Introduction
Glioma is one of the most common types of tumor of
the central nervous system, accounting for ~44.6% of cases
(1). It was previously reported
that the incidence of glioma is 3–10/100,000 people, among whom
70–80% are diagnosed with malignant glioma (2). Due to incomplete differentiation and
infiltrative growth in deep brain tissue, it is rarely possible to
remove the glioma completely through surgery. Although radiotherapy
and chemotherapy are used post-operatively, patients exhibit a
recurrence rate of ~98% and a median overall survival of <1 year
(2). As the second most common
cause of mortality in patients with cancer <34 years of age, and
the third most common in patients between 35 and 54 years of age,
malignant glioma leads to 180,000–600,000 annual mortalities in
young and middle-aged people worldwide (2). Therefore, glioma-associated research
is a global focus in neurosurgery, investigating the basic
characteristics, biological behavior and treatment of glioma.
C-C motif chemokine 2 (CCL2), additionally termed
monocyte chemoattractant protein 1, is a β-chemokine located on
chromosome 17q11 (3). Human CCL2
was first cloned in the supernatant of a glioma culture in 1989
(4). CCL2 was initially considered
to be a tumor-derived chemokine, and further research demonstrated
abnormal expression of CCL2 in various types of cancer, including
breast, ovarian, prostate, cervical, esophageal, gastric and
nasopharyngeal cancer (5).
Functional CCL2, combined with the surface receptor C-C chemokine
receptor type 2 (CCR2), is able to activate phospholipase C and
kinase C, resulting in the release of calcium ions (6). Among the primary functions of CCL2 is
chemotaxis and the sequestration of monocytes and macrophages to
contribute to the inflammatory response. In addition, it has been
reported that CCL2-mediated chemotaxis and the induction of
histamine release from basophils is associated with immune
regulation (3).
However, although the importance of the CCL2/CCR2
axis in the occurrence and development of cancer has been
demonstrated in previous research, the biological function of CCL2
in glioma has rarely been investigated. Clinical research has
exerted little impact on the recurrence rate, mortality rate and
survival time of patients with glioma. In the present study,
cellular and molecular biology methods were used to investigate the
function and mechanism of the CCL2/CCR2 axis, in the recruitment of
tumor-associated macrophages (TAMs), infiltrative growth of glioma
and angiogenesis in tumors using in vitro and clinical data.
The effect of CCL2 small interfering (si)RNA on the proliferation
of the glioma cell line U251 was demonstrated using a Cell Counting
kit-8 (CCK8) assay. Cell cycle distribution and cellular apoptosis
were determined using flow cytometry. The expression levels of the
biological pathway-associated proteins caspase-3, caspase-7, tumor
necrosis factor receptor superfamily member 10C (TNFRSF10C), growth
regulated α protein (CXCL1), C-X-C motif chemokine 2 (CXCL2), C-X-C
chemokine receptor type 2 (CXCR2), vascular endothelial growth
factor (VEGF) A, VEGFB and VEGF were measured using western
blotting and the reverse transcription-quantitative polymerase
chain reaction (RT-qPCR), in order to investigate the biological
functions involved in the CCL2/CCR2 axis. Glioma cells treated with
the CCR2 inhibitor RS-102895 were used to investigate the mechanism
underlying the apoptosis of the glioma cell line U251.
Materials and methods
Patients and tissue samples
The data on CCL2 expression levels were collected
from The Cancer Genome Atlas database (TCGA; cancergenome.nih.gov). In order to investigate the
mechanism involved in the pathogenesis of glioma through the CCL2
pathway, 30 patients with glioma (average age of 43.23±4.84; 14
male and 16 female) were recruited from Huzhou Central Hospital,
with complete clinical and pathological follow-up data, and were
enrolled in the present study. Additionally, 20 non-neoplastic
brain tissue samples were obtained from surgical procedures for
epilepsy. Ethical approval for the present study was provided by
the independent ethics committee, Shanghai Tongren Hospital
(Shanghai, China). Informed written consent was obtained from all
patients or their advisers according to the guidelines of the
ethics committee.
Transfection
U251 cells (5×105/well) were seeded into
6-well plates containing antibiotic-free DMEM (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) the day prior to
transfection. The cells were transfected with 50 nmol/l CCL2 siRNA
or the negative control using Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. The sequence of the CCL2 siRNA was
5′-CTCGCGAGCTATAGAAGAA-3′.
A total of 48 h subsequently, the transfected cells
were harvested and processed for proliferation, cell cycle
distribution, cellular apoptosis, western blot and RT-qPCR
assays.
Cell proliferation assay using
CCK8
The glioma cell line U251, purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China), was cultured in DMEM (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin streptomycin combination (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) at 37°C in an
atmosphere containing 5% CO2. Cultured cells were
inoculated in three 96-well plates (5×103 cells/well)
and incubated at 37°C for 12 h. A plate was designated to be the
control group, and the other two plates were transfected with empty
plasmid or CCL2 siRNA as the negative control and interference
groups, respectively. CCK-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) and DMEM were mixed at the ratio 1:10 and added to
the plates (100 µl/well) at 0, 24, 48 and 72 h post-transfection,
and the plates were incubated at 37°C in 5% CO2 for 1 h.
The cell viability was determined by the optical density at 450 nm,
measured using a spectrophotometer.
Cell cycle distribution and cellular
apoptosis assays using flow cytometry
CCL2 siRNA-transfected U251 cells were digested
following culturing for 48 h using 0.25% trypsin, followed by
suspension in PBS. The cells were fixed with −20°C pre-cooling in
ethanol and RNA removed with 1 mg/ml RNase A. The samples were
stained with propidium iodide (PI) for 10 min at room temperature
and the cell cycle distribution was measured using flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) at 488 nm
and analyzed using FlowJo version 7.6.1 (FlowJo LLC, Ashland, OR,
USA). DNA content was used to determine the proportion of cells in
each stage of cell cycle.
