Introduction
Pathophysiological mechanisms underlying
atherosclerosis and its thrombotic complications are extremely
complex. Luminal thrombosis-induced acute ischemic events are often
triggered by the sudden rupture of a vulnerable plaque, which is
characterized by having a large lipid core, a thin fibrous cap,
high plaque burden and the presence of inflammatory cells (1). Other key high-risk features include:
Neovascularization, inflammation and intraplaque hemorrhage
(2).
Among the complex mechanisms that may cause plaque
rapture, the upregulation of intraplaque angiogenesis and the
proliferation of adventitial vasa vasorum have attracted much
interest. Intraplaque angiogenesis is closely related to the
inflammatory process and may result in erosion of the extracellular
matrix, which may subsequently be replaced with fragile
neovasculature, resulting in weakened arterial walls or plaque
rupture (3). Proliferation of the
adventitial vasa vasorum is linked with early plaque formation and
extensive neoangiogenesis has previously been associated with
vulnerable plaque features (4,5). The
mechanisms by which the vasa vasorum may contribute to plaque
instability include their roles in leukocyte recruitment and
intraplaque hemorrhage (3).
Intraplaque neoangiogenesis were demonstrated to originate from the
adventitial vasa vasorum network and have been associated with
inflammatory infiltrates subjacent to advanced plaques (5,6).
Owing to their immature and fragile nature, neomicrovessels may
locally promote the extravasation of plasma proteins and
erythrocytes, an important source of free cholesterol with
consequent macrophage infiltration; intraplaque extravasations may
be involved in the expansion of the necrotic core, which may
subsequently cause abrupt lesion progression (7). Therefore, the direct visualization of
neomicrovessels and vasa vasorum may be important for detecting of
plaque vulnerability, and the identification of reliable biomarkers
that are targeted to plaque neoangiogenesis and the selection of
proper imaging modalities are of great importance.
There are numerous candidate biomarkers for
molecular imaging of neoangiogenesis, including selectins,
integrins, vascular cell adhesion molecule (VCAM) and vascular
endothelial growth factor (VEGF). VEGF and VEGF receptors (VEGFRs)
serve unique roles in the mediation and promotion of intraplaque
neoangiogenesis (8). VEGF is a 45
kDa glycoprotein that is secreted in the vascular wall by
endothelial and smooth muscle cells and is a main mediator of
angiogenesis and vascular permeability (9). A previous study has confirmed that
endothelial cells of novel intraplaque microvessels express higher
concentrations of VEGF compared with those in the main arterial
lumen, which in turn promotes angiogenesis (10). In addition, VEGFR-2 (a tyrosine
kinase receptor), has been reported to be highly expressed on the
activated endothelial cell layer, particularly in neonatal blood
vessels, which covers the atherosclerotic plaque; whereas the
healthy vascular wall expresses low levels of VEGFR-2 on the
luminal endothelial cells (11).
VEGFR-2 has been revealed to be a key component of the signaling
system that regulates proliferation and migration of endothelial
and vascular smooth muscle cells, and promotes neovascularization
(12). Therefore, VEGFR-2 may be a
promising candidate for molecular imaging of intraplaque
neoangiogenesis.
Previous studies have focused on VEGFR-2 as a target
in preclinical trials for tumor imaging and block tumor
angiogenesis, which led to significantly suppressed tumor growth
(13,14). However, studies on the imaging of
intraplaque neovasculature with VEGFR-2-targeted agents are limited
(15). Additionally, despite there
being several noninvasive imaging modalities available for the
molecular imaging of intraplaque neoangiogenesis, such as magnetic
resonance (MR), ultrasonography (US), nuclear techniques, computed
tomography angiography (16–18),
most studies focused on the use of only one imaging modality,
thereby failing to provide complementary insights into
neoangiogenesis.
Recent advances in the fabrication of nanomaterials
has led to their wide clinical application in diagnostics (19). Perfluorooctyl bromide (PFOB) with
its exceptional properties including low surface tension, inertness
and biocompatibility, has been encapsulated in biocompatible and
biodegradable polymeric shells to form ultrasound contrast agents
with significant echogenicity (20). Superparamagnetic iron oxide (SPIO),
which is a T2-weighted MRI contrast agent, can offer an intensive
negative contrast enhancement of the target lesion in MRI due to
its biocompatibility and high sensitivity (21).
The present study hypothesized that intensive
intraplaque neoangiogenesis is a biomarker for impending plaque
rupture, and VEGFR-2 may be a promising candidate that reflects
neoangiogenesis closer to vascular pathological conditions.
Intraplaque angiogenesis is associated with plaque rupture, and
VEGFR-2 has been confirmed as highly expressed on the activated
endothelial cells within plaque (11). The levels of VEGFR-2 may therefore
reflect neoangiogenesis in plaque. Therefore, the present study
aimed to: i) Examine the reliability of the VEGFR-2-targeted
nanocapsule (VTNC) with dual probes of SPIO and PFOB as a contrast
agent for molecular imaging of intraplaque neovasculature; and ii)
to explore the feasibility and superiority of combined US and MR
for in vivo bimodal molecular imaging of atherosclerotic
neovasculature by using VTNC.
Materials and methods
Materials and reagents
1-(3-Dimethylaminopropyl)-3-ethyl carbodiimide
hydrochloride (EDC), N-hydroxysuccinimide (NHS) perfluorooctyl
bromide (PFOB) and ethyldiisopropylamine (DIPEA) were purchased
from J & K Scientific Ltd. (Beijing, China).
Poly-lactic-co-glycolic acid (PLGA-COOH; 50:50; molecular weight
15,000) was purchased from Daigang Biomaterial Co., Ltd. (Jinan,
China); polylactic acid (80k MW) was purchase from Shandong
Institute of Medical Instruments (Shandong, China); polyvinyl
alcohol (PVA; 86–89% hydrolyzed; low molecular weight; Alfa Aesar;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Rabbit
polyclonal VEGFR-2 primary antibody (ab39256) was from Abcam
(Cambridge, MA, USA); secondary fluorescein isothiocyanate
(FITC)-labeled goat anti-rabbit immunoglobulin (IgG; zf-0511) was
from OriGene Technologies, Inc. (Beijing, China); monoclonal CD31
primary antibody (MAB-0031) was from Maixin Biotech. Co., Ltd
(Fuzhou, China); 2-(N-morpholino) ethanesulfonic acid hydrate (MES)
was from Best-reagent Co. (Chengdu, China); primary bovine aortic
endothelial cells (BAECs) were from Health Science Research
Resources Bank (Osaka, Japan); Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were purchased from Gibco
(Thermo Fisher Scientific, Inc.) and deionized (DI) water (18.2
MΩ-cm) was obtained from Milli-Q Gradient System (Merck KGaA,
Darmstadt, Germany).
Iron oxide nanoparticle
preparation
Oleic acid-stabilized superparamagnetic iron oxide
nanoparticles (SPIOs) were generated by a following a previously
described co-precipitation method (22). Briefly,
FeCl3-6H2O (0.5 M) and
FeSO4-7H2O (0.5 M) were dissolved in DI water
(100 ml) in a 4-necked flask, followed by the addition of
NH3-H2O solution (30 ml) at room temperature
under N2 atmosphere. The aqueous solution was heated to
80°C for 30 min, and oleic acid (0.891–0.896 g/ml) was added with
vigorous stirring for an additional 30 min. The precipitate was
isolated with a neodymium magnet, washed 3 times with DI water and
3 times with acetone, and dispersed in hexane for subsequent
use.
SPIO-embedded PFOB nanocapsule (NC)
fabrication
Polymer PLA (100 mg), PLGA-COOH (1.1 mg) and PFOB
(0.1 ml) were dissolved in methylene chloride (3.5 ml), and
combined with oleic acid-stabilized SPIO in hexane (20 mg
ml−1; 0.5 ml) and well mixed. SPIO-embedded PFOB NCs
were prepared by an adapted oil-in-water (O/W) emulsion solvent
evaporation process as previously described (20). Briefly, the O/W emulsion was
generated by adding the oil phase from the oleic acid stabilized
SPIO-embedded PFOB NCs to an aqueous solution of PVA (2% w/v; 20
ml), followed by continuous probe sonication for 120 sec with a
1.27 cm diameter titanium alloy horn (Sonicator S-4000; Misonix,
Inc.; Qsonica, Newton, CT, USA) using an output amplitude setting
of 80%. Subsequently, the emulsion was magnetically stirred at room
temperature for 2 h to evaporate most of the methylene chloride,
and the NCs were collected by centrifugation (Avanti J-25
high-speed centrifuge; Beckman Coulter, Inc. Brea, CA, USA) at
21,000 × g at 15°C for 5 min, washed and dispersed with DI water
and stored at 4°C until use.
