Introduction
Prostate cancer is one of the most common frequent
malignant tumor in urinary system. Its morbidity and mortality are
higher in developing countries than those of developed countries.
In spite of the decrease in mortality due to the improvements in
early detection and treatments, it is still the second leading
cause of cancer death. The majority of patients are diagnosed with
clinically localized cancer which can be treated effectively with
radical prostatectomy, radiotherapy or androgen deprivation
therapy. However, advanced androgen dependent prostate cancer
patients, which were treated with androgen deprivation therapy,
almost invariably progressed to castration-resistant prostate
cancer stage with the poor prognosis and high mortality. Therefore,
it's important to expedite the development of effective therapeutic
targets for prostate cancer.
T-box gene family is a transcription factor that
plays an important role in the regulation of embryonic development
and organ formation. TBX2 gene is one of the members of T-BOX gene
family. TBX2 gene is involved in the formation of a variety of
tissues and organs as well, including the heart, lungs, kidney,
limb buds, mammary glands, gastrulation and cranial structures.
TBX2 have been shown to function as transcriptional repressors.
Recent studies have found that TBX2 gene has abnormal expression in
many kinds of malignant tumors, and is associated with the cell
proliferation, apoptosis and cell invasion. In breast cancer, TBX2
displays high expression in a cohort of primary breast cancers.
Increased TBX2 expression is associated with metastases, and tumors
with altered TBX2 expression also show poor overall survival
(1). Simultaneously, the study has
demonstrated that TBX2 is a strong cell-autonomous inducer of the
epithelial-mesenchymal transition (EMT), and in the malignant human
breast carcinoma cell lines MDA-MB-435 and MDA-MB-157, down
regulation of endogenous TBX2 expression leads to a restitution of
epithelial characteristics with reciprocal loss of mesenchymal
markers and then inhibition of capacity of tumor cell migration and
invasion (2). In colorectal
cancer, the results showed significant correlations between tumor
TBX2 overexpression and pTstage, distant metastasis, advanced AJCC
stage and relapse. These data suggested that up regulation of TBX2
expression might contribute to tumor invasion and metastasis
(3). In human melanoma cells, the
activity of endogenous TBX2 are critically required to maintain
proliferation and suppress senescence (4). Similarly, the recent study indicated
that in contrast to normal tissues, TBX2 was involved in the
carcinogenesis of endometrial adenocarcinoma, and was also
associated with lymph node metastasis. Therefore, it might be a
candidate therapeutic target for treatment of endometrial
adenocarcinoma (5).
However, the role of TBX2 gene in prostate cancer
cells remains largely unknown. In our study, to explore whether
TBX2 participates in the development and progression of prostate
cancer, small interfering RNA (siRNA) targeted on TBX2 were
transfected to silence the TBX2 expression in prostate cancer PC3
and LNCaP cells, and then the changes of biological function after
decrease in expression of TBX2 were examined.
Materials and methods
Cell cultures
The human prostate cancer cell lines PC3 and LNCaP
were purchased from Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). Two kinds of Cells were both grown in
RPMI-1640 (HyClone, Logan, UT, USA) medium supplemented with 10%
fetal bovine serum (FBS; Invitrogen, Shanghai, China) and 1%
penicillin-streptomycin (Beyotime Biotech, Jiangsu, China). Cells
were kept at 37°C in a humidified atmosphere with 5%
CO2.
Antibodies and reagents
TBX2 polyclonal antibodies were purchased from
Bioworld Technology, Inc., St. Louis Park, MN, USA. Polyclonal
mouse antibodies against E-cadherin, N-cadherin, Vimentin and
Fibronectin were purchased from BD Biosciences (Franklin Lakes, NJ,
USA). Snail and Twist polyclonal rabbit antibodies were purchased
from ABclonal Biotechnology Co., Ltd., Cambridge, MA, US. Mouse
monoclonal antibody against Mdm2, p53, Bax, Akt, p-Akt, VEGF and
polyclonal rabbit antibodies against Bcl-2 and β-actin were all
purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. The
secondary antibodies including goat anti-rabbit IgG and goat
anti-mouse IgG were obtained from Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China. The standard of dilution
was referenced in accordance to the antibody specification. TBX2
siRNA and negative control siRNA were designed, synthesized by
GenePharma (Suzhou, China). Cell proliferation was measured by Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology,
Nantong, China). Cell migration and Cell invasion ability were
tested using transwell chambers (Corning Incorporated, Corning, NY,
USA). Cell apoptosis was assessed by Annexin V-FITC/propidium
iodide (PI) Apoptosis Detection kit (KeyGen, Nanjing, China).
SiRNA and transfection
The cells were divided into two groups: Transfection
of TBX2 silenced by TBX2 siRNA as the experimental interference
(TBX2 siRNA) group and transfection of negative control siRNA as
the negative control group. When PC3 and LNCaP Cells cultured
regularly were grown at a density of 60–70%, TBX2 siRNA and
negative control siRNA were respectively transfected into cells
with serum-free medium by SiLentFect™ Lipid Reagent according to
each reagent instruction. Six hours later, serum-free medium
containing SiLentFect™ Lipid Reagent was removed from petri dish.
The medium was replaced with the complete medium, and continued to
culture for 48 h, cells were gathered for the next assays.
Western blot analysis
Two kinds of Prostate cancer cells were collected
after transfection for 48 h and washed three times with
phosphate-buffered saline (PBS). Then by using cell lysis buffer
the proteins were extracted at 4°C at 15,000 g for 5 min. The total
protein concentration was determined by BCA protein assay kit
(Beyotime Biotechnology). Equal amounts of protein were separated
on 15% sodium dodecyl sulfate-polyacrylamide gelelectrophoresis
(SDS-PAGE; Beyotime Biotechnology), and then transferred onto
nitrocellulose filter membranes (Millipore, Billerica, MA, USA).
After the membranes were blocked in 5% non-fat milk for 2 h at room
temperature, they were incubated with corresponding primary
antibodies with following dilution and β-actin antibody as internal
reference at 4°C overnight (TBX2 1:500 dilution; E-cadherin,
N-cadherin 1:2,000 dilution; Vimentin 1:5,000 dilution; Fibronectin
1:8,000 dilution; Snail, Twist 1:1,200 dilution; MDM2, Bcl-2, p53,
Bax, 1:200 dilution; p-Akt, Akt 1:100 dilution; VEGF 1:200
dilution). After the completion of the previous step, the membranes
were washed with washing buffer 3 times for 15 min, and then
incubated with secondary antibody (β-actin 1:10,000 dilution) at
room temperature. The membranes were washed with washing buffer
adding Tween-20 3 times again 2 h later. Finally, the bound
antibodies were visualized by an enhanced chemiluminescence (ECL)
reagent (Tanon Science and Technology Co., Ltd., Shanghai, China)
and analysed using ImageJ software. This assay was performed in
triplicate.
