Introduction
Diabetic nephropathy (DN) is one of most serious
complications of both type 1 and type 2 diabetes, and frequently
results in end-stage renal disease (ESRD), which eventually
requires renal replacement therapy or renal transplantation
(1). Initiation and development of
DN is closely associated with podocyte injury and loss, and
involved mechanisms include podocyte apoptosis, detachment,
hypertrophy, effacement and loss of foot process proteins (2–5).
Among them, podocyte apoptosis is a hotspot of research. Increasing
data has that revealed endoplasmic reticulum (ER) stress serves an
important role in the podocyte apoptosis (6,7). It
is understood that ER stress is a kind of adaptive response of the
cell itself. Under normal physiological conditions, aggregated
protein does not amass in cells partially due to the presence of
cellular ‘quality control’ mechanisms (8). However, the unfolded protein response
(UPR), whose role is restoring ER homeostasis and normal function,
will activate a series of signaling molecules and induce the
apoptosis cascade when the ER is exposed in a harmful environment
for an extended period of time (9,10).
The UPR is characterized by the activation of three ER
transmembrane effector proteins: PKR-like ER kinase (PERK),
inositol requiring enzyme 1 (IRE1) and activating transcription
factor-6 (ATF-6) (11). One major
pathway of UPR is the suppression of most protein translations
through phosphorylation of eukaryotic translation initiation factor
2 subunit a (eIf2a) by PERK (12).
Another pathway is the upregulated expression of ER-localized
molecular chaperones, such as glucose-regulated protein 78
(GRP78/Bip), GRP94 and other molecular chaperones like heat shock
proteins (13). After a series of
target protein activation, activation of ER resident protein
caspase-12 will eventually lead to the cellular apoptosis (14).
Epigallocatechin-3-gallate (EGCG), the most abundant
catechin in green tea, has been demonstrated to exert its
anti-inflammatory, antioxidant and antitumor properties in chronic
diseases including cancer (15,16),
neurodegenerative disease (17),
diabetes (18), heart disease
(19) and autoimmune arthritis
(20). EGCG can prevent DN
progression through decreasing reactive oxygen species (ROS)
expression (21). However, the
concrete mechanism of EGCG on high glucose-induced podocyte injury
remains uncertain. The current study aimed to investigate the
effects of EGCG on proliferation and apoptosis of mouse podocytes
cultured in high glucose, and to provide a theoretical basis of the
mechanisms involved in ER stress.
Materials and methods
Cell culture
The conditionally immortalized mouse podocytes
donated by Dr Peter Mundel (Massachusetts General Hospital and
Harvard Medical School, Charlestown, MA, USA) were maintained in
RPMI1640 medium supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10 U/ml
interferon-γ (PeproTech, Inc., Rocky Hill, NJ, USA) and 100 U/ml
antibiotics (penicillin and streptomycin). Frozen podocytes were
firstly cultivated at 33°C in a 5% CO2 incubator. Then
cells were cultured in medium at 37°C without interferon-γ to
induce differentiation for at least 2 weeks. Cells were cultured in
serum-free medium for 24 h to synchronize the cell growth before
each experiment.
Cell viability assay
Podocytes were seeded at a density of 1×10^4 cells
per well in 100 ul RPMI1640 complete medium on 96-well plates for
24 h. Cells were divided into 10 groups as follows: 5.6 mM
D-glucose (group N), 5.5 mM D-glucose and 24.5 mM D-mannitol (group
M), 30 mM D-glucose (group H), 1 µM EGCG (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and 30 mM D-glucose (group E1), 10 µM
EGCG and 30 mM D-glucose (group E10), 20 µM EGCG and 30 mM
D-glucose (group E20), 40 µM EGCG and 30 mM D-glucose (group E40),
60 µM EGCG and 30 mM D-glucose (group E60), 80 µM EGCG and 30 mM
D-glucose (group E80), and 100 µM EGCG and 30 mM D-glucose (group
E100). Cells were exposed to different conditions of reagents for
24, 48 or 72 h. At each time point, cells were cultured with fresh
medium with 10 µl Cell Counting kit-8 (CCK-8; BD Biosciences,
Franklin Lakes, NJ, USA) at 37°C for 3 h. Cell proliferation was
measured using a microplate reader (Model 680, Bio-Rad
Laboratories, Hercules, CA, USA) at a wavelength of 450 nm. Each
individual experiment was repeated at least three times.
