Introduction
Metabolic syndrome (MetS) represents a cluster of
cardiovascular and metabolic injuries that include mainly the
presence of arterial hypertension, hyperglycaemia, insulin
resistance, dyslipidaemia and obesity (1,2).
Currenlty, there is much concern regarding this pathology, as its
incidence has markedly increased over the past 10 years, worldwide.
There is increasing evidence to indicate that mortality due to
cardiovascular events, including stroke, different cardiovascular
diseases and myocardial infarction is highly influenced by the
presence of metabolic imbalances (3,4).
Data from animal models, as well as from clinical studies have
shown the strong association between diet, chronic exposure to
xenobiotics and pesticides, and the high levels of oxidative stress
and the emergence of MetS (5–10).
Extensive research is currently being conducted in order to assess
all the possible mechanisms underlying the etiopathogenesis of
MetS.
Osteoprotegerin (OPG) has recently been highlighted
as a key factor of the biochemical mechanisms underlying the
association between MetS and cardiovascular risk (2,11–16).
OPG is a soluble glycoprotein of the tumour necrosis factor
receptor (TNFR) superfamily implicated in bone remodelling. It
functions as a decoy receptor of receptor activator for nuclear
factor κB ligand (RANKL) and TNF-related apoptosis-inducing ligand
(TRAIL). For its particular ability to block RANKL, OPG was
initially identified as a key regulator in bone turnover (17–21).
The expression of OPG at the RANK level translates into the
inhibition of bone resorption by blocking osteoclastogenesis and
reducing the activity of mature osteoclasts. At the osteoblastic
level, OPG biosynthesis and secretion is stimulated by various
cytokines, such as interleukins (ILs) (IL-1 and IL-6) and TNF-α,
while parathyroid hormone (PTH), 25-hydroxy vitamin D [25(OH)
vitamin D] and prostaglandin E2 decrease the expression of OPG
(3,12,22–24).
This inflammatory cytokine receptor is expressed in vivo by
vascular smooth muscle cells, hepatic cells and osteoblasts, and
its assessment is valuable for understanding the link between bone
mineralization and vascular pathology (25–29).
Clinical studies have suggested that OPG may also be a risk factor
for progressive atherosclerotic cardiovascular disease (9,16,27),
mainly by mediating different processes in cells known to be
implicated in the atherogenic process.
Another important discovery is that the
pro-inflammatory environment that characterises MetS, leads to the
secretion of OPG by endothelial tissue (2). Thus, endothelial cells express and
release OPG under conditions of hyperglycaemia, hyperinsulinaemia,
oxidative stress and various inflammatory stimuli (1,2,13,28–30).
The aim of this study was to evaluate the value of
OPG as a predictive marker for cardiovascular and metabolic risk in
osteoporotic patients, in the constellate clinical millieu of
MetS.
Materials and methods
The present study comprised 71 randomly selected
women (48 post-menopausal and 23 pre-menopausal women) diagnosed
with osteoporosis that were referred to the Endocrine Department of
Elias Hospital (Bucharest, Romania).
The diagnosis of osteoporosis was made based on the
WHO criteria, with a T score expressed in standard deviations (SD),
either lumbar spine or neck, of ≤-2.5. Bone densitometry was
evaluated using a GE Lunar DXA (dual X-ray absorptiometry) for
lumbar (L1-L4) scan and femoral (neck) scans based on the DXA
evaluation SDs. The results were expressed as bone mineral density
(BMD) at lumbar and femoral neck sites, T score (SD resulting from
the comparison to the young adult database) and Z score (SD
resulting from the comparison to age- and sex-matched database).
All these parameters were recorded for further analyses (31,32).
Data collection consisted of clinical evaluation,
physical exam and biochemical routine blood tests together with the
assessment of bone turnover parameters required for the
differential diagnosis of osteoporosis.
In this respect, we determined the levels of calcium
in serum and urine, total alkaline phosphates (total ALP), PTH,
25(OH) vitamin D, serum osteocalcin, OPG and beta crosslaps. Serum
osteocalcin, OPG and 25(OH) vitamin D levels were assessed using
immunological ELISA methods, PTH was analysed through a
chemiluminometric method, while calcium and ALP levels were
measured using spectrophotometric methods. Blood tests included the
measurement of total serum cholesterol, HDL cholesterol, LDL
cholesterol, triglyceride (TG) and blood glucose levels. The
weight, height and waist circumference of the patients were also
measured, as well as blood pressure, as previously described
(1,4,33–38).
All these methods used are described below:
ELISA
The serum levels osteocalcin, OPG 25(OH) vitamin D
and beta crosslaps were assessed by ELISA on a Chemwell 2010 ELISA
system (Awareness Technology Inc., Palm City, FL, USA) using
commercially available kits provided by Immunodiagnostic Systems
(The Boldons, UK).
N-MID® Osteocalcin ELISA is based upon
the application of 2 highly specific monoclonal antibodies (Mabs)
against human osteocalcin. An antibody recognizing the midregion
(amino acids 20–29) was used as the capture antibody, and for
detection a peroxidase conjugated antibody recognizing the
N-terminal region (amino acids 10–16) was used. In addition to
intact osteocalcin (amino acid 1–49) the N-terminal-Mid fragment
(amino acids 1–43) was also detected.
A sandwich-type ELISA assay was used for the direct
determination of OPG in serum. In this assay, 2 highly specific
antibodies against OPG were used. The binding antibody was attached
to the wells of the microtiter plate, and the detection antibody
was labelled with biotin. A sandwich-type complex was found
consisting of the binding antibody on the plate, OPG and the
biotinylated detection antibody.
