Introduction
Homocysteine, a thiol-containing amino acid, is
generated by the demethylation of methionine (1,2). It
has been established that an elevated level of circulating
homocysteine is an independent risk factor for Alzheimer's disease
(AD) (3–8), and there is increasing evidence that
homocysteine directly causes neurotoxicity in multiple neuronal
types (9–11). In addition, it is known that
endoplasmic reticulum (ER) stress is closely associated with the
development and pathology of AD, which has the typical
characteristics of inclusion bodies, abnormal formation and
misfolded protein aggregation (12–14).
It has also been reported that homocysteine leads to ER stress in
neuronal cells (15–18), which suggests that ER
stress-mediated homocysteine-induced neurotoxicity may be vital in
the pathogenesis of AD. Therefore, the suppression of ER stress may
provide a promising approach for the treatment of
homocysteine-dependent neurodegenerative diseases.
Hydrogen sulfide (H2S) is considered to
be a novel endogenous neuroprotectant (19–23).
Of note, data from our previous study demonstrated that the
disturbance of endogenous H2S generation was involved in
the neurotoxicity of homocysteine (24), and that H2S ameliorated
homocysteine induced-neurotoxicity (25), indicating the potential of
H2S-based prevention and treatment for neuronal injury
induced by homocysteine exposure. Based on the importance of ER
stress in the neurotoxicity of homocysteine, the present study
aimed to expand on current understanding of the protective effects
of H2S in homocysteine-elicited neurotoxicity by
examining the effects of H2S on homocysteine-induced ER
stress and the underlying mechanisms.
Sirtuins are nicotinamide adenine
dinucleotide-dependent histone deacetylases, which counter aging,
having a broad spectrum of metabolic and stress-tolerance
functions. Emerging evidence has confirmed that silent mating type
information regulator 2 homolog 1 (SIRT-1), one of the seven
mammalian sirtuins, is directly involved in the neuronal protective
effect against cellular damage and stressful perturbations in
neurological diseases, including AD (26–29),
amyotrophic lateral sclerosis (28), Huntington's disease (30,31)
and Parkinson's disease (32).
Furthermore, it has been reported that SIRT-1 mediates the
neuroprotective effect of paliperidone against MK-801-induced
neuronal damage (33) and
hyperbaric oxygen preconditioning-induced ischemic tolerance in the
rat brain (34). Of note, previous
studies have suggested that SIRT-1 exhibits its beneficial effects
in neuroprotection via alleviation of the ER stress response
(35,36). Therefore, the present study
investigated whether SIRT-1 contributes to the protective effects
of H2S against homocysteine-induced ER stress.
The results of the present study revealed that
H2S prevented homocysteine-induced ER stress and
increased the protein expression of SIRT-1 in PC12 cells. Sirtinol,
a specific inhibitor of SIRT-1, eliminated the inhibitory effects
of H2S against homocysteine-induced ER stress in the
PC12 cells. These findings indicated that H2S protects
PC12 cells against homocysteine-induced ER stress via upregulating
the expression of SIRT-1.
Materials and methods
Materials
Sodium hydrosulfide (NaHS), an exogenous donor of
H2S, homocysteine and sirtinol, a specific inhibitor of SIRT-1,
were supplied by Sigma-Aldrich (cat. no. S7942; Merck KGaA,
Darmstadt, Germany). Specific antibody against SIRT-1 (cat. no.
ab110304) was purchased from Abcam (Cambridge, UK). Specific
antibody against glucose-regulated protein 78 (GRP78; cat. no.
S1931) was obtained from Epitomics (Burlingame, CA, USA). Specific
antibody against cleaved caspase-12 (cat. no. C7611) was supplied
by Sigma-Aldrich (Merck KGaA); β-actin polyclonal antibody (cat.
no. 20536-1-AP) and goat anti-rat immunoglobulin (Ig)G (cat. no.
SA00001-2) or goat anti-mouse IgG (cat. no. SA00001-1) antibody
were obtained from ProteinTech Group, Inc. (Chicago, IL, USA).
RPMI-1640 medium, fetal bovine serum (FBS) and horse serum were
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Cell culture
The PC12 cells (American Type Culture Collection;
CRL-1721), provided by Sun Yat-sen University Experimental Animal
Center (Guangzhou, China), were cultured in RPMI-1640 medium
supplemented with 10% (v/v) heat-inactivated horse serum and 5% FBS
(v/v) at 37°C, in an atmosphere containing 5% CO2 and
95% air. The culture medium was replaced every 2–3 days.