Podocyte injury and apoptosis
Cells of 4 groups (N, M, H and E20) in the above
conditions were used for the following experiments. Mouse podocytes
were inoculated at a density of 1×105 cells/well on
cover slides in six-well plates. Then cells were washed with
phosphate buffer saline (PBS) and incubated with 0.1% Triton X-100
at room temperature for 5 min. Hoechst 33258 (Sigma-Aldrich; Merck
KGaA) was added on the cells and incubated in the dark for 20 min.
Images were captured under a fluorescence microscope (Olympus DP72,
Olympus Corporation, Tokyo, Japan).
Flow cytometry
Cells were selected for planting in the petri dishes
with treatment of normal glucose, mannitol, high glucose or high
glucose with 20 µmol/l EGCG. Cells were collected after 72 h and
the cell concentration was adjusted to 1×10^6 in 500 µl binding
buffer (BD Biosciences). Annexin V-fluorescein isothiocyanate (5
µl) and propidium iodide (PI; 10 µl) were added into each sample
and incubated at 37°C for 30 min without bright light. The
apoptosis data was acquired by a flow cytometer (Epics XL, Beckman
Coulter, Inc., Kreefeld, Germany).
Western blotting
Cells were treated with different conditions and
then lysed in radioimmunoprecipitation buffer containing protease
inhibitor. After centrifugation at 24,000 × g and 4°C for 30 min,
supernatants were collected and analyzed for protein concentration
with a Bicinchoninic Acid protein assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Total protein (30–50 µg) was
diluted in sample buffer and boiled for 5 min for denaturation.
Proteins in each sample were separated by 10% SDS-PAGE and
transferred to a nitrocellulose filter membrane. After blocking
with 5% milk in PBS with Tween-20 for 1 h, the membrane was
incubated at 4°C overnight with the following primary antibodies:
Anti-GAPDH (1:1,000; cat. no. ab9485), anti-Nephrin (1:1,000; cat.
no. ab136894), anti-Wilms tumor protein 1 (WT-1; 1:1,000; cat. no.
ab180840), anti-GRP78 (1:2,000; cat. no. ab21685), anti-caspase-12
(1:2,000; cat. no. ab62484) (all from Abcam, Cambridge, UK),
anti-PERK (1:1,000; cat. no. sc-13073) and anti-phosphorylated
(p)-PERK (1:1,000; cat. no. sc-32577) (both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The membranes were washed
and incubated with a goat anti-rabbit horseradish
peroxidase-conjugated IgG secondary antibody (1:5,000; cat. no.
ZB-2301, Origene Technologies, Inc., Rockville, MD, USA) at 24°C
for 1 h. Protein expression was detected with a chemiluminescence
detection kit (EMD Millipore, Billerica, MA, USA). Data were
analyzed using a western-blot analyzer (FluorChen E ProteinSimple
Inc., San Jose, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data were analyzed using SPSS 18.0 software (SPSS Inc.,
Chicago, IL, USA). Data were analyzed by one-way analysis variance
followed by Dunnetts multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
EGCG promotes podocyte
proliferation
Cells were cultured with normal glucose (N),
D-mannitol (M) and high glucose (H) for 24, 48 and 72 h,
respectively. CCK-8 reagent was used to detect podocyte
proliferation. Optical density 450 values were used to represent
cell proliferation. D-mannitol was used to observe the influence of
hypertonicity on cells. As presented in Fig. 1A, there was no difference in cell
proliferation among groups when cells were incubated for 24 h
(P>0.05). Cell proliferation in H group was significantly
decreased compared with N and M groups when cells were incubated
for 48 or 72 h (P<0.05). There were no significant differences
between N and M group. Interestingly, when EGCG with different
concentrations was added to high glucose treated cells, EGCG
especially at a concentration of 20 µmol/l (E20) significantly
promoted cell proliferation (P<0.01, Fig. 1B). However, its effect gradually
decreased with increasing EGCG concentration (Fig. 1B).