A competitive ELISA technique with a selected
monoclonal antibody recognizing 25(OH)-vitamin D was employed. For
a reliable determination of 25(OH)-vitamin D, it is necessary to
release it from the 25(OH)-vitamin D-DBP-complex. Standards,
controls and patient samples, which are assayed for 25(OH)-vitamin
D, were incubated with the releasing reagent. The pre-incubated
solutions were then transferred to the microplate coated with
25(OH)-vitamin D, and an anti-25(OH)-vitamin D antibody was added.
Tetramethylbenzidine (TMB) was used as a peroxidase substrate and
the intensity of the yellow color was inversely proportional to the
concentration of 25(OH)-vitamin D.
The Serum CrossLaps® ELISA used, is based
on 2 highly specific monoclonal antibodies against the amino acid
sequence of EKAHD-β-GGR, where the aspartic acid residue (D) is
β-isomerized. In order to obtain a specific signal in the Serum
CrossLaps® ELISA, 2 chains of EKAHD-β-GGR must be cross
linked.
Chemiluminometric methods
Serum PTH was assessed using the COBAS E 411
analyzer which is an automated, random-access, multichannel
analyzer for immunological analysis. The development of ECL
immunoassays is based on the use of a ruthenium chelate as the
complex for the development of light. The chemiluminescent
reactions that lead to the emission of light from the ruthenium
complex are initiated electrically. This is achieved by applying a
voltage to the immunological complexes (including the ruthenium
complex) that are attached to Streptavidin-coated micro
particles.
Spectrophotometric methods
ALP and total serum calcium were measured by
spectrophotometric methods on a Jasco V 650 spectrometer. All
reagents were supplied by Sigma-Aldrich.
Alkaline phosphatase in the sample catalyzes the
hydrolysis of colorless p-nitrophenyl phosphate (p-NPP) to yield
p-nitrophenol and inorganic phosphate. At the pH of the assay
(alkaline), the p-nitrophenol is in the yellow phenoxide form. The
rate of absorbance increase at 404 nm is directly proportional to
the alkaline phosphatase activity in the sample.
Total serum calcium was measured using the
arsenazo-III dye that reacts with calcium in an acid solution to
form a blue-purple complex. The color developed is measured at 660
nm and is proportional to the calcium concentration in the
sample.
The age of the onset of menopause and the first
menstruation were recorded. Fractures and exposure to risk factors
for fracture were also recorded. All other associated diseases were
noted and recorded.
The diagnosis of MetS was made based on the
International Diabetes Federation (IDF) 2007 guidelines as follows:
a waist circumference >84 cm for women as a mandatory criteria
along with a minimum of two other conditions: low HDL cholesterol
(<40 mg/dl), high TG levels (>150 mg/dl), high blood pressure
(>130 mmHg systolic, >80 mmHg diastolic) and high blood
glucose (>100 mg/dl) levels (3,12,27).
Ethics statement
Our research involved human participants and was
approved by the Elias Hospital Ethics Committee, Bucharest,
Romania. All clinical investigations were conducted according to
the principles expressed in the Declaration of Helsinki, and all
patients provided written and signed informed consent prior to
enrollment.
Statistical analysis
Data analysis was performed using SPSS for Windows
(version 20; IBM SPSS, Armonk, NY, USA). Variables were tested for
normal distribution across study groups using the Shapiro-Wilk test
and for comparison between mean and median values. Descriptive
summary measures of the study variables were computed as
appropriate: Mean and SD in normal distributed variables and median
and interquartile range (IQR) in other variables. Pearson
correlation coefficients were computed for bivariate correlation
and significant correlations were discussed based on the P-value. A
Student's t-test was used to compare variables across normally
distributed variables. A non-parametric Mann Withney U test was
used to compare continuous variables. The Canonical Discriminant
Function coefficients were calculated in SPSS in order to assess
the predictive value of different variables. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
The demographic parameters of all the subjects are
illustrated in Table I. From the
71 patients, 48 were post-menopausal and 23 pre-menopausal. As an
overall view, the period of time spent after menopause varied
significantly and correlated with the age. We found that some women
underwent menopause at an earlier age. The mean BMI in our subjects
was under the normal limits, with maximum values not exceeding the
limit for severe obesity.
 | Table I.Descriptive baseline parameters for
all the subjects with measured osteoprotegerin levels. |
Table I.
Descriptive baseline parameters for
all the subjects with measured osteoprotegerin levels.
|
| Age (years) | Years since
menopause | BMI
(kg/m2) |
|---|
|
|
|
|
|
|---|
| Median | Min | Max | IQR | Median | Min | Max | IQR | Mean | Min | Max | SD |
|---|
| 65.50 | 22.00 | 82.00 | 15.00 | 43.00 | 0.00 | 55.00 | 48.00 | 26.47 | 15.60 | 34.00 | 4.00 |
Routine blood test parameters in the study group
revealed a wide range of blood glucose levels, suggesting that the
study group also included subjects with diabetes mellitus and
probably insulin-treated patients, the last situation explaining
the very low glucose level in some cases. The lipid parameters also
suggested a wide variety of dyslipidaemia in our subjects, as
depicted in Table II.
 | Table II.Descriptive statistics for lipid and
glucose blood parameters. |
Table II.
