Introduction
Periodontitis is a chronic inflammatory disease that
destroys the periodontal ligament and alveolar bone, and
constitutes a major cause of tooth loss in adults (1,2). It
is caused by bacterial plaque, which leads to impaired immune and
inflammatory responses (1).
Currently, investigations have primarily focused on defining the
complex regulatory interactions between the immune system and bone
remodeling, which is widely known as ‘osteoimmunology’ (3).
Mounting evidence indicates that the novel
CD4+ T-cell subset termed ‘Th17’ and the signature
pro-inflammatory cytokine interleukin (IL)-17 have a potentially
important role in gingival inflammation and subsequent bone
destruction in the pathogenesis of periodontitis (4). Recent clinical studies reported that
the proportion of Th17 cells in the peripheral blood, plasma IL-17
levels and IL-17 expression in gingival lesions are significantly
increased in patients with chronic periodontitis compared with
healthy controls (5,6); da Costa et al (7) additionally observed increased
accumulation of IL-17+ and tumor necrosis factor-related
activation protein+ cells in periodontal lesions,
indicating that high numbers of osteoclasts in local tissues may be
associated with the presence of IL-17+ cells.
By contrast, Yu et al (8) demonstrated that IL-17 exerts a
profound bone-protective effect on bone loss in periodontal disease
via IL-17 receptor A (IL-17RA) signaling. A recent study reported
that the Tannerella forsythia wecC deletion mutant TFM-ED1
increases the Th17 response without enhancing osteoclastic
activity, suggesting a protective role for Th17/IL-17 in the
pathogenesis of periodontitis (9).
A previous study indicated that osteogenic cells may be responsive
to IL-17, and IL-17 modulates osteoclast activity (10). However, the role of IL-17A in bone
protection is poorly understood.
In a previous study, it was demonstrated that RAC-β
serine/threonine protein kinase (AKT2) knockdown weakened the
osteogenic effects of preosteoblastic MC3T3-E1 cells, with reduced
osteocalcin (OCN) expression and calcified deposits (11). Mukherjee et al (12) reported that AKT2 promoted bone
morphogenetic protein 2-mediated osteoblast differentiation. RAC-α
serine/threonine protein kinase (AKT) is activated by
phosphatidylinositol 3-kinase (PI3K), resulting in the
phosphorylation of other host proteins that affect cell
proliferation, growth, the cell cycle and survival (13,14).
Furthermore, a complex relationship exists between IL-17 and
PI3K/AKT signaling, which triggers multiple actions: IL-17A
regulation in stimulated T-B cell co-culture is preferentially
associated with the PI3K pathway (15); IL-17-producing natural killer T
cells are essential for homeostasis and survival via PI3K/AKT
signaling (16); and
Porphyromonas gingivalis (a periodontopathogen)
lipopolysaccharide is involved in periodontal disease-induced bone
destruction and may mediate IL-17 and IL-23 release from human
periodontal ligament cells, with PI3K/AKT signaling serving a role
in this process (17). However, it
remains unclear whether the PI3K/AKT pathway may be activated by
IL-17A in the process of osteogenesis. In addition, no reports
assessing the involvement of AKT2 in osteoblast differentiation and
calcification in association with IL-17A have been published.
Therefore, the purpose of the present study was to
examine the effects of IL-17A on the proliferation, differentiation
and calcification of preosteoblastic MC3T3-E1 cells and to examine
the associated signaling pathways. In a previous study, AKT2
knockdown (AKT2−/−) cells were obtained by RNA
interference (RNAi) following transfection with an effective
AKT2-specific RNAi plasmid (11).
The present study further investigated whether AKT2 was implicated
in IL-17A-mediated osteoblast differentiation and calcification by
examining cell proliferation in addition to the expression of early
and late osteogenic markers. The results of the present study
provided novel insights regarding the role of AKT2 in
IL-17A-mediated osteogenesis and may help elucidate the mechanism
of bone destruction in periodontitis.
Materials and methods
Materials
Mouse IL-17A was from Peprotech Inc. (Rocky Hill,
NJ, USA). Dexamethasone, L-ascorbic acid, β-glycerophosphate, and
dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). All cell culture media and supplements
were from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Reagents for the reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) were obtained from Takara Bio, Inc. (Otsu,
Japan). MTT was purchased from Amresco, LLC (Solon, OH, USA).
Rabbit anti-PI3K (cat. no. 4292), anti-phosphorylated (p)-PI3K
(cat. no. 4228) and anti-GAPDH (cat. no. 2118) monoclonal
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Goat anti-rabbit immunoglobulin G secondary
antibodies (cat. no. BA1054) were obtained from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). Enhanced
chemiluminescence (ECL) detection reagent was purchased from Thermo
Fisher Scientific, Inc. Alkaline phosphatase (ALP) activity kit was
provided by Beyotime Institute of Biotechnology (Haimen,
China).
Cell culture
MC3T3-EI cells, a murine calvarial preosteoblastic
cell line with the capacity to differentiate into osteoblasts and
deposit minerals in vitro (18), were acquired from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China),
and maintained in α-Minimum Essential Medium containing 10% fetal
bovine serum and 1% penicillin-streptomycin (normal medium, NM) at
37°C under 5% (v/v) CO2 in a humidified atmosphere. For
osteogenic induction, MC3T3-E1 cells were incubated in osteogenic
induction medium (Ind.) containing 10 nM dexamethasone, 50 µg/ml
L-ascorbic acid and 10 mM β-glycerophosphate. IL-17A was tested at
a concentration of 50 ng/ml, with the medium being replaced every
48 h.
AKT2 knockdown by RNAi in MC3T3-E1
cells
The knockdown of AKT2 in MC3T3-E1 cells was
described in detail in a previous report (11).
Cell proliferation assay
Cells were plated at 1×103 cells/well in
96-well plates in serum-free medium for 24 h. In order to assess
the effects of IL-17A on cell proliferation, the control (wild type
MC3T3-E1 cells) and SAKT2 (AKT2−/− MC3T3-E1 cells)
groups were incubated in NM with or without IL-17A for 24, 48, 72
and 96 h, respectively. Cell viability was assessed using an MTT
assay, according to the manufacturer's protocol. A total of 50 µl
MTT (5 mg/ml) was added to each well post-treatment. After 4 h of
incubation in a 5% CO2 incubator at 37°C, the culture
medium was carefully aspirated, and 150 µl DMSO added to each well.
