Introduction
Atherosclerosis is a disorder in which the intima
retains lipid, triggering a chronic inflammatory disease mediated
by innate and adaptive immune responses (1,2).
Dendritic cells (DCs) constitute a family of specialized
antigen-presenting cells (APCs) that play a critical role as a link
between the innate and adaptive immune responses (3). DCs have been identified in both the
normal and pathological intima, thus they play a role in all stages
of atherosclerosis, with multiple functions such as lipid
accumulation, foam cell formation, pro-inflammatory cytokine
secretion and antigen presentation (4–7). In
most tissues, DCs are present in an immature state and the process
of maturation is triggered in response to inflammatory stimulation.
Upon maturation, DCs exhibit reduced antigen capture and uptake
capacity, but increased antigen presentation ability and surface
expression of major histocompatibility complex (MHC) class II and
co-stimulatory molecules such as CD40, CD80, and CD86 (8,9).
Therefore, the maturation of DCs is the key process in the
activation of naïve T cells and mediation of the inflammatory
cascade.
TLR signal pathways have been described as a crucial
checkpoint for DC maturation by recognizing pathogen-associated
molecular patterns (PAMPs) and inducing this signal pathway that
ultimately causes cytokine production and upregulation of MHC II
and co-stimulatory molecules by DCs (10). Previously, we demonstrated that in
comparison with immature DCs, mature DCs expressed much higher
levels of Toll-like receptor 4 (TLR4) mRNA in vitro and were
better at stimulating T cell proliferation (in press). However, the
question of whether DCs contribute to inflammation in vivo
similar to their effect in vitro should be further
investigated. In this study, we transferred bone marrow-derived DCs
(BMDCs) from wild mice into ApoE−/− mice. These
transferred exogenous DCs induced endogenous DC maturation in
vivo by upregulation of TLR-4, which further increased the
inflammatory response and accelerated atherosclerosis.
Materials and methods
Cells and animals
The principal method of generating BMDCs was adapted
from that published by Son et al (11) with minor modifications and involved
culturing bone marrow from C57BL/6 wild-type mice with 1,000 U/ml
of granulocyte macrophage colony stimulating factor (GM-CSF) and
interleukin (IL)-4 (R&D Systems, Minneapolis, MN, USA).
CD11c+ DCs were selected using magnetic beads (Miltenyi
Biotec, Auburn, CA, USA) according to the manufacturer's
instructions. To stimulate DCs, 1 µg/ml of lipopolysaccharide (LPS;
Sigma-Aldrich, St. Louis, MO, USA) was added from day 8 to day 9 to
obtain mature DCs. The expression of surface molecules on DCs was
analyzed by fluorescence-activated cell sorting (FACS, FACS Aria
II; Becton Dickinson Immunocytometry Systems). ApoE−/−
mice on a C57BL/6 background were purchased from Jackson Laboratory
(Bar Harbor, ME, USA). Eight week-old ApoE−/− mice (male
or female) were randomly assigned to one of three experimental
groups (n=6–7). One group was injected subcutaneously inside the
thigh with 1×106 purified DCs and then fed normal chow
(NC), the second group received an equivalent volume of
phosphate-buffered saline (PBS) and a diet of normal chow, while
the third group was left untreated but fed a western diet (WD).
Mice were kept under specific pathogen-free conditions, housed in a
pathogen-free environment at Dalian Medical University and injected
with DCs or PBS weekly for a total of 12 weeks. The investigation
conformed to the National Institutes of Health guide for the Care
and Use of Laboratory Animals (no. 8523, revised 1996; NIH
Publications). They were then used in accordance with protocols
approved by the Institutional Animal Care and Use Committee of
Dalian Medical University (L2014030). All mice were sacrificed at
about 20 weeks of age. After a 12 h overnight fast, animals were
heavily sedated, and blood samples were drawn from the orbita
immediately. For extraction of tissues, the spleen and the aorta
from the aortic valve to the femoral bifurcation were removed and
snap frozen at −80°C for later RNA extraction. In addition, some
aorta tissues were stored in 10% buffered formalin at 4°C for
histological staining.
Cytokines and plasma lipid
analysis
Mouse plasma and spleen levels of interleukin
(IL)-6, tumor necrosis factor (TNF)-α, IL-12p40 and IFN
(interferon)-γ were determined by ELISA following the
manufacturer's instructions (Abcam, Cambridge, UK). Briefly, plasma
or tissue samples were obtained as indicated above and stored at
−80°C prior to use. Microplate wells were respectively coated with
purified rat anti-mouse IL-6, TNF-α, IL-12p40 or IFN-γ, followed by
incubation with blocking buffer at room temperature for 1 h and
subsequent washing with PBS. Subsequently, mouse plasma or tissue
samples were added to the microplate wells and incubated for 2 h at
room temperature, followed by incubation with biotin-labeled
anti-mouse for IL-6, TNF-α, IL-12p40 or IFN-γ 1 h at room
temperature. The absorbance at 450 nm was measured with a
microplate reader (Thermo Fisher Scientifc, Inc., Waltham, MA,
USA). Plasma total cholesterol and triglyceride levels as well as
lipoprotein fractions were also determined following the
manufacturer's instructions (Abcam).
Flow cytometric analysis
The expression of surface molecules on DCs in
peripheral blood was analyzed by flow cytometry. At each step of
the staining, 1×106 cells were stained with specific Abs
for 1 h at 4°C in 100 µl of PBS containing 2% bovine serum albumin.
We used fluorescein isothiocyanate (FITC) or phycoerythrin
(PE)-labeled monoclonal Abs for staining of MHC class II (FITC),
CD40 (FITC), CD80 (FITC), CD83 (FITC), CD86 (FITC), TLR-4 (FITC)
and CD11c (PE). FITC- or PE-labeled IgG was substituted for
isotype-matched controls. All Abs were purchased from BioLegend
(San Diego, CA, USA).