Cell proliferation assay
Two kinds of prostate cancer cells selected in
logarithmic growth phase were seeded into 96-well plates with 100
µl medium and continued to grow at 37°C. PC3 cells were incubated
with 4×103 cells per well, and LNCaP cells were
incubated with 5×103 cells per well. When the cells were
cultured at 24, 48, and 72 h, 10 µl CCK-8 solutions were added into
every well, followed by cultivation at 37°C for 2 h. The optical
density (OD) values were determined at 450 nm through an ELX-800
spectrometer reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
All six wells were tested in each experimental group, and this
assay was performed in triplicate.
Cell migration assay
Two kinds of prostate cancer cells were gathered 48
h after transfection and then plated into the upper chamber. PC3
cells were at a density of 5×104 in 200 µl per well, and
LNCaP cells were 10×104 per well. Meanwhile, 600 µl
RPMI-1640 medium including 10% FBS was added into the lower
chamber. After 24 h cultivation, the medium in the upper chamber
was removed and the cells on the side of the membrane were
carefully wiped with cotton swabs. The cells that had been located
at the lower surface of membrane were fixed with paraformaldehyde
for 30 min, and then stained by crystal violet for 15 min. After
the completion of the above operations, the upper chamber was
washed using PBS, and the residual cells on the side of the
membrane were wiped again. At last, the cells migrating through the
chamber were photographed and counted (×200 magnifications). The
average number of migrated cells was determined by counting four
random areas of every chamber. This assay was repeated three
times.
Cell invasion assay
Before conducting this assay, every upper chamber
was precoated with 40 µl Matrigel (BD Biosciences) diluted by
serum-free medium (1:7 dilution). The next day, two kinds of
prostate cancer cells were gathered and seeded into the upper
transwell chamber with 600 µl RPMI-1640 medium containing 10% FBS.
The remaining experimental procedure was the same as the migration
assay. This assay too was repeated three times.
Cell apoptosis assay
After transfection with TBX2 siRNA and negative
control siRNA for 48 h, two kinds of prostate cancer cells were
gathered, centrifuged, and resuspended in 500 µl binding buffer.
Annexin V-FITC (5 µl) and PI (5 µl) were added and incubated at
room temperature in the dark for 15 min, and then this assay was
conducted within 1 h on a flow cytometry (BD Biosciences).
Vascular tube formation assay
The 80 µl Matrigel was thawed and taken into 48-well
plates evenly. After being incubated at 37°C for 1 h, human
umbilical vein endothelial cells (HUVECs) were resuspended using
the supernatant containing TBX2 siRNA or negative control siRNA
were transferred to the 48-well plates with a density of
4×104 per well respectively, and then continued to be
incubated for 6 h at 37°C. The cells were photographed to observe
tube formation. Tube formed was quantified by counting the number
of tube formed in every well.
Patients and samples
Fifty-three archived formalin-fixed,
paraffin-embedded prostate cancer tissue specimens and tumor
adjacent tissue specimens were collected in The Affiliated Hospital
of Xuzhou Medical University during 2011–2016, and all of these
cases were confirmed by pathological examination. None of the
patients received preoperative adjuvant chemotherapy or
radiotherapy. The age of all patients ranged from 50 to 77 (the
mean age 67.81±6.09). The patients were staged according to the
2012 American Association of Cancer (AJCC) TNM staging system: 6
were stage I, 27 were stage II, 17 were stage III, 3 were stage IV.
Pathology was graded according to the Gleason grading system with
the ISUP 2005 modification: Well differentiation (Gleason2-6): 19
cases; moderate differentiation (Gleason7): 23 cases; poor
differentiation (Gleason8-10): 11 cases. Other histological cell
types were not included in our study.
Immunohistochemistry
Immunoperoxidase tissue sections were dewaxed for 2
h at 65°C, and then washed with xylene for 30 min. The sections
were blended in 100, 95, 85, 75 and 50% ethanol in sequence. With
the help of a microwave, citrate was heated to boiling, and then
the sections were placed in it for 2 min at high heat and 8 min at
medium heat. When the above operation was completed, citrate buffer
needed to be cooled down to room temperature. Then after being
washed with PBS for 15 min, hydrogen peroxide was added to the
sections in order to block endogenous peroxidase activity. 30 min
later, the sections were incubated with TBX2 polyclonal antibody
(1:50 dilution) at 4°C overnight after being washed with PBS again.
The slices were rewarmed at room temperature, and then incubated
with secondary antibody and streptavidin-peroxidase for 30 min
successively. After being washed with PBS, the slices were stained
by DAB, counterstained with hematoxylin, and mounted with neutral
balsam. At last, the result of each section was evaluated by light
microscopy. Evaluation of immunohistochemical staining: Staining
intensity (0: no color; 1: light yellow; 2: brownish yellow; 3:
chocolate brown) and the percent of positive cells (0: <10%; 1:
11–30%; 2: 30–60%; 3: >60%), and the summation of the two gave
the final score (−: 0–1; +: 2–4; ++: 5–8; +++: 9).