Absorbance was read at 490 nm using a spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell cycle analysis
Cells were inoculated at 5×104 cells/well
in 6-well plates, and starved in serum-free medium for 24 h.
Following treatment with 50 ng/ml IL-17A in NM and incubation at
37°C for 48 h, the cells were resuspended to ~106
cells/ml in PBS. Subsequently, 1 ml cell suspension was transferred
to 3 ml 70% ethanol by slowly pipetting, vortexed at full speed,
and incubated at 4°C overnight. Following centrifugation at 1,200 ×
g for 5 min at room temperature, the cell pellets were resuspended
in 2 ml PBS and rehydrated for 15 min, and mixed with 1 ml cell
cycle staining solution containing propidium ioidide [Multi
Sciences (Lianke) Biotech Co., Ltd., Hangzhou, China]. Cell cycle
distribution was analyzed by flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) following 30 min of incubation at room
temperature. Data were analyzed using CellQuest Pro software,
version 5.1 (BD Biosciences).
Western blotting
Control and SAKT2 group cells were cultured in NM
and Ind., separately. Cells in Ind. were treated with or without
IL-17A, and the grouping was the same in the following experiments.
A total of 48 h following incubation of 5×104 cells/well
in 6-well plates, radioimmunoprecipitation assay cell lysate buffer
(Beyotime Institute of Biotechnology) was used for cell lysis.
Equal amounts of total protein (60 µg), as quantified by a
bicinchoninic acid kit (Thermo Fisher Scientific, Inc.), were
resolved by SDS-PAGE on a 10% gel and transblotted onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). Subsequently, the membranes were blocked with 5% non-fat milk
for 2 h at room temperature. Anti-p-PI3K (1:1,000), PI3K (1:1,000),
and GAPDH (1:5,000) were incubated overnight at 4°C. Membranes were
washed and incubated with appropriate horseradish
peroxidase-conjugated secondary antibodies (1:5,000) at room
temperature for 1.5 h. Following washing, immunoreactive bands were
visualized using an ECL kit (Thermo Fisher Scientific, Inc.). Data
were analyzed using Image J software, version 1.48 (National
Institutes of Health, Bethesda, MD, USA).
RT-qPCR analysis
RT-qPCR was used to assess the gene expression of
IL-17RA and osteogenic markers, including Runt-related
transcription factor-2 (Runx-2), ALP, and osteocalcin (OCN). Cells
were prepared as described above and total cellular RNA was
isolated following treatment with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). A total of 1 µg total RNA was
reverse-transcribed to generate single-stranded cDNA using a
PrimeScript™ RT Master Mix kit (Takara Bio, Inc.). The reverse
transcription parameters were as follows: 37°C for 15 min, 85°C for
5 sec, and terminating at 4°C. The expression levels of target
genes were quantified with a SYBR® Premix Ex Taq™ II kit
(Takara Bio, Inc.) on a StepOnePlus Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
were as follows: Forward, 5′-GCCCACTACCCTCACATCTT-3′, and reverse,
5′-TCCTGTGCTCTCTCAAGTGC-3′ for IL-17RA; forward,
5′-TTCTCCAACCCACGAATGCAC-3′, and reverse,
5′-CAGGTACGTGTGGTAGTGAGT-3′ for Runx-2; forward,
5′-TGACCTTCTCTCCTCCATCC-3′, and reverse, 5′-CTTCCTGGGAGTCTCATCCT-3′
for ALP; forward, 5′-TGCTTGTGACGAGCTATCAG-3′, and reverse,
5′-GAGGACAGGGAGGATCAAGT-3′ for OCN; and forward,
5′-GACTTCAACAGCAACTCCCAC-3′, and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′ for GAPDH. Amplification was performed
for 40 cycles at 95°C (30 sec), 95°C (5 sec), and 60°C (30 sec).
Relative expression levels of target genes were derived by the
relative quantitative method (2−ΔΔCq) (19), and normalized to the mouse GAPDH
gene.
ALP activity assay
To assess the early differentiation of MC3T3-E1
cells, ALP activity was analyzed using a specific kit, according to
the manufacturer's protocol. Cells were lysed in lysis buffer
(Beyotime Institute of Biotechnology) on day 14. Subsequently, 50
µl cell lysate was mixed with 50 µl freshly prepared
para-nitrophenylphosphate (pNPP) substrate and incubated at 37°C
for 30 min. The reaction was terminated by addition of 100 µl stop
solution, and the amounts of pNPP produced were quantified by
measuring the absorbance at 405 nm on a spectrophotometer. ALP
activity was normalized to the total protein concentration.
Analysis of cell calcification
For calcification assessment, MC3T3-E1 cells were
cultured (1×104 cells/well in 6-well plates) under the
same culture conditions as detailed above for 14 days. Alizarin Red
staining of matrix mineralization was performed. Cells were fixed
in 4% paraformaldehyde for 10 min at room temperature and stained
with 0.1% Alizarin Red (Sigma-Aldrich; Merck KGaA)/Tris-HCl (pH
8.3) for 30 min at 37°C, air-dried and photographed under a light
microscope at ×100 magnification (4 images/well; ZEISS Axio VertA1,
Zeiss AG, Oberkochen, Germany). The calcified nodules were
quantized by integrated optical density using Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Each experiment was performed in triplicate.
Quantitative data are presented as the mean ± standard deviation.