In addition, the effect of CCL2 siRNA on the
cellular apoptosis of U251 cells was evaluated using flow
cytometry. CCL2 siRNA-transfected cells were digested using trypsin
and re-suspended in PBS following 24 h of culturing. Cells were
centrifuged at 1,000 × g for 5 min at room temperature. The
supernatant was discarded and the cells were incubated with the
Annexin V fluorescein isothiocyanate (FITC) apoptosis detection kit
(BD Biosciences) for 10 min at room temperature in the dark.
Cellular apoptosis rate was measured and the data was obtained
using FlowJo version 7.6.1 (FlowJo LLC) at a wavelength of 488
nm.
Western blot analysis
Control and CCL2 siRNA-transfected U251 cells were
washed with PBS. Besides, cultured U251 cells were pre-treated with
5 µM CCR2 inhibitor RS-102895 (MCE, USA) at room temperature. Cells
were fully lysed in radioimmunoprecipitation assay buffer (Beijing
Solarbio Science & Technology Co., Ltd.) for 10 min and
centrifuged at 12,000 × g for a further 10 min at 4°C. A total of
35 µg supernatant was subjected to 15% SDS-PAGE following
quantification using bicinchoninic acid protein assay reagent
(Sangon Biotech Co., Ltd., Shanghai, China). A nitrocellulose
filter membrane (Merck KGaA, Darmstadt, Germany) was used to
transfer the blots following soaking in transfer buffer (48 mM
Tris, 39 mM glycine, 0.04% SDS and 20% methyl alcohol) for 10 min.
The membrane was blocked with 5% skimmed milk (BD Biosciences) at
room temperature for 1 h. Anti-caspase-3 (Ab32351; 1:5,000; Abcam
Cambridge, UK), caspase-7 (Ab32522, 1:1,000; Abcam), vascular
endothelial growth factor (VEGF) A (Ab115961; 1:1,000; Abcam),
VEGFB (Ab185696; 1:500; Abcam), VEGF (Ab46154, 1:1,000; Abcam),
CXCL1 (Santa, Sc-130316, 1:200), CXCL2 (Abcam, Ab139115, 1:100),
CXCR2 (Abcam, Ab65968, 1:200; Abcam), TNFRSF10C (Sc-26462, 1:200;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p38 (9212;
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated (p)-p38 (9211; 1:1,000; Cell Signaling Technology,
Inc.), extracellular signal-related kinase (ERK)1/2 (4695; 1:1,000;
Cell Signaling Technology, Inc.), p-ERK1/2 (4376; 1:1,000; Cell
Signaling Technology, Inc.) and GAPDH (5174; 1:1,500; Cell
Signaling Technology, Inc.) were diluted and added to blocking
buffer (Merck KGaA), followed by incubation at 4°C for 12 h. The
membrane was subsequently incubated with Goat anti-rabbit (A0208;
1:1,000; Beyotime Institute of Biotechnology, Haimen, China) and
anti-mouse (A0216; 1:1,000; Beyotime Institute of Biotechnology)
secondary antibodies tagged with horseradish peroxidase at 37°C for
1 h. The image was developed using enhanced chemiluminescence
reagents (Merck KGaA) and Tanon-5200 (Tanon Science and Technology
Co., Ltd., Shanghai, China).
RT-qPCR
The gene expression level of CCL2 in glioma cell
lines T98G (Thermo Fisher Scientific, Inc.), U251, and SHG44 (Type
Culture Collection of the Chinese Academy of Sciences), was
measured using RT-qPCR. TRIzol reagent (Gibco; Thermo Fisher
Scientific, Inc.) was used to extract and quantify total mRNA from
U251 cells. RT was performed to synthesize cDNA using the
Thermoscript RT-PCR System (Thermo Fisher Scientific, Inc.) at a
total volume of 25 µl (12 µl RNA-primer mix, 5 µl 5X RT reaction
buffer, 1 µl 25 mM dNTPs, 1 µl 25 U/µl RNase inhibitor, 1 µl 200
U/µl M-MLV Rtase, 1 µl Oligo(dt)18 and 4 µl DNase-free
ddH2O).
A total PCR mix (Thermo Fisher Scientific, Inc.) of
25 µl (12.5 µl SYBR-Green mix, 0.5 µl forward primer, 0.5 µl
reverse primer, 9.5 µl ddH2O and 2 µl cDNA template) was
amplified via 40 cycles of denaturing at 95°C for 15 sec, annealing
at 60°C for 30 sec and elongation at 60°C for 45 sec. Amplification
kinetic curves were obtained and the data was analyzed using ABI
Prism 7300 SDS built-in software (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Primers used in the real-time PCR analysis are
listed in Table I.