Descriptive statistics for lipid and
glucose blood parameters.
| Parameter | Median | Minimum | Maximum | Interquartile
range |
|---|
| Blood glucose
(mg/dl) | 89 | 54 | 255 | 15 |
| Cholesterol total
(mg/dl) | 211.22 | 205.31 | 383.58 | 57.8 |
| HDL cholesterol
(mg/dl) | 57.13 | 15.74 | 125.35 | 68.51 |
| LDL cholesterol
(mg/dl) | 126.34 | 119.62 | 145.74 | 65.02 |
| TG (mg/dl) | 240.76 | 151.81 | 524.96 | 97.83 |
| Serum calcium
(mg/dl) | 9.6 | 3 | 11.6 | 0.7 |
| Urine calcium
(mg/24 h) | 147.19 | 24.7 | 560.74 | 152.39 |
| Total ALP
(UI/l) | 88.5 | 21 | 184 | 97 |
| PTH (pg/ml) | 68.50 | 57.3 | 191.21 | 45.49 |
| 25(OH) vitamin D
(ng/ml) | 9.7 | 6.6 | 18.6 | 4.5 |
| Osteocalcin
(ng/ml) | 10.40 | 2 | 13.1 | 9.21 |
| Crosslaps
(ng/ml) | 0.185 | 0.015 | 1.704 | 0.35 |
| Osteoprotegerin
(pmol/l) | 5.26 | 2.9 | 7.62 | 3.6 |
Total serum calcium statistical parameters indicated
that most of the values were aggregated near the normal values;
however, patients with extremely low calcium levels were also found
among our patients. A low blood calcium level is not a common
feature in osteoporosis, but is rather a similar situation as low
glucose levels, with patients being admitted to the endocrine
department following an acute episode of hypocalcaemia.
Hypercalcaemia is more frequently observed in
patients diagnosed with osteoporosis, primary hyperparathyroidism
being one of the most important secondary causes of osteoporosis
(20,39). Consequently, the measurement of
calcium and PTH is a mandatory step in the process for the
differential diagnosis of osteoporosis. Failing to identify the
secondary cause in osteoporosis will decrease the treatment
efficacy and will allow the untreated condition to evolve and lead
to complications.
Among other markers, 25(OH) vitamin D may be
considered by some specialised departments as a routine test for
the differential diagnosis of osteoporosis (38,40,41).
In our patients, we found very low levels of 25(OH) vitamin D,
somehow confirming other reports; the median value was <30
ng/ml, which indicated that most of our subjects had inadequate
serum 25(OH) vitamin D levels (Table
II). Osteocalcin and crosslaps, as biochemical markers of bone
turnover, had a specific value in differentiating specific
situations or monitoring treatment, the absolute baseline value
being not very specific.
Correlation coefficients and the statistical
significance between calcium, the lipidic and glucidic profiles and
bone turnover parameters were computed and are presented in
Table III.
 | Table III.Pearson correlation's coefficients
between calcium, specific bone parameters and metabolic
biomarkers. |
Table III.
Pearson correlation's coefficients
between calcium, specific bone parameters and metabolic
biomarkers.
| Parameter | Age (years) | BMI
(kg/m2) | Years since
menopause | Blood glucose
(mg/dl) | Total cholesterol
(mg/dl) | HDL cholesterol
(mg/dl) | LDL cholesterol
(mg/dl) | TG (mg/dl) |
|---|
| Serum total calcium
(mg/dl) | 0.151 | −0.176 | −0.546a | −0.200 | −0.169 | 0.395 | −0.283 | −0.233 |
| Calcium urine
excretion (mg/24 h) | −0.098 | 0.268 | 0.373 | 0.080 | −0.544 | −0.425 | −0.181 | 0.008 |
| Total ALP
(UI/l) | 0.310 | 0.090 | 0.103 | 0.211 | 0.134 | −0.103 | 0.090 | 0.143 |
| PTH (pg/ml) | 0.065 | 0.128 | 0.090 | 0.491b | 0.127 | 0.225 | 0.030 | 0.268a |
| 25(OH) vitamin D
(ng/ml) | −0.358b | −0.100 | 0.261 | −0.152 | −0.170 | −0.101 | −0.128 | −0.267 |
| Osteocalcin
(ng/ml) | −0.194 | −0.116 | 0.206 | −0.011 | 0.035 | 0.344a | −0.035 | −0.136 |
| Crosslaps
(ng/ml) | −0.598 | 0.458 | 0.212 | −0.101 | 0.040 | 0.830b | −0.586 | −0.008 |
| Osteoprotegerin
(pmol/l) | 0.101 | 0.078 | −0.079 | 0.245 | 0.246 | 0.333a | 0.264 | 0.023 |
Serum total calcium correlated with the time spent
in the post-menopausal period in these patients, justified probably
by the well-known increase in hyperparathyroidism in the forth and
sixth decades of life in women. Calcaemia also significantly
correlated with crosslaps, a marker of bone turnover; this
indicates that even in the case of small variations, serum total
calcium correlates with increased bone turnover.
On the other hand, serum total calcium (plasmatic
Ca2+) was also found to inversely correlate with the
urinary calcium excretion; this association is normal mostly in
high resorbtive states (20). As
previously described (39),
calcium excretion into urine is dependent on bone resorption and
calcium intake; consequently it is higher in increased bone
resorption states. Since all the women in our study group had
osteoporosis and most of them had low vitamin D levels, calciuria
exhibited an inverse correlation with blood calcium levels due to
the PTH effect on the kidney function. No significant correlations
were found with bone densitometry parameters (Table V).
 | Table V.Pearson correlation's coefficients
between calcium, bone and densitometric parameters. |
Table V.