Groups were compared using one-way analysis of variance, followed
by the least significant difference post hoc test, using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of IL-17A and AKT2 knockdown
on MC3T3-E1 cell proliferation
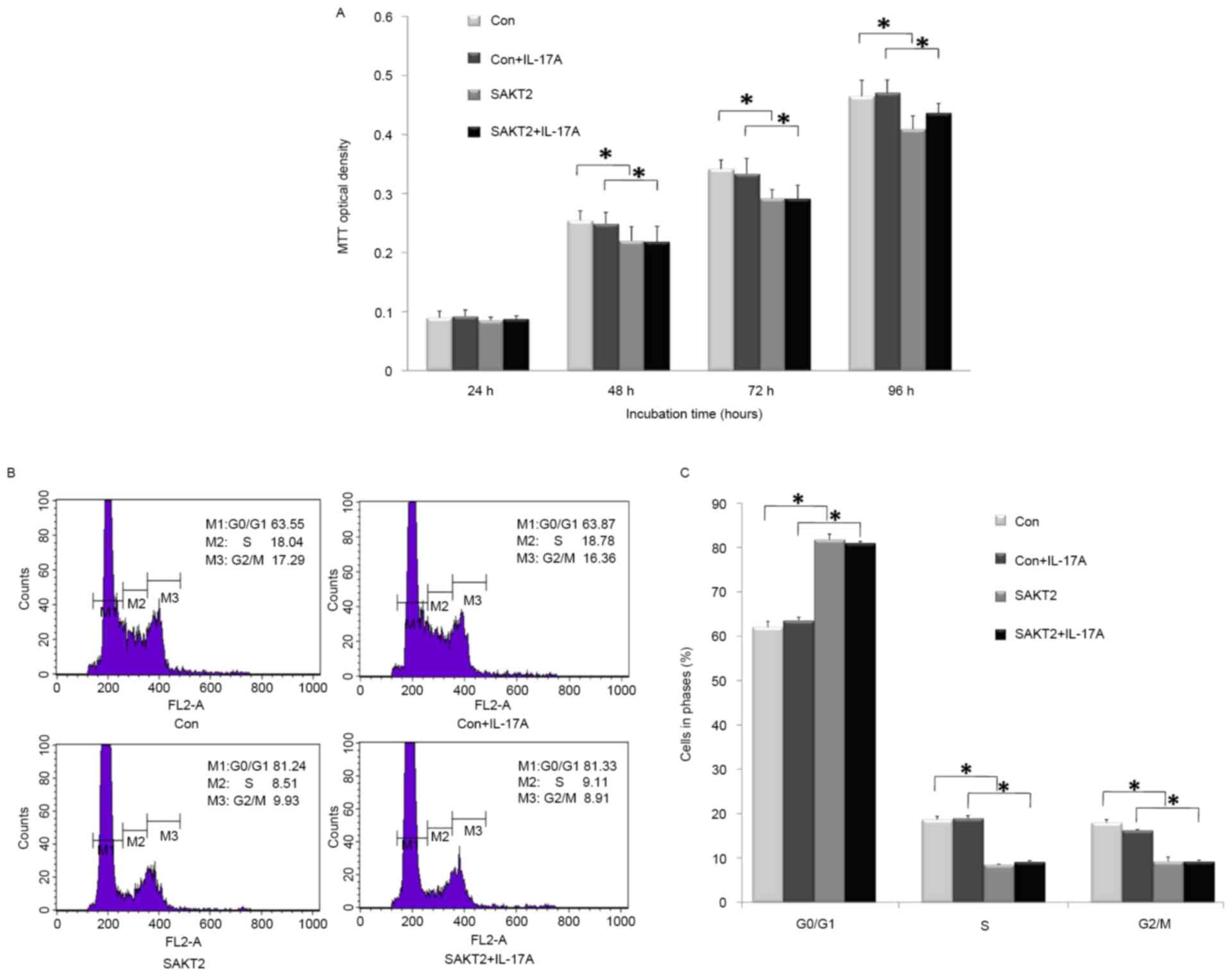
A time-dependent assay was performed to assess the
cell proliferation of wild type and AKT2−/− MC3T3-E1
cells treated with or without IL-17A. At the 24 h time point, there
was no statistically significant difference (P>0.05) among all 4
groups (Fig. 1A). However,
progressive and significant increases were obtained at 48, 72 and
96 h. Notably, cell numbers were decreased upon AKT2−/−
treatment, regardless of stimulation with IL-17A (P<0.05;
Fig. 1A). IL-17A elicited a
similar effect, with a slight difference in proliferation, on wild
type and AKT2−/−cells (P>0.05; Fig. 1A). For cell cycle distribution
(Fig. 1B and C), AKT2 knockdown
induced a significant increase in the number of cells arrested in
G0/G1 (P<0.05), while reducing the numbers of S and G2/M cells
(P<0.05). IL-17A exerted no significant effect on
AKT2−/− cells compared with the control group
(P>0.05).
IL-17A promotes differentiation and
calcification in MC3T3-E1 cells
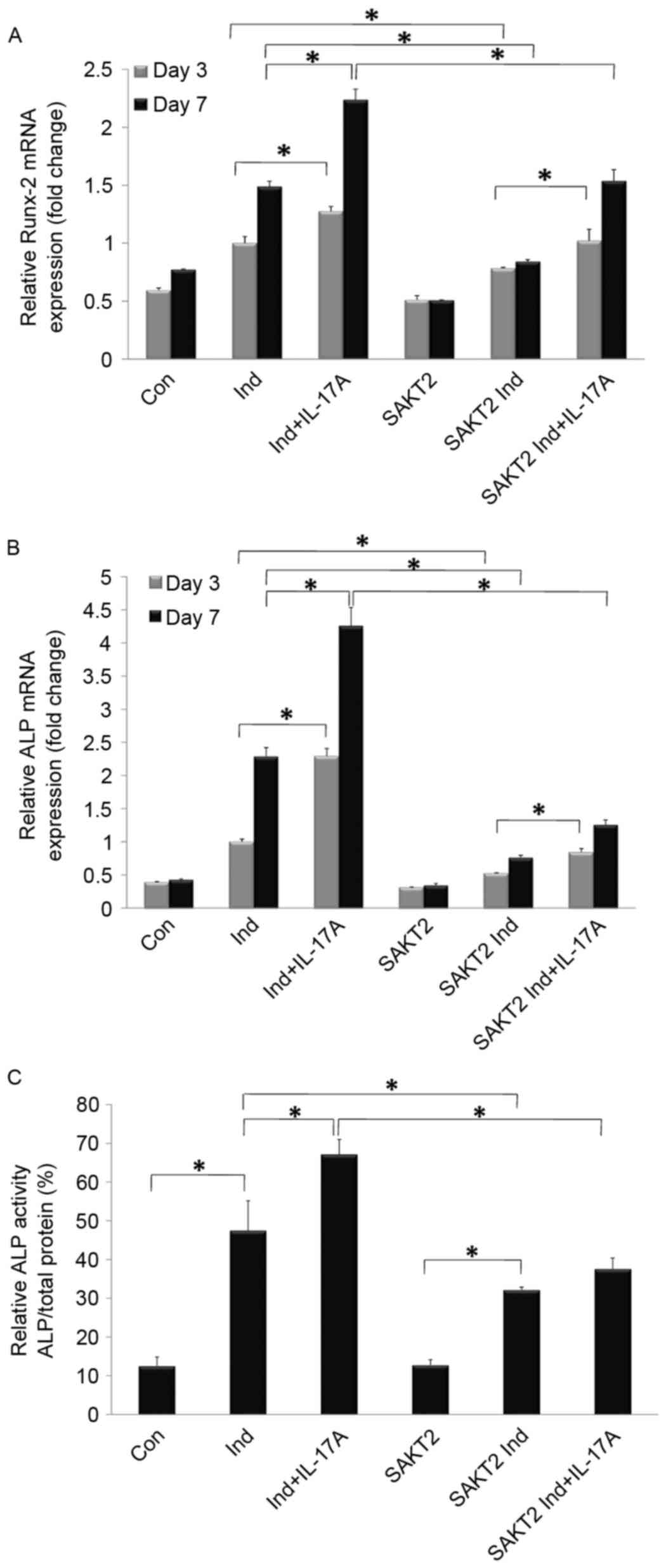
ALP and Runx-2 are important genes required for
early osteoblast differentiation (20,21).