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence | Species | Amplicon size
(bps) |
|---|
| CXCL1 | Forward:
5′ATGCTGAACAGTGACAAATC3′ | Homo | 147 |
|
| Reverse:
5′AAACACATTAGGCACAATCC3′ |
|
|
| CXCL2 | Forward:
5′CCCAAACCGAAGTCATAGC3′ | Homo | 188 |
|
| Reverse:
5′GGAACAGCCACCAATAAGC3′ |
|
|
| CXCR2 | Forward:
5′CCTGTCTTACTTTTCCGAAGGAC3′ | Homo | 82 |
|
| Reverse:
5′TTGCTGTATTGTTGCCCATGT33′ |
|
|
| VEGFA | Forward:
5ATTTCTGGGATTCCTGTAG3′ | Homo | 157 |
|
| Reverse:
5′CAGTGAAGACACCAATAAC3′ |
|
|
| VEGFB | Forward:
5′GGATAGCCCAGTCAATACAG3′ | Homo | 127 |
|
| Reverse:
5′CAAGCAAGGTCACTCAGTAG3′ |
|
|
| Caspase-3 | Forward:
5′GTTTGAGCCTGAGCAGAGAC3′ | Homo | 120 |
|
| Reverse:
5′TGGCAGCATCATCCACAC3′ |
|
|
| Caspase-7 | Forward:
5′AGTGACAGGTATGGGCGTTC3′ | Homo | 164 |
|
| Reverse:
5′CGGCATTTGTATGGTCCTCTT3′ |
|
|
| CCL2 | Forward:
5′AACCGAGAGGCTGAGACTAAC3′ | Homo | 125 |
|
| Reverse:
5′GGAATGAAGGTGGCTGCTATG3′ |
|
|
| TNFRSF10C | Forward:
5′ACCAACGCTTCCAACAATGAA3′ | Homo | 173 |
|
| Reverse:
5′CTAGGGCACCTGCTACACTTC3′ |
|
|
| GAPDH | Forward:
5GTCGGTGTGAACGGATTTG3′ | Homo | 181 |
|
| Reverse:
5′TCCCATTCTCAGCCTTGAC3′ |
|
|
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using t-tests and analysis of variance. GraphPad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA) was used to perform all statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
Screening of glioma cell lines with
high expression level of CCL2
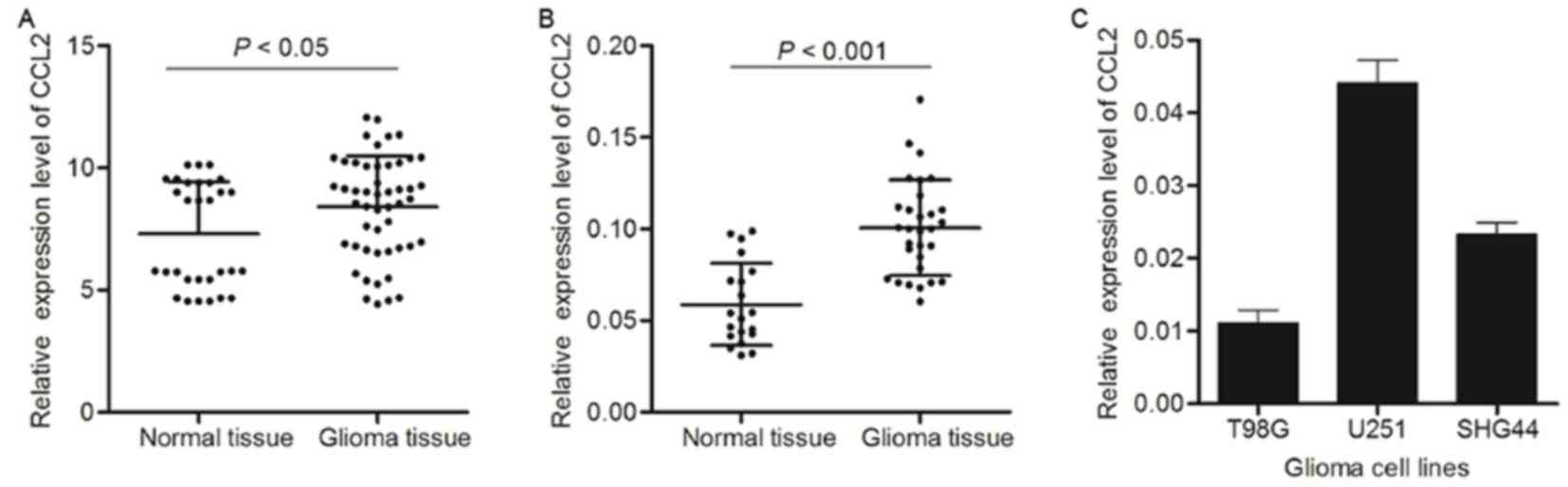
According to the data on CCL2 expression level
obtained from TCGA, glioma tissue exhibited an increase in CCL2
expression compared with healthy tissue (Fig. 1A). RT-qPCR analysis was also
performed to detect the gene expression level of CCL2 in glioma
tissue and healthy tissue. Similarly, compared with healthy
samples, CCL2 expression level was significantly increased in the
glioma tissue samples (Fig. 1B).
Due to the significant alteration of CCL2 expression between glioma
tissue and normal tissue, three glioma cell lines, T98G, U251 and
SHG44, were used to detect the expression level of CCL2 using
western blot analysis. As presented in Fig. 1C, CCL2 exhibited increased
expression levels in U251 cells compared with the other cell lines.
Therefore, the glioma cell line U251 was used for further
investigation of the possible mechanism underlying siRNA
interference in glioma.