Pearson correlation's coefficients
between calcium, bone and densitometric parameters.
| Parameter | Lumbar BMD
(g/cm2) | Lumbar T score
(SD) | Lumbar Z score
(SD) | Femoral BMD
(g/cm2) | Femoral T score
(SD) | Femoral Z score
(SD) |
|---|
| Serum total calcium
(mg/dl) | −0.213 | 0.167 | −0.249 | −0.157 | 0.266 | 0.215 |
| Calcium urine
excretion (mg/24 h) | 0.034 | −0.280 | 0.097 | −0.322 | −0.207 | −0.374 |
| Total ALP
(UI/l) | 0.143 | −0.290 | −0.011 | −0.055 | −0.311 | −0.090 |
| PTH (pg/ml) | −0.041 | −0.140 | −0.039 | −0.098 | −0.091 | −0.084 |
| 25(OH) vitamin D
(ng/ml) | 0.163 | −0.267 | −0.162 | 0.060 | −0.091 | −0.244 |
| Osteocalcin
(ng/ml) | 0.000 | −0.007 | −0.037 | 0.056 | 0.031 | 0.010 |
| Crosslaps
(ng/ml) | −0.002 | 0.646 | −0.072 | 0.749 | 0.415 | 0.106 |
| Osteoprotegerin
(pmol/l) | −0.025 | −0.032 | −0.100 | −0.001 | −0.079 | −0.073 |
Serum total ALP levels (as presented in Table IV) exhibited a significant
correlation with serum PTH levels, illustrating the direct effect
of PTH on the renal handling of calcium: PTH decreases the renal
loss of calcium into urine. The direct correlation with OPG may be
explained by the link with bone resorption: both parameters, total
ALP and OPG may be increased in relation to the increased
osteoclast activity in the bone.
 | Table IV.Pearson correlation's coefficients
between calcium and bone parameters. |
Table IV.
Pearson correlation's coefficients
between calcium and bone parameters.
| Parameter | Serum total calcium
(mg/dl) | Calcium urine
excretion (mg/24 h) | Total ALP
(UI/l) | PTH (pg/ml) | 25(OH) D vitamin
(ng/ml) | Osteocalcin
(ng/ml) | Crosslaps
(ng/ml) |
|---|
| Serum total calcium
(mg/dl) | 1 | −0.610a | 0.054 | 0.245 | −0.293 | 0.074 | 1.000b |
| Calcium urine
excretion (mg/24 h) | −0.610a | 1 | 0.41 | 0.284 | 0.376 | −0.351 | −0.78 |
| Total ALP
(UI/l) | 0.050 | −0.509 | 1 | 0.782a | −0.156 | −0.054 | 0.654 |
| PTH (pg/ml) | 0.245 | 0.284 | 0.45 | 1 | −0.155 | 0.172 | −0.222 |
| 25(OH) vitamin D
(ng/ml) | −0.293 | 0.376 | −0.011 | −0.155 | 1 | 0.023 | 0.366 |
| Osteocalcin
(ng/ml) | 0.074 | −0.351 | −0.034 | 0.172 | 0.023 | 1 | −0.103 |
| Crosslaps
(ng/ml) | 1.000b | −0.213 | 0.654 | −0.222 | 0.366 | −0.103 | 1 |
| Osteoprotegerin
(pmol/l) | 0.300 | −0.460 | 0.78a | 0.332b | 0.006 | 0.068 | −0.413 |
The correlations of the PTH serum levels with age
(Table III) or the serum 25(OH)
vitamin D levels (Table IV) were
not significant probably due to the low number of cases.
Nevertheless, PTH did correlate with the blood glucose and serum TG
levels (Table III). These
correlations sustain the hypothesis of metabolic and cardiovascular
risk associated even with normocalcemic primary
hyperparathyroidism.
The serum 25(OH) vitamin D levels were found to
negatively correlate with age (Table
III) in our study group. Increased age is a well-known risk
factor for low 25(OH) vitamin D levels, which was confirmed in our
data. Several factors found in elderly individuals may be the cause
of this aspect: low sun exposure due to invalidity, skin
alterations with age and the decreased activation of vitamin D in
the kidneys. The inverse correlation between PTH and 25(OH) vitamin
D levels was not significant; an explanation for this may be the
lower case number or the lower prevalence of vitamin D deficiency
in this group which alleviates it.
Serum osteocalcin levels were found to directly
correlate with HDL cholesterol in the OPG group (Table III), a correlation explained by
the utility of osteocalcin in the evaluation of cardiovascular
risk, an intensively explored aspect in the past years. Osteocalcin
did not correlate with blood calcium levels, PTH levels and serum
crosslaps (Table IV). The utility
and significance of osteocalcin levels measured in serum as a
reliable marker of bone turnover, regarding both resorption and
formation, are based on larger group analysis (34,39).
This aspect led to complications in the individual patient value of
osteocalcin measurements. This biomarker is not yet clinically
validated as a diagnostic tool for bone turnover, and therefore it
is mainly used for research purposes.
The OPG serum levels were found to directly and
significantly correlate with HDL cholesterol, total ALP and serum
PTH levels (Tables III and
IV). These correlations sustain
the protective role of the OPG for cardiovascular diseases and, in
the same time, the link to the bone metabolism.
No significant correlation was found between
biochemical calcium levels, bone markers and densitometric
parameters in our population (as depicted in Table V). This may be explained by the
independent value of these markers in the fracture risk evaluation;
it is well known that the correlation between densitometric and
biochemical aspects in osteoporosis is not linear and is somehow
divergent (31,32,36).
Correlation between serum OPG levels
and MetS
Women with measured OPG serum levels were also
segregated into two subgroups considering the presence of Mets: a
control group without Mets and a study group with Mets (as
presented in Table VI).
 | Table VI.Comparison between study subgroups
according to the MetS presence criteria: Clinical, bone and
metabolic parameters. |
Table VI.