VTNC fabrication
Anti-VEGFR-2 antibodies were covalently attached
onto the NC surfaces to create VTNC using a previously described
carbodiimide technique (23).
Briefly, the NCs were washed with MES buffer (pH 6.0), collected by
centrifugation (96 × g at room temperature for 3 min), activated
with a mixture of EDC and NHS (the molar ratio of PLGA-COOH to EDC
to NHS was 1:10:20) in MES buffer (pH 6.0) for 30 min at room
temperature. The NCs were washed twice with MES (pH 6.0) followed
by one wash with MES (pH 8.0), centrifuged 96 × g at room
temperature for 3 min to eliminate the remaining unreacted EDC/NHS
mixture, and dispersed in MES buffer (pH 8.0). Subsequently, an
excess of anti-VEGFR-2 antibodies (molar ratio of PLGA-COOH to
anti-VEGFR-2 was 50:1) were immersed into the mixture to ensure
that the antibodies fully bound to the PLGA-COOH located on the
surface of the nanocapsules. The reaction was incubated for 2 h at
room temperature and stopped by washing twice with phosphate
buffered saline (PBS; pH 7.0). The immuno-NCs were collected via
centrifugation (96 × g at room temperature for 3 min) and
redispersed in 100 µl PBS for use (24).
NC characterization and cell
culture
The size distribution of NCs was measured by dynamic
light scattering using a Nicomp 380 ZLS particle size distribution
analyzer (Particle Sizing Systems, Inc., Port Richey, FL, USA). NC
morphology was observed and measured with a JEM-100CXII
transmission electron microscope (TEM; JEOL, Ltd., Tokyo, Japan).
To test if the antibodies were able to covalently attach to the
NCs, primary bovine aortic endothelial cells (BAECs) were used.
BAEC cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum at 37°C in
5% CO2. BAECs (1×106) were treated for 24 h
at 37°C with or without glucose (33 mmol/l) to induce VEGFR-2
expression, as previously described (25). Following the induction of VEGFR-2
expression, cells were incubated with VTNC (30 mg/ml) at 4°C in 300
µl PBS for 1 h. The cells were centrifuged at 96 × g at room
temperature for 3 min and washed several times with PBS to remove
unbound NCs. Cells were incubated with FITC-conjugated goat
anti-rabbit IgG antibodies in the dark for 2 h at room temperature
to determine whether the surfaces of fluorescent-labeled immuno-NCs
appeared green under fluorescent microscope.
Atherosclerotic animal model
Male Sprague Dawley rats (weight, 246±12 g, n=28)
were obtained from The Experimental Animal Center of Fujian Medical
University (Fuzhou, China) and kept in a pathogen-free facility
with a constant humidity (45%) and temperature (26°C) with a 12-h
light/dark cycle and with free access to food and water. A rat
model with vulnerable plaque was established as previously
described (26). Briefly,
following one week of feeding on standard rat chow, all rats were
anesthetized with pentobarbital sodium (0.5 ml/100 g; 1% w/v in
0.9% saline) and underwent abdominal aorta balloon-injury (ABI)
(n=20) or sham operation (n=8). The left common carotid artery was
dissected and ligated, and an over-the-wire balloon catheter with a
balloon of 1.5 mm was advanced through the left common carotid
artery until the abdominal aorta (~10 cm). The balloon was inflated
to a pressure of 12 atm (1.216 MPa) followed by pulling back 5 cm
(corresponding to the target segment from the celiac brunch to
iliac artery); this step was repeated five times for ABI. The sham
operation was performed by dissection and ligation of the left
common carotid artery without ABI. The ABI rats were fed 3%
cholesterol chow for 120 days to create the atherosclerotic model,
and the Sham control rats were fed standard chow for 120 days.
Experimental protocol
The study protocol was approved by The Animal
Studies Committee, Fujian Medical University Union Hospital
(Fuzhou, China). All animal experiments were performed in
accordance with the relevant laws and institutional guidelines. The
cholesterol-fed rats with ABI (atherosclerotic rats) were randomly
placed into 2 groups (n=8/group): i) The atherosclerotic/targeted
group, which received an intravenous injection of VTNC (30 mg
ml−1; 200 µl); and ii) the atherosclerotic/nontargeted
group, which received an intravenous injection of nontargeting NC
(30 mg ml−1; 200 µl). In addition, the Sham control rats
(n=8) received an injection of VTNC (30 mg ml−1; 200 µl;
control/targeted group).
In vitro imaging of VTNC
For US imaging, NCs were prepared in PBS at
different concentrations (0, 1×104, 1×105,
1×106, 1×107 and 1×108
particles/ml), injected into a latex tube and imaged using an Aplio
500 Sonographic System (Toshiba Corporation, Tokyo, Japan) with a
12L-5 Linear-Array Transducer (12L-5, Toshiba Corporation). Both
pulse inversed harmonic imaging (PIHI) with a mechanical index (MI)
of 0.72 and B-mode imaging were performed to obtain the transverse
sectional images of the tube. The video intensity was analyzed by
the program provided by the US system.
For MRI, the NCs were prepared in PBS at different
concentrations (0, 5, 10, 20, 40 and 80 µM), scanned with
T2-weighted (T2W) imaging with a 3.0 Tesla (T) GE Discovery MR750
MRI scanner (GE Healthcare, Chicago, IL, USA) with a small animal
coil using the following settings: Repetition time (TR)=5,000 msec;
echo time (TE) between 10.6 and 53 msec; field of view (FOV)=25×62
mm; and slice thickness (ST)=1.0 mm. All images were stored for
off-line analysis for calculating the T2 relaxivity using the
analytical software provided with the MRI instrument.
In vivo imaging of VTNC
At the end of day 120, rats were anesthetized with
pentobarbital sodium (0.5 ml/100 g; 1% w/v in 0.9% saline) through
intraperitoneal administration.
For US imaging, the rat abdominal aorta below the
celiac branch to the iliac artery was interrogated using a Vivid E7
Ultrasound System with a ML6-15 US probe (GE Vingmed Ultrasound AS,
Horten, Norway). Tissue harmonic imaging (THI) modality was used
with the following consistent settings: MI=1.6; frequency=15 MHz;
gain=52; and depth=2 cm. A set of images were obtained prior to
injection and at 2.5, 5, 15, 30 and 60 min, and at 2, 4 and 24 h
post-NC injection of NCs suspension (30 mg ml−1, 200
µl). The mean grayscale intensity (GSI) in the region of interest
(ROI) was measured prior to and following contrast enhancement by
using the histogram tool in Photoshop version 7.01 (Adobe Systems,
Inc., Beijing, China), and the change in GSI at each time point was
calculated to assess the relative retention of NCs in the
plaques.
For MRI, the rat abdominal aorta below the celiac
branch to the iliac artery was scanned using a 3.0T GE Discovery
MR750 MRI scanner with a knee coil. T2W scanning was used under the
following 3D time-of-flight sequence parameters: TR/TE=22/5.7 msec;
flip=15; FOV=8×6 cm; matrix=512×512; and ST=1.2 mm. A set of images
was recorded prior to injection and at 1, 4 and 24 h post-injection
of NCs. Following acquisition of the T2W images, recognition of the
aortic wall and signal intensity in ROI were performed with the
analysis programs provided by the MRI scanner. The threshold of NCs
was defined to be 3 standard deviations below the mean signal
intensity of the pre-contrast abdominal aorta on signal intensity
histograms. The threshold was applied to sequential abdominal aorta
MRI slices to determine the mean area of NC low-signal contrast.
The contrast-to-noise ratio (CNR) of each ROI was compared prior to
and following contrast. CNR was calculated as previously described
(27): CNR=(blood pool
signal-lesion signal)/standard deviation of the noise.
Histological analysis
Following US and MR imaging, rats were sacrificed,
abdominal aorta were removed, cut into 2.5 mm serial sections,
fixed overnight in 4% paraformaldehyde at 4°C, with a second
overnight tissue processing and dehydration, and embedded in
paraffin. The slides were stained with hematoxylin and eosin. The
luminal surface was observed by microscopy (Olympus Corporation,
Tokyo, Japan).