As presented in Fig. 2, the levels
of both genes were significantly increased in the induction group,
while a more significant upregulation was detected at 7 days
compared with 3 days. In addition, ALP and Runx-2 mRNA expression
was markedly enhanced by IL-17A treatment (P<0.05; Fig. 2A and B). OCN mRNA expression and
Alizarin Red staining were quantified to assess osteoblast
mineralization. Notably, OCN expression and calcification areas
were increased in the induction group, and markedly promoted by
IL-17A addition (P<0.05; Fig.
3A).
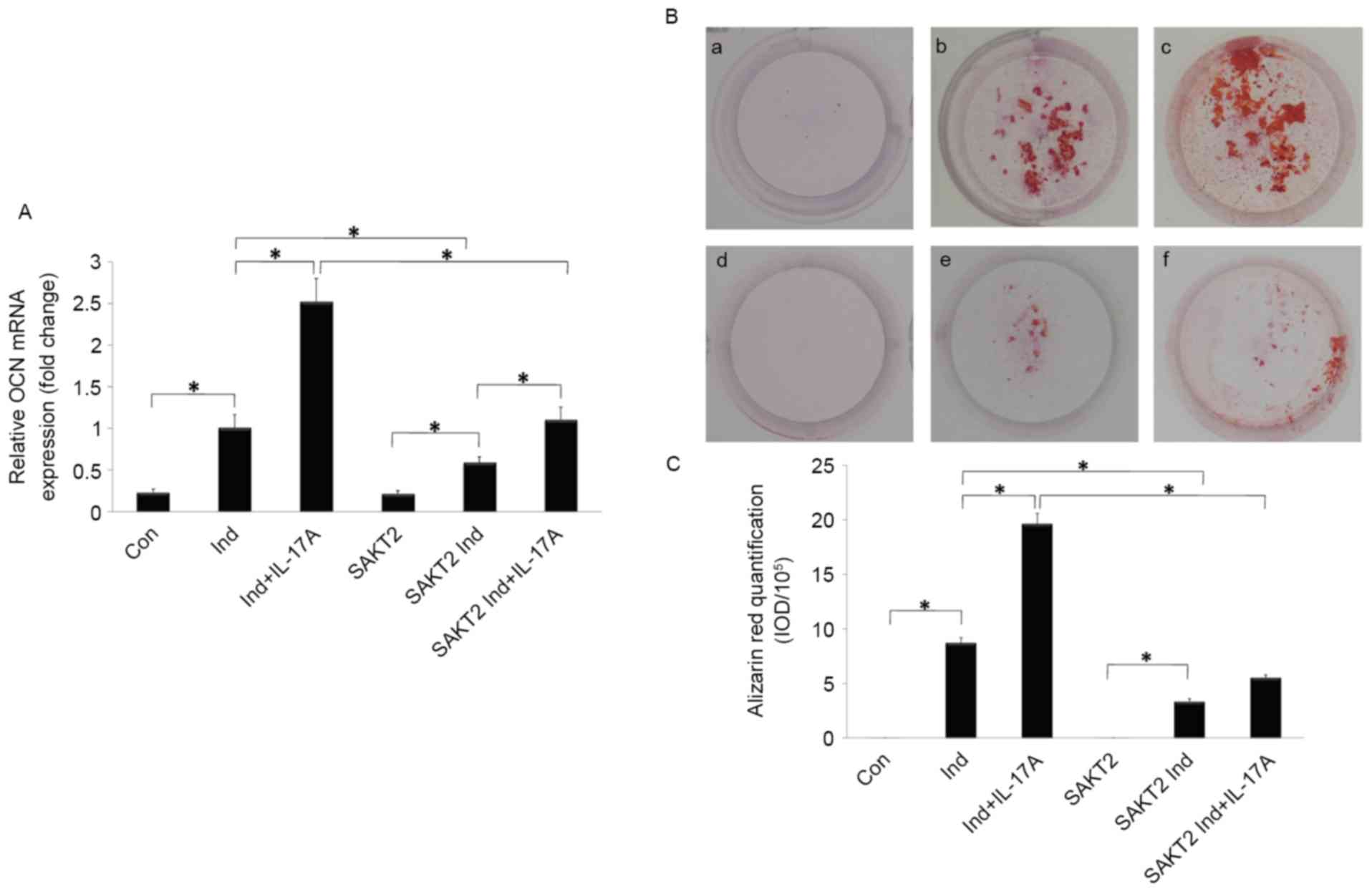 | Figure 3.Effect of IL-17A and AKT2 on MC3T3-E1
cell calcification during the late osteogenesis stage. (A) IL-17A
stimulated the expression of OCN, which was decreased by AKT2
knockdown. (B) Assessment of matrix mineralization of MC3T3-E1
cells by Alizarin Red S staining. Typical observations depicting
mineralization. a, Con; b, Ind; c, Ind+IL-17A; d, SAKT2; e, SAKT2
Ind; f, SAKT2 Ind+IL-17A. (C) Quantitation of mineralization (mean
± standard deviation). *P<0.05. AKT2, RAC-β serine/threonine
protein kinase; SAKT2, Akt2 −/−cells; IL-17A,
interleukin 17A; OCN, osteocalcin; Ind, osteogenic induction; Con,
control. |
AKT2 signaling is involved in IL-17A
induced early osteogenesis
IL-17A increased ALP and Runx-2 mRNA expression
levels, particularly at 7 days, although these genes were
downregulated following AKT2 knockdown (P<0.05; Fig. 2A and B). ALP protein levels were
additionally detected at 7 days of incubation. Cells in the AKT2
knockdown and wild type groups exhibited markedly increased
relative ALP activities in Ind. compared with NM. In addition, ALP
activity was markedly increased by IL-17A (P<0.05), an effect
which was inhibited following AKT2 knockdown (P<0.05; Fig. 2C). IL-17A exerted no overt
promotive effects on ALP activity with AKT2 knockdown (P>0.05;
Fig. 2C). The results of the
present study indicated that AKT2 signaling was required for the
IL-17A-mediated promotion of osteogenic differentiation, while
other signaling pathways may additionally be involved (Fig. 2).