Western blot analysis
The PC12 cells, treated as described above, were
homogenized in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Shanghai, China) containing
phenylmethylsulphonyl fluoride (1 mM) for 30 min 4°C and the
supernatants was obtained by centrifugation at 5,000 × g for 10 min
at 4°C. Protein concentrations were determined using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Equivalent quantities of protein (50 µg) were
separated by SDS-PAGE on a 12% gel. The proteins were then
transferred onto polyvinylidene fluoride membranes, and the
membranes were blocked with 5% skim milk in Tris-buffered saline
containing 0.1% Tween-20 (TBST) for 2 h at room temperature. The
membranes were then incubated with primary antibodies specific for
blocking solution, containing primary antibodies against SIRT-1
(1:2,000), GRP78 (1:2,000), cleaved caspase-12 (1:2,000) and
β-actin (1:5,000) overnight at 4°C. Following washing with TBST
three times, the membranes with SIRT-1 were incubated in
peroxidase-conjugated affinipure goat anti-mouse IgG (1:5,000) and
others were incubated in anti-rabbit secondary antibodies (1:5,000)
in blocking solution for 2 h at 25°C and then washed in TBST
buffer. The bands of protein were visualized using an enhanced
chemiluminescence reaction solution (solution 1:0.1 M Tris-HCl,
luminol and p-coumaric acid; solution 2:0.1 M Tris-HCl and hydrogen
peroxide) for 2 min, and quantified using an image analysis system
equipped withBIO-1D software (v4.62; VilberLourmat,
Marne-la-Vallée, France).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The significance of differences in different groups was
assessed using one-way analyses of variance followed by the least
significant difference test using SPSS version 19.0 (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
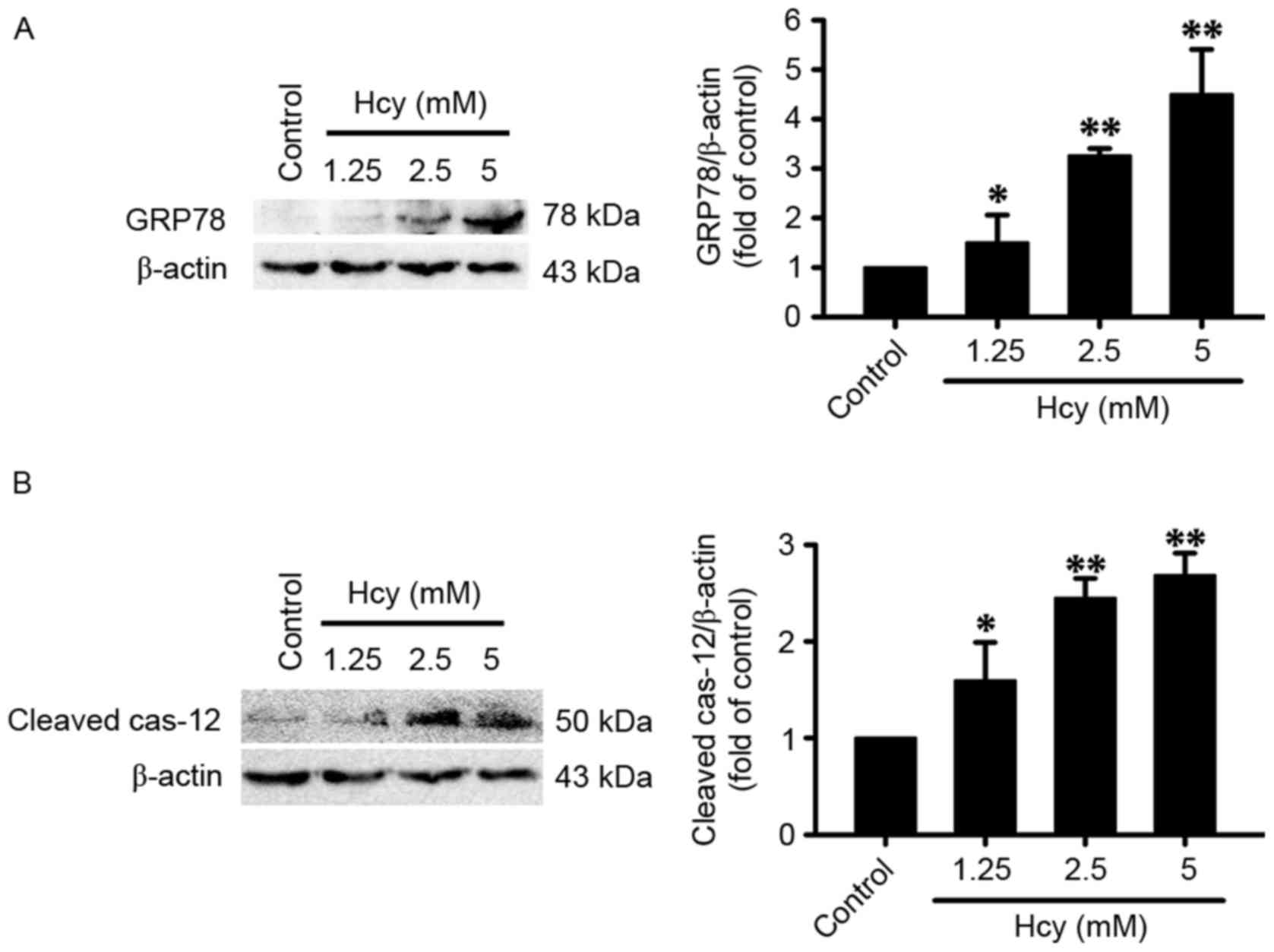
Homocysteine induces ER stress in PC12
cells
To investigate whether homocysteine induces ER
stress in PC12 cells, the present study measured the expression
levels of ER stress-related proteins, including GRP78 and cleaved
caspase-12, in homocysteine-treated PC12 cells using western blot
analysis. It was found that treatment with homocysteine (1.25, 2.5
or 5 mM for 24 h) significantly increased the expression levels of
GRP78 (Fig. 1A) and cleaved
caspase-12 (Fig. 1B) in the PC12
cells, which indicated that homocysteine-induced ER stress in the
PC12 cells.