Histological analysis
To assay the burden of lipid in the aorta, aorta
samples were opened longitudinally and stained with Oil red O
solution. Aortic roots were embedded in 10% buffered formalin for
serial sections, 5 µm thick. Hematoxylin and eosin (H&E)
staining was used to analyze lesions. Masson's trichrome was used
to delineate the fibrous area. Immunohistochemistry was performed
on paraffin sections of mouse aortic roots using rabbit anti-murine
CD4 IgG (10 µg/ml) and rabbit anti-murine TLR4 IgG (10 µg/ml); DCs
were identified by immunostaining with FITC-conjugated primary
antibody against CD11c (5 µg/ml), and foam cells were identified by
Alexa Fluor® 647-conjugated primary antibody against
CD68 (5 µg/ml) (all from Abcam). Control immunostaining was
performed using the respective non-immune IgG; no specific staining
was observed. Quantification of positive area was performed using
Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD,
USA).
Real-time RT-PCR analysis
Total RNA in aorta or spleen was extracted using the
RNAiso Reagent Plus (Takara Bio Inc., Shiga, Japan), and
complementary DNA (cDNA) was synthesized using an RT-PCR kit
(Takara Bio Inc.) according to the manufacturer's recommendations.
Table I shows the primer sequences
and expected sizes of the products. Relative changes in mRNA
expression were normalized with GAPDH and calculated using
the 2−ΔΔCq method.
 | Table I.Primer sequences for real-time
RT-PCR. |
Table I.
Primer sequences for real-time
RT-PCR.
| Gene | Accession no. | Sequence (5′3′) | Size (bp) |
|---|
| IL-6 | NC_000071.6 |
F:TAGTCCTTCCTACCCCAATTTCC | 76 |
|
|
|
R:TTGGTCCTTAGCCACTCCTTC |
|
| IL-12p40 | NC_000077.6 |
F:AGACCCTGCCCATTGAACTG | 70 |
|
|
|
R:GAAGCTGCTGCTGTAGTTCTCATATT |
|
| TNF-α | NC_000083.6 |
F:TAGCCCACGTCGTAGCAAAC | 170 |
|
|
|
R:GCAGCCTTGTCCCTTGAAGA |
|
| IFN-γ | NC_000076.6 |
F:TCAAGTGGCATAGATGTGGAAGAA | 95 |
|
|
|
R:TGGCTCTGCAGGATTTTCATG |
|
| TLR4 | NC_000070.6 |
F:GCCTTTCAGGGAATTAAGCTCC | 114 |
|
|
|
R:GATCAACCGATGGACGTGTAAA |
|
| GAPDH | NC_000072.6 |
F:TGGCCTTCCGTGTTCCTAC | 178 |
|
|
|
R:GAGTTGCTGTTGAAGTCGCA |
|
Statistical analysis
Statistical analyses were performed with GraphPad
Prism version 5.0 (GraphPad Software, San Diego, CA, USA). Data are
expressed as mean ± standard deviation. Statistical significance
was determined by single factor analysis of variance (ANOVA) with
Bonferroni correction. P<0.05 was considered to indicate a
statistically significant result.
Results
Characterization of cultured DCs
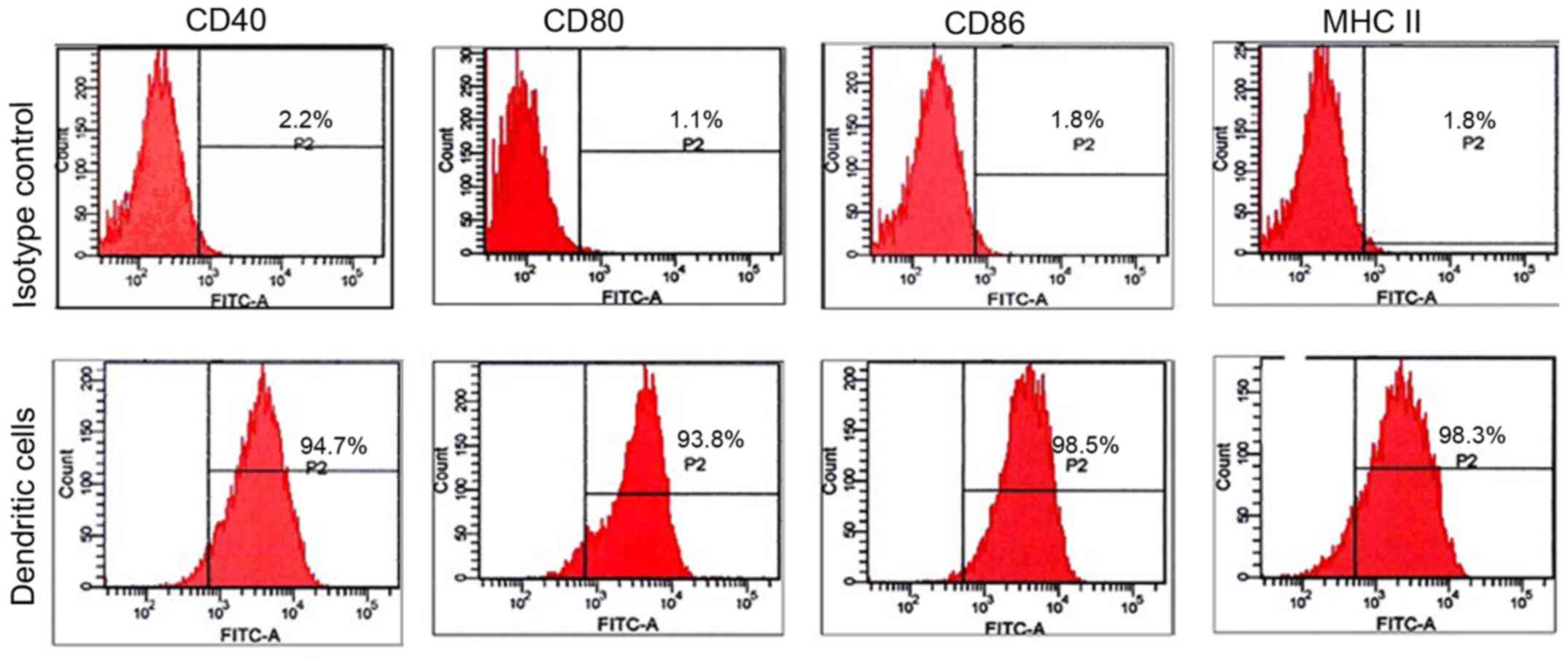
BMDCs were cultured with GM-CSF and IL-4, and
stimulated with LPS to obtain mature DCs. More than 90% of the
purified cells expressed the surface markers of mature DCs: MHC II,
CD40, CD80 and CD86 (Fig. 1).