Comparison between study subgroups
according to the MetS presence criteria: Clinical, bone and
metabolic parameters.
| Parameter | Control group (no
MetS) | Study group (MetS
present) |
|---|
| Age
(years)a | 61 (19) | 70 (17.5) |
| BMI
(kg/m2)b | 24.5 (4.7) | 26.3 (3.7) |
| Years since
menopausea | 40 (45) | 41 (48) |
| Blood glucose
(mg/dl)a | 87 (12) | 107 (17) |
| Total cholesterol
(mg/dl)b | 200 (53.8) | 231.8 (42.89) |
| HDL cholesterol
(mg/dl)a | 56 (18) | 58 (17) |
| LDL cholesterol
(mg/dl)b | 119 (43.3) | 138 (43.1) |
| Triglycerides
(mg/dl)b | 94.4 (35.1) | 146.1 (52.5) |
| Serum total Ca
(mg/dl)b | 9.3 (0.5) | 10 (0.8) |
| 24 h urine calcium
(mg/24 h)a | 158 (151) | 118 (168) |
| Total ALP
(UI/l)a | 87 (90) | 134 (109) |
| PTH
(pg/ml)a | 77.2 (13.3) | 80.6 (16.9) |
| 25(OH) vitamin D
(ng/ml)a | 30 (39) | 17.4 (11.1) |
| Osteocalcin
(ng/ml)a | 10.4 (12.4) | 10.1 (10.1) |
| Crosslaps
(ng/ml)a | 0.448 (0.137) | 0.132 (0.389) |
| Osteoprotegerin
baseline (pmol/l)b | 5.15 (0.54) | 5.6 (0.68) |
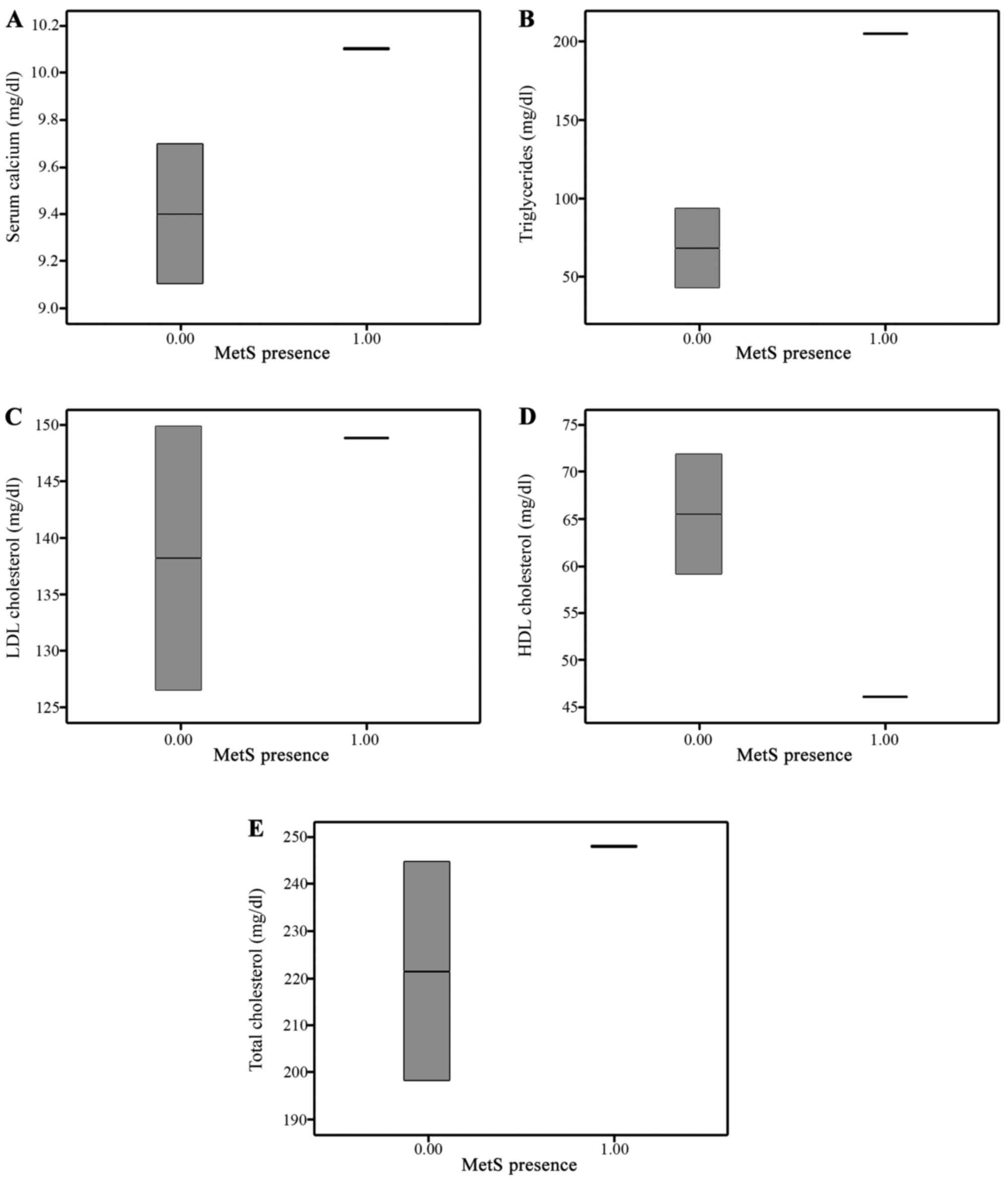
There were some significant differences across these
two groups as regards age and blood glucose, total and HDL
cholesterol and serum TG levels (Fig.