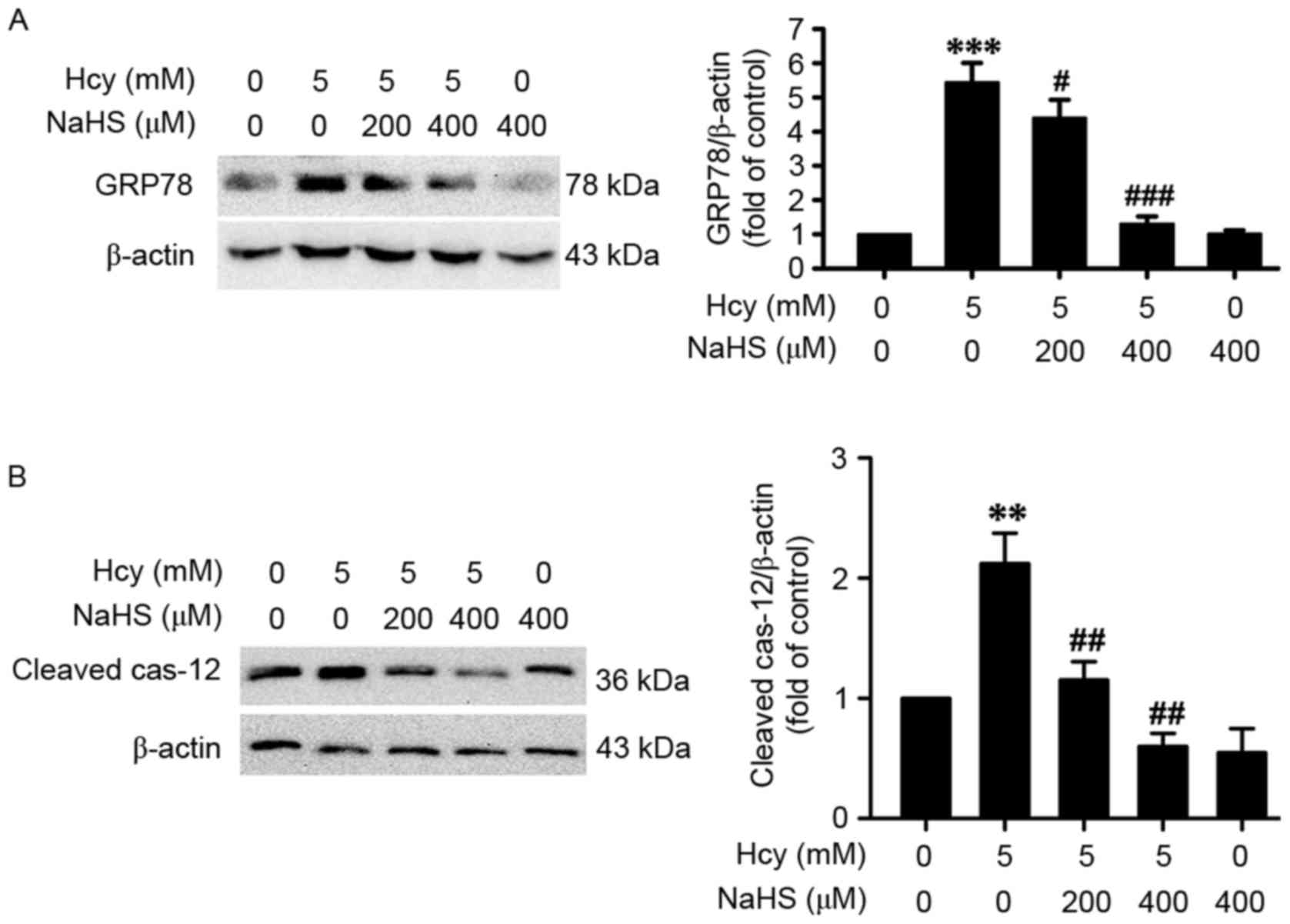
H2S protects PC12 cells
from homocysteine-induced ER stress
To determine whether H2S protects PC12 cells against
homocysteine-induced ER stress, the present study examined the
effects of H2S on the protein levels of GRP78 and cleaved
caspase-12 in homocysteine-exposed PC12 cells. As shown in Fig. 2, cotreatment of the PC12 cells with
NaHS (200 or 400 µM) significantly downregulated the expression
levels of GRP78 (Fig. 2A) and
cleaved caspase-12 (Fig. 2B),
compared with the cells treated with 5 mM of homocysteine for 24 h.
This indicated that H2S had a protective effect in
homocysteine-induced ER stress.