Statistical analysis
The data were expressed as mean ± standard deviation
(SD). Statistical analyses were conducted via independent samples t
test using the software SPSS version 19.0 (SPSS, Chicago, USA). The
relationship between TBX2 staining and clinicopathologic parameters
of the patients was assessed by χ2 test and exact
probability method. Every assay was carried out at least three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
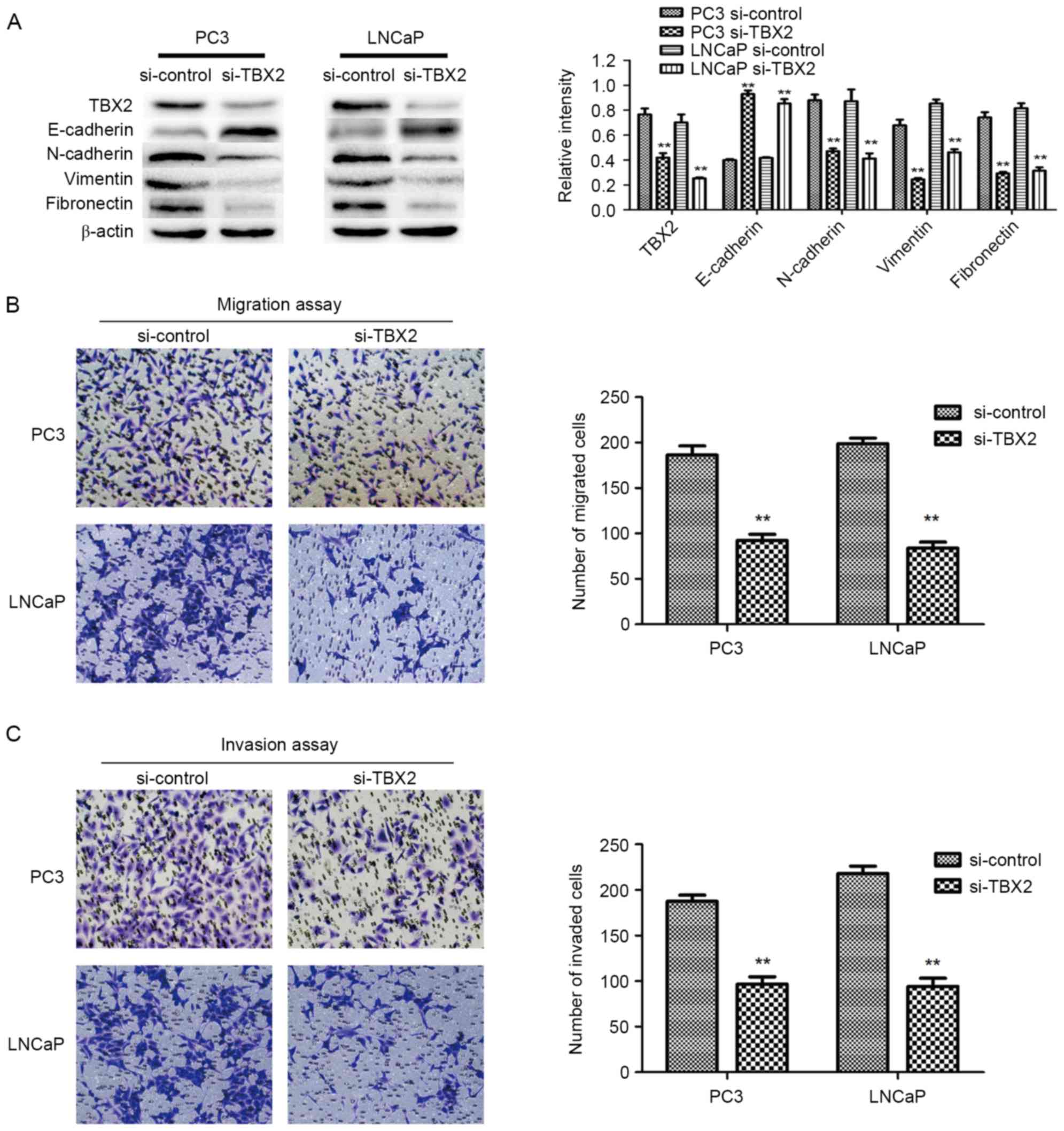
TBX2 siRNA decreases the expression of
TBX2 in PC3 and LNCaP cells
The expression of TBX2 after transfection of TBX2
siRNA in PC3 and LNCaP cells was measured by western blot analysis.
The results demonstrated that the protein expression of TBX2 was
markedly reduced after silencing of TBX2 in prostate cancer cell
lines (P<0.01; Fig. 1A).
Silencing of TBX2 represses the
migration and invasion of prostate cancer cells
In order to evaluate the capacities of both two
types of prostate cancer cells after knockdown of TBX2, Transwell
migration and invasion assays were conducted. The migration results
showed that the number of migrated cells in experimental
interference (TBX2 siRNA) group were significantly reduced than
that in the negative control group (P<0.01; P<0.01; Fig. 1B). Meanwhile, the invasion assay
showed the similar results that the number of cells that penetrated
through the matrigel-coated chambers in the TBX2 siRNA group were
less than that in the negative control group (P<0.01; P<0.01;
Fig. 1C). Based on the above data,
we believed that silencing of TBX2 inhibited the abilities of
migration and invasion of PC3 and LNCaP cell lines.
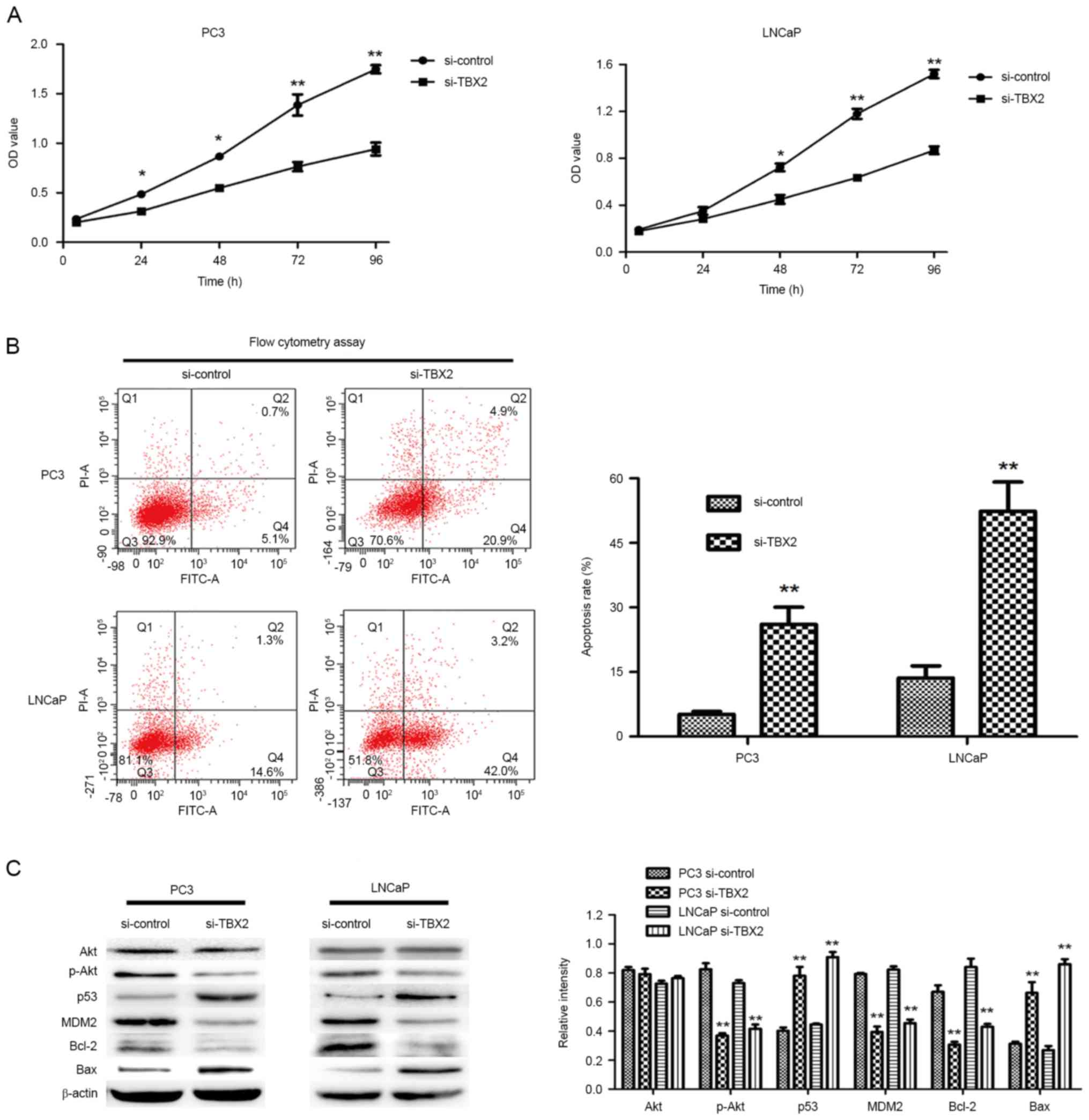
Downregulation of TBX2 inhibits the
proliferation and promotes the apoptosis of prostate cancer
cells
In order to evaluate the effect of TBX2 siRNA on
cell growth, we performed the CCK-8 assay. Compared with the
negative control group, the ability of cell proliferation in TBX2
siRNA group was much weaker (Fig.