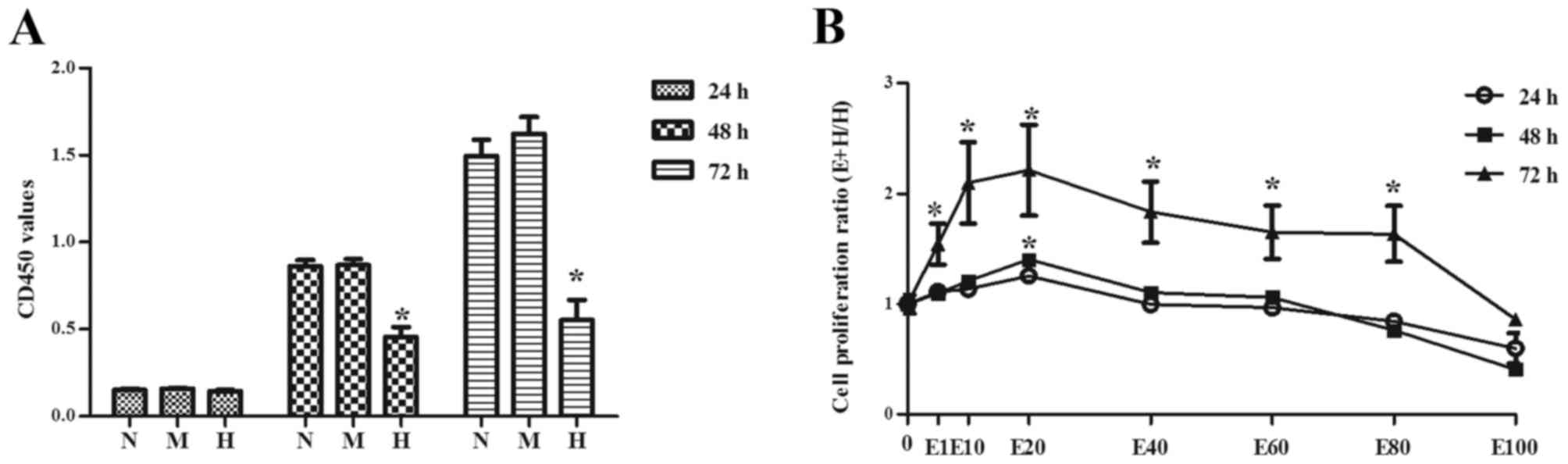 | Figure 1.Podocytes were treated with varying
concentrations of glucose and the cell proliferation was evaluated
by Cell Counting kit-8 assay. (A) The OD 450 values of different
groups. (B) Cell proliferation ratio of different concentration
gradient of EGCG group to high D-glucose group. Data are presented
as the mean ± standard deviation of at least three experiments in
each group. *P<0.05 vs. N. N: 5.6 mM D-glucose, M: 5.5 mM
D-glucose and 24.5 mM D-mannitol, H: 30 mM D-glucose. E1: 1 µM EGCG
and 30 mM D-glucose, E10: 10 µM EGCG and 30 mM D-glucose, E20: 20
µM EGCG and 30 mM D-glucose, E40: 40 µM EGCG and 30 mM D-glucose,