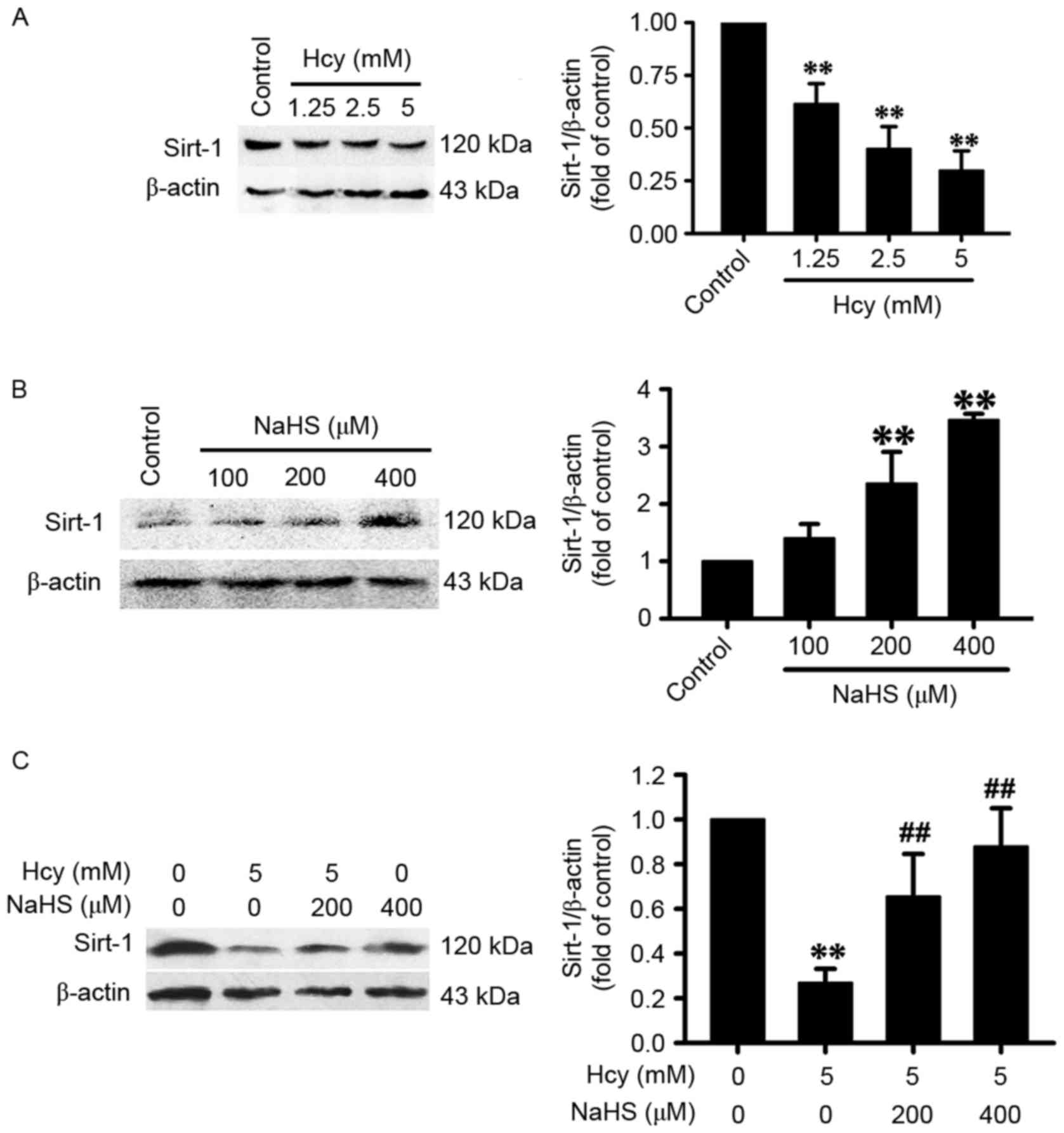
H2S upregulates the
expression of SIRT-1 in PC12 cells
As shown in Fig.
3A, treatment with homocysteine (1.25, 2.5 or 5 mM, for 24 h)
significantly decreased the expression levels of SIRT-1 in the PC12
cells, which indicated that homocysteine downregulated the protein
expression of SIRT-1 in PC12 cells. Treatment with NaHS at
concentrations of 200 and 400 µM for 24 h dose-dependently
increased the expression of SIRT-1 in the PC12 cells (Fig. 3B), and also significantly increased
the expression of SIRT-1 in the homocysteine-exposed (5 mM for 24
h) PC12 cells (Fig. 3C), which
indicated the promoting effect of H2S on the expression of SIRT-1
in PC12 cells.
Inhibition of SIRT-1 eliminates the
beneficent effect of H2S against homocysteine-induced ER
stress in PC12 cells
To further investigate whether the protective effect
of H2S on homocysteine-induced ER stress in PC12 cells was through
the upregulation of SIRT-1, the present study used sirtinol, a
specific inhibitor of SIRT-1, to examine the effect of H2S on ER
stress under homocysteine treatment. The PC12 cells were pretreated
with sirtinol (15 µM) for 30 min prior to the administration of
NaHS (200 µM). The results demonstrated that sirtinol (15 µM)
treatment inhibited the NaHS (200 µM)-induced suppression of GRP78
(Fig. 4A) and cleaved caspase-12
(Fig. 4B) in the
homocysteine-exposed PC12 cells. Treatment with sirtinol alone did
not affect the expression of these two proteins. These results
indicated that the inhibition of SIRT-1 eliminated the protective
effect of H2S against homocysteine-induced ER stress.