Immunohistochemistry
Immunohistochemical staining for VEGFR-2 and CD31
were performed as follows. Following fixation with 4%
paraformaldehyde at 4°C for 12 h, abdominal aorta samples were
processed for paraffin-embedded slides. The slides were subjected
to antigen retrieval, and the endogenous peroxidase activity was
quenched with hydrogen peroxide. After blocking nonspecific
proteins with normal serum in PBS (0.1% Tween 20), the slides were
incubated with rabbit polyclonal antibodies against VEGFR-2 and
CD31 (all at 1:300 dilution). Following washing with PBS, the
slides were incubated with biotinylated secondary antibody followed
by conjugated horseradish peroxidase (HRP)-labelled streptavidin
(Dako; P039701-2; 1:400; Agilent Technologies, Inc., Santa Clara,
CA, USA) at 37°C for 60 min and then washed with PBS. The slides
were then incubated with diamino-benzidine (Sigma-Aldrich; Merck
KGaA) as the chromogen at 37°C for 10 min, followed by
counterstaining with diluted Harris hematoxylin (Sigma-Aldrich;
Merck KGaA). Images of the stained tissue sections were captured
with an optical microscope (Olympus Corporation, Tokyo, Japan) for
computerized image analysis with Image-Pro Plus version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). The brown-stained areas in
the tissue sections were defined as CD31+ or
VEGFR-2+, and were easily identified from the other
tissues. The percent positive area (PPA) of CD31+ or
VEGFR-2+ expression (PPACD31+ or
PPAVEGFR-2+; respectively) was calculated by [(positive
area/total area)x100%] and adopted as a parameter for reflection of
neoangiogenesis as previously described (28).
Control of analytic quality
Consideration of the comparability and consistency
required for parametric analysis, a similar ROI was used for US/MR
imaging, and histopathological sections were selected at the same
anatomical sites with the most severe plaque formation. All data
were analyzed by two observers blinded to each other's results and
the results among imaging or histological measurements.
Statistical analysis
All analysis was performed with SPSS 19.0 software
package (IBM Corp., Armonk, NY, USA). Continuous data were
expressed as the mean ± standard deviation. One-way of analysis of
variance was used to analyze differences among groups, followed by
Fisher's least significant difference test between groups if
significant. Correlations between imaging and histological
parameters were assessed by Pearson's correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
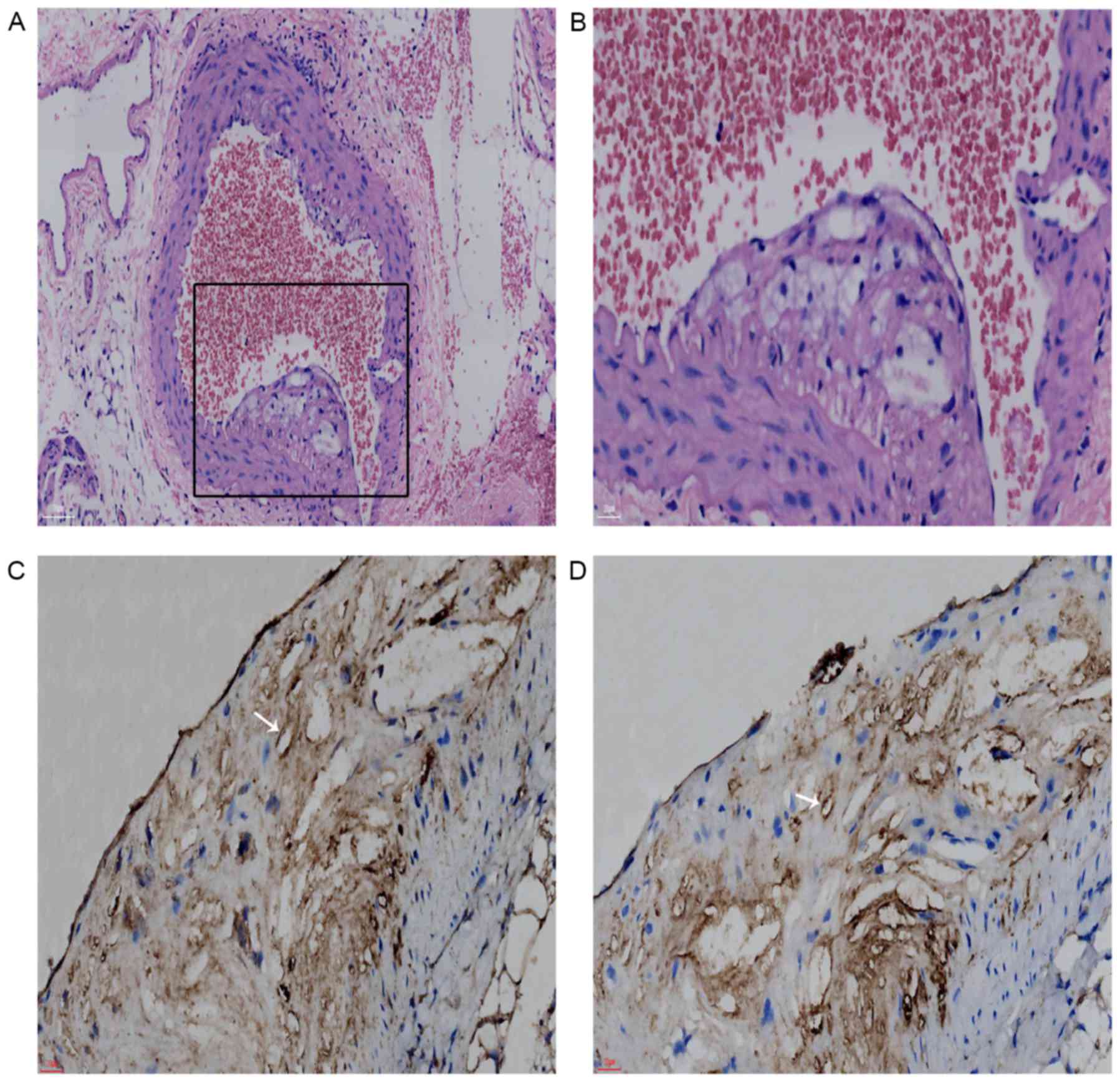
Confirmation of vulnerable
plaques
Of the 20 rats that underwent ABI surgery, 1 failed
to recover from anesthesia and 3 perished during the operation. The
remaining 16 rats were fed 3% cholesterol chow for 120 days to
develop aortic plaques, most of which were vulnerable plaques with
a thin cap and large lipid core filled with foam cells, confirmed
pathologically and immunohistochemically (Fig. 1A-D). Sham rats fed standard chow
did not develop notable plaques (data not shown).