AKT2 signaling is involved in
IL-17A-induced late osteogenesis
The increased OCN gene expression induced by IL-17A
was reduced by AKT2 knockdown (P<0.05; Fig. 3A). As presented in Fig. 3B and C, no calcification deposits
were detected in cells cultured in NM alone, although they were
observed in SAKT2 and control group cells cultured in Ind. The
latter exhibited higher levels of calcification area, with a more
pronounced increase upon IL-17A addition. However, AKT2 knockdown
significantly weakened this effect (P<0.05). IL-17A exerted no
overt calcification-promoting effects following AKT2 knockdown
(P>0.05).
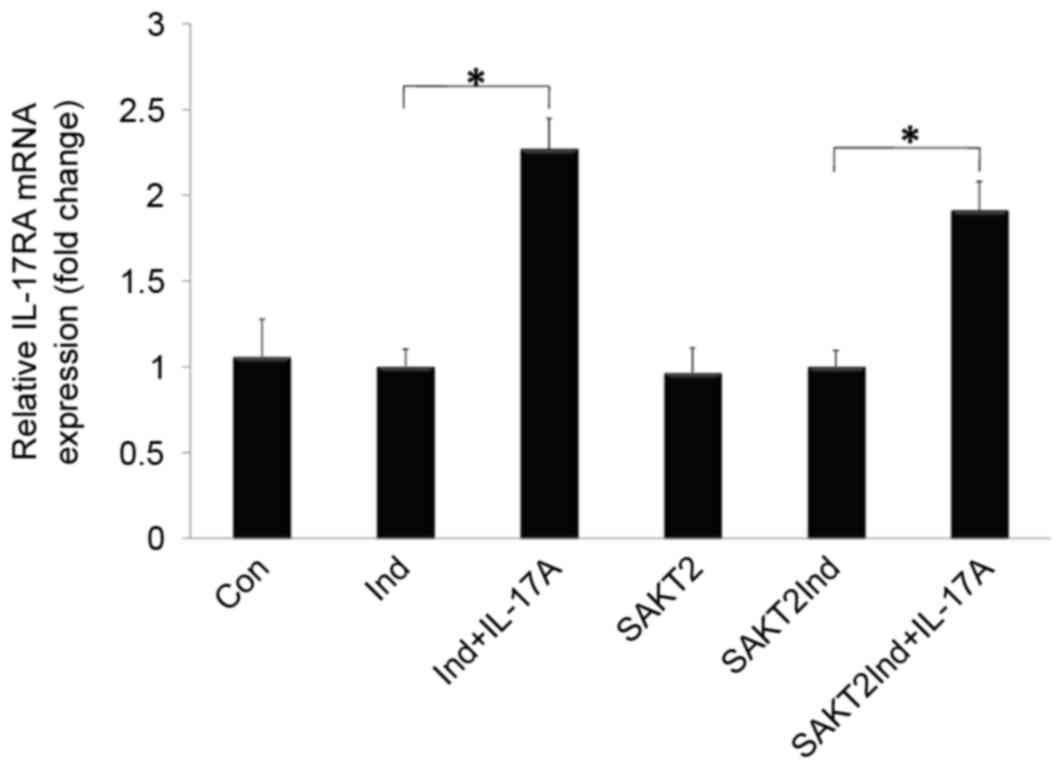
Effects of IL-17A and AKT2 knockdown
on IL-17RA mRNA expression in MC3T3-E1 cells
As presented in Fig.
4, IL-17RA mRNA was detected at baseline, and significantly
increased following IL-17A stimulation (P<0.05). In addition,
there was no significant difference in IL-17RA mRNA expression
between AKT2 knockdown and wild type MC3T3-E1 cells when induced by
IL-17A (P>0.05).