Transfer of DCs increases DC
maturation
To investigate the contribution of exogenous DCs to
augmentation of the population of mature DCs in the peripheral
blood of recipients, the percentage of CD11c+/CD40+, CD11c+/CD80+,
CD11c+/CD86+, CD11c+/CD83+, and CD11c+/MHC II+ cells were analyzed
by FACS. The results showed that mice treated with DCs showed
higher expression of CD83 and co-stimulatory molecules compared to
mice treated with PBS (cells positive for CD83+/CD11c+,
CD11c+/CD40+, CD11c+/CD86+, and CD11c+/CD80+ made up 1.3, 3.8, 2.8,
and 8.5%, respectively, vs. 0.3, 1.6, 1.6, and 3.5% in the mice
treated with PBS, P<0.05, Fig.
2). Although the expression of MHC II in the mice receiving DCs
did not differ significantly from that of the mice receiving PBS,
the level was higher in the DC-treated mice. When compared with
mice fed a WD, mice treated with DCs showed no significant
difference in the expression of co-stimulatory molecules, MHC II or
CD83. Thus, only ApoE−/− mice treated with exogenous DCs
showed an increase in the maturation of DCs.
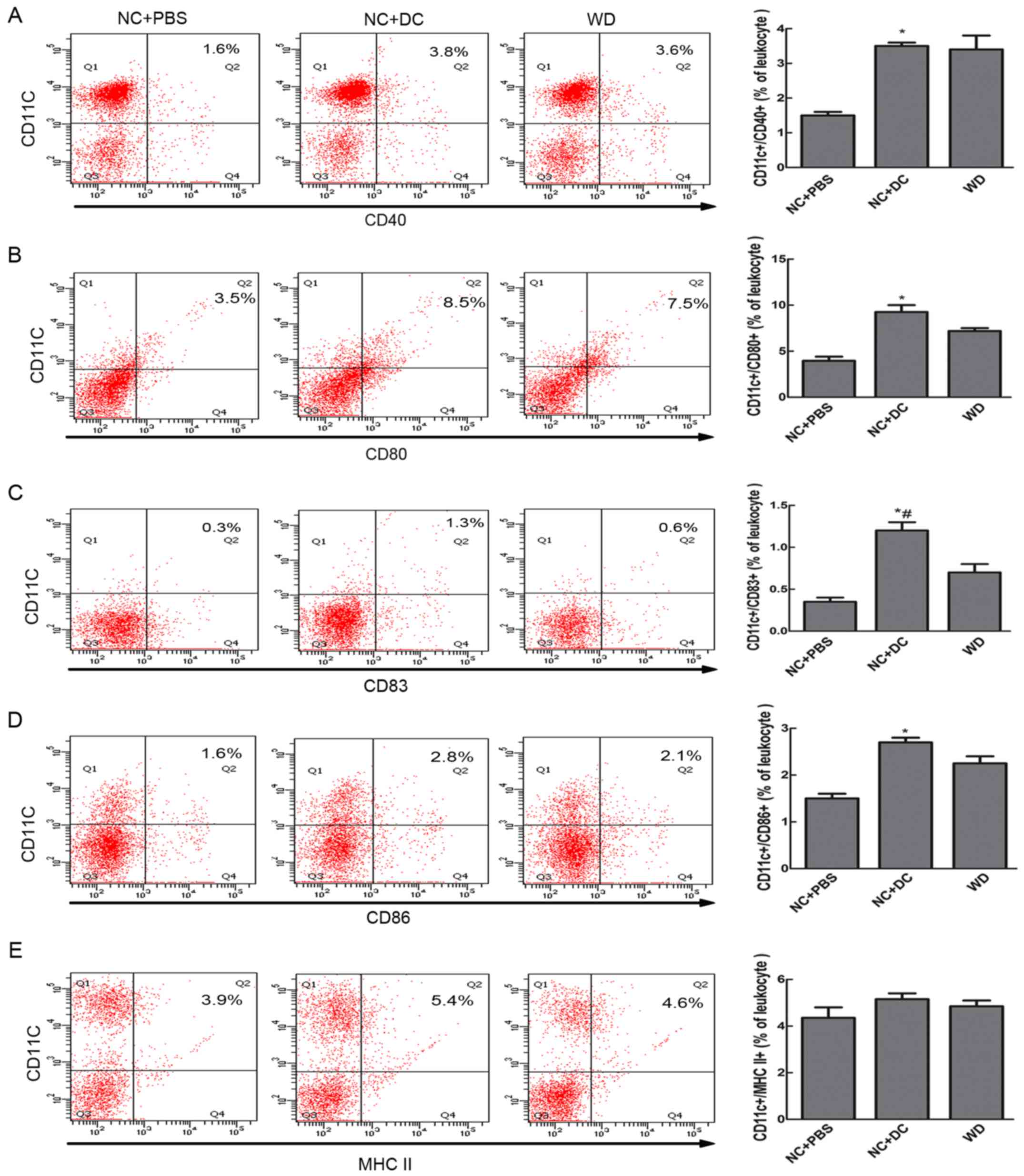 | Figure 2.Transfer of DCs increased DC
maturation in peripheral blood. Eight-week-old ApoE−/−
mice on a C57BL/6 background were injected subcutaneously inside
the thigh with 1×106 purified DCs and then fed normal
chow, a second group received an equivalent volume of PBS and a
diet of NC, while a third group was left untreated but fed a WD.
The mice were sacrificed at about 20 weeks of age, peripheral blood
leukocytes were harvested and the expression of surface markers on
DCs was assessed by flow cytometry. Cells in the gated PBLs were
examined for expression of CD11c and for combination with CD40 (A),
CD80 (B), CD83 (C), CD86 (D) and MHC II (E) from mice treated with
PBS (n=6), mice treated with DCs (n=7) and mice fed a western diet
(n=6); and the bar graph of each showing DCs as a percentage of
leukocytes. Data are expressed as mean ± SD. *P<0.05, mice
treated with DCs vs. mice treated with PBS. #P<0.05
mice treated with DCs vs. mice fed a western diet. DCs, dendritic
cells; NC, normal chow; PBS, phosphate-buffered saline; WD, western
diet; MHC, major histocompatibility complex. |
Transfer of DCs induces inflammatory
activation in the recipients
As we report previously, maturation of DCs could
activate naïve T cells and the inflammatory cascade. Next, we
measured the concentrations of the cytokines IL-6, TNF-α, IFN-γ and
IL-12p40 in the circulation and in atherosclerotic lesions. The
plasma levels of IL-6, TNF-α, IFN-γ and IL-12p40 in the mice
treated with DCs were all markedly higher compared to mice treated
with PBS or those fed a WD (Fig.