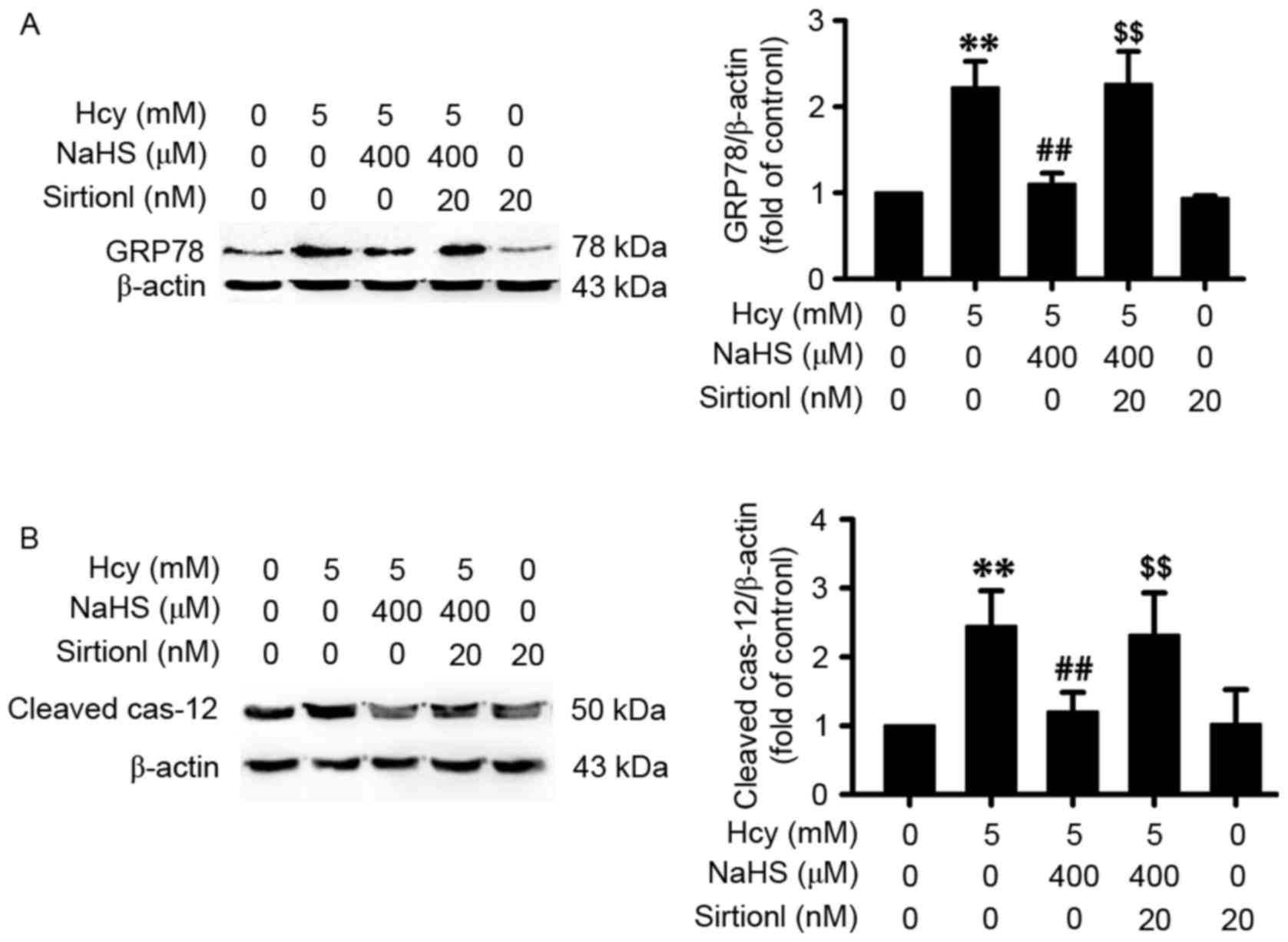 | Figure 4.Sirtinol, a specific SIRT-1
inhibitor, reverses hydrogen sulfide-induced endoplasmic reticulum
stress in Hcy-treated PC12 cells. PC12 cells were pre-incubated
with sirtinol (15 µM) for 30 min prior to cotreatment with NaHS
(400 µM) and Hcy (5 mM) for 24 h. The protein expression levels of
(A) GRP78 and (B) cleaved caspase-12 in PC12 cells were measured
using western blot analysis, respectively. β-actin was used as a
loading control. The results were normalized to the percentage of
β-actin and expressed as the fold of the control group. Values are
expressed as the mean ± standard error of the mean of three
independent experiments. **P<0.01, compared with the control
group; ##P<0.01, compared with the Hcy only group;
$$P<0.01, compared with the NaHS and Hcy cotreatment
group. Hcy, homocysteine; NaHS, sodium hydrosulfide; SIRT-1 silent
mating type information regulator 2 homolog 1; GRP78,
glucose-regulated protein 78; cas-12, caspase-12. |
Discussion
In our previous study, it was demonstrated that
H2S had a protective effect against homocysteine-induced
neurotoxicity (25). In addition,
the involvement of abnormal ER stress has been shown to be
prominent in the neurotoxicity of homocysteine (15,16).
Therefore, the present study was designed to investigate whether
the protective role of H2S in the neurotoxicity of
homocysteine was associated with regulating neuronal ER stress, and
the underlying mechanisms were investigated. The main findings of
the present study were as follows: i) H2S markedly
inhibited homocysteine-induced ER stress in the PC12 cells; ii)
H2S enhanced the protein level of SIRT-1 in the presence
or absence of homocysteine treatment; and iii) sirtinol, an
inhibitor of SIRT-1, eliminated the inhibitory effect of
H2S on homocysteine-induced ER stress. These findings
suggested the protective role of H2S against
homocysteine-induced ER stress by enhancing the expression of
SIRT-1.
Increasing evidence has confirmed the neurotoxic
effects of homocysteine (37–40),
which are associated with ER stress (15,16).
It is known that GRP78 is an ER-chaperone protein involved in the
modulation of ER dynamic homeostasis (41,42).
Pro-caspase-12 is located on the cytoplasmic region of ER and is
proteolytically activated during excess ER stress (43–45).
GRP78 and caspase-12 are two important markers of ER stress. In the
present study, the effects of homocysteine on the protein
expression levels of GRP78 and cleaved caspase-12 in PC12 cells
were examined. It was demonstrated that homocysteine upregulated
the protein levels of GRP78 and cleaved caspase-12 in the PC12
cells. These results indicated that homocysteine was able to
elevate ER stress in the PC12 cells. It is known that ER stress is
involved in the pathogenic effects of homocysteine in several
diseases, including cardiovascular disease (46), apoptosis of osteoblastic cells
(47), insulin resistance of
adipose tissue (48), and type 2
diabetes mellitus (49).
Therefore, ER stress may be a common intermediate pathway in the
homocysteine-induced pathogenic effects in tissues and cells.