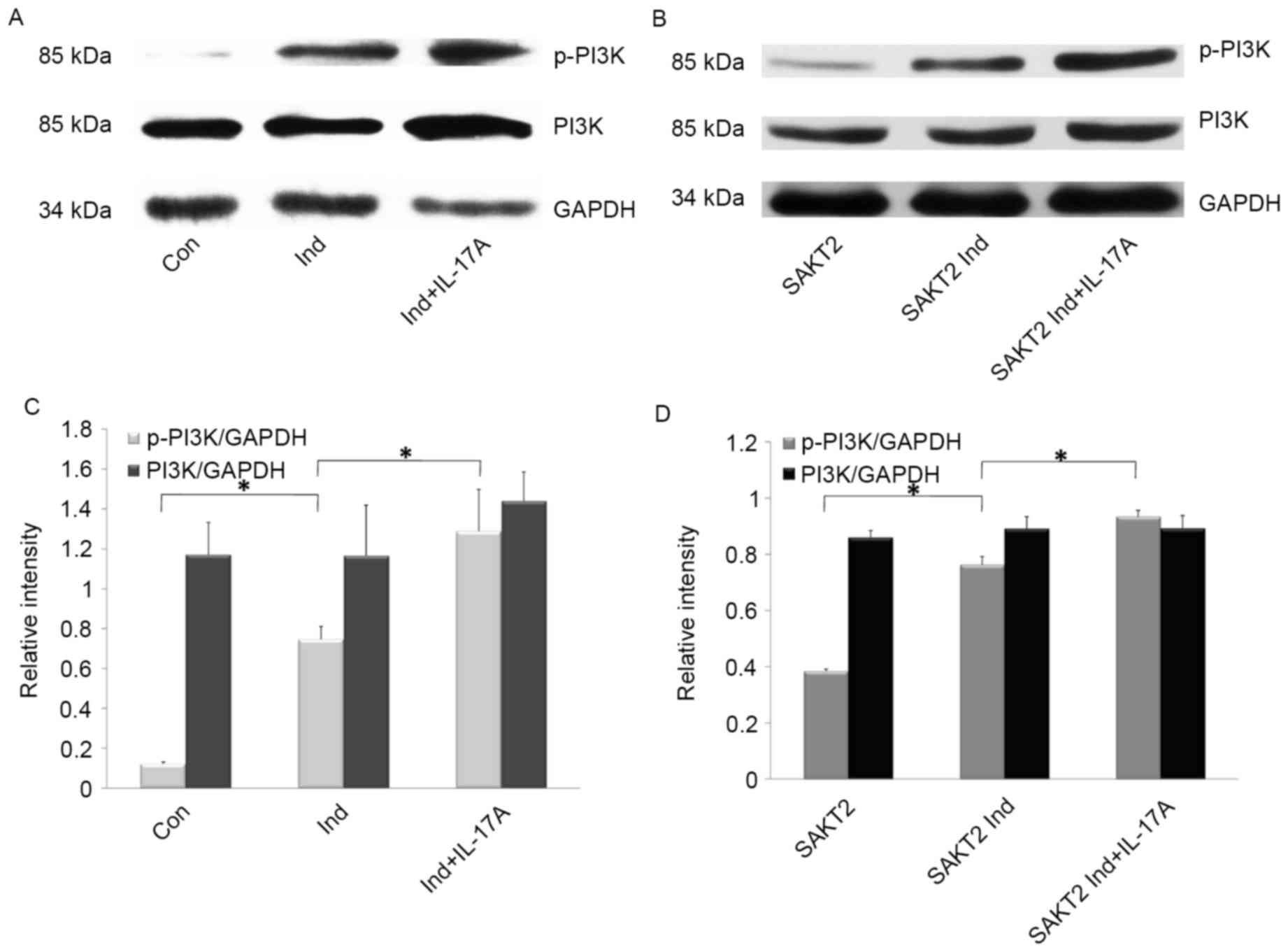
Effects of IL-17A and AKT2 knockdown
on p-PI3K and PI3K expression in MC3T3-E1 cells
To further investigate whether IL-17A-induced
MC3T3-E1 cell alterations were mediated by the PI3K signaling
pathway, the effect of IL-17A on p-PI3K and PI3K (p85) expression
was assessed (Fig. 5). PI3K
exhibited increased phosphorylation levels in Ind. compared with
NM, in AKT2 knockdown and wild type cells (P<0.05; Fig. 5B and D). However, p-PI3K amounts
were significantly increased following treatment with IL-17A
(P<0.05; Fig. 5B and D); total
PI3K and GAPDH were used as internal controls (Fig. 5).
Discussion
IL-17 regulates local inflammation in addition to
osteoclastogenic mediators in gingival resident cells during the
progression of periodontitis (4);
indeed, the gingival concentration of IL-17 is above normal in
severe attachment loss sites in periodontitis (22), and IL-17A amounts are significantly
higher in periodontal lesions adjacent to bone destruction
(23). These findings indicated
that IL-17 exhibits pleiotropic biological activities, and is
associated with the severity of inflammation in periodontal
lesions.
Additional insights into elucidating distinct
functions for AKT2 have been proposed in cellular activity,
demonstrating a significant role for AKT2 as a central signaling
factor in the modulation of bone morphogenetic protein-stimulated
osteogenic differentiation (12).
Vascular smooth muscle cell differentiation appears to require the
activation of AKT2 by PI3K (24).
In a previous study, osteoclast bone resorption medium was
demonstrated to promote differentiation and calcification in
MC3T3-E1 cells, while AKT2-specific knockdown weakened the
osteogenic effects (11). These
previous findings provided a basis for understanding the importance
of AKT2 signaling in osteoblast activity.
The functional association between bone cell
proliferation and the initiation and progression of events
associated with differentiation was proposed (25). Therefore, preosteoblastic MC3T3-E1
cell proliferation may support its osteogenic activity throughout
the bone cellular metabolism process. Concomitantly, a number of
downstream signaling pathways of PI3K affect cell growth and
survival, including serine/threonine protein kinases (AKT and
3-phosphoinositide-dependent protein kinase 1) and protein tyrosine
kinases (Tec family) (14). In
this context, it was observed that cells lacking AKT2 exhibited
growth deficiency, with slower proliferation. Additionally, AKT2
signaling regulates cancer cell growth and survival (13), and the suppression of PI3K/AKT
signaling using specific inhibitors results in decreased osteoblast
proliferation (26–28). Cell cycle distribution was assessed
in the present study. Notably, AKT2 knockdown regulated cell
proliferation by inducing cell cycle arrest in the G0/G1 phase and
decreasing the number of cells in the S and G2/M phases. The
results of the present study provided additional evidence that
PI3K/AKT activity is required during a number of phases of the cell
cycle to allow for entry into mitosis (29). However, no apparent alteration was
observed following treatment with IL-17A in cell proliferation or
cell cycle distribution, in wild type and AKT2 knockdown cells. The
precise function of IL-17A in cell growth and survival remain
controversial. A previous study indicated that IL-17A stimulated
cardiac fibroblast proliferation and migration via activation of
certain pathways (30), while
Nograles et al (31)
observed no apparent effect of IL-17 on keratinocyte proliferation
in vitro, indicating that this matter merits further
investigation.
The expression levels of Runx-2 and ALP were
assessed as specific early markers of osteogenic differentiation.
OCN expression and extracellular matrix mineralization (Alizarin
Red staining) occur during the late stages of osteogenesis. Runx-2,
an important transcription factor of the Runx family, is expressed
in the early stage of osteogenesis, and potently initiates the
expression of major bone matrix protein genes (32). ALP, generated early, stimulates
osteoblast precursor cell differentiation (20). OCN, a terminal osteoblast
differentiation marker, is an osteoblast-secreted hormone
regulating insulin secretion and sensitivity (33). The gene expression levels of ALP,
Runx-2, and OCN, in addition to calcification areas, were increased
in the induction group, and further enhanced by IL-17A, indicating
that IL-17A promoted differentiation and calcification in MC3T3-E1
cells. Previous findings suggested that IL-17F, the amino acid
sequence most homologous to IL-17A (both located on the same
chromosome) (34), directly
rescued impaired healing and promoted bone synthesis in
vitro in a model of early fracture repair (35), demonstrating a protective role in
bone remodeling.