3A), which is consistent with expression of those cytokines at
the mRNA level in atherosclerotic lesions (Fig. 3B). Moreover, CD4+ T cell
accumulation was markedly increased with DC treatment compared with
the PBS treatment group (Fig. 4C).
Strikingly, the mice treated with DCs exhibited increased spleen
size compared to that of the mice treated with PBS (Fig. 3C). An intensified inflammatory
reaction occurred in recipient mice after injection of exogenous
DCs, and since inflammatory cells typically infiltrate and
proliferate in the spleen, this may be thought to account for the
splenomegaly. Next, we evaluated the content of cytokines at both
protein and mRNA levels in spleen tissue. The protein and mRNA
levels of IL-6, TNF-α, IFN-γ and IL-12p40 were all significantly
upregulated in the mice treated with DCs compared with those
treated with PBS (Fig. 3D and E).
These data suggest that transfer of DCs induces inflammatory
activation in the recipients.
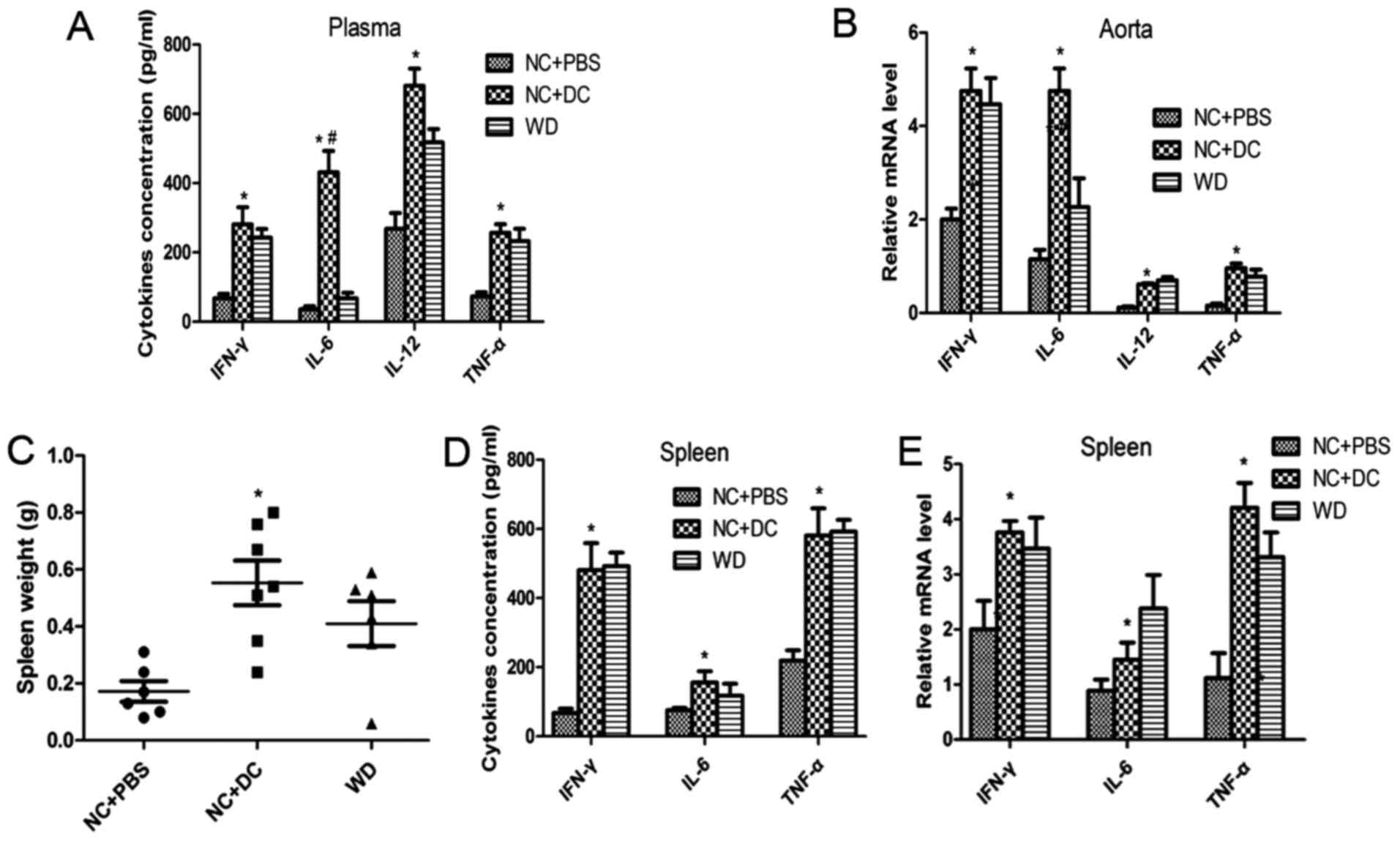 | Figure 3.Transfer of DCs induces inflammatory
activation in the recipients. (A) ELISA detecting the level of
IL-6, TNF-α, IFN-γ and IL-12p40 in plasma from mice treated with
PBS, injected with DCs or fed a western diet; (B) quantitative
real-time RT-PCR analyzing the level of these cytokines in
atherosclerotic lesions in the three groups; (C-E) spleens of all
mice were harvested and weighed after sacrifice, then the protein
and mRNA levels of IL-6, TNF-α, and IFN-γ were measured by ELISA
and qPCR. Data are expressed as mean ± SD (n=6–7). *P<0.05, mice
treated with DCs vs. mice treated with PBS, #P<0.05
mice treated with DCs vs. mice fed a western diet. DCs, dendritic