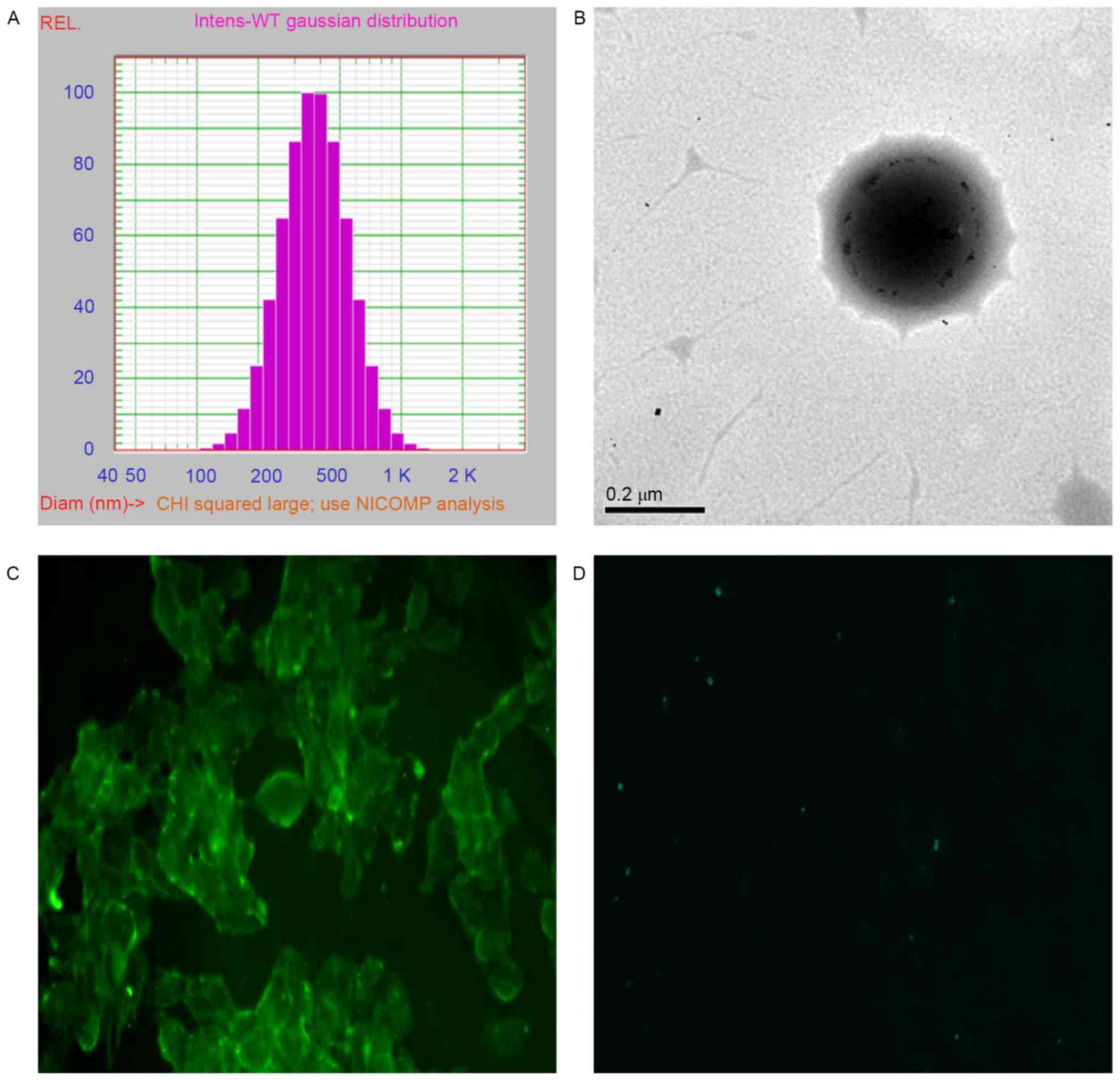
Characterization of VTNC
Measurement of NC particle size distribution
indicated a monodispersion with an average diameter of 404.4±153.7
nm (Fig. 2A). TEM imaging of NCs
demonstrated dark grey spots within the NCs, which indicated the
successful encapsulation of SPIO within the NCs (Fig. 2B). Immunodetection with a
fluorescent microscope revealed that the targeted VTNCs were able
to selectively target VEGFR-2-expression BAECs, in which high or
low expression was noted with or without high glucose treatment
(Fig. 2C and D, respectively).
These results indicated the successful and specific conjunction of
anti-VEGFR-2 antibodies onto NCs.
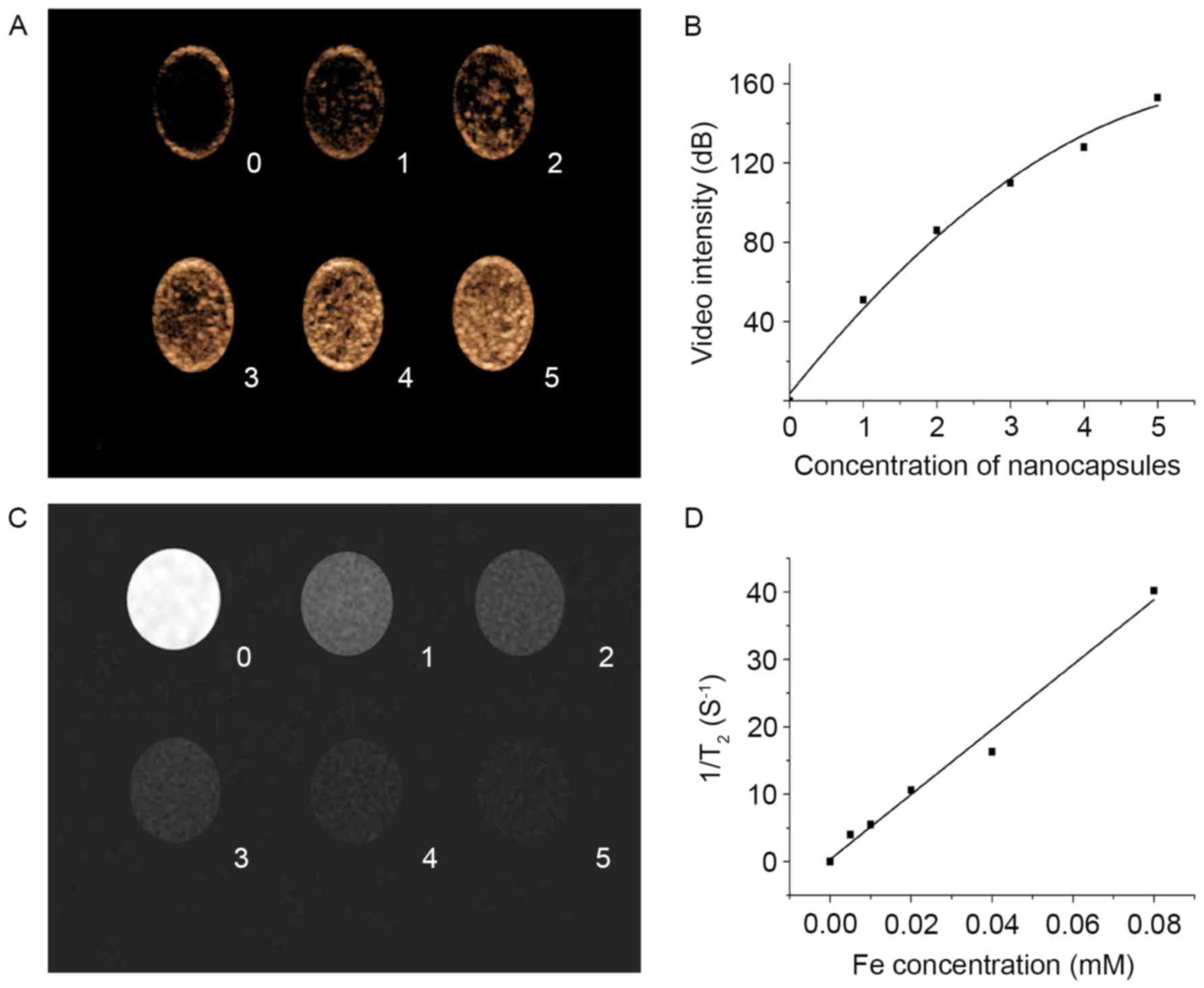
In vitro acoustic or magnetic feature
of NCs
US and MR imaging were performed to test the bimodal
imaging capability of SPIO-embedded PFOB NCs loaded in the latex
tubes. Contrast enhancement in a dose-dependent manner was detected
with US-PIHI imaging (Fig. 3A and
B); contrast attenuation in a dose-dependent manner was
detected with MR-T2W scanning (Fig. 3C
and D). In addition to excellent echo-enhancement, high T2
relativity of 421 mM−1 s−1, which is more
than double the commercial SPIO-based MR agent Resovist (185.8±9.3
Mm−1s−1) (29), enabled in vitro bimodal
detection of SPIO-embedded PFOB NCs with US and MR imaging.