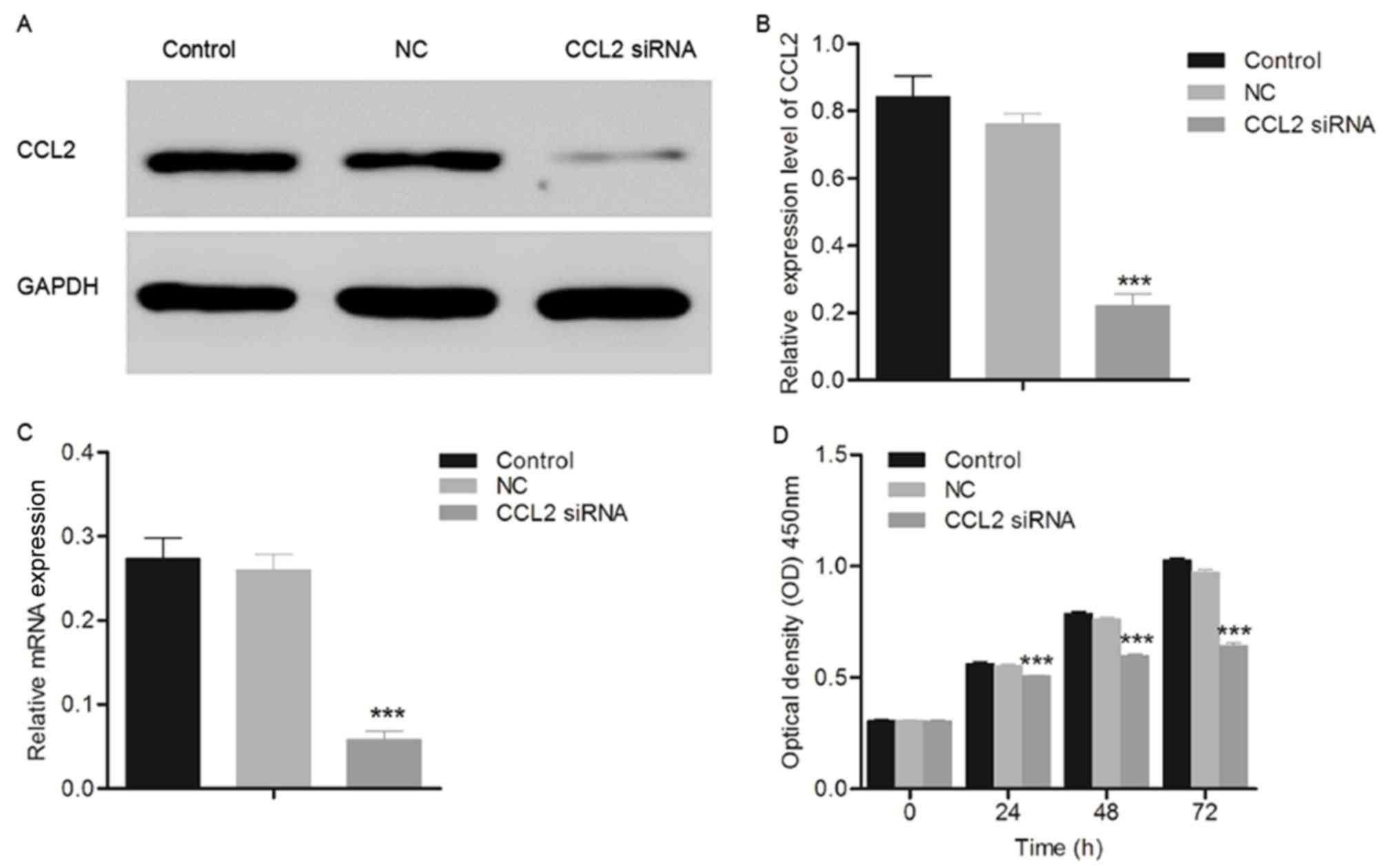
CCL2 siRNA inhibits cell
proliferation
The glioma cell line U251 was transfected with CCL2
siRNA and the results are presented in Fig. 2. The expression level of CCL2 was
significantly decreased in U251 cells transfected with CCL2 siRNA
compared with the control group, demonstrating the interference
ability of CCL2 siRNA in glioma cell lines. Additionally, the data
presented in Fig. 2D and E
demonstrated that the proliferation of U251 cells transfected with
CCL2 siRNA decreased in a time-dependent manner compared with the
control group, measured using a CCK8 assay. Although a significant
difference was observed between the negative control and control
groups at 48 and 72 h post-transfection, it was hypothesized that
this was due to over-proliferation and not the effect of the empty
plasmid.
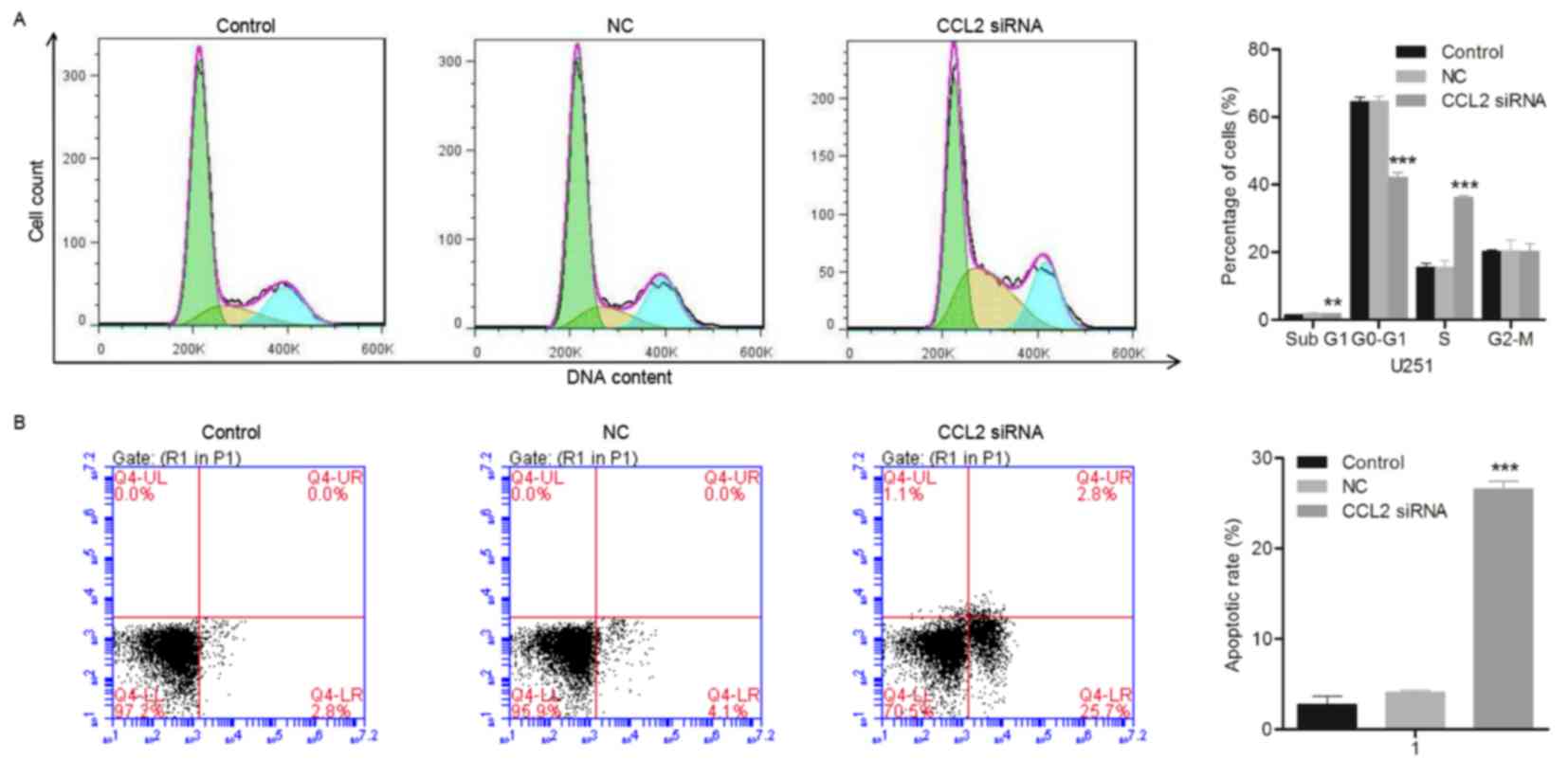
CCL2 siRNA causes cell cycle
arrest
The cell cycle distribution of the glioma cell line
U251 was detected 48 h following transfection with CCL2 siRNA using
flow cytometry. The cell cycle of U251 cells was observed to be
arrested at the S phase. As presented in Fig. 3A, the percentage of G0-G1 cells in
the siRNA-treated U251 group decreased significantly from
64.52±1.47 to 42.16±1.80% (n=3; P<0.001) compared with the
control group, while the percentage of cells in the S phase
increased from 15.59±1.65 to 36.36±1.71% (n=3; P<0.001),
compared with the control group. The significant increase in the
percentages of cells in S phase indicated that the cell cycle of
U251 cells was arrested at S phase due to the treatment with CCL2
siRNA.
CCL2 siRNA induces cellular
apoptosis
The effect of CCL2 siRNA on the cellular apoptosis
of the glioma cell line U251 was evaluated using flow cytometry,
following treatment with CCL2 siRNA for 48 h. As presented in
Fig. 3B, the cellular apoptosis
induced by CCL2 siRNA in U251 cells was detected visually using
Annexin V FITC/PI double staining. The percentages of early
apoptotic cells located in the lower right quadrant of the
histograms were recorded as the apoptotic rate and are presented in
Fig. 3B. The apoptotic rate of