cells; PBS, phosphate-buffered saline; TNF, tumor necrosis factor,
IFN, interferon. |
Transfer of DCs aggravates aortic
atherosclerosis
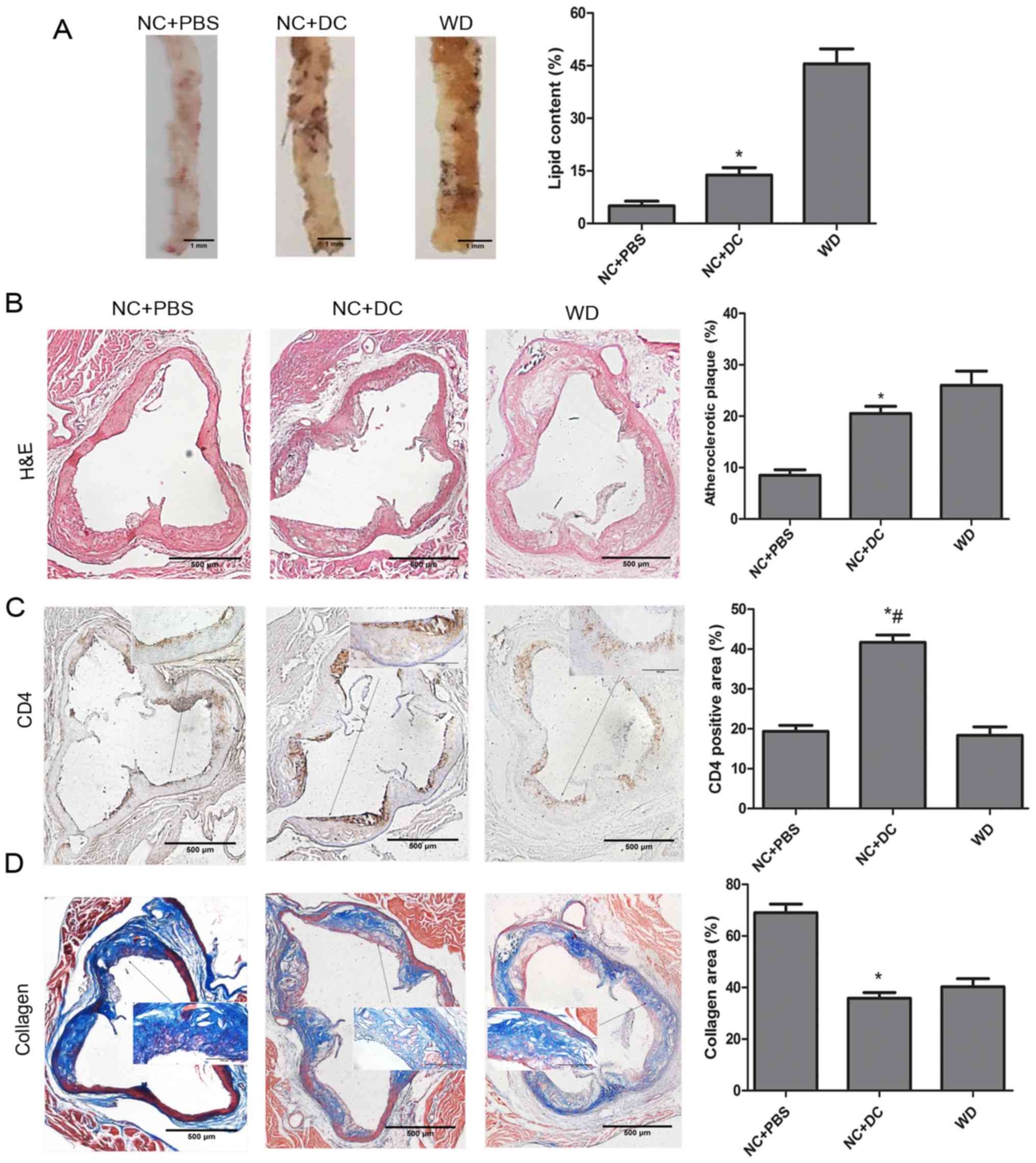
To evaluate the effect of transferring DCs on
atherosclerosis, we first measured plasma lipid and lipoprotein
levels. Compared with animals treated with PBS, mice treated with
DCs exhibited significant increases in all lipid components except
high-density lipoprotein (HDL) (Table
II). Next, we analyzed total lipid burden by using en
face preparation of the aorta and Oil red O staining.
Computer-assisted quantitative histomorphometric analysis revealed
a significant increase of the total aortic plaque burden in mice
injected with exogenous DCs compared with those receiving PBS.
Meanwhile, the mice fed a WD showed a serious lipid burden
(Fig. 4A). Similarly, the
atherosclerotic plaque area as a percentage of total aortic area
was determined from H&E-stained histological sections of the
aorta, which showed that, in mice treated with DCs, the area was
remarkably increased compared to the mice treated with PBS
(Fig. 4B). In addition, CD4+ T
cell accumulation was markedly increased with DC treatment compared
with the PBS treatment group (Fig.
4C). In contrast, collagen content, assessed by Masson's
trichrome staining, was decreased by treatment with DCs compared
with PBS (Fig. 4D). In summary,
these results indicate serious aggravation of atherosclerosis after
DC treatment in ApoE−/− mice.
 | Table II.Intervention effects on total
cholesterol, triglycerides and lipoprotein fractions. |
Table II.
Intervention effects on total
cholesterol, triglycerides and lipoprotein fractions.