1). All these differences are completely explained by the
selection criteria: the diagnosis of MetS is based on decreased HDL
cholesterol levels, increased blood glucose levels and increased TG
levels. On the other hand, the prevalence of MetS is increasing
with age; consequently, increased age in the study group was a
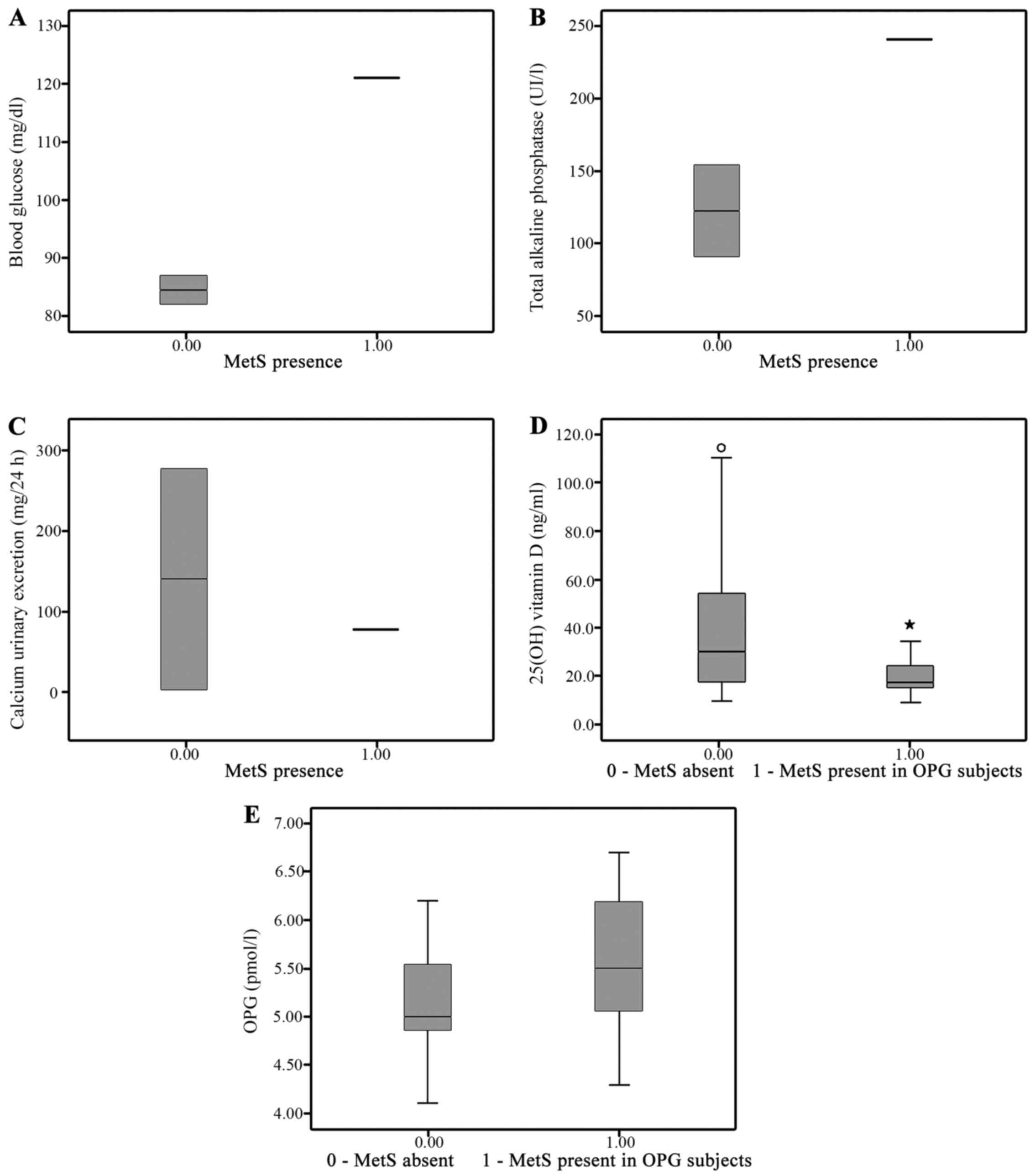
normal finding. Among the calcium and bone biochemical markers, the
25(OH) vitamin D and OPG serum levels were significantly different
across the study groups (Fig.
2).
Discussion
The serum level of 25(OH) vitamin D (Fig. 1) was significantly lower in the
Mets group, in accordance with the reports of increased
cardiovascular risk associated with vitamin D deficiency (38,40).
Vitamin D has been shown in several studies to be
implicated in the maintenance of the good function of the immune
system, vitamin D deficiency has been associated with neoplasia and
diabetes, as well as atherosclerosis and insulin resistance
(22,42,43).
We found a significant difference in terms of serum
25(OH) vitamin D levels between the two groups, differences
suggesting that vitamin D deficiency may be a risk factor for the
development of MetS and its related complications, such as diabetes
and atherosclerosis.
The OPG levels were higher in the Mets group
(Fig. 2). It has been suggested
that increased levels of OPG in patients with cardiovascular risk
are due to the protective effect of OPG; however, the underlying
mechanisms are still under debate (2,4,11–16).
Correlations between OPG and 25(OH) vitamin D levels
in the presence of MetS as an indicator of cardiovascular risk were
further analysed in order to exclude other co-founding factors. Due
to the correlations between OPG and total ALP levels, as well as
PTH and HDL cholesterol, suggesting that the differences in OPG
levels between the Mets groups may be mediated by one of these
parameters, a discriminant analysis (SPSS discriminant function
analysis) was performed. The results revealed a significant
predictive value for OPG and 25(OH) vitamin D for Mets (P<0.05).
PTH was not significantly predictive (P>0.05) (Table VII). Cholesterol parameters were
not included in this analysis, as the significant difference found
between the Mets subgroups were due to the selection criteria (Mets
definition) (13–15).
 | Table VII.Standardized Canonical Discriminant
Function Coefficients and significance in predicting Mets
occurance. |
Table VII.
Standardized Canonical Discriminant
Function Coefficients and significance in predicting Mets
occurance.
| Parameter | Coefficient | P-value |
|---|
| PTH (pg/ml) | 0.144 | 0.193 |
| OPG (pmol/l) | 0.673 | 0.022 |
| 25(OH) Vit D
(ng/ml) | −0.765 | 0.009 |
The levels of serum total calcium, calcium
excretion, serum PTH and bone resorption markers correlated with
each other in our patients with osteoporosis, sustaining their
relevance for bone turnover evaluation in osteoporosis.