H2S is a protective gaseous signaling
molecule. In our previous study, it was demonstrated that
H2S prevented homocysteine-induced neurotoxicity in PC12
cells (25). To improve current
understanding of the protective role of H2S in the
neurotoxicity of homocysteine, the present study investigated
whether H2S suppresses the homocysteine-induced
upregulatory effect on the expression levels of GRP78 and cleaved
caspase-12. The results showed that NaHS (the donor of
H2S) downregulated the expression levels of GRP78 and
cleaved caspase-12 in the homocysteine-exposed PC12 cells, which
indicated that H2S was able to suppress
homocysteine-induced ER stress. Previous studies have demonstrated
that H2S prevents ER stress in doxorubicin-induced
cardiotoxicity (50) and
6-hydroxydopamine-induced neurotoxicity (51). These previous findings offer a
reasonable explanation for the results obtained in the present
study. Furthermore, Wei et al (46) revealed the protective effect of
H2S against homocysteine-induced cardiomyocytic ER
stress. In the present study, the inhibitory role of H2S
in homocysteine-induced ER stress was further confirmed in the PC12
cells. Therefore, the regulation of ER stress offers insights into
the protective effect of H2S against homocysteine
neurotoxicity.
The present study also examined the possible
underlying signaling mechanisms for the protective effect of
H2S against homocysteine-induced ER stress. SIRT-1is
involved in lifespan modulation (52–54)
and orchestrates diverse biological processes, including cell
survival, differentiation and metabolism (55,56).
SIRT-1 is considered to be a vital modulator of cellular defenses
and survival in response to stress (57,58).
SIRT-1 is also expressed in the brain. Accumulating evidence has
indicated that the upregulation of SIRT-1 rescues neurons in acute
and chronic neurological diseases (28,31,59).
It has also been found that impaired SIRT1-deacetylation induces ER
stress (60) and that the
overexpression of SIRT-1 attenuates ER stress (35,36).
This suggests that SIRT-1 is important in counteracting ER stress,
therefore, the present study focused on the effect of homocysteine
on the expression of SIRT-1, and the role of H2S in the
expression of SIRT-1 in PC12 cells treated with or without
homocysteine. The results showed that the expression of SIRT-1 was
downregulated in the homocysteine-exposed PC12 cells, which
indicated the involvement of downregulated SIRT-1 in the increase
of ER stress induced by homocysteine. In addition, NaHS was found
to increase the protein expression of SIRT-1 in PC12 cells. It was
also found that NaHS inhibited the homocysteine-induced decrease in
the protein expression of SIRT-1 in PC12 cells. These results
suggested that the upregulation of SIRT-1 contributed to the
beneficent effects of H2S on homocysteine-induced ER
stress. To confirm whether SIRT-1 mediates the protective effect of
H2S against homocysteine-induced ER stress, the present
study examined whether the inhibition of SIRT-1 eliminates the
protective effect of H2S against ER stress induced by
homocysteine. The results confirmed that the inhibition of SIRT-1
inhibited the reversal effect of H2S on the
homocysteine-increased protein expression of GRP78 and cleaved
caspase-12 in PC12 cells. Taken together, these results suggested
that the upregulation of SIRT-1 mediated the H2S-induced
protective effects against homocysteine-induced ER stress.
In conclusion, the present study demonstrated that
H2S was able to overcome homocysteine-induced ER stress
and increases in the protein expression of SIRT-1 in PC12 cells.
Inhibiting SIRT-1 reversed the protective effect of H2S
against ER stress elicited by homocysteine in PC12 cells. These
results suggested that H2S had the ability to inhibit
homocysteine-induced ER stress and that the upregulation of SIRT-1
mediated this protective effect of H2S. These findings
shed light on the molecular mechanism underlying the protective
role of H2S in the neurotoxicity of homocysteine.
Homocysteine is an independent risk factor for AD (3–7) and
ER stress is a crucial process in the pathogenesis of AD (12–14).
Therefore, the results of the present study suggested that positive
intervention of SIRT-1 may have profound therapeutic benefits
against homocysteine-dependent neurodegenerative diseases.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (grant nos. 81400881 and 81471310) and the
Zhengxiang Scholar Program of University of South China (grant no.
2014-004).
References
|
1
|
Prudova A, Bauman Z, Braun A, Vitvitsky V,
Lu SC and Banerjee R: S-adenosylmethionine stabilizes cystathionine
beta-synthase and modulates redox capacity. Proc Natl Acad Sci USA.
103:6489–6494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Selhub J: Homocysteine metabolism. Annu
Rev Nutr. 19:217–246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Dam F and Van Gool WA:
Hyperhomocysteinemia and Alzheimer's disease: A systematic review.
Arch Gerontol Geriatr. 48:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seshadri S, Beiser A, Selhub J, Jacques
PF, Rosenberg IH, D'Agostino RB, Wilson PW and Wolf PA: Plasma
homocysteine as a risk factor for dementia and Alzheimer's disease.
N Engl J Med. 346:476–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller JW: Homocysteine and Alzheimer's
disease. Nutr Rev. 57:126–129. 1999.PubMed/NCBI
|
|
6
|
Clarke R, Smith AD, Jobst KA, Refsum H,
Sutton L and Ueland PM: Folate, vitamin B12, and serum total
homocysteine levels in confirmed Alzheimer disease. Arch Neurol.