2A). The results suggested that silencing of TBX2 inhibited the
proliferation of PC3 and LNCaP cells. Meanwhile, in order to
determine whether the reduced proliferation was due to the increase
of apoptosis, we carried out cell apoptosis assay by flow cytometry
analysis. The assay results indicated that cells in TBX2 siRNA
group had higher apoptosis rate in both two types of prostate
cancer cells (P<0.01, P<0.01, Fig. 2B). Therefore, the above results
suggested that knockdown of TBX2 promoted prostate cancer cell
senescence.
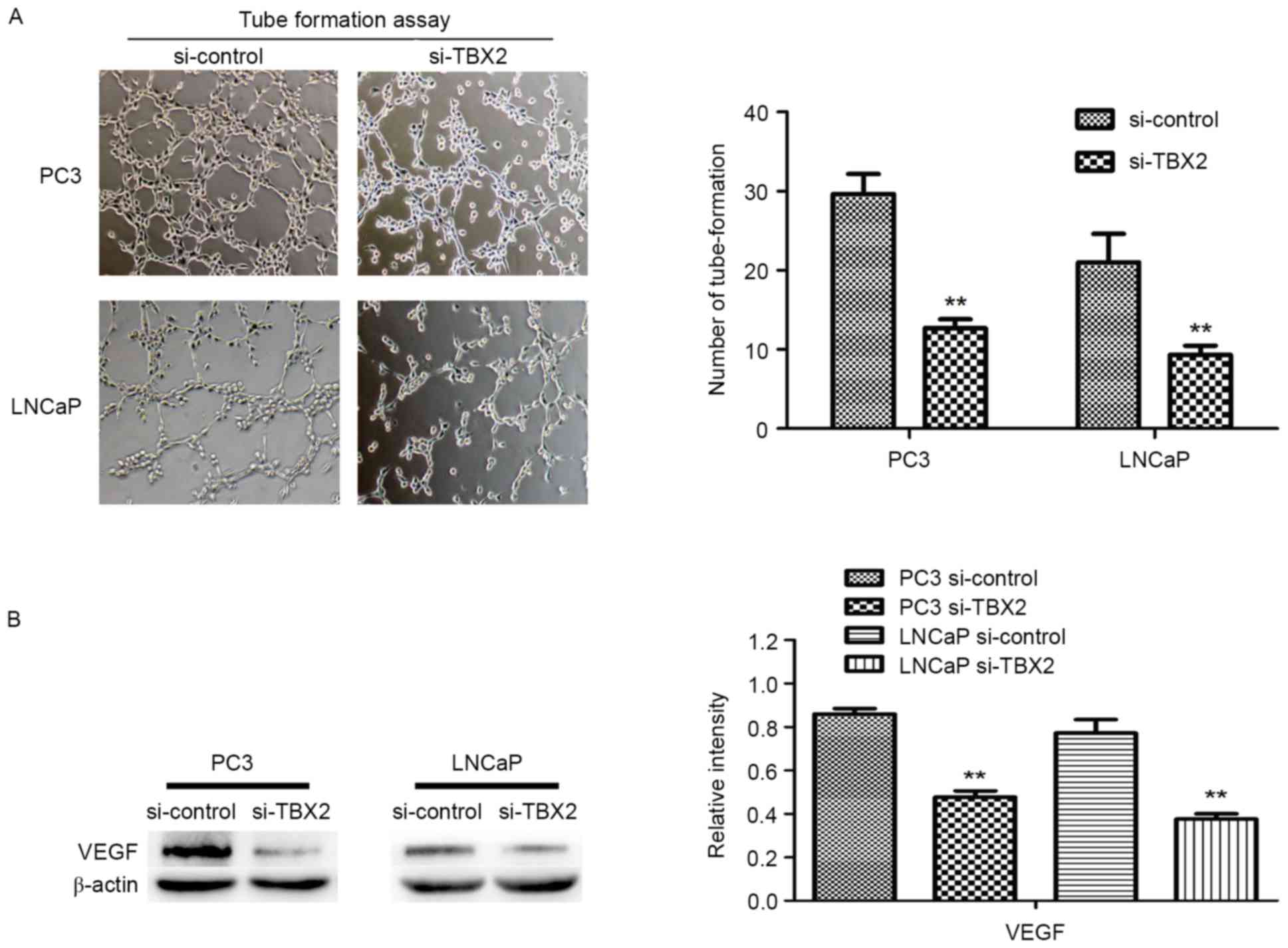
Downregulation of TBX2 inhibits the
tube formation of HUVECs
To evaluate effect of TBX2 siRNA on tube formation,
we performed the vascular tube formation assay. Compared to the
negative control siRNA, HUVECs with TBX2 siRNA showed a markedly
reduction in the number of tube formed (P<0.01, P<0.01,
Fig. 3A). Then we used western
blot analysis to determine the expression of VEGF in both two kinds
of prostate cancer cells. The results showed that VEGF was
decreased in TBX2 siRNA group, which indicated that low expression
of VEGF, caused by silencing of TBX2 exerted an effect on
endothelial cells (P<0.01; Fig.
3B).