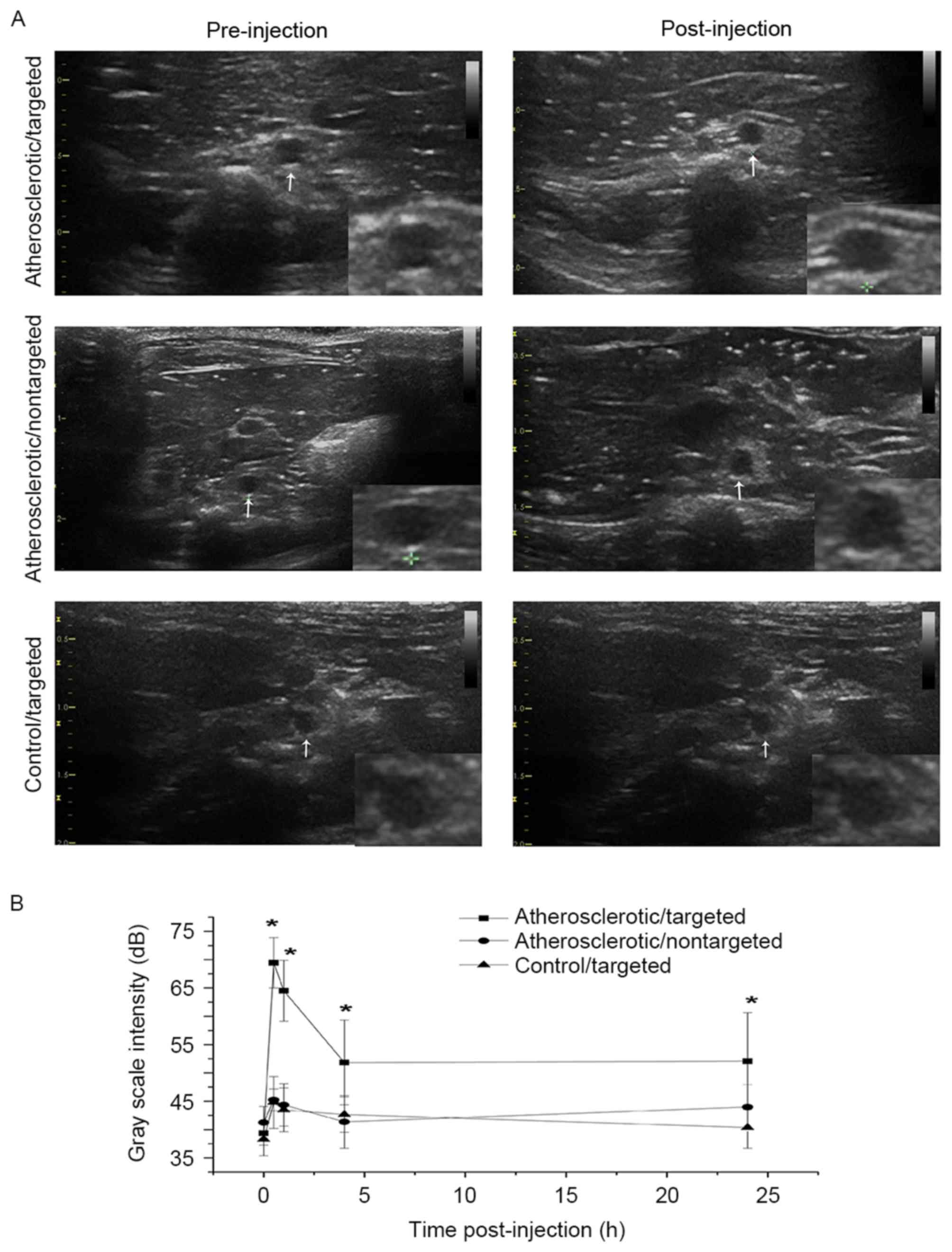
In vivo US imaging of VTNC
US-THI imaging was performed in rats to interrogate
contrast enhancement in the targeted segment of rat abdominal aorta
prior to and 10, 20, 30, 40, 50, 60 min and at 2, 4 and 24 h
following the administration of NCs. Hyperechoic plaques were
detected in all atherosclerotic rats, although notable enhancement
of echoic intensity and massive retention of VTNC in
atherosclerotic plaques were observed only in the
atherosclerotic/targeted group (Fig.
4A). The baseline GSI was compared among the three groups,
administration of NCs significantly increased the mean and peak GSI
in the atherosclerotic/targeted group, but not in the
atherosclerotic/nontargeted or control/targeted group (Table I). Time-intensity curve analysis
clearly indicated that there was higher peak intensity and longer
duration of contrast enhancement in the atherosclerotic/targeted
group compared with the atherosclerotic/nontargeted and the
control/targeted groups (Fig.
4B).
 | Table I.Comparison of the average and peak
GSI and CNR among the three groups. |
Table I.
Comparison of the average and peak
GSI and CNR among the three groups.
| Measurement |
Atherosclerotic/targeted |
Atherosclerotic/nontargeted |
Control/targeted |
|---|
| GSI (dB) |
|
|
|
|
Baseline |
39.37±2.14 |
41.25±2.82 |
38.29±2.89 |
|
Post-injection mean |
65.09±6.21a |
46.66±4.01b |
46.02±4.19b |
|
Peak |
69.44±4.45a |
45.24±1.92b |
44.78±4.59b |
| CNR |
|
|
|
|
Baseline |
42.06±6.03 |
47.53±4.19 |
44.58±3.63 |
|
Post-injection mean |
74.02±11.46a |
48.86±4.07b |
46.88±4.31b |
|
Peak |
87.51±6.63a |
49.22±3.19b |
47.12±5.19b |
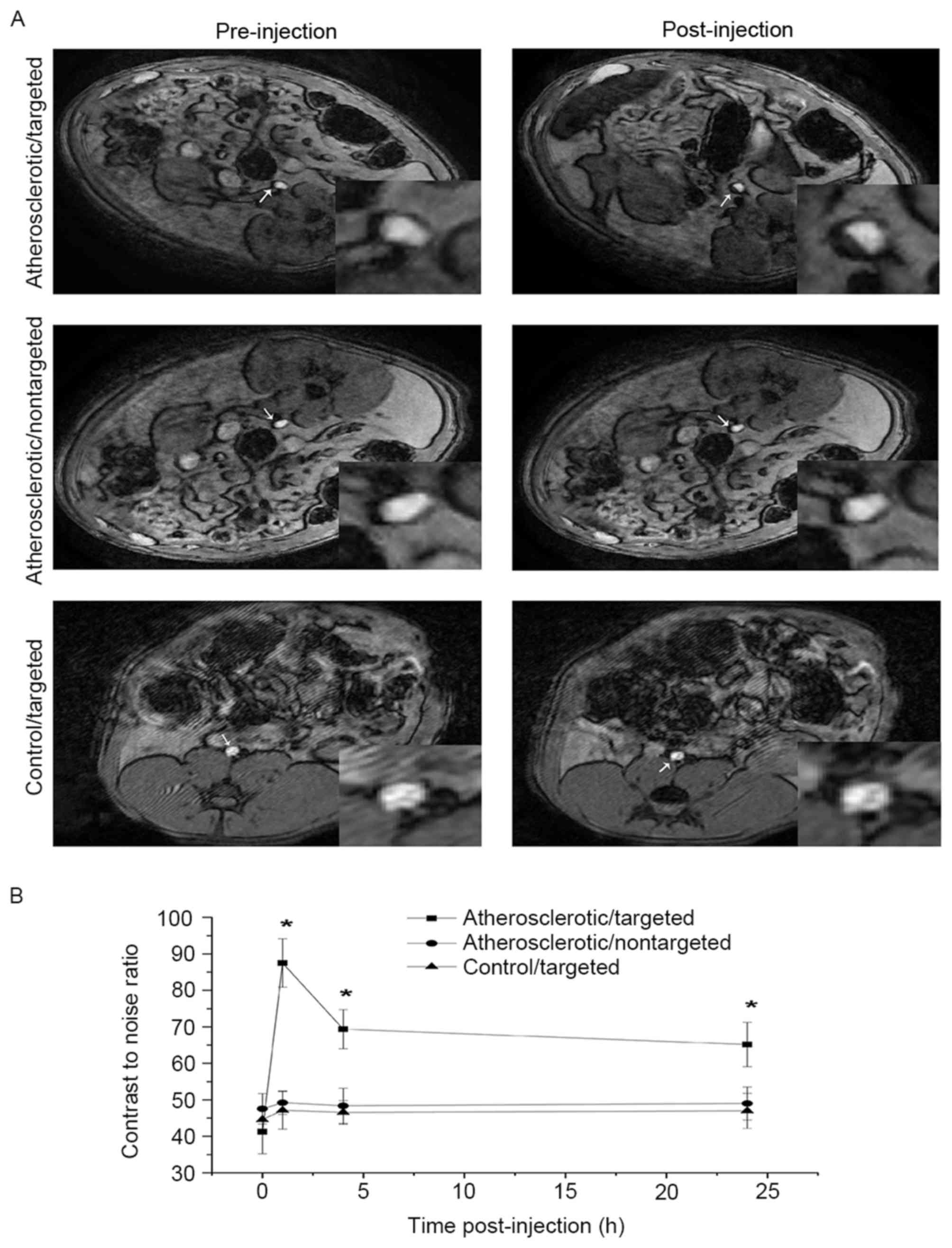
In vivo MRI of VTNC
T2W imaging was used to scan the targeted segment of
rat abdominal aorta prior to and 1, 4 and 24 h following injection
of NCs. Aortic plaques were identified in all atherosclerotic rats
but not control rats, whereas significant T2W signal attenuation
was only detected in rats in the atherosclerotic/targeted group
(Fig. 5A). Comparing the baseline
CNR among the three groups revealed that the injection of NCs
significantly attenuated the mean and the peak CNR in the
atherosclerotic/targeted group, but not in the
atherosclerotic/nontargeted or control/targeted group (Table I). Time-attenuation curve analysis
indicated a higher peak CNR and longer duration of T2W signal
attenuation in the atherosclerotic/targeted group compared with the
atherosclerotic/nontargeted or the control/targeted group (Fig. 5B).