U251 cells transfected with CCL2 siRNA increased between 2.73±0.89
and 26.57±1.14% (n=3; P<0.001) compared with the control group,
demonstrating that cellular apoptosis of U251 cells was induced by
treatment with CCL2 siRNA. Additionally, western blot and RT-qPCR
analyses were used to evaluate the alteration in cellular
apoptosis-associated proteins caspase-3, caspase-7 and TNFRSF10C.
The expression levels of caspase-3 and caspase-7 were upregulated
in U251 cells compared with the control, while TNFRSF10C exhibited
a significant decrease in expression level (Fig. 4A-C). Consistent with the results
obtained in the western blot assay, the gene expression levels
measured using RT-qPCR demonstrated up- and downregulation of the
caspase proteins and TNFRSF10C, respectively. The results of the
present study demonstrated that the increase in the expression
levels of apoptosis-associated proteins caspase-3 and caspase-7
resulted in the cellular apoptosis of U251 cells.
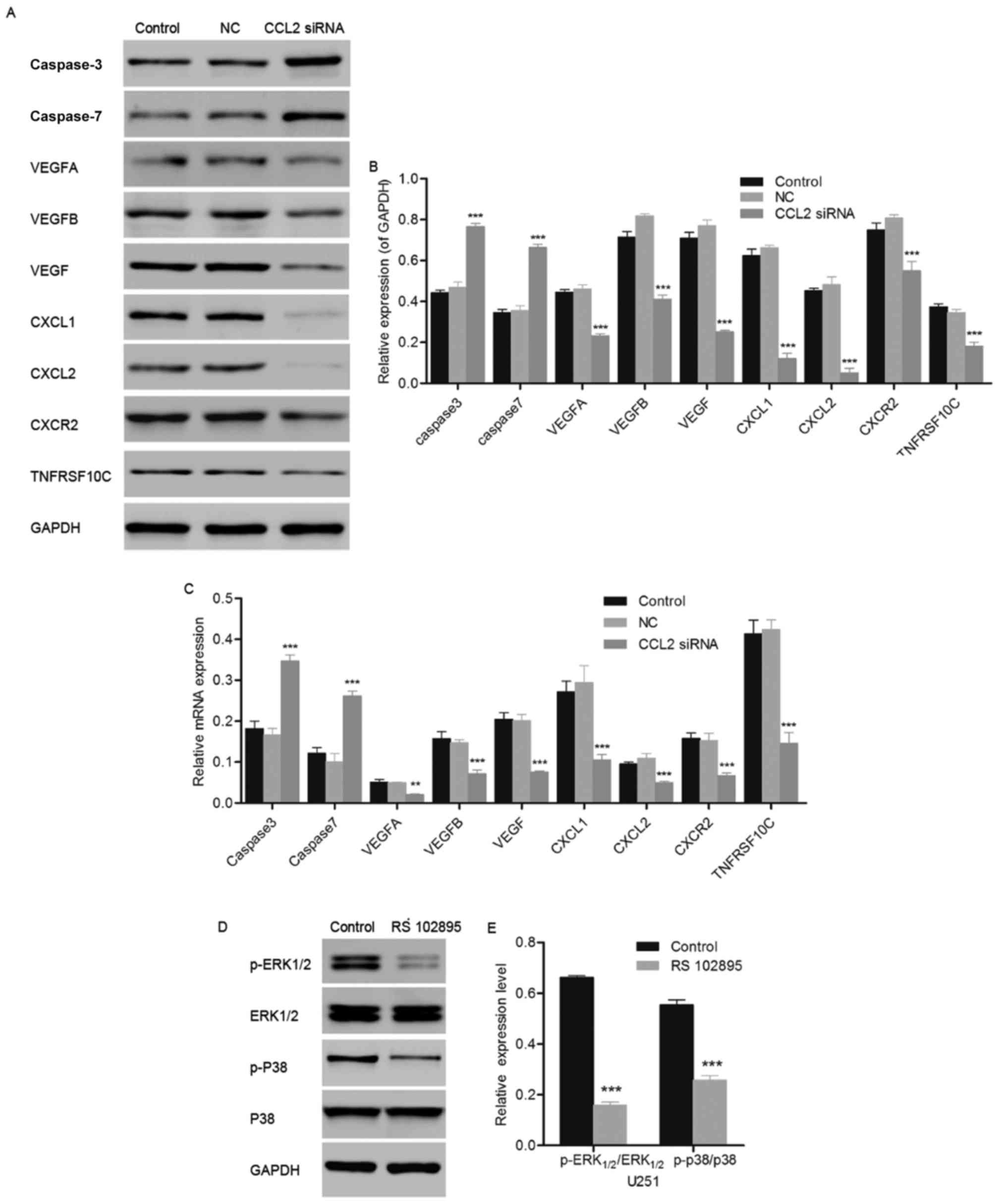 | Figure 4.Effect of CCL2 siRNA on the expression
levels of caspase-3, caspase-7, TNFRSF10C, VEGFA, VEGFB, VEGF,
CXCL1, CXCL2 and CXCR2. (A) Western blot analysis of the protein
expression of caspase-3, caspase-7, TNFRSF10C, VEGFA, VEGFB, VEGF,
CXCL1, CXCL2 and CXCR2 was performed using SDS-PAGE. (B)
Quantification of the western blotting used to measure the protein
expression level of apoptosis- and angiogenesis-associated proteins
in the glioma cell line U251 following transfection with CCL2 siRNA
for 48 h. (C) Gene expression of apoptosis- and
angiogenesis-associated proteins in the glioma cell line U251
following transfection with CCL2 siRNA for 48 h was measured using
the reverse transcription-quantitative polymerase chain reaction.
(D) Western blot analysis of the protein expression of ERK1/2,
p-ERK1/2, p38 and p-p38 was performed using SDS-PAGE. (E)
Quantification of the western blotting used to detect the
phosphorylation of ERK1/2, p-ERK1/2, p38 and p-p38. n=3;
**P<0.01, ***P<0.001 vs. control; n=3. CCL2, C-C motif
chemokine 2; siRNA, small interfering RNA; NC, negative control;
TNFRSF10C, tumor necrosis factor receptor superfamily member 10C;