55:1449–1455. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dwyer BE, Raina AK, Perry G and Smith MA:
Homocysteine and Alzheimer's disease: A modifiable risk? Free Radic
Biol Med. 36:1471–1475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma GS, Kumar T, Dar TA and Singh LR:
Protein N-homocysteinylation: From cellular toxicity to
neurodegeneration. Biochim Biophys Acta. 1850:2239–2245. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin N, Qin S, Luo S, Cui S, Huang G and
Zhang X: Homocysteine induces cytotoxicity and proliferation
inhibition in neural stem cells via DNA methylation in vitro. FEBS
J. 281:2088–2096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abushik PA, Niittykoski M, Giniatullina R,
Shakirzyanova A, Bart G, Fayuk D, Sibarov DA, Antonov SM and
Giniatullin R: The role of NMDA and mGluR5 receptors in calcium
mobilization and neurotoxicity of homocysteine in trigeminal and
cortical neurons and glial cells. J Neurochem. 129:264–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park YJ, Ko JW, Jang Y and Kwon YH:
Activation of AMP-activated protein kinase alleviates
homocysteine-mediated neurotoxicity in SH-SY5Y cells. Neurochem
Res. 38:1561–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoozemans JJ, Veerhuis R, Van Haastert ES,
Rozemuller JM, Baas F, Eikelenboom P and Scheper W: The unfolded
protein response is activated in Alzheimer's disease. Acta
Neuropathol. 110:165–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salminen A, Kauppinen A, Suuronen T,
Kaarniranta K and Ojala J: ER stress in Alzheimer's disease: A
novel neuronal trigger for inflammation and Alzheimer's pathology.
J Neuroinflammation. 6:412009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cornejo VH and Hetz C: The unfolded
protein response in Alzheimer's disease. Semin Immunopathol.
35:277–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Althausen S and Paschen W:
Homocysteine-induced changes in mRNA levels of genes coding for
cytoplasmic- and endoplasmic reticulum-resident stress proteins in
neuronal cell cultures. Brain Res Mol Brain Res. 84:32–40. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chigurupati S, Wei Z, Belal C, Vandermey
M, Kyriazis GA, Thiruma V, Arumugam TV and Chan SL: The
homocysteine-inducible endoplasmic reticulum stress protein
counteracts calcium store depletion and induction of CCAAT
enhancer-binding protein homologous protein in a neurotoxin model
of Parkinson disease. J Biol Chem. 284:18323–18333. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HJ, Cho HK and Kwon YH: Synergistic
induction of ER stress by homocysteine and beta-amyloid in SH-SY5Y
cells. J Nutr Biochem. 19:754–761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park YJ, Jang Y and Kwon YH: Protective
effect of isoflavones against homocysteine-mediated neuronal
degeneration in SH-SY5Y cells. Amino Acids. 39:785–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou CF and Tang XQ: Hydrogen sulfide and
nervous system regulation. Chin Med J (Engl). 124:3576–3582.
2011.PubMed/NCBI
|
|
20
|
Łowicka E and Beltowski J: Hydrogen
sulfide (H2S)-the third gas of interest for pharmacologists.
Pharmacol Rep. 59:4–24. 2007.PubMed/NCBI
|
|
21
|
Kimura H, Shibuya N and Kimura Y: Hydrogen
sulfide is a signaling molecule and a cytoprotectant. Antioxid
Redox Signal. 17:45–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu LF, Lu M, Wong PT Hon and Bian JS:
Hydrogen sulfide: Neurophysiology and neuropathology. Antioxid
Redox Signal. 15:405–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu K, Lee SW, Bian JS, Low CM and Wong PT:
Hydrogen sulfide: Neurochemistry and neurobiology. Neurochem Int.
52:155–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang XQ, Shen XT, Huang YE, Chen RQ, Ren
YK, Fang HR, Zhuang YY and Wang CY: Inhibition of endogenous
hydrogen sulfide generation is associated with homocysteine-induced
neurotoxicity: Role of ERK1/2 activation. J Mol Neurosci. 45:60–67.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang XQ, Shen XT, Huang YE, Ren YK, Chen
RQ, Hu B, He JQ, Yin WL, Xu JH and Jiang ZS: Hydrogen sulfide
antagonizes homocysteine-induced neurotoxicity in PC12 cells.
Neurosci Res. 68:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Zhou Y, Mueller-Steiner S, Chen
LF, Kwon H, Yi S, Mucke L and Gan L: SIRT1 protects against
microglia-dependent amyloid-beta toxicity through inhibiting
NF-kappaB signaling. J Biol Chem. 280:40364–40374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen
L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al: Neuronal
SIRT1 activation as a novel mechanism underlying the prevention of
Alzheimer disease amyloid neuropathology by calorie restriction. J
Biol Chem. 281:21745–21754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim D, Nguyen MD, Dobbin MM, Fischer A,
Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et
al: SIRT1 deacetylase protects against neurodegeneration in models
for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J.
26:3169–3179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donmez G, Wang D, Cohen DE and Guarente L:
SIRT1 suppresses beta-amyloid production by activating the
alpha-secretase gene ADAM10. Cell. 142:320–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong H, Cohen DE, Cui L, Supinski A,
Savas JN, Mazzulli JR, Yates JR III, Bordone L, Guarente L and
Krainc D: Sirt1 mediates neuroprotection from mutant huntingtin by
activation of the TORC1 and CREB transcriptional pathway. Nat Med.
18:159–165. 2012. View
Article : Google Scholar
|
|
31
|
Jiang M, Wang J, Fu J, Du L, Jeong H, West
T, Xiang L, Peng Q, Hou Z, Cai H, et al: Neuroprotective role of
Sirt1 in mammalian models of Huntington's disease through
activation of multiple Sirt1 targets. Nat Med. 18:153–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Donmez G, Arun A, Chung CY, McLean PJ,
Lindquist S and Guarente L: SIRT1 protects against α-synuclein
aggregation by activating molecular chaperones. J Neurosci.