Intraplaque VEGFR-2 expression and
neovascularization
Immunohistochemical staining indicated that there
were notable upregulation of VEGFR-2 expression and proliferation
of neonatal vascular endothelial cells within the plaques in the
atherosclerotic rats (Fig. 1C and
D). Further analysis of the positive expressions revealed that
PPAVEGFR-2+ was 44.5±2.9% in the
atherosclerotic/targeted group and 43.1±3.2% in the
atherosclerotic/nontargeted group (P>0.05). Similarly,
PPACD31+ was 46.0±3.0% in the atherosclerotic/targeted
group and 44.6±3.5% in the atherosclerotic/nontargeted group
(P>0.05). The control/targeted group did not develop
atherosclerotic plaques (data not shown).
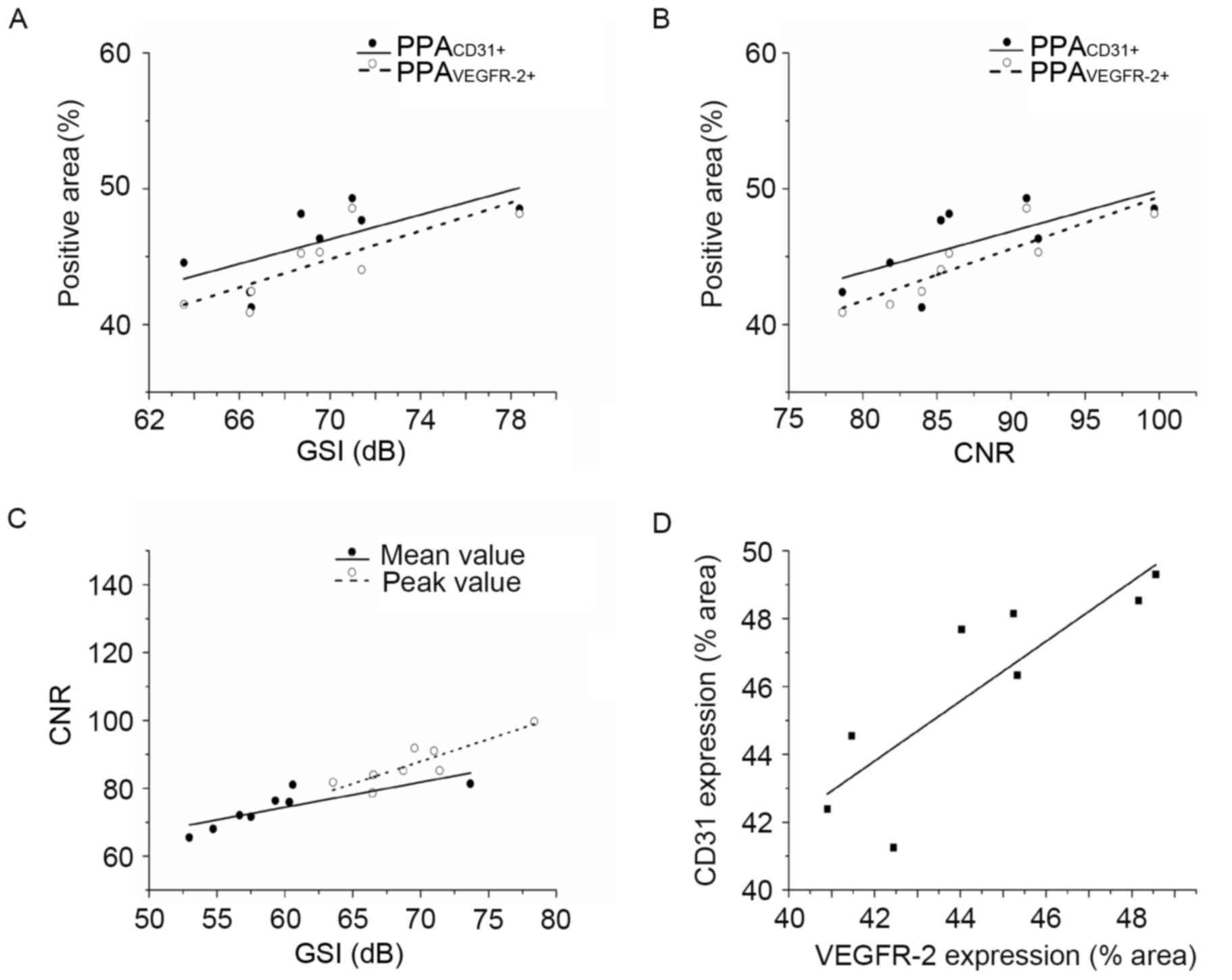
Correlations between in vivo US/MR
imaging and immunostaining parameters
In the atherosclerotic/targeted group, there were
strong correlations between peak GSI and PPAVEGFR-2+
(r=0.81; P<0.05) and between GSI and PPACD31+
(r=0.73; P<0.05; Fig. 6A).
Correlations were also detected between peak CNR and
PPAVEGFR-2+ (r=0.89; P<0.01) and CNR vs.
PPACD31+ (r=0.72; P<0.05; Fig. 6B). Peak GSI exhibited a strong
correlation with CNR (r=0.87; P<0.01) and the mean GSI with CNR
(r=0.82; P<0.05; Fig. 6C).
PPAVEGFR-2+ and PPACD31+ expression also
exhibited a strong correlation (r=0.84; P<0.01; Fig. 6D). Conversely, no correlations were
detected between the imaging and histological parameters in the
atherosclerotic/nontargeted group or the control/targeted
group.
Discussion
Over the past few decades an increasing number of
studies from clinical and basic research have improved our
knowledge and understanding of the pathophysiological mechanisms
that underlie atherosclerosis and the associated thrombotic
complications. The focus on stenotic severity of atherosclerotic
plaque has been replaced with a new concept that suggested that
clinical complications of atherosclerosis, particularly thrombotic
complications, are mainly determined by the intramural plaque
composition rather than the luminal stenotic severity itself
(1,2). The identification of potential
molecular targets that may accompany plaque vulnerability and an
improvement in the detection capabilities of these molecular
targets are gaining more interest (30–32).
Although there are several noninvasive molecular imaging modalities
available, including US, MR, computed tomography (CT), electrical
capacitance tomography and positron emission tomography (PET),
determining the optimal molecular targets with suitable detecting
techniques remains challenging owing to multifaceted mechanisms
that are associated with plaque vulnerability. Nonetheless,
intraplaque angiogenesis with proliferation of the adventitial vasa
vasorum, one of major mechanisms of plaque vulnerability, is a much
easier to target (4).
The present study was the first, to the best of the
authors' knowledge, to create VTNC with two probes (PFOB and SPIO)
for bimodality US and MR molecular imaging of intraplaque
neovasculature. Three main results were obtained from the present
study: i) VTNC exhibited a strong capability with highly specific
binding efficiency to BAECs expressing VEGFR-2 in vitro; ii)
VTNC had a robust ability to target intraplaque neomicrovessels
expressing VEGFR-2 with long-term accumulation in vivo; and
iii) two-probe VTNC was able to be detected by either US or MR
imaging with a broader detectable time-window. Based on the present
results, VTNC combined with US and MR imaging provided an optimal
combination, which enabled reliable imaging of intraplaque
neovasculature and more precise prediction plaque
vulnerability.