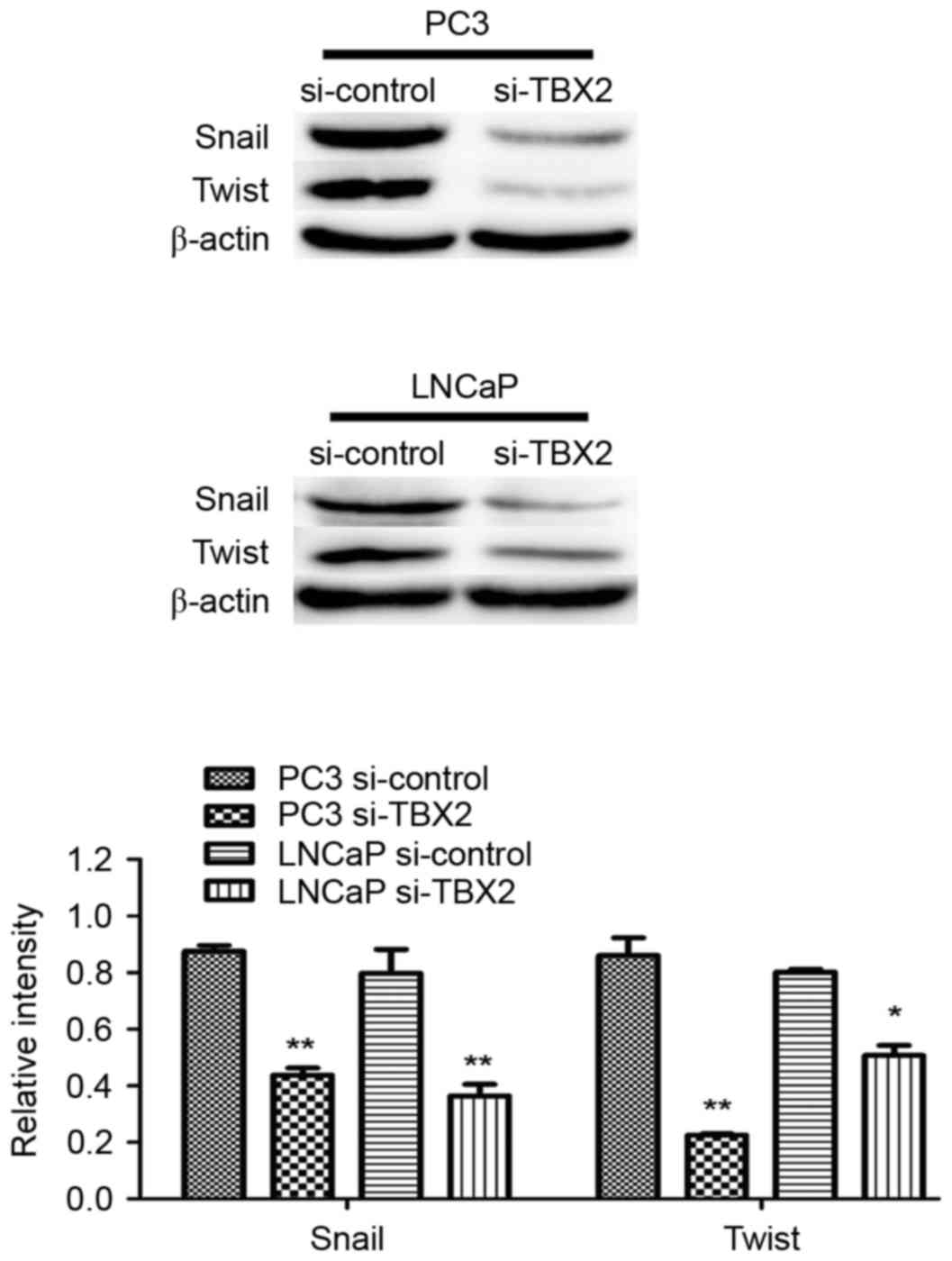
Silencing of TBX2 blocks the process
of EMT in part through downregulation of Snail/Twist in the
prostate cancer cells
Based on fore-mentioned views, we performed the
increasing studies, in which knockdown of TBX2 had effect on EMT
that played a crucial role in tumor metastasis in prostate cancer
cells. Western blot analysis showed that epithelial biomarker
E-cadherin protein expression in the experimental interference
(siRNA) group was significantly higher than that in the negative
control group, while the mesenchymal biomarkers N-cadherin,
Vimentin and Fibronectin in TBX2 siRNA group were lower compared
with that of the negative control group (Fig. 1A). the expression of EMT related
transcription factors Snail and Twist in the experimental
interference (TBX2 siRNA) group was significantly reduced compared
with the negative control group. There were significant differences
between TBX2 siRNA group and negative control group both in two
types of prostate cancer cell lines (Fig. 4A).
Silencing of TBX2 impacts on the
expression of the proteins related in PI3K/AKT signaling
pathway
Increasing studies have demonstrated that PI3K/AKT
signaling plays a critical function during cancer cell
proliferation and cell apoptosis, so we detected the expressions of
Akt, MDM2, p53, Bax and Bcl-2 via silencing of TBX2 in PC3 and
LNCaP cells. Western blot analysis showed that TBX2 knockdown led
to the lower expression of p-Akt MDM2 and Bcl-2, while the increase
of p53 and Bax. however, There was no significant difference in
protein level of Akt between the two groups (Fig. 2C). These findings revealed that
TBX2 might be involved in cell proliferation and apoptosis in
prostate cancer cells.