32:124–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu D, Zhang J, Wu J, Li G, Yao W, Hao J
and Sun J: Paliperidone protects SH-SY5Y cells against
MK-801-induced neuronal damage through inhibition of Ca(2+) influx
and regulation of SIRT1/miR-134 signal pathway. Mol Neurobiol.
53:2498–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C
and Xiong L: SirT1 mediates hyperbaric oxygen
preconditioning-induced ischemic tolerance in rat brain. J Cereb
Blood Flow Metab. 33:396–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Xu S, Giles A, Nakamura K, Lee JW,
Hou X, Donmez G, Li J, Luo Z, Walsh K, et al: Hepatic
overexpression of SIRT1 in mice attenuates endoplasmic reticulum
stress and insulin resistance in the liver. FASEB J. 25:1664–1679.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung TW, Lee KT, Lee MW and Ka KH: SIRT1
attenuates palmitate-induced endoplasmic reticulum stress and
insulin resistance in HepG2 cells via induction of oxygen-regulated
protein 150. Biochem Biophys Res Commun. 422:229–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuszczyk M, Gordon-Krajcer W and
Lazarewicz JW: Homo-cysteine-induced acute excitotoxicity in
cerebellar granule cells in vitro is accompanied by PP2A-mediated
dephosphorylation of tau. Neurochem Int. 55:174–180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oldreive CE and Doherty GH: Neurotoxic
effects of homocysteine on cerebellar Purkinje neurons in vitro.
Neurosci Lett. 413:52–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lipton SA, Kim WK, Choi YB, Kumar S,
D'Emilia DM, Rayudu PV, Arnelle DR and Stamler JS: Neurotoxicity
associated with dual actions of homocysteine at the
N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA.
94:5923–5928. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhatia P and Singh N: Homocysteine excess:
Delineating the possible mechanism of neurotoxicity and depression.
Fundam Clin Pharmacol. 29:522–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ni M, Zhang Y and Lee AS: Beyond the
endoplasmic reticulum: Atypical GRP78 in cell viability, signalling
and therapeutic targeting. Biochem J. 434:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee AS: The ER chaperone and signaling
regulator GRP78/BiP as a monitor of endoplasmic reticulum stress.
Methods. 35:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de la Cadena SG, Hernandez-Fonseca K,
Camacho-Arroyo I and Massieu L: Glucose deprivation induces
reticulum stress by the PERK pathway and caspase-7- and
calpain-mediated caspase-12 activation. Apoptosis. 19:414–427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei H, Zhang R, Jin H, Liu D, Tang X, Tang
C and Du J: Hydrogen sulfide attenuates
hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum
stress in rats. Antioxid Redox Signal. 12:1079–1091. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y, Zhang H, Jiang C, Xu M, Pang Y, Feng
J, Xiang X, Kong W, Xu G, Li Y and Wang X: Hyperhomocysteinemia
promotes insulin resistance by inducing endoplasmic reticulum
stress in adipose tissue. J Biol Chem. 288:9583–9592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zbidi H, Redondo PC and López JJ, Bartegi
A, Salido GM and López JJ: Homocysteine induces caspase activation
by endoplasmic reticulum stress in platelets from type 2 diabetics
and healthy donors. Thromb Haemost. 103:1022–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park SJ, Kim KJ, Kim WU, Oh IH and Cho CS:
Involvement of endoplasmic reticulum stress in homocysteine-induced
apoptosis of osteoblastic cells. J Bone Miner Metab. 30:474–484.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang XY, Yang CT, Zheng DD, Mo LQ, Lan AP,
Yang ZL, Hu F, Chen PX, Liao XX and Feng JQ: Hydrogen sulfide
protects H9c2 cells against doxorubicin-induced cardiotoxicity
through inhibition of endoplasmic reticulum stress. Mol Cell
Biochem. 363:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie L, Tiong CX and Bian JS: Hydrogen
sulfide protects SH-SY5Y cells against 6-hydroxydopamine-induced
endoplasmic reticulum stress. Am J Physiol Cell Physiol.
303:C81–C91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Canto C and Auwerx J: Caloric restriction,
SIRT1 and longevity. Trends Endocrinol Metab. 20:325–331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bordone L and Guarente L: Calorie
restriction, SIRT1 and metabolism: Understanding longevity. Nat Rev
Mol Cell Biol. 6:298–305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Satoh A, Brace CS, Rensing N, Cliften P,
Wozniak DF, Herzog ED, Yamada KA and Imai S: Sirt1 extends life
span and delays aging in mice through the regulation of Nk2
homeobox 1 in the DMH and LH. Cell Metab. 18:416–430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tang BL: SIRT1, neuronal cell survival and
the insulin/IGF-1 aging paradox. Neurobiol Aging. 27:501–505. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stress-dependent regulation of FOXO transcription factors by
the SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Milner J: Cellular regulation of SIRT1.
Curr Pharm Des. 15:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Herskovits AZ and Guarente L: SIRT1 in
neurodevelopment and brain senescence. Neuron. 81:471–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ghemrawi R, Pooya S, Lorentz S, Gauchotte
G, Arnold C, Gueant JL and Battaglia-Hsu SF: Decreased vitamin B12
availability induces ER stress through impaired SIRT1-deacetylation
of HSF1. Cell Death Dis. 4:e5532013. View Article : Google Scholar : PubMed/NCBI
|