A number of nontargeted contrast agents have been
investigated in previous studies, although they were reported to
only enhance the capability for detection of intraplaque
neovasculature to a certain but limited extent (33). A major reason for the poor ability
to detect neoangiogenesis is that such agents possess only
first-pass rather than retention or binding effects, thus affording
a very narrow detectable time-window. For these reasons, targeted
contrast agents are becoming more commonly used. There are numerous
biomarkers, such as P-selectin, αvβ3 integrin, VCAM-1, ICAM-1 or
Profilin-1, that have been used to construct targeted agents for
molecularly imaging atherosclerosis (34–37).
However, most of these biomarkers offered only a single-imaging
modality of MR, CT or PET-CT, and fail to provide complementary
information into the atherosclerotic process. Although some
previous studies have committed to the bimodal imaging of
atherosclerosis by combining MR and either nuclear or fluorescence
imaging, they were eventually demonstrated to be costly,
radioactive and non-real time (14,37,38),
and none of them used VEGFR-2 as a candidate to image intraplaque
neovasculature with bimodal imaging.
Intraplaque angiogenesis has been demonstrated to be
mainly promoted by VEGF, which is overexpressed as atherosclerosis
progresses and is primarily mediated by VEGFR-2 (39). VEGFR-2 is widely expressed in
neovascular endothelial cells and functions by binding its dominant
ligand VEGF, which causes the proliferation of endothelial and
vascular smooth muscle cells (40). Accordingly, construction of
nanoparticles with detectable probes targeted to VEGFR-2 was
theoretically superior and technically promising, and enabled the
present study to detect neovascular endothelial cells or, more
precisely, to molecularly interrogate intraplaque
neovasculature.
As verified in the in vitro experiment, the
created VTNCs were monodispersed with a mean diameter of ~400 nm,
exhibited strong targeting capability with highly specific binding
efficiency to BAECs, and had substantial contrast enhancement under
US-PIHI or MR-T2W imaging. Notably, results from the in vivo
study revealed that the VTNC exhibited excellent retention ability,
as indicated by the strong peak or mean intensity with favorable
contrast-enhancing duration in the time-intensity curves. These
results suggested that the VTNCs possess not only first-pass
effect, but also high binding efficiency and affinity targeted to
the neovascular endothelial cells, thus making VTNCs more suitable
for molecularly targeted imaging of intraplaque neovasculature.
In addition to having the properties of a targeted
biomarker, the detectable probes carried by the VTNCs and the
detection tools available are also crucial for precisely detecting
intraplaque neoangiogenesis. It is desirable not only to obtain
information as accurate as possible in technical view, but also to
acquire data as convenient as possible from a practical point.
Therefore, the use of at least two imaging modalities would be
expected to create an efficient combination to detect their
respective probes. Although US exhibits suboptimal spatial
resolution, it is the more attractive modality of the noninvasive
imaging techniques, owing to its convenient, real-time and low-cost
features (41); conversely, MRI
possesses excellent spatial resolution, but has low temporal
resolution, is costly and examination is time-consuming (42). Integration of US and MR imaging may
satisfy the requirements for precisely detecting intraplaque
neoangiogenesis by compensating for their individual weaknesses.
The proper selection of detectable probes for each imaging modality
may present another issue. Among the numerous available probes,
PFOB with hyperechoic property and SPIO with superparamagnetic
features have been proven to be the most suitable for US or MR
detecting, respectively (21,43).
As demonstrated by the in vivo experiments in
the present study, both US-derived mean and peak GSI and MR-derived
mean and peak CNR were much higher in the atherosclerotic/targeted
group compared with the atherosclerotic/nontargeted or
control/targeted group. Of all parameters examined, these were
highly correlated with intraplaque VEGFR-2 expression and
neovascular formation, which suggested that the bimodalities of US
and MR imaging may be sensitive and specific for detecting PFOB and
SPIO carried by VTNC, respectively. Additionally, the present study
demonstrated that there was a broader detectable time-window (at
least 24 h) for the detection of VTNC using US imaging, which
favored continuous and repeated examination and may improve
accuracy for imaging intraplaque neovasculature.
The results of the present study may offer a number
of benefits; however, there are still some limitations. Although
the use of the dual-probe VTNC was feasible for US and MR bimodal
imaging of intraplaque neovasculature in the abdominal aorta, it
remains difficult to detect coronary plaques due to the constant
beating of the heart. In addition, although merging of the images
of the same section from the two imaging modalities may improve the
accuracy of detection, this remains technically difficult based on
the current techniques. This current technique bottleneck may soon
be resolved with the introduction of fast high-field MRI scanners
and ultrasonographs with high spatial and temporal resolution.
In conclusion, VTNC was associated with robust
targeting capability and excellent intraplaque retention ability.
The use of VTNCs carrying two probes (PFOB and SPIO) was
demonstrated to be technically feasible with a broader detectable
time-window for bimodal US and MR molecular imaging of intraplaque
neovasculature, which may offer complementary information for a
more reliable prediction of plaque vulnerability.
Glossary
Abbreviations
Abbreviations:
|
US
|
ultrasonography
|
|
MR
|
magnetic resonance
|
|
VEGFR-2
|
vascular endothelial growth factor
receptor 2
|
|
PFOB
|
perfluorooctyl bromide
|
|
SPIO
|
superparamagnetic iron oxide
|
|
VTNC
|
VEGFR-2-targeted nanocapsule
|
References
|
1
|
Hansson GK, Libby P and Tabas I:
Inflammation and plaque vulnerability. J Intern Med. 278:483–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falk E, Nakano M, Bentzon JF, Finn AV and
Virmani R: Update on acute coronary syndromes: The pathologists'
view. Eur Heart J. 34:719–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haasdijk RA, Den Dekker WK, Cheng C,
Tempel D, Szulcek R, Bos FL, Hermkens DM, Chrifi I, Brandt MM, Van
Dijk C, et al: THSD1 preserves vascular integrity and protects
against intraplaque haemorrhaging in ApoE−/− mice.
Cardiovasc Res. 110:129–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolodgie FD, Gold HK, Burke AP, Fowler DR,
Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, et al:
Intraplaque hemorrhage and progression of coronary atheroma. N Engl
J Med. 349:2316–2325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moreno PR, Purushothaman KR, Zias E, Sanz
J and Fuster V: Neovascularization in human atherosclerosis. Curr
Mol Med. 6:457–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taruya A, Tanaka A, Nishiguchi T, Matsuo
Y, Ozaki Y, Kashiwagi M, Shiono Y, Orii M, Yamano T, Ino Y, et al:
Vasa Vasorum Restructuring in Human Atherosclerotic Plaque
Vulnerability: A clinical optical coherence tomography study. J Am
Coll Cardiol. 65:2469–2477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang SH, Feng D, Nagy JA, Sciuto TE,
Dvorak AM and Dvorak HF: Vascular permeability and pathological
angiogenesis in caveolin-1-null mice. Am J Pathol. 175:1768–1776.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michel JB, Martin-Ventura JL, Nicoletti A
and Ho-Tin-Noe B: Pathology of human plaque vulnerability:
Mechanisms and consequences of intraplaque haemorrhages.
Atherosclerosis. 234:311–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brogi E, Wu T, Namiki A and Isner JM:
Indirect angiogenic cytokines upregulate VEGF and bFGF gene
expression in vascular smooth muscle cells, whereas hypoxia
upregulates VEGF expression only. Circulation. 90:649–652. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmad S, Hewett PW, Wang P, Al-Ani B,
Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D
and Ahmed A: Direct evidence for endothelial vascular endothelial
growth factor receptor-1 function in nitric oxide-mediated
angiogenesis. Circ Res. 99:715–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belgore F, Blann A, Neil D, Ahmed AS and
Lip GY: Localisation of members of the vascular endothelial growth
factor (VEGF) family and their receptors in human atherosclerotic
arteries. J Clin Pathol. 57:266–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perrot-Applanat M and Di Benedetto M:
Autocrine functions of VEGF in breast tumor cells: Adhesion,
survival, migration and invasion. Cell Adh Migr. 6:547–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi HS, Kim MK, Lee K, Lee KM, Choi YK,
Shin YC, Cho SG and Ko SG: SH003 represses tumor angiogenesis by
blocking VEGF binding to VEGFR2. Oncotarget. 7:32967–32979.