|
| NC+PBS | NC+DC | WD |
|---|
| Weight (g) |
29.6±1.4 |
30.1±1.6 |
28.7±1.9 |
| TC (mg/dl) |
465±78 |
566±92a |
678±156 |
| TG (mg/dl) |
80±16 |
101±22a |
114±45 |
| HDL-C (mg/dl) |
61±14 |
67±18 |
74±32 |
| LDL-C/VLDL-C
(mg/dl) |
359±54 |
475±42a |
502±50 |
Transfer of DCs increases DC
recruitment and foam cell number in lesions
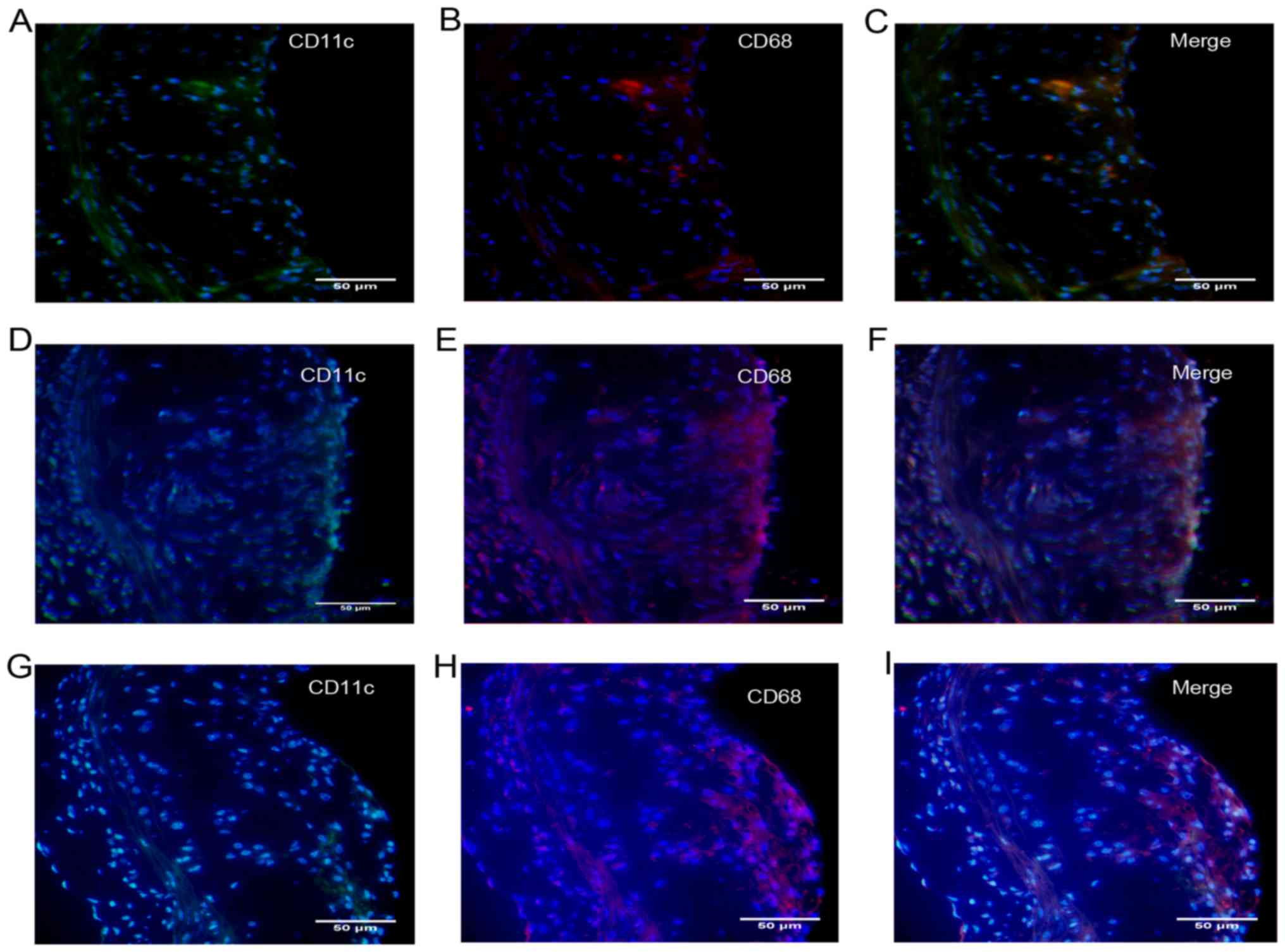
To further explore the characteristics of
atherosclerosis following treatment with DCs, we quantified the
number of DCs recruited into plaques. In addition, like
macrophages, DCs have been shown to form foam cells in
atherosclerotic lesions, most likely via the uptake of lipoproteins
and lipid-laden apoptotic cells (12), so we also quantified the number of
DC-derived foam cells in atherosclerotic plaques. Foam cells are
derived primarily from monocytes/macrophages and a single
monocyte/macrophage marker, such as CD68, has generally been used
(13). In this study, DC-derived
foam cells were co-stained with CD11c and CD68. After transfer of
DCs, a large amount of CD11c/CD68-positive content was found in
atherosclerotic lesions (Fig. 5),
so we presumed that DCs in situ or those from the
circulation had migrated into atherosclerotic lesions, become foam
cells and been deposited in the lipid pool after
disintegration.
Transfer of DCs upregulates expression
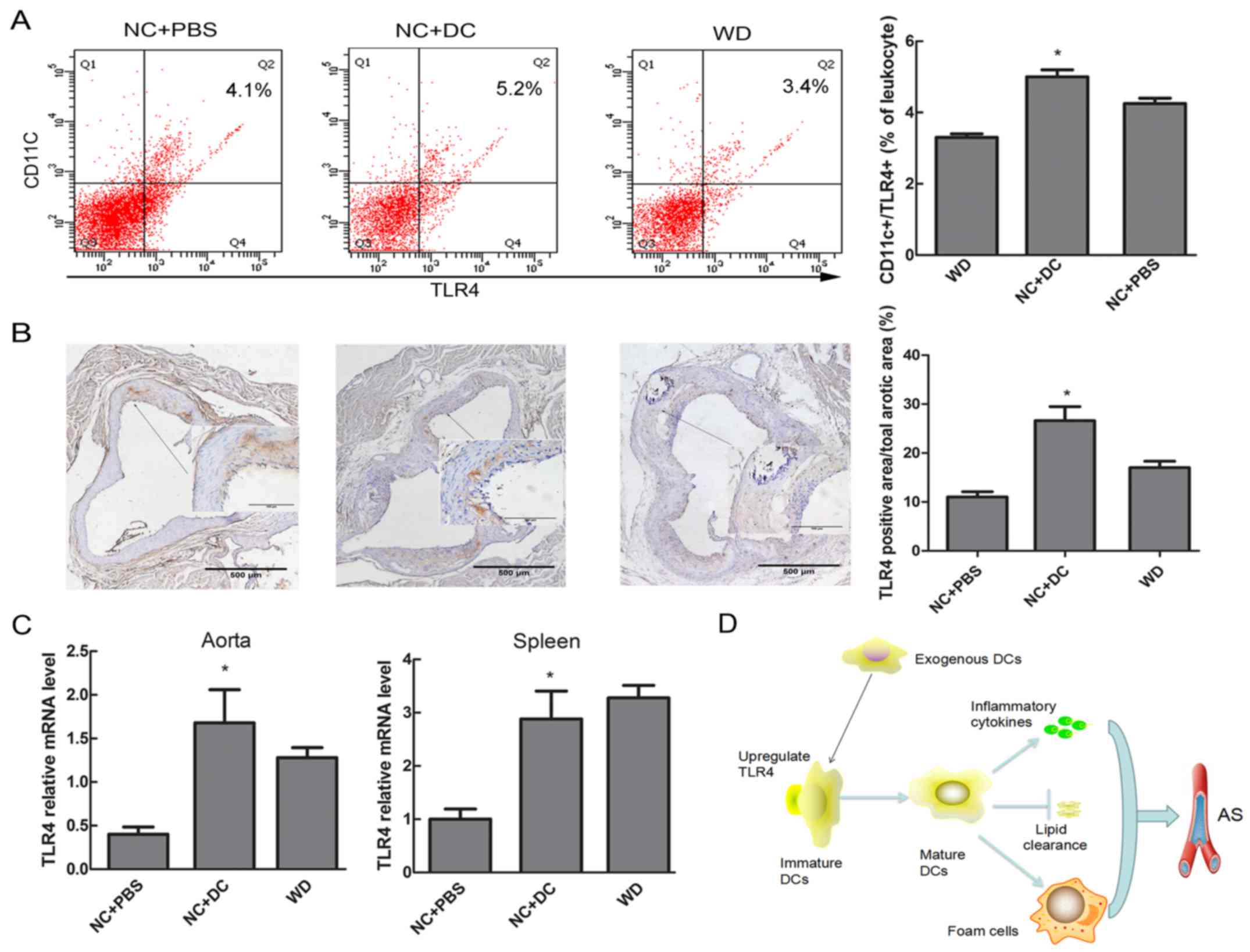
of TLR4 in DCs
It is well established that activation of TLRs has
been implicated in triggering the release of cytokines and the
differentiation of immature to mature DCs, linking the innate
immune system to the adaptive immune response (14). Thus, we explored whether expression
of TLR4 in DCs coincided with similar changes of inflammatory
cytokines and atherosclerotic lesions. Flow cytometric analysis
showed that the expression of TLR-4 on DCs in peripheral blood
tended to be higher in mice injected with DCs compared with mice
treated with PBS (5.2% vs. 3.4%, P<0.05, Fig. 6A). Similarly, a significant
increase in TLR4 expression at both protein and mRNA level was
observed by immunohistochemistry and real-time RT-PCR in aortic
tissue after transfer of DCs, as well as TLR4 mRNA level in spleen
tissue (Fig. 6B and C).
Discussion
Accumulating evidence indicates a fundamental link
between the immune system and atherosclerosis, but thus far this
evidence has been mostly indirect (15). Depletion of plasmacytoid dendritic
cells (pDCs) by injection of PDCA-1 antibody or mice with
conditional depletion of DCs (DTR-CD11c transgenic mice) showed
significantly reduced atherosclerotic plaque formation (16,17).
Herein, we demonstrate that transferred exogenous BMDCs can
increase atherosclerosis in ApoE−/− mice. DC maturation
is associated with the secretion of pro-inflammatory cytokines and
possesses the ability to robustly activate naïve T cells in an
antigen-presenting manner, both of which are known to accelerate
atherosclerosis (18,19). In our study we showed that
expression of the costimulatory molecules, CD83 and MHC II, on
circulating DCs was upregulated after intervention, indicating that
the content of mature DCs was increased. Thereby, because of the
noticeable ability of transferred DCs to activate inflammation,
levels of the cytokines IL-6, TNF-α, IFN-γ and IL-12p40 were