VEGF, vascular endothelial growth factor; CXCL1, growth regulated
alpha protein; CXCL2, C-X-C motif chemokine 2; CXCR2, C-X-C
chemokine receptor type 2; ERK, extracellular signal related
kinase; p38, p38 mitogen-activated protein kinase; p,
phosphorylated. |
CCL2 siRNA regulates biological
pathway-associated proteins
The expression levels of CCL2-associated
pathway-associated proteins, including CXCL1, CXCL2, CXCR2, VEGFA,
VEGFB and VEGF, were analyzed using western blot and RT-qPCR
analysis following treatment with CCL2 siRNA for 48 h. As presented
in Fig. 4A and B, the protein
expression of CXCL1, CXCL2 and CXCR2, which are responsible for the
proliferative and invasive capabilities of tumor cells, decreased
significantly compared with the control (P<0.001). Similarly,
the protein expression of vascular endothelium
generation-associated proteins VEGFA, VEGFB and VEGF were observed
to be significantly decreased compared with the control group
(P<0.001). Consistent with the results obtained using western
blotting, the gene expression levels of VEGFA (P<0.01), and
CXCL1, CXCL2, CXCR2, VEGFB and VEGF (all P<0.001) exhibited a
decrease compared with the control (Fig. 4C). The downregulation of biological
pathway-associated proteins demonstrated the inhibitory ability of
CCL2 siRNA against the proliferation and invasion of the glioma
cell line U251.
Inhibition of CCL2/CCR2 signaling
downregulates the MAPK/ERK signaling pathway
The glioma cell line U251 was treated with the CCR2
inhibitor RS-102895, and the phosphorylation of p38 and ERK1/2 was
measured using western blotting. The inhibition of CCR2 led to the
downregulation of the expression of apoptosis-associated proteins
p38 and ERK1/2. As presented in Fig.
4D and E, the phosphorylation of ERK1/2 and p38 decreased
significantly compared with the control in U251 cells, due to the
inhibition of CCR2 (P<0.001). The inhibition of CCR2 resulted in
the downregulation of p38 and ERK1/2 expression levels, leading to
cellular apoptosis in U251 cells and demonstrating the important
role of the CCL2/CCR2 signaling pathway in the proliferation of the
glioma cell line U251.