Expression of TBX2 in prostate cancer
tissues and correlations of clinical stage and pathological grade
in prostate cancer
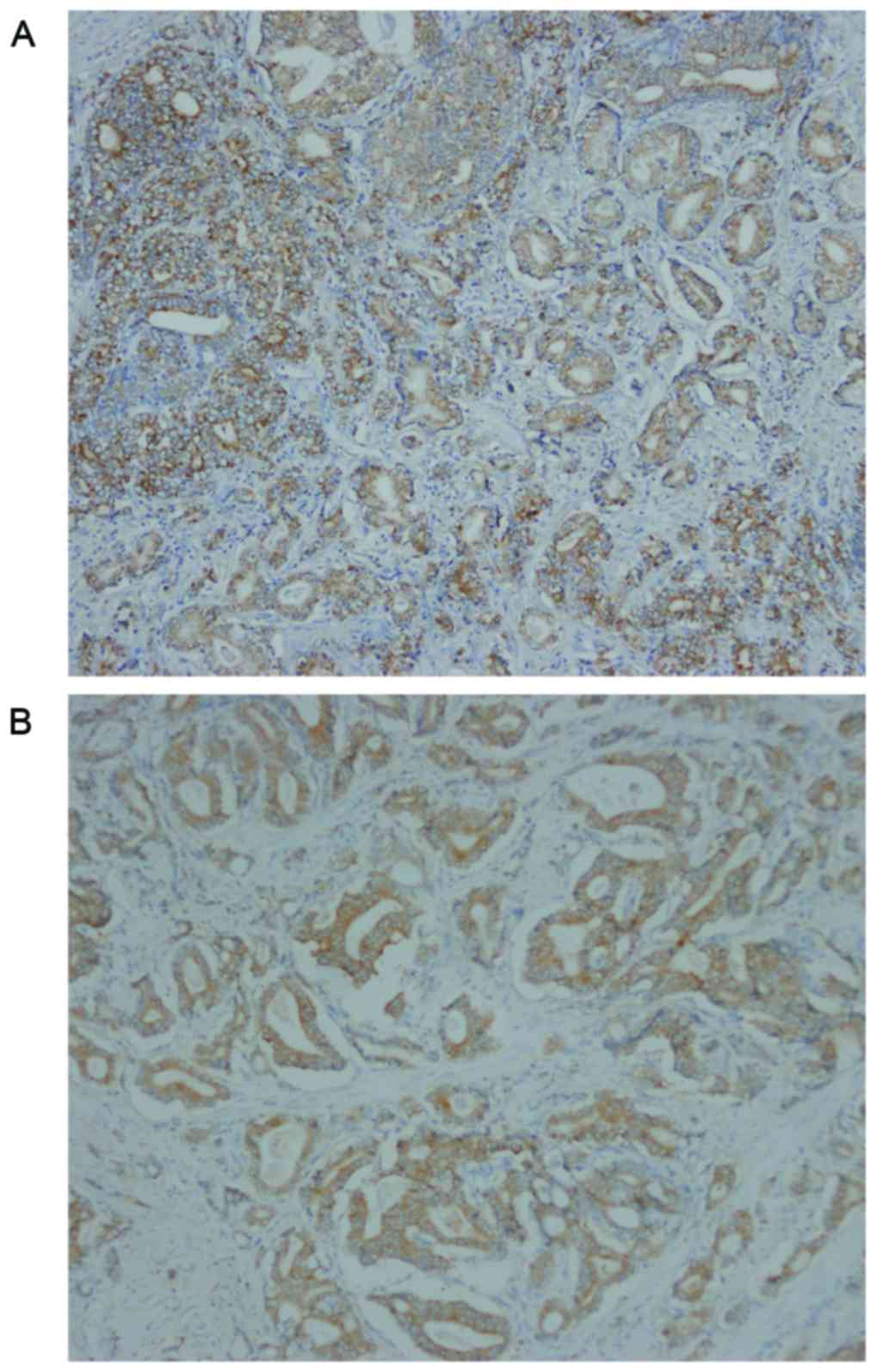
TBX2 was located in the cytoplasm of prostate cancer
cells (Fig. 5). The expression
rates of TBX2 were 75.47% (40/53) in prostate cancer tissue, and
were significantly higher than these of tumor adjacent tissue. A
valuable difference in TBX2 positive staining was discovered
between prostate cancer tissue and tumor adjacent tissue (Table I). Investigation of the
relationship between TBX2 expression and clinicopathologic
parameters were summarized in Table
II. In prostate cancer, patients with poor differentiation
showed more frequent expression of TBX2 than those with well and
moderate differentiation. Meanwhile, TBX2 expression was positively
correlated with clinical stage. However, TBX2 had no association
with age and PSA (Table II).
 | Table I.The expression rates of TBX2 in
prostatic cancer tissue and tumor adjacent tissue. |
Table I.
The expression rates of TBX2 in
prostatic cancer tissue and tumor adjacent tissue.
|
|
| TBX2 |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Patients, n | + | − | χ2 | P-value |
|---|
| Prostatic cancer
tissue | 53 | 40 | 13 | 12.589 | <0.01 |
| Tumor adjacent
tissue | 53 | 22 | 31 |
|
|
 | Table II.Clinicopathological parameters for
TBX2 in prostatic cancer. |
Table II.
Clinicopathological parameters for
TBX2 in prostatic cancer.
|
|
| TBX2 |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathologic
parameters | Patients, n | + | − | χ2 | P-value |
|---|
| Age, years |
|
|
|
| 1.000 |
|
<67 | 18 | 14 | 4 | 0.003 |
|
| ≥67 | 35 | 26 | 9 |
|
|
| Clinical stage
(TMN) |
|
|
|
| 0.025 |
| I,
II | 33 | 21 | 12 | 5.031 |
|
| III,
IV | 20 | 19 | 1 |
|
|
| Pathological
grade |
|
|
|
| 0.047a |
| Well
and moderate | 42 | 29 | 13 |
|
|
|
Poor | 11 | 11 | 0 |
|
|
| PSA (ng/ml) |
|
|
|
| 0.331 |
|
<10 | 13 | 8 | 5 | 0.947 |
|
|
≥10 | 40 | 32 | 8 |
|
|
Discussion
As one of the members of T-box gene family, TBX2 has
been shown to play a critical role in embryonic development and
organogenesis according to previous studies. Due to the
overexpression of TBX2 in embryonic development, it can guide
embryonic and tissue axial development, and play an inhibitory role
in the process of regulation of development. Besides, the recent
researches have explained TBX2 can be involved in cell cycle
regulation, cell apoptosis and aggressive tumor growth through a
variety of signaling pathways, as well as participate in EMT.
Firstly, TGF-β1 pathway has the ability to inhibit the
proliferation of epithelial cells but promote their migration.
Baldwin et al (6) have
demonstrated that the TGF-β1-mediated growth arrest could be
bypassed by TBX2 overexpression. While the following study has
shown that the downregulation of TBX2 through the direct binding of
TBX3 to a half T-element in the TBX2 promoter, or the de-repression
of the TBX2 target gene, p21, activates TGF-β1 signaling pathway to
exert its anti-proliferative effects (7). Furthermore,
p19Arf-MDM2-p53 pathway is an important axis in
vivo which is associated with cell senescence. TBX2 represses
transcription from the Arf tumor suppressor promoter, which
decreases p53 activity by dampening the ability of
p19Arf (8), and
consequently bypasses the normal senescence default mechanisms to
restrain tumor cell apoptosis (9).
In addition, E-cadherin, as a tumor suppressor, whose loss is
implicated in EMT and metastatic tumor progression, is also a
direct TBX2 target gene. The previous study has confirmed that
RNAi-mediated silencing of TBX2 in two kinds of aggressive human
breast carcinoma cell lines lead to re-expression of E-cadherin,
and the concomitant loss of mesenchymal N-cadherin, Vimentin, and
Fibronectin expression, which accordingly inhibit tumor cell
migration, invasion, and EMT processes (2).