Significant correlations between classical
biochemical bone and calcium parameters, such as osteocalcin and
PTH with lipid and glucose metabolism, were found in our patients,
sustaining the crosstalk between calcium, bone parameters and
cardiovascular risk.
The OPG serum level was proven to have a significant
and independent predictive value for MetS as a cardiovascular risk
factor in osteoporotic patients. The OPG serum levels were
increased in patients with MetS as a protective response against
atherosclerotic lesions.
The serum levels of 25(OH) vitamin D had significant
and independent predictive values for the cardiovascular and
metabolic risk in our subjects sustaining the wide role of the
vitamin D outside the bone metabolism.
References
|
1
|
Suliburska J, Bogdanski P, Gajewska E,
Kalmus G, Sobieska M and Samborski W: The association of insulin
resistance with serum osteoprotegerin in obese adolescents. J
Physiol Biochem. 69:847–853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Ciriza Perez C Villacampa:
Osteoprotegerin: A promising biomarker in the metabolic syndrome -
New perspectives. Biochem Anal Biochem. 5:1–3. 2016.
|
|
3
|
Fuentes E, Fuentes F, Vilahur G, Badimon L
and Palomo I: Mechanisms of chronic state of inflammation as
mediators that link obese adipose tissue and metabolic syndrome.
Mediators Inflamm. 2013:1365842013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monseu M, Dubois S, Boursier J, Aubé C,
Gagnadoux F, Lefthériotis G and Ducluzeau PH: Osteoprotegerin
levels are associated with liver fat and liver markers in
dysmetabolic adults. Diabetes Metab. 42:364–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Docea AO, Vassilopoulou L, Fragou D,
Arsene AL, Fenga C, Kovatsi L, Petrakis D, Rakitskii VN, Nosyrevh
AE, Izotovh BN, et al: CYP polymorphisms and pathological
conditions related to chronic exposure to organochlorine
pesticides. Toxicol Rep. 4:335–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drăgoi CM, Nicolae AC, Grigore C,
Dinu-Pîrvu CE and Arsene AL: Characteristics of glucose homeostasis
and lipidic profile in a hamster metabolic syndrome model, after
the co-administration of melatonin and irbesartan in a
multiparticulate pharmaceutical formulationThe Second International
Conference on Interdisciplinary Management of Diabetes Mellitus and
its Complications, INTERDIAB 2016. 3–5–March. Diabetes Mellitus as
Cardiovascular Disease, Ed.Niculescu, Bucharest: pp. 221–229.
2016;
|
|
7
|
Hernández AF, Parrón T, Tsatsakis AM,
Requena M, Alarcón R and López-Guarnido O: Toxic effects of
pesticide mixtures at a molecular level: Their relevance to human
health. Toxicology. 307:136–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mrema EJ, Rubino FM, Brambilla G, Moretto
A, Tsatsakis AM and Colosio C: Persistent organochlorinated
pesticides and mechanisms of their toxicity. Toxicology. 307:74–88.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aguilera AA, Diaz GH, Barcelata ML,
Guerrero OA and Ros RM: Effects of fish oil on hypertension, plasma
lipids, and tumor necrosis factor-alpha in rats with
sucrose-induced metabolic syndrome. J Nutr Biochem. 15:350–357.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klöting N, Blüher M and Klöting I: The
polygenetically inherited metabolic syndrome of WOKW rats is
associated with insulin resistance and altered gene expression in
adipose tissue. Diabetes Metab Res Rev. 22:146–154. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Augoulea A, Vrachnis N, Lambrinoudaki I,
Dafopoulos K, Iliodromiti Z, Daniilidis A, Varras M, Alexandrou A,
Deligeoroglou E and Creatsas G: Osteoprotegerin as a marker of
atherosclerosis in diabetic patients. Int J Endocrinol.
2013:1820602013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernardi S, Fabris B, Thomas M, Toffoli B,
Tikellis C, Candido R, Catena C, Mulatero P, Barbone F, Radillo O,
et al: Osteoprotegerin increases in metabolic syndrome and promotes
adipose tissue proinflammatory changes. Mol Cell Endocrinol.
394:13–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bjerre M: Osteoprotegerin (OPG) as a
biomarker for diabetic cardiovascular complications. Springerplus.
2:6582013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gordin D, Soro-Paavonen A, Thomas MC,
Harjutsalo V, Saraheimo M, Bjerre M, Forsblom C, Flyvbjerg A and
Groop PH; FinnDiane Study Group, : Osteoprotegerin is an
independent predictor of vascular events in Finnish adults with
type 1 diabetes. Diabetes Care. 36:1827–1833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo C, Hu F, Zhang S, Wang Y and Liu H:
Association between osteoprotegerin gene polymorphisms and
cardiovascular disease in type 2 diabetic patients. Genet Mol Biol.
36:177–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montagnana M, Lippi G, Danese E and Guidi
GC: The role of osteoprotegerin in cardiovascular disease. Ann Med.
45:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baud'huin M, Duplomb L, Teletchea S,
Lamoureux F, Ruiz-Velasco C, Maillasson M, Redini F, Heymann MF and
Heymann D: Osteoprotegerin: Multiple partners for multiple
functions. Cytokine Growth Factor Rev. 24:401–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Xue J, Shen T, Mu S and Fu Q:
Curcumin alleviates glucocorticoid-induced osteoporosis through the
regulation of the Wnt signaling pathway. Int J Mol Med. 37:329–338.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ndip A, Wilkinson FL, Jude EB, Boulton AJ
and Alexander MY: RANKL-OPG and RAGE modulation in vascular
calcification and diabetes: Novel targets for therapy.