Discussion
Chemokines, which belong to the superfamily of small
molecular weight secreted proteins, are cytokines with chemotactic
abilities, containing 70–100 amino acids (7). The receptors of chemokines are
G-protein-coupled receptors located in the cell membrane of immune
cells, endothelial cells and tumor cells (7). It was previously reported that
chemokines and their receptors are involved in cell growth,
differentiation, apoptosis and tissue damage in somatic cells, and
cell proliferation and migration in tumor cells (7). Chemokines and their receptors have
been demonstrated to exhibit a dual role in tumor biological
behavior. Chemokines exhibit anticancer functions through
chemotaxis and activation of immune cells, or the inhibition of
angiogenesis; in addition, they promote growth, invasion and
migration, through the stimulation of tumor cells and induction of
chemotaxis (8). Therefore,
research focused on chemokines and their receptors has investigated
the tumorigenesis, progression and potential treatment of cancer
(9,10).
CXCL1 and CXCL2, regulated by CCL2, have been
demonstrated to be involved in the growth, proliferation,
metastasis, invasion and angiogenesis of tumors via specific
binding with the G-protein-coupled receptor CXCR2 (11). The expression of CXCL1 was
previously observed in melanoma, and colorectal, breast, bladder
and epithelial ovarian cancer, while no CXCL1 was observed in
healthy melanocytes (11).
Additionally, melanocytes with continuously-expressed CXCL1 exhibit
the potential to transform into tumor cells (12). In a previous study, mice carrying
CXCL1-expressed melanocytes were treated with serum containing a
CXCR2 inhibitor. Tumor growth was observed to be inhibited with a
decrease in angiogenesis, demonstrating the facilitating effect of
CXCL1 in the proliferation and growth of tumor cells (13). In addition, the expression of
metastasis-associated proteins including, matrix metalloproteinase
2 and integrin β1, were demonstrated to be upregulated in the cells
with overexpressed CXCL1 (14).
The invasive capability of five human uveal melanoma cell lines was
observed to be promoted by CXCL1, interleukin 8, stromal
cell-derived factor 1 and hepatocyte growth factor in vivo.
CXCL1, and its receptor CXCR2, were observed to be expressed in all
of the five cell lines with the highest invasion rates, indicating
that invasion and metastasis of tumor cells is promoted by CXCL1
(15). Invasion and metastasis of
tumor cells is an active and organized process with specific
underlying mechanisms (16). The
infiltrating growth of glioma is one of the most important factors
influencing the surgical removal of glioma. The effect of the
CCL2/CCR2 axis on stem cells, including bone marrow stromal cells
and neural stem cells, has been well-studied, while the effect of
CCL2/CCR2 on the chemotaxis and growth facilitation of tumor cells
has rarely been investigated. The present study demonstrated the
association between decreased expression of CXCL1 and inhibited
cell viability of glioma cells due to CCL2 siRNA, exhibiting the
positive regulatory effect of CXCL1 and CXCR2 on cell proliferation
in tumor cells.
Increased angiogenesis and overexpressed CXCL1 has
been observed in rat cornea tissue, and the angiogenic response was
inhibited following treatment with anti-CXCL1 antibody (17). It was previously reported that
CXCL1 regulates angiogenesis via epidermal growth factor and ERK1/2
in vitro, in addition to the recruitment of TAMs, which was
regulated by VEGF (18). VEGF,
also termed vascular permeability factor, is a type of specific
heparin-binding growth factor which is expressed in vascular
endothelial cells (19). The
process of angiogenesis, including the proliferation and migration
of endothelial cells, the degradation of the basement membrane and
the formation of the lumen, is a complex process regulated by
numerous positive and negative regulatory factors (20). Tumor angiogenesis factor serves a
role in the generation of tumor vessels, the uncontrolled growth of
which is an important characteristic of tumor cells. VEGF, VEGFA
and VEGFB were previously demonstrated to induce angiogenesis
through the recruitment of TAMs, which serve an important role in
the angiogenesis and growth of tumor cells in gastric cancer
(21). The association between
increased expression of VEGF and metastasis, angiogenesis and
survival rate in rectal cancer has been previously investigated
(22). VEGF promotes angiogenesis
through direct induction of CCL2 protein expression. The capillary
lumen was able to be formed in CCL2-treated umbilical vein
endothelial cells, demonstrating the regulatory effect of CCL2 on
the expression of VEGF, which results in an increased angiogenic
response in tumor tissues (23).
In the present study, the phosphorylation levels of
ERK1/2 and p38 were measured using western blotting, in order to
investigate the mechanism underlying cellular apoptosis in U251
cells. Mitogen activated protein kinases, including p38, are
involved in the activation of cellular apoptosis regulated by
mitochondria, and induce the translocation of apoptosis regulator
BAX to the mitochondria, followed by the release of cytochrome
c. This process results in the activation of caspase-3 and
the death of cells via the formation of apoptosomes (24). ERK1/2, activated by dual
specificity mitogen-activated protein kinase kinase, promotes the
survival of cells by inducing the expression of protein C-ets-1,
ETS domain-containing protein ELK1 and Myc proto-oncogene protein
(25). In the present study, the
phosphorylation levels of p38 and ERK1/2 were observed to be
downregulated following treatment with the CCR2 inhibitor
RS-102895, demonstrating that the involvement of the MAPK/ERK
pathway in cellular apoptosis is induced by CCL2 siRNA, which was
associated with the increased expression levels of caspase-3 and
−7. Additionally, the downregulated expression level of TNFRSF10C
was consistent with the result obtained in the western blot
analysis.
In conclusion, the results of the present study
demonstrated the regulatory effect of CCL2 on the proliferation and
angiogenesis of the glioma cell line U251, by inhibiting the
expression of CCL2 with CCL2 siRNA. The MAPK/ERK signaling pathway
was identified in the cellular apoptosis of U251 cells. The present
study may provide a theoretical basis for further in vivo
research and clinical treatment for glioma.