In the present study, the gene expression levels of
Runx-2, ALP and OCN, in addition to relative ALP activity and
calcification areas in AKT2 knockdown MC3T3-E1 cells, were reduced
compared with values obtained for wild type cells under inducing
conditions. Additionally, Runx-2 and ALP mRNA expression levels
peaked at 7 days, with a time-dependent increase, corroborating
findings by Luan et al (36). A specific role for AKT2 as a
gatekeeper of osteogenic differentiation through regulation of
Runx-2 gene expression was previously demonstrated (12); in addition, PI3K/AKT signaling is
involved in Runx-2-dependent osteoblast differentiation, and the
association between them is mutually dependent, further indicating
that Runx-2 is involved in osteoblast differentiation (37). ALP and OCN regulation occurs at
their promoter regions via interaction with Runx-2 (21). As early markers of osteoblast
differentiation, these effectors may signal the beginning of matrix
mineralization.
In addition, expression levels of osteoblast
precursor cell differentiation markers at the early (Runx-2 and
ALP) and late (OCN and Alizarin Red staining) stages in the control
group were markedly increased following treatment with IL-17A,
compared with values obtained for Akt2−/− MC3T3-E1 cells
under the same conditions, indicating that IL-17A promoted
differentiation and calcification, while AKT2 signaling is required
for such enhancement. However, compared with Akt2−/−
MC3T3-E1 cells under osteogenic induction conditions, IL-17A
continued to increase Runx-2, ALP and OCN mRNA expression levels,
likely to be due to other signaling pathways which may participate
in the IL-17A-mediated induction of osteogenic differentiation and
calcification. Differences at the gene level were noted in
Akt2−/− MC3T3-E1 cells following IL-17A treatment, while
the relative ALP activity level and calcification areas exhibited
no alterations. However, significant differences were obtained for
all these parameters between the control and IL-17A treatment
groups. It is plausible that such effects are influenced by
numerous factors and cellular signaling pathways, including the
AKT2 signaling mentioned above and other pathways to be
determined.
To clarify how IL-17A regulated osteoblast
differentiation and calcification by AKT2 signaling, the present
study identified osteoblastic MC3T3-E1 cells as target cells for
IL-17A due to the increased expression of IL-17RA mRNA,
corroborating van Bezooijen et al (10) who first described IL-17 as a new
bone-acting cytokine in vitro. IL-17RA, the most
highly-expressed member of the IL-17R family, was necessary for
signal transduction mediated by IL-17A (38). However, no significant difference
in IL-17RA mRNA expression was observed between AKT2 knockdown and
wild type MC3T3-E1 cells when both induced by IL-17A, suggesting
that AKT2 may lie downstream of IL-17RA, and there were no apparent
variations in the expression of IL-17RA, even in the absences of
AKT2. The results of the present study additionally demonstrated
that IL-17A was able to upregulate p-PI3K expression, consistent
with others studies reporting that PI3K signaling serves an
important role in bone development and growth (12,39).
These findings suggested that IL-17A is involved in regulating
osteoblast activity. Therefore, IL-17A-induced differentiation and
calcification in MC3T3-E1 cells may be mediated primarily through
binding to IL-17RA, followed by the activation of PI3K and
downstream AKT2 signaling.
In conclusion, the present study identified
important roles for AKT2 in MC3T3-E1 cell proliferation and the
cell cycle, additionally demonstrating that IL-17A promoted
osteogenic differentiation and calcification, an effect involving
AKT2 signaling. The results of the present study provided novel
insights regarding the regulatory mechanism of IL-17A in alveolar
bone remodeling, in addition to a basis for designing therapeutic
targets for clinical periodontal disease. However, these
preliminary data do not define accurately the complex association
of bone remodeling with the immune system. Future investigations
are required to examine how IL-17A regulates PI3K/AKT2 signaling to
promote osteogenic differentiation and calcification, and whether
other IL-17A-associated pathways, including mitogen-activated
protein kinase and adapter protein CIKS/TNF receptor-associated
factor 6 may be involved (38);
further understanding of the biochemical and molecular mechanisms
underlying ‘osteoimmunology’ are a prerequisite for periodontitis
research.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81271142, 81400510
and 81300881), the Natural Science Foundation of Zhejiang Province
(grant no. LY16H140002) and the Doctoral Program Foundation of the
Ministry of Education of China (grant no. 20130101110011).
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartold PM, Cantley MD and Haynes DR:
Mechanisms and control of pathologic bone loss in periodontitis.
Periodontology. 53:55–69. 2000. View Article : Google Scholar
|
|
3
|
Arron JR and Choi Y: Bone versus immune
system. Nature. 408:535–536. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng WC, Hughes FJ and Taams LS: The
presence, function and regulation of IL-17 and Th17 cells in
periodontitis. J Clin Periodontol. 41:541–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XT, Tan JY, Lei LH and Chen LL:
Cytokine levels in plasma and gingival crevicular fluid in chronic
periodontitis. Am J Dent. 28:9–12. 2015.PubMed/NCBI
|
|
6
|
Chen XT, Chen LL, Tan JY, Shi DH, Ke T and
Lei LH: Th17 and Th1 lymphocytes are correlated with chronic
periodontitis. Immunol Invest. 45:243–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
da Costa TA, Silva MJ, Alves PM, Chica JE,
Barcelos EZ, Giani MA, Garlet GP, da Silva JS, Júnior V Rodrigues,
Rodrigues DB and Cardoso CR: Inflammation biomarkers of advanced
disease in nongingival tissues of chronic periodontitis patients.