E60: 60 µM EGCG and 30 mM D-glucose, E80: 80 µM EGCG and 30 mM
D-glucose, E100: 100 µM EGCG and 30 mM D-glucose. OD, optical
density; EGCG, epigallocatechin-3-gallate. |
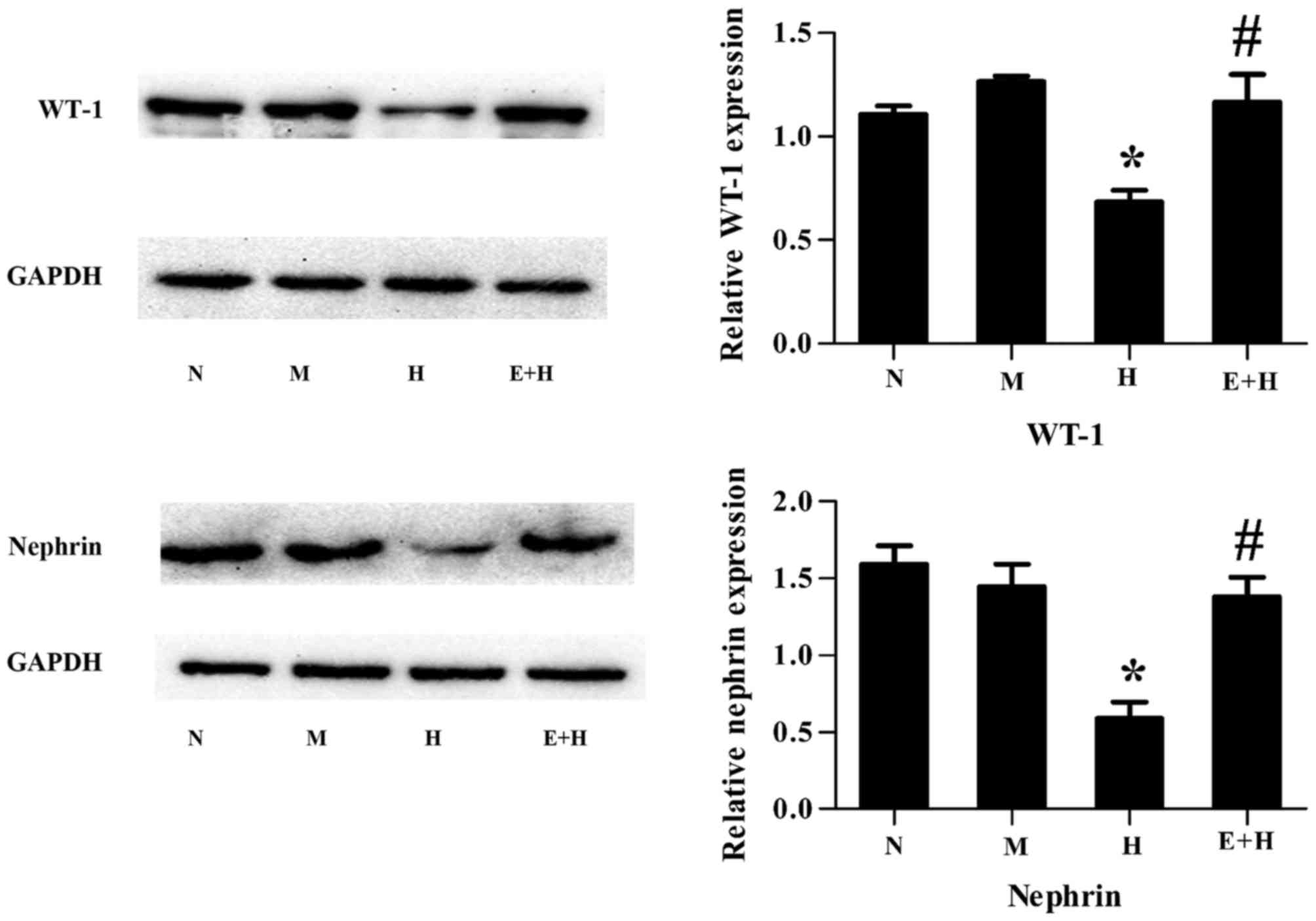
EGCG attenuates high glucose-induced
podocyte injury
WT-1 and nephrin are well-known podocyte-specific
protein markers and thus are used to evaluate podocyte damage. It
was demonstrated that both WT-1 and nephrin protein expression
levels were decreased in podocytes stimulated with high glucose
compared with those with normal glucose or mannitol (P<0.05,
Fig. 2). However, this
significantly increased following treatment of EGCG (20 µmol/l) and
high glucose, compared with high glucose alone (P<0.05; Fig. 2).