2016.
|
|
14
|
Mancini M, Greco A, Salvatore G, Liuzzi R,
Di Maro G, Vergara E, Chiappetta G, Pasquinelli R, Brunetti A and
Salvatore M: Imaging of thyroid tumor angiogenesis with
microbubbles targeted to vascular endothelial growth factor
receptor type 2 in mice. BMC Med Imaging. 13:312013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tekabe Y, Kollaros M, Zerihoun A, Zhang G,
Backer MV, Backer JM and Johnson LL: Imaging VEGF receptor
expression to identify accelerated atherosclerosis. EJNMMI Res.
4:412014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Pan D, Schmieder AH, Senpan A,
Caruthers SD, Cui G, Allen JS, Zhang H, Shen B and Lanza GM:
Atherosclerotic neovasculature MR imaging with mixed
manganese-gadolinium nanocolloids in hyperlipidemic rabbits.
Nanomedicine. 11:569–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schinkel AF, van den Oord SC, Van Der
Steen AF, van Laar JA and Sijbrands EJ: Utility of
contrast-enhanced ultrasound for the assessment of the carotid
artery wall in patients with Takayasu or giant cell arteritis. Eur
Heart J Cardiovasc Imaging. 15:541–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romero JM, Pizzolato R, Atkinson W, Meader
A, Jaimes C, Lamuraglia G, Jaff MR, Buonanno F, Almandoz J Delgado
and Gonzalez RG: Vasa vasorum enhancement on computerized
tomographic angiography correlates with symptomatic patients with
50 to 70% carotid artery stenosis. Stroke. 44:3344–3349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hrkach J, Von Hoff D, Ali M Mukkaram,
Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M,
Horhota A, et al: Preclinical development and clinical translation
of a PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128ra392012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pisani E, Tsapis N, Galaz B, Santin M,
Berti R, Taulier N, Kurtisovski E, Lucidarme O, Ourevitch M, Doan
BT, et al: Perfluorooctyl Bromide Polymeric Capsules as Dual
Contrast Agents for Ultrasonography and Magnetic Resonance Imaging.
Adv Funct Mater. 18:2963–2971. 2008. View Article : Google Scholar
|
|
21
|
Ghosh D, Lee Y, Thomas S, Kohli AG, Yun
DS, Belcher AM and Kelly KA: M13-templated magnetic nanoparticles
for targeted in vivo imaging of prostate cancer. Nat Nanotechnol.
7:677–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Ma Y, Yue X, Cao Z and Dai Z:
One-pot construction of doxorubicin conjugated magnetic silica
nanoparticles. New J Chem. 33:2414–2418. 2009. View Article : Google Scholar
|
|
23
|
Liu J, Li J, Rosol TJ, Pan X and Voorhees
JL: Biodegradable nanoparticles for targeted ultrasound imaging of
breast cancer cells in vitro. Phys Med Biol. 52:4739–4747. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hermanson GT: Bioconjugate Techniques. I.
Academic Press; London: pp. 169–172. 1996, View Article : Google Scholar
|
|
25
|
Tian C, Zhang R, Ye X, Zhang C, Jin X,
Yamori Y, Hao L, Sun X and Ying C: Resveratrol ameliorates
high-glucose-induced hyperpermeability mediated by caveolae via
VEGF/KDR pathway. Genes Nutr. 8:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fonseca FA, Paiva TB, Silva EG, Ihara SS,
Kasinski N, Martinez TL and Filho EE: Dietary magnesium improves
endothelial dependent relaxation of balloon injured arteries in
rats. Atherosclerosis. 139:237–242. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan F, Yang W, Li X, Liu H, Nan X, Xie L,
Zhou D, Xie G, Wu J, Qiu B, et al: Magnetic resonance imaging of
atherosclerosis using CD81-targeted microparticles of iron oxide in
mice. Biomed Res Int. 2015:7586162015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo S, Shen S, Wang J, Wang H, Li M, Liu
Y, Hou F, Liao Y and Bin J: Detection of high-risk atherosclerotic
plaques with ultrasound molecular imaging of glycoprotein IIb/IIIa
receptor on activated platelets. Theranostics. 5:418–430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reimer P and Balzer T: Ferucarbotran
(Resovist): A new clinically approved RES-specific contrast agent
for contrast-enhanced MRI of the liver: Properties, clinical
development, and applications. Eur Radiol. 13:1266–1276.
2003.PubMed/NCBI
|
|
30
|
Wiesner P, Tafelmeier M, Chittka D, Choi
SH, Zhang L, Byun YS, Almazan F, Yang X, Iqbal N, Chowdhury P, et
al: MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in
human plasma. J Lipid Res. 54:1877–1883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu WY, Chao YW, Tsai YL, Lien CC, Chang
CF, Deng MC, Ho LT, Kwok CF and Juan CC: Resistin induces
monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1
expression in endothelial cells via p38MAPK-dependent pathway. J
Cell Physiol. 226:2181–2188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fotis L, Agrogiannis G, Vlachos IS,
Pantopoulou A, Margoni A, Kostaki M, Verikokos C, Tzivras D,
Mikhailidis DP and Perrea D: Intercellular adhesion molecule
(ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early
stages of atherosclerosis in a rat model. In Vivo. 26:243–250.
2012.PubMed/NCBI
|
|
33
|
Deyama J, Nakamura T, Takishima I, Fujioka
D, Kawabata K, Obata JE, Watanabe K, Watanabe Y, Saito Y, Mishina H
and Kugiyama K: Contrast-enhanced ultrasound imaging of carotid
plaque neovascularization is useful for identifying high-risk
patients with coronary artery disease. Circ J. 77:1499–1507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Bauer W, Israel I, Kreissl MC,
Weirather J, Richter D, Bauer E, Herold V, Jakob P, Buck A, et al:
Targeting P-selectin by gallium-68-labeled fucoidan positron
emission tomography for noninvasive characterization of vulnerable
plaques: Correlation with in vivo 17.6T MRI. Arterioscler Thromb
Vasc Biol. 34:1661–1667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Daeichin V, Kooiman K, Skachkov I, Bosch
JG, Theelen TL, Steiger K, Needles A, Janssen BJ, Daemen MJ, Van
Der Steen AF, et al: Quantification of Endothelial αvβ3 expression
with high-frequency ultrasound and targeted Microbubbles: In vitro
and In vivo Studies. Ultrasound Med Biol. 42:2283–2293. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi CM, Du L, Wu WH, Li DY, Hao J, Gong L,
Deng L, Zhang T, Zhang C and Zhang Y: Detection of Vulnerable
Atherosclerotic Plaques in Experimental Atherosclerosis with the
USPIO-Enhanced MRI. Cell Biochem Biophys. 73:331–337. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Chen J, Yang B, Qiao H, Gao L, Su
T, Ma S, Zhang X, Li X, Liu G, et al: In vivo MR and Fluorescence
Dual-modality Imaging of Atherosclerosis Characteristics in Mice
Using Profilin-1 Targeted Magnetic Nanoparticles. Theranostics.
6:272–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Tilborg GA, Vucic E, Strijkers GJ,
Cormode DP, Mani V, Skajaa T, Reutelingsperger CP, Fayad ZA, Mulder
WJ and Nicolay K: Annexin A5-functionalized bimodal nanoparticles
for MRI and fluorescence imaging of atherosclerotic plaques.
Bioconjug Chem. 21:1794–1803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arnott C, Punnia-Moorthy G, Tan J,
Sadeghipour S, Bursill C and Patel S: The Vascular Endothelial
Growth Factor Inhibitors Ranibizumab and Aflibercept Markedly
Increase Expression of Atherosclerosis-Associated Inflammatory
Mediators on Vascular Endothelial Cells. PLoS One. 11:e01506882016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kiessling F, Gaetjens J and Palmowski M:
Application of molecular ultrasound for imaging integrin
expression. Theranostics. 1:127–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eckert MA, Vu PQ, Zhang K, Kang D, Ali MM,
Xu C and Zhao W: Novel molecular and nanosensors for in vivo
sensing. Theranostics. 3:583–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Díaz-López R, Tsapis N and Fattal E:
Liquid perfluorocarbons as contrast agents for ultrasonography and
(19)F-MRI. Pharm Res. 27:1–16. 2010. View Article : Google Scholar : PubMed/NCBI
|