Oord JJ: Practical suggestions for successful immunoenzyme
double-staining experiments. Histochem J. 25:1–13. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casco VH, Veinot JP, de Kuroski Bold ML,
Masters RG, Stevenson MM and de Bold AJ: Natriuretic peptide system
gene expression in human coronary arteries. J Histochem Cytochem.
50:799–809. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kiemer AK and Vollmar AM: Effects of
different natriuretic peptides on nitric oxide synthesis in
macrophages. Endocrinology. 138:4282–4290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agui T, Yamada T, Legros G, Nakajima T,
Clark M, Peschel C and Matsumoto K: Expression of receptors for
atrial natriuretic peptide on the murine bone marrow-derived
stromal cells. Endocrinology. 130:2487–2494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCormack RT, Nelson RD, Chenoweth DE and
LeBien TW: Identification and characterization of a unique
subpopulation (CALLA/CD10-negative) of human neutrophils
manifesting a heightened chemotactic response to activated
complement. Blood. 70:1624–1629. 1987.PubMed/NCBI
|
|
26
|
Braun MP, Martin PJ, Ledbetter JA and
Hansen JA: Granulocytes and cultured human fibroblasts express
common acute lymphoblastic leukemia-associated antigen. Blood.
61:718–725. 1983.PubMed/NCBI
|
|
27
|
Tran-Paterson R, Boileau G, Giguère V and
Letarte M: Comparative levels of CALLA/neutral endopeptidase on
normal granulocytes, leukemic cells, and transfected COS-1 cells.
Blood. 76:775–782. 1990.PubMed/NCBI
|
|
28
|
McCormack RT, Nelson RD, Solem LD and
LeBien TW: Decreased expression of the common acute lymphoblastic
leukemia antigen (CALLA/CD10) on neutrophils from patients with
thermal injury. Br J Haematol. 69:189–195. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perchansky L, Pirrotta V and Kaplan S:
Flow cytometric study of the expression of neutral endopeptidae
(CD10/CALLA) on the surface of newborn granulocytes. Modern Pathol.
6:414–418. 1993.
|
|
30
|
Martens A, Eppink GJ, Woittiez AJ, Eidhof
H and de Leij LF: Neutrophil function capacity to express CD10 is
decreased in patients with septic shock. Crit Care Med. 27:549–553.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
el Mtairag M, Houard X, Rais S, Pasquier
C, Oudghiri M, Jacob MP, Meilhac O and Michel JB: Pharmacological
potentiation of natriuretic peptide limits polymorphonuclear
neutrophil-vascular cell interactions. Arterioscler Thromb Vasc
Biol. 22:1824–1831. 2002. View Article : Google Scholar : PubMed/NCBI
|