Based on the above data about its effects on cell
migration, invasion and apoptosis in various cancers, we assumed
that TBX2 gene also had the effect on cell migration, invasion and
apoptosis in prostate cancer. Prior to this there were no
researches about the TBX2 gene in prostate cancer including PC3 and
LNCaP cells. Therefore, in order to determine the expression of
TBX2 gene in PC3 and LNCaP cells, TBX2 siRNA and negative control
siRNA were transfected into two types of prostate cancer cell lines
respectively. Western blot analysis showed that the protein level
of TBX2 was obviously repressed in TBX2 siRNA group, which
demonstrated that the expression of TBX2 was effectively inhibited
in cells transfected with TBX2 siRNA in PC3 and LNCaP cells.
However, then we used the immunostaining to detect the expression
of TBX2 in prostate cancer tissue and tumor adjacent tissue. The
results showed that the expression rates of TBX2 in prostate cancer
tissue were markedly higher than these in tumor adjacent tissue.
Furthermore, TBX2 expression was correlated with clinical stage and
pathological grade. In other words, the TBX2 expression was higher
than in patients with poor differentiation or high clinical stage.
The positive expression rates of TBX2 increased with the decrease
of Gleason Score. On one hand, this might be due to the inhibition
of p19Arf by TBX2 expression blocked cell senescence
(10). On the other hand, the
interference of PRb transcription via the expression of TBX2 might
be influence on the normal operation of cell cycle and
differentiation (11). Clinical
staging is one of the important indexes, which could judge the
prognosis of cancer sufferers. The patients with high clinical
stage has greater probability of distant metastasis and poor
prognosis. Our studies revealed that the activation of TBX2 gene
might occur in the terminal stage of prostate cancer, which made it
easier for prostate cancer to develop in the distance and increased
the ability of invasion to progress toward a more malignant
direction. Therefore, TBX2 gene has the remarkable characteristics
of oncogene and is closely related to tumor invasion, which
provides a new key factor for the prognosis of prostate cancer.
Then we preliminarily conducted the cell
proliferation assay by CCK-8 in order to test the cell
proliferation among experimental interference (TBX2 siRNA) group
and negative control group in the first place. The assay revealed
that silencing of TBX2 led to reducing the ability of prostate
cancer cell proliferation. Simultaneously, in order to determine
the effect of downregulation of TBX2 gene for prostate cancer cell
apoptosis, we also performed cell apoptosis assay by flow
cytometry. The result showed that silencing TBX2 expression
heightened apoptosis rate in both two kinds of prostate cancer
cells. Next, we checked potential target genes involved in
apoptosis of tumor cells in order to interpret the above
experimental results and regulatory mechanisms of apoptosis caused
by knockdown of TBX2 expression. We revealed that Bcl-2, MDM2 and
p-Akt were downregulated, while Bax, p53 were upregulated. Bcl-2
family includes anti-apoptotic proteins such as Bcl-2, Bcl-XL, and
pro-apoptotic proteins such as Bax, Bad, Bid. The Bcl-2 family
regulates apoptosis through Bcl-2 as an inhibitor or Bax as an
activator (12,13). In previous studies, we have
confirmed Bcl-2 and Bax, whose activity is responsible for cell
apoptosis (14), is dysregulated
in many cancers (15). In our
research, we got similar results that TBX2 siRNA might regulate
Bcl-2 and Bax to reduce the ability of cell apoptosis in prostate
cancer. Furthermore, as we know, PI3K/AKT signaling plays a
critical function during cancer cell proliferation and cell
survival as well as invasiveness, angiogenesis and metastasis
(16,17). Akt activation might be a crucial
promoter, not only leading to regulate other gene expression, but
inhibiting cancer cell senescence and improving its growth.
Phosphorylation of Akt and inhibition of androgen receptor via
activation of Akt block the apoptosis of androgen dependent
prostate cancer cells (18).
Conditional activation of Akt has also been proved to promote the
occurrence of androgen independent prostate cancer (19). MDM2, which participates in an
auto-regulatory loop with p53, has been shown to function as a
ubiquitin ligase that targets p53 for degradation. However, the
activation and stabilization of p53 have also influence on function
of MDM2, where any deletions in the pathways are determined to
affect MDM2 expression in the cancer cells (20). Our results revealed that TBX2 might
be a contributor, which directly regulated phosphorylation of Akt.
On one hand, low expression of p-Akt by silencing of TBX2 gene
inhibited MDM2 activation, which sequentially enhanced the ability
of p53, and then promoted cancer cell senescence. On the other
hand, we showed that knockdown of TBX2 gene initiated cell
senescence mechanism and inhibited cell progression through
inhibition of phosphorylation of Akt, whose activity directly
promoted Bax expression.
The epithelial-mesenchymal transition is major
mechanism that is related to cancer cell invasion and metastasis.
It has reported that when the EMT process occurs, the phenotype of
epithelial cells is transformed into the phenotype of mesenchymal
cells, which weakens the ability of cell-cell adhesion, and then
invades adjacent tissues to form metastases (21). It is marked by decreased expression
of epithelial marker such as E-cadherin, while increased expression
of mesenchymal markers such as N-cadherin, Vimentin and
Fibronectin. Wang et al (2)
has clarified that TBX2 was related to invasion, migration and
regulation of the progression of EMT in the development of
malignant breast epithelial cells. Based on above results, we
designed a similar experiment firstly in prostate cancer PC3 and
LNCaP cells, and obtained similar results. We revealed that
silencing of TBX2 increased E-cadherin expression and decreased
N-cadherin, Vimentin and Fibronectin. In the meanwhile, cell
migration and invasion assay also revealed that the number of cells
of migration and invasion in the TBX2 siRNA group were
significantly lower than that in the negative control group
respectively. Our study indicated that TBX2 might be a target gene
that directly regulated the expression of E-cadherin, and TBX2
knockdown was involved in the progression of EMT and reversed it
through increasing E-cadherin expression.
In the increasing studies, we tried to determine
whether TBX2 knockdown had any effect on expression of Snail and
Twist, which both played a crucial role in EMT. Snail, which is
essentially a transcription repressor, is a major regulatory
protein in EMT. By combining with the E-Box element located at
E-cadherin promoter, Snail can directly inhibit the expression of
E-cadherin, and then EMT can be induced to enhance cancer invasion
and metastasis (22). Twist has
been described as an oncogene due to its anti-apoptotic effects,
but in recent studies, it has reported that Twist is highly
expressed in various epithelial, mesenchymal and hematological
malignant tumors, and is closely related to cell differentiation,
lymph node metastasis and poor prognosis (23,24).