Diabetologia. 57:2251–2260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong J, Piemontese M, Thostenson JD,
Weinstein RS, Manolagas SC and O'Brien CA: Osteocyte-derived RANKL
is a critical mediator of the increased bone resorption caused by
dietary calcium deficiency. Bone. 66:146–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Shao J, Wang Z, Yang T, Liu S,
Liu Y, Fan X and Ye W: Aqueous extract of pomegranate seed
attenuates glucocorticoid-induced bone loss and hypercalciuria in
mice: A comparative study with alendronate. Int J Mol Med.
38:491–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bischoff-Ferrari HA and Staehelin HB:
Importance of vitamin D and calcium at older age. Int J Vitam Nutr
Res. 78:286–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Brien EA, Williams JH and Marshall MJ:
Osteoprotegerin is produced when prostaglandin synthesis is
inhibited causing osteoclasts to detach from the surface of mouse
parietal bone and attach to the endocranial membrane. Bone.
28:208–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nan R, Grigorie D, Cursaru A, Șucaliuc A,
Drăguț R, Rusu E, Mușat M and Radulian G: Bisphosphonates - A good
choice for women with type 2 diabetes and postmenopausal
osteoporosis? Farmacia. 64:257–261. 2016.
|
|
25
|
Cima LN and Fica S: The use of anabolic
therapy in patients with betathalassemia major-induced osteoporosis
- review of the literature. Farmacia. 65:167–172. 2017.
|
|
26
|
Lee CJ, Wang JH, Chen ML, Yang CF, Chen YC
and Hsu BG: Serum osteoprotegerin is associated with arterial
stiffness assessed according to the cardio-ankle vascular index in
hypertensive patients. J Atheroscler Thromb. 22:304–312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pérez de Ciriza C, Moreno M, Restituto P,
Bastarrika G, Simón I, Colina I and Varo N: Circulating
osteoprotegerin is increased in the metabolic syndrome and
associates with subclinical atherosclerosis and coronary arterial
calcification. Clin Biochem. 47:272–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu G, Ji X, Jin J and Bu S: Association of
serum and vitreous concentrations of osteoprotegerin with diabetic
retinopathy. Ann Clin Biochem. 52:232–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu M, Fang X, Zhou S, Li W and Guan S:
Indirect co culture of vascular smooth muscle cells with bone
marrow mesenchymal stem cells inhibits vascular calcification and
downregulates the Wnt signaling pathways. Mol Med Rep.
13:5141–5148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ST, Xu JM, Wang M, Chen FL and Ding
G: Increased plasma osteoprotegerin concentrations in type 1
diabetes with albuminuria. Clin Nephrol. 79:192–198. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borman P, Babaoğlu S, Gur G, Bingol S and
Bodur H: Bone mineral density and bone turnover in patients with
psoriatic arthritis. Clin Rheumatol. 27:443–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanis JA, McCloskey EV, Johansson H,
Cooper C, Rizzoli R and Reginster JY: Scientific Advisory Board of
the European Society for Clinical and Economic Aspects of
Osteoporosis and Osteoarthritis (ESCEO) and the Committee of
Scientific Advisors of the International Osteoporosis Foundation
(IOF): European guidance for the diagnosis and management of
osteoporosis in postmenopausal women. Osteoporos Int. 24:23–57.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Christgau S, Rosenquist C, Alexandersen P,
Bjarnason NH, Ravn P, Fledelius C, Herling C, Qvist P and
Christiansen C: Clinical evaluation of the Serum CrossLaps One Step
ELISA, a new assay measuring the serum concentration of
bone-derived degradation products of type I collagen
C-telopeptides. Clin Chem. 44:2290–2300. 1998.PubMed/NCBI
|
|
34
|
Grădinaru D, Mitrea N, Margină D, Arsene
AL, Gruia V, Drăgoi C, Nicolae A, Borşa C and Gherasim P:
Evaluation of serum osteocalcin in eldery patients with type-2
diabetes mellitus. Farmacia. 57:331–338. 2009.
|
|
35
|
Hamann KL and Lane NE: Parathyroid hormone
update. Rheum Dis Clin North Am. 32:703–719. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henriksen K, Karsdal MA and Martin TJ:
Osteoclast-derived coupling factors in bone remodeling. Calcif
Tissue Int. 94:88–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Starup-Linde J, Eriksen SA, Lykkeboe S,
Handberg A and Vestergaard P: Biochemical markers of bone turnover
in diabetes patients - a meta-analysis, and a methodological study
on the effects of glucose on bone markers. Osteoporos Int.
25:1697–1708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamineni V, Latha AP and Ramathulasi K:
Association between serum 25-hydroxyvitamin D levels and bone
mineral density in normal postmenopausal women. J Midlife Health.
7:163–168. 2016.PubMed/NCBI
|
|
39
|
Rasheed N Wael, Barbu CG, Florea S,
Branceanu G, Fica S, Mitrea N, Dragoi CM, Nicolae AC and Arsene AL:
Biochemical markers of calcium and bone metabolism in the
monitoring of osteoporosis treatment. Farmacia. 62:728–736.
2014.
|
|
40
|
Ohta H, Uemura Y, Nakamura T, Fukunaga M,
Ohashi Y, Hosoi T, Mori S, Sugimoto T, Itoi E, Orimo H, et al
Adequate Treatment of Osteoporosis (A-TOP) Research Group, : Serum
25-hydroxyvitamin D level as an independent determinant of quality
of life in osteoporosis with a high risk for fracture. Clin Ther.
36:225–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zugravu CA, Soptica F, Tarcea M and Cucu
A: Pertinence of vitamin D supplementation in the adult Romanian
population. Farmacia. 64:467–472. 2016.
|
|
42
|
Heaney RP: The vitamin D requirement in
health and disease. J Steroid Biochem Mol Biol. 97:13–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holick MF: Vitamin D: A millenium
perspective. J Cell Biochem. 88:296–307. 2003. View Article : Google Scholar : PubMed/NCBI
|