remarkably increased, which coincided with augmentation of the
accumulation of CD4+ T cells in atherosclerotic lesions.
In addition, we studied the role of transferred DCs on
atherosclerotic plaques. As anticipated, our results with
ApoE−/− mice after injection of exogenous DCs displayed
a significant increase in atherosclerotic lesions with less
collagen content, which can contribute to plaque instability.
The functional significance, if any, of lipid
accumulation by DCs is not known and will be discussed below. For
example, in WD-fed LDLR−/− and ApoE−/−
background mice in which the lifespan of DCs was enhanced by
CD11c-specific transgenic expression of the anti-apoptotic protein
Bcl-2, there was a marked decrease in plasma cholesterol (20). However, we observed that elevation
in the matured DC population after treatment with exogenous DCs led
to markedly increased plasma cholesterol levels. This unexpected
effect could be a consequence of a reduction of lipoprotein uptake
and clearance of DCs from the circulation by maturation.
Accumulation of foam cells in atherosclerotic plaques is a hallmark
of atherogenesis (21). Moreover,
dead foam cells have the effect of activating inflammation and
promoting the growth of the necrotic area in a plaque, leading to
plaque rupture and the occurrence of clinical events (22,23).
As Paulson et al reported, lipid accumulation could be found
within vascular DCs in the arterial intima of LDLR−/−
mice after a few days of hypercholesterolemia, and the foam
cell-like DCs may constitute the formation of atherosclerotic
plaques (12). We observed similar
results, with CD11c/CD68-positive content being abundant in the
necrotic core of atherosclerotic plaques, which coincided with the
severity of atherosclerosis. The deposition of foam cells in
plaques has been implicated as a source of inflammatory cytokines,
contributing to plaque rupture with hemorrhage and formation of
thrombi.
As described, DCs are present in the immature state
prior to antigen exposure, characterized by the ability to capture
antigen but not expressing high levels of T cell costimulatory
molecules or MHC II, while undergoing the progress of maturation,
which is associated with downregulation of the capacity of antigen
capture, upregulation of MHC II and CD40, CD80, CD86 and migration
to draining lymph nodes or other immunization sites for antigen
presentation to T cells (8). There
is evidence that the TLR signaling pathway is a critical mediator
of inflammation during atherosclerosis. More specifically,
stimulation of DCs with TLR ligands induces DC maturation and
activation in vivo and in vitro (24). In our previous study,
monocyte-derived DCs were isolated and cultured from human
peripheral blood, and shown to have increased levels of CD1a, CD80,
CD83 and CD86 after stimulation appeared to coincide with the level
of TLR4 (in press). Evidence for the involvement of TLR4 in the
development of atherosclerosis is substantial, albeit largely
indirect. However, one study reported that TLR4-deficient mice
appeared to develop more severe atherosclerosis, perhaps due to the
mechanism of impaired regulatory T cell infiltration, and
especially, a reduction of DC IL-10, which is a regulatory T cell
polarizing cytokine (25).