Twist has a similar mechanism of action with Snail. The deletion of
Twist can lead to the loss of stem cells in tumor cells, which
significantly inhibits the ability to initiate tumor (23). Consistent with these findings, we
found that the protein expression of Snail and Twist markedly
decreased in TBX2 siRNA group, which suggested that silencing of
TBX2 might interfere with Snail/Twist signaling pathway. After TBX2
expression was knocked down in TBX2 siRNA group, Snail and Twist
might have been unable to combine with the E-Box element of
E-cadherin promoter, making it unable to initiate EMT, or even
reverse EMT.
Neoangiogenesis, the formation of new blood vessels,
is stimulated through growth factors, such as vascular endothelial
growth factor, VEGF. These factors cause endothelial cells into the
tumor tissue, where they form new capillaries to support tumor
progression (25). In recent
studies, we found that the function of VEGF might not only be
limited to angiogenesis, but also play an important role in the
survival, proliferation and migration of tumor cells (26). Therefore, we used western blot
analysis to measure the VEGF expression level both in TBX2 siRNA
group and negative control group. The result showed that the
expression of VEGF was downregulated via silencing of TBX2 in TBX2
siRNA group, which indicated that downregulation of VEGF expression
inhibited the formation of new blood vessels, and then prevented
tumor progression.
Taken together, our assays revealed that TBX2 gene
may be a promoter of malignant tumor through induction of EMT
process and inhibition of cell senescence. Specifically, in our
studies downregulation of TBX2 suppressed the migration, invasion,
proliferation, as well as EMT process, while promoting the
apoptosis in PC3 and LNCaP cells. Meanwhile, according to the high
expression of TBX2 in prostate cancer, TBX2 can help improve the
accuracy in the clinical diagnosis of prostate cancer. The present
study firstly demonstrated the effect of TBX2 gene in the prostate
cancer, and based on our results, TBX2 might be a potential
beneficial therapeutic target for prostate cancer treatment.
References
|
1
|
D'Costa ZC, Higgins C, Ong CW, Irwin GW,
Boyle D, McArt DG, McCloskey K, Buckley NE, Crawford NT,
Thiagarajan L, et al: TBX2 represses CST6 resulting in uncontrolled
legumain activity to sustain breast cancer proliferation: A novel
cancer-selective target pathway with therapeutic opportunities.
Oncotarget. 5:1609–1620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang B, Lindley LE, Fernandez-Vega V,
Rieger ME, Sims AH and Briegel KJ: The T box transcription factor
TBX2 promotes epithelial-mesenchymal transition and invasion of
normal and malignant breast epithelial cells. PLoS One.
7:e413552012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han Y, Tu WW, Wen YG, Yan DW, Qiu GQ, Peng
ZH and Zhou CZ: Increased expression of TBX2 is a novel independent
prognostic biomarker of a worse outcome in colorectal cancer
patients after curative surgery and a potential therapeutic target.
Med Oncol. 30:6882013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vance KW, Carreira S, Brosch G and Goding
CR: Tbx2 is overexpressed and plays an important role in
maintaining proliferation and suppression of senescence in
melanomas. Cancer Res. 65:2260–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu WK, Jiang XY and Zhang ZX: Expression
of PSCA, PIWIL1, and TBX2 in endometrial adenocarcinoma. Onkologie.
33:241–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baldwin RL, Tran H and Karlan BY: Loss of
c-myc repression coincides with ovarian cancer resistance to
transforming growth factor beta growth arrest independent of
transforming growth factor beta/Smad signaling. Cancer Res.
63:1413–1419. 2003.PubMed/NCBI
|
|
7
|
Li J, Ballim D, Rodriguez M, Cui R, Goding
CR, Teng H and Prince S: The anti-proliferative function of the
TGF-β1 signaling pathway involves the repression of the oncogenic
TBX2 by its homologue TBX3. J Biol Chem. 289:35633–35643. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jerome-Majewska LA, Jenkins GP, Ernstoff
E, Zindy F, Sherr CJ and Papaioannou VE: Tbx3, the ulnar-mammary
syndrome gene, and Tbx2 interact in mammary gland development
through a p19Arf/p53-independent pathway. Dev Dyn. 234:922–933.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Redmond KL, Crawford NT, Farmer H, D'Costa
ZC, O'Brien GJ, Buckley NE, Kennedy RD, Johnston PG, Harkin DP and
Mullan PB: T-box 2 represses NDRG1 through an EGR1-dependent
mechanism to drive the proliferation of breast cancer cells.
Oncogene. 29:3252–3262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowley M, Grothey E and Couch FJ: The role
of Tbx2 and Tbx3 in mammary development and tumorigenesis. J
Mammary Gland Biol Neoplasia. 9:109–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vance KW, Shaw HM, Rodriguez M, Ott S and
Goding CR: The retinoblastoma protein modulates Tbx2 functional
specificity. Mol Biol Cell. 21:2770–2779. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balogová L, Maslaňáková M, Dzurová L,
Miškovský P and Stroffeková K: Bcl-2 proapoptotic proteins
distribution in U-87 MG glioma cells before and after hypericin
photodynamic action. Gen Physiol Biophys. 32:179–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hojabrpour P, Waissbluth I, Ghaffari M,
Cox ME and Duronio V: CaMKII-γ mediates phosphorylation of BAD at
Ser170 to regulate cytokine-dependent survival and proliferation.
Biochem J. 442:139–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chao DT and Korsmeyer SJ: BCL-2 family:
Regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bertram J, Peacock JW, Tan C, Mui AL,
Chung SW, Gleave ME, Dedhar S, Cox ME and Ong CJ: Inhibition of the
phosphatidylinositol 3′-kinase pathway promotes autocrine
Fas-induced death of phosphatase and tensin homologue-deficient
prostate cancer cells. Cancer Res. 66:4781–4788. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan XL, Guo L and Wang GH: Polyporus
umbellatus inhibited tumor cell proliferation and promoted tumor
cell apoptosis by downregulating AKT in breast cancer. Biomed
Pharmacother. 83:526–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radisky DC and LaBarge MA:
Epithelial-mesenchymal transition and the stem cell phenotype. Cell
Stem Cell. 2:511–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonavida B and Baritaki S: Inhibition of
Epithelial-to-Mesen chymal Transition (EMT) in cancer by nitric
oxide: Pivotal roles of nitrosylation of NF-κB, YY1 and Snail. For
Immunopathol Dis Therap. 3:125–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferrara N: VEGF as a therapeutic target in
cancer. Oncology 69 Suppl. 3:11–16. 2005. View Article : Google Scholar
|
|
26
|
Kowanetz M and Ferrara N: Vascular
endothelial growth factor signaling pathways: Therapeutic
perspective. Clin Cancer Res. 12:5018–5022. 2006. View Article : Google Scholar : PubMed/NCBI
|