Acknowledgements
The present study was supported by the Medical
Science and Technology Project of Zhejiang Province (grant no.
2014ZDA025), and the Science and Technology Project of Zhejiang
Province (grant no. 2015FB048).
References
|
1
|
Ohgaki H: Epidemiology of brain tumors.
Methods Mol Biol. 472:323–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Sarkar S, Cua R, Zhou Y, Hader W
and Yong VW: A dialog between glioma and microglia that promotes
tumor invasiveness through the CCL2/CCR2/interleukin-6 axis.
Carcinogenesis. 33:312–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshimura T, Yuhki N, Moore SK, Appella E,
Lerman MI and Leonard EJ: Human monocyte chemoattractant protein-1
(MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated
blood mononuclear leukocytes, and sequence similarity to mouse
competence gene JE. FEBS Lett. 244:487–493. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dagouassat M, Suffee N, Hlawaty H, Haddad
O, Charni F, Laguillier C, Vassy R, Martin L, Schischmanoff PO,
Gattegno L, et al: Monocyte chemoattractant protein-1 (MCP-1)/CCL2
secreted by hepatic myofibroblasts promotes migration and invasion
of human hepatoma cells. Int J Cancer. 126:1095–1098.
2010.PubMed/NCBI
|
|
6
|
Cho YA and Kim J: Association of
polymorphisms in the MCP-1 and CCR2 genes with the risk of cancer:
A meta-analysis. Cytokine. 64:213–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horuk R: Chemokines.
ScientificWorldJournal. 7:224–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nyberg P, Salo T and Kalluri R: Tumor
microenvironment and angiogenesis. Front Biosci. 13:6537–6553.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kruizinga RC, Bestebroer J, Berghuis P, de
Haas CJ, Links TP, de Vries EG and Walenkamp AM: Role of chemokines
and their receptors in cancer. Curr Pharm Des. 15:3396–3416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Wang H, Brown J, Daikoku T, Ning
W, Shi Q, Richmond A, Strieter R, Dey SK and DuBois RN: CXCL1
induced by prostaglandin E2 promotes angiogenesis in colorectal
cancer. J Exp Med. 203:941–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balentien E, Mufson BE, Shattuck RL,
Derynck R and Richmond A: Effects of MGSA/GRO alpha on melanocyte
transformation. Oncogene. 6:1115–1124. 1991.PubMed/NCBI
|
|
13
|
Haghnegahdar H, Du J, Wang D, Strieter RM,
Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R and
Richmond A: The tumorigenic and angiogenic effects of MGSA/GRO
proteins in melanoma. J Leukoc Biol. 67:53–62. 2000.PubMed/NCBI
|
|
14
|
Zhou Y, Zhang J, Liu Q, Bell R, Muruve DA,
Forsyth P, Arcellana-Panlilio M, Robbins S and Yong VW: The
chemokine GRO-alpha (CXCL1) confers increased tumorigenicity to
glioma cells. Carcinogenesis. 26:2058–2068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Cesare S, Marshall JC, Logan P, Antecka
E, Faingold D, Maloney SC and Burnier MN Jr: Expression and
migratory analysis of 5 human uveal melanoma cell lines for CXCL12,
CXCL8, CXCL1, and HGF. J Carcinog. 6:22007.PubMed/NCBI
|
|
16
|
Pham K, Luo D, Liu C and Harrison JK:
CCL5, CCR1 and CCR5 in murine glioblastoma: Immune cell
infiltration and survival rates are not dependent on individual
expression of either CCR1 or CCR5. J Neuroimmunol. 246:10–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Owen JD, Strieter R, Burdick M,
Haghnegahdar H, Nanney L, Shattuck-Brandt R and Richmond A:
Enhanced tumor-forming capacity for immortalized melanocytes
expressing melanoma growth stimulatory activity/growth-regulated
cytokine beta and gamma proteins. Int J Cancer. 73:94–103. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyake M, Goodison S, Urquidi V, Giacoia E
Gomes and Rosser CJ: Expression of CXCL1 in human endothelial cells
induces angiogenesis through the CXCR2 receptor and the ERK1/2 and
EGF pathways. Lab Invest. 93:768–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuroda T, Kitadai Y, Tanaka S, Yang X,
Mukaida N, Yoshihara M and Chayama K: Monocyte chemoattractant
protein-1 transfection induces angiogenesis and tumorigenesis of
gastric carcinoma in nude mice via macrophage recruitment. Clin
Cancer Res. 11:7629–7636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urushihara M, Ohashi N, Miyata K, Satou R,
Acres OW and Kobori H: Addition of angiotensin II type 1 receptor
blocker to CCR2 antagonist markedly attenuates crescentic
glomerulonephritis. Hypertension. 57:586–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Futagami S, Tatsuguchi A, Hiratsuka T,
Shindo T, Horie A, Hamamoto T, Ueki N, Kusunoki M, Miyake K, Gudis
K, et al: Monocyte chemoattractant protein 1 and CD40 ligation have
a synergistic effect on vascular endothelial growth factor
production through cyclooxygenase 2 upregulation in gastric cancer.
J Gastroenterol. 43:216–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saji H, Koike M, Yamori T, Saji S, Seiki
M, Matsushima K and Toi M: Significant correlation of monocyte
chemoattractant protein-1 expression with neovascularization and
progression of breast carcinoma. Cancer. 92:1085–1091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rafei M, Deng J, Boivin MN, Williams P,
Matulis SM, Yuan S, Birman E, Forner K, Yuan L, Castellino C, et
al: A MCP1 fusokine with CCR2-specific tumoricidal activity. Mol
Cancer. 10:1212011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang CH and Tsai CC: CCL2 increases MMP-9
expression and cell motility in human chondrosarcoma cells via the
Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol.
83:335–344. 2012. View Article : Google Scholar : PubMed/NCBI
|