Similarly, deficiency of MyD88 was associated with systemic defects
in regulatory T cell development by blocking DC maturation, leading
to severe atherosclerosis (26).
These intriguing results may be due to the role of regulatory T
cells, a subset of T cells that is well known to be
atheroprotective by inhibiting pro-atherogenic Th1 cell response
and activating macrophages or DCs by secreting anti-inflammatory
cytokines such as IL-10 and TGF-β (27,28).
However, our study suggested that the upregulation of TLR4 was
correlated in general with the maturation of DCs, as well as the
extent of atherosclerosis; thus, we speculate that activation of
the TLR4 pathway has a dominant effect on the pro-atherogenic role
of DCs in activating T cells over the atheroprotective role of
regulatory T cells in our model.
In this study, we report that injection of exogenous
DCs could upregulative the expression of TLR4 on DCs, leading to
activation of inflammatory mediators in the circulation and
enhancing the number of DCs infiltrating into lesions, both of
which aggravate atherosclerosis development. Our work represents an
important step in establishing the important role of DCs in
atherosclerosis and identifying a link between TLR4 signaling and
atherosclerosis in vivo. However, further investigation will
be required to elucidate the functions of DCs during the
progression of atherosclerosis. Given the pro- and
anti-inflammatory functions of DCs, it is conceivable that their
role in atherosclerosis is complex.
Acknowledgements
The authors thank Dr Dai Liu and Dr Shengnan Zhu in
the Department of Cardiology of the First Affiliated Hospital of
Dalian Medical University for animal care, cell isolation and
culture. This study was supported by the National Natural Science
Foundation of China (81100220 and 8167020454).
References
|
1
|
Libby P: Inflammation in atherosclerosis.
J Associat Physicians India. 48:265–266. 2012.
|
|
2
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bobryshev YV and Watanabe T: Subset of
vascular dendritic cells transforming into foam cells in human
atherosclerotic lesions. Cardiovasc Pathol. 6:321–331. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chistiakov DA, Sobenin IA, Orekhov AN and
Bobryshev YV: Dendritic cells in atherosclerotic inflammation: The
complexity of functions and the peculiarities of pathophysiological
effects. Front Physiol. 5:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi J, Do Y, Cheong C, Koh H, Boscardin
SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG and Steinman RM:
Identification of antigen-presenting dendritic cells in mouse aorta
and cardiac valves. J Exp Med. 206:497–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koltsova EK and Ley K: How dendritic cells
shape atherosclerosis. Trends Immunol. 32:540–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Son YI, Egawa S, Tatsumi T, Redlinger RE
Jr, Kalinski P and Kanto T: A novel bulk-culture method for
generating mature dendritic cells from mouse bone marrow cells. J
Immunol Methods. 262:145–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paulson KE, Zhu SN, Chen M, Nurmohamed S,
Jongstra-Bilen J and Cybulsky MI: Resident intimal dendritic cells
accumulate lipid and contribute to the initiation of
atherosclerosis. Circ Res. 106:383–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rong JX, Shapiro M, Trogan E and Fisher
EA: Transdifferentiation of mouse aortic smooth muscle cells to a
macrophage-like state after cholesterol loading. Proc Natl Acad Sci
USA. 100:13531–13536. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling GS, Bennett J, Woollard KJ, Szajna M,
Fossati-Jimack L, Taylor PR, Scott D, Franzoso G, Cook HT and Botto
M: Integrin CD11b positively regulates TLR4-induced signalling
pathways in dendritic cells but not in macrophages. Nat Commun.
5:30392014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chávez-Sánchez L, Espinosa-Luna JE,
Chávez-Rueda K, Legorreta-Haquet MV, Montoya-Díaz E and
Blanco-Favela F: Innate immune system cells in atherosclerosis.
Arch Med Res. 45:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Macritchie N, Grassia G, Sabir SR,
Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M,
McInnes IB, Brewer JM, et al: Plasmacytoid dendritic cells play a
key role in promoting atherosclerosis in apolipoprotein E-deficient
mice. Arterioscler Thromb Vasc Biol. 32:2569–2579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weeks MF, Rampersad RR and Liu P:
Dendritic cells and macrophages contribute differently to the
initiation of atherosclerosis. Circulation. 126:A157152012.
|
|
18
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mempel TR, Henrickson SE and Von Andrian
UH: T-cell priming by dendritic cells in lymph nodes occurs in
three distinct phases. Nature. 427:154–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gautier EL, Huby T, Saint-Charles F,
Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum
JL, Chapman MJ and Lesnik P: Conventional dendritic cells at the
crossroads between immunity and cholesterol homeostasis in
atherosclerosis. Circulation. 119:2367–2375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glass CK and Witztum JL: Atherosclerosis:
The road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamada M, Nakamura M, Tran MT, Moriguchi
T, Hong C, Ohsumi T, Dinh TT, Kusakabe M, Hattori M, Katsumata T,
et al: MafB promotes atherosclerosis by inhibiting foam-cell
apoptosis. Nat Commun. 5:31472014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SW, Qiao SB, Yuan JS and Liu DQ:
Association of plasma visfatin levels with inflammation,
atherosclerosis and acute coronary syndromes (ACS) in humans. Clin
Endocrinol (Oxf). 71:202–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Kleijn D and Pasterkamp G: Toll-like
receptors in cardiovascular diseases. Cardiovasc Res. 60:58–67.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayashi C, Papadopoulos G, Gudino CV,
Weinberg EO, Barth KR, Madrigal AG, Chen Y, Ning H, LaValley M,
Gibson FC III, et al: Protective role for TLR4 signaling in
atherosclerosis progression as revealed by infection with a common
oral pathogen. J Immunol. 189:3681–3688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Subramanian M, Thorp E, Hansson GK and
Tabas I: Treg-mediated suppression of atherosclerosis requires
MYD88 signaling in DCs. J Clin Invest. 123:179–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alroqi FJ and Chatila TA: T regulatory
cell biology in health and disease. Curr Allergy Asthma Rep.
16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|