Mediators Inflamm. 2015:9837822015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu JJ, Ruddy MJ, Wong GC, Sfintescu C,
Baker PJ, Smith JB, Evans RT and Gaffen SL: An essential role for
IL-17 in preventing pathogen-initiated bone destruction:
Recruitment of neutrophils to inflamed bone requires IL-17
receptor-dependent signals. Blood. 109:3794–3802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Settem RP, Honma K, Nakajima T, Phansopa
C, Roy S, Stafford GP and Sharma A: A bacterial glycan core linked
to surface (S)-layer proteins modulates host immunity through Th17
suppression. Mucosal Immunol. 6:415–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Bezooijen RL, Farih-Sips HC,
Papapoulos SE and Löwik CW: Interleukin-17: A new bone acting
cytokine in vitro. J Bone Miner Res. 14:1513–1521. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LL, Huang M, Tan JY, Chen XT, Lei LH,
Wu YM and Zhang DY: PI3K/AKT pathway involvement in the osteogenic
effects of osteoclast culture supernatants on preosteoblast cells.
Tissue Eng Part A. 19:2226–2232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukherjee A, Wilson EM and Rotwein P:
Selective signaling by Akt2 promotes bone morphogenetic protein
2-mediated osteoblast differentiation. Mol Cell Biol. 30:1018–1027.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melton AC, Melrose J, Alajoki L, Privat S,
Cho H, Brown N, Plavec AM, Nguyen D, Johnston ED, Yang J, et al:
Regulation of IL-17A production is distinct from IL-17F in a
primary human cell co-culture model of T cell-mediated B cell
activation. PLoS One. 8:e589662013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Webster KE, Kim HO, Kyparissoudis K,
Corpuz TM, Pinget GV, Uldrich AP, Brink R, Belz GT, Cho JH, Godfrey
DI and Sprent J: IL-17-producing NKT cells depend exclusively on
IL-7 for homeostasis and survival. Mucosal Immunol. 7:1058–1067.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park YD, Kim YS, Jung YM, Lee SI, Lee YM,
Bang JB and Kim EC: Porphyromonas gingivalis lipopolysaccharide
regulates interleukin (IL)-17 and IL-23 expression via SIRT1
modulation in human periodontal ligament cells. Cytokine.
60:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang D, Christensen K, Chawla K, Xiao G,
Krebsbach PH and Franceschi RT: Isolation and characterization of
MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo
differentiation/mineralization potential. J Bone Miner Res.
14:893–903. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN,
Jin YH, Ryoo HM, Choi JY, Yoshida M, Nishino N, et al: Bone
morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem.
281:16502–16511. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lester SR, Bain JL, Johnson RB and Serio
FG: Gingival concentrations of interleukin-23 and −17 at healthy
sites and at sites of clinical attachment loss. J Periodontol.
78:1545–1550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohyama H, Kato-Kogoe N, Kuhara A,
Nishimura F, Nakasho K, Yamanegi K, Yamada N, Hata M, Yamane J and
Terada N: The involvement of IL-23 and the Th17 pathway in
periodontitis. J Dent Res. 88:633–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin KA, Merenick BL, Ding M, Fetalvero
KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL,
et al: Rapamycin promotes vascular smooth muscle cell
differentiation through insulin receptor
substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling.
J Biol Chem. 282:36112–36120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stein GS and Lian JB: Molecular mechanisms
mediating proliferation/differentiation interrelationships during
progressive development of the osteoblast phenotype. Endocr Rev.
14:424–442. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vinals F, Lopez-Rovira T, Rosa JL and
Ventura F: Inhibition of PI3K/p70 S6K and p38 MAPK cascades
increases osteoblastic differentiation induced by BMP-2. FEBS Lett.
510:99–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin SK, Fitter S, Bong LF, Drew JJ,
Gronthos S, Shepherd PR and Zannettino AC: NVP-BEZ235, a dual pan
class I PI3 Kinase and mTOR inhibitor, promotes osteogenic
differentiation in human mesenchymal stromal cells. J Bone Miner
Res. 25:2126–2137. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dangi S, Cha H and Shapiro P: Requirement
for phosphatidylinositol-3 kinase activity during progression
through S-phase and entry into mitosis. Cell Signal. 15:667–675.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valente AJ, Yoshida T, Gardner JD, Somanna
N, Delafontaine P and Chandrasekar B: Interleukin-17A stimulates
cardiac fibroblast proliferation and migration via negative
regulation of the dual-specificity phosphatase MKP-1/DUSP-1. Cell
Signal. 24:560–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nograles KE, Zaba LC, Guttman-Yassky E,
Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, Khatcherian A,
Gonzalez J, Pierson KC, White TR, et al: Th17 cytokines interleukin
(IL)-17 and IL-22 modulate distinct inflammatory and
keratinocyte-response pathways. Br J Dermatol. 159:1092–1102.
2008.PubMed/NCBI
|
|
32
|
Komori T: Runx2, a multifunctional
transcription factor in skeletal development. J Cell Biochem.
87:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mundy GR and Elefteriou F: Boning up on
ephrin signaling. Cell. 126:441–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong C: Diversification of T-helper-cell
lineages: Finding the family root of IL-17-producing cells. Nat Rev
Immunol. 6:329–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nam D, Mau E, Wang Y, Wright D, Silkstone
D, Whetstone H, Whyne C and Alman B: T-lymphocytes enable
osteoblast maturation via IL-17F during the early phase of fracture
repair. PLoS One. 7:e400442012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luan J, Cui Y, Zhang Y, Zhou X, Zhang G
and Han J: Effect of CXCR4 inhibitor AMD3100 on alkaline
phosphatase activity and mineralization in osteoblastic MC3T3-E1
cells. Biosci Trends. 6:63–69. 2012.PubMed/NCBI
|
|
37
|
Fujita T, Azuma Y, Fukuyama R, Hattori Y,
Yoshida C, Koida M, Ogita K and Komori T: Runx2 induces osteoblast
and chondrocyte differentiation and enhances their migration by
coupling with PI3K-Akt signaling. J Cell Biol. 166:85–95. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gaffen SL: Structure and signalling in the
IL-17 receptor family. Nat Rev Immunol. 9:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghosh-Choudhury N, Abboud SL, Nishimura R,
Celeste A, Mahimainathan L and Choudhury GG: Requirement of
BMP-2-induced phosphatidylinositol 3-kinase and Akt
serine/threonine kinase in osteoblast differentiation and
Smad-dependent BMP-2 gene transcription. J Biol Chem.
277:33361–33368. 2002. View Article : Google Scholar : PubMed/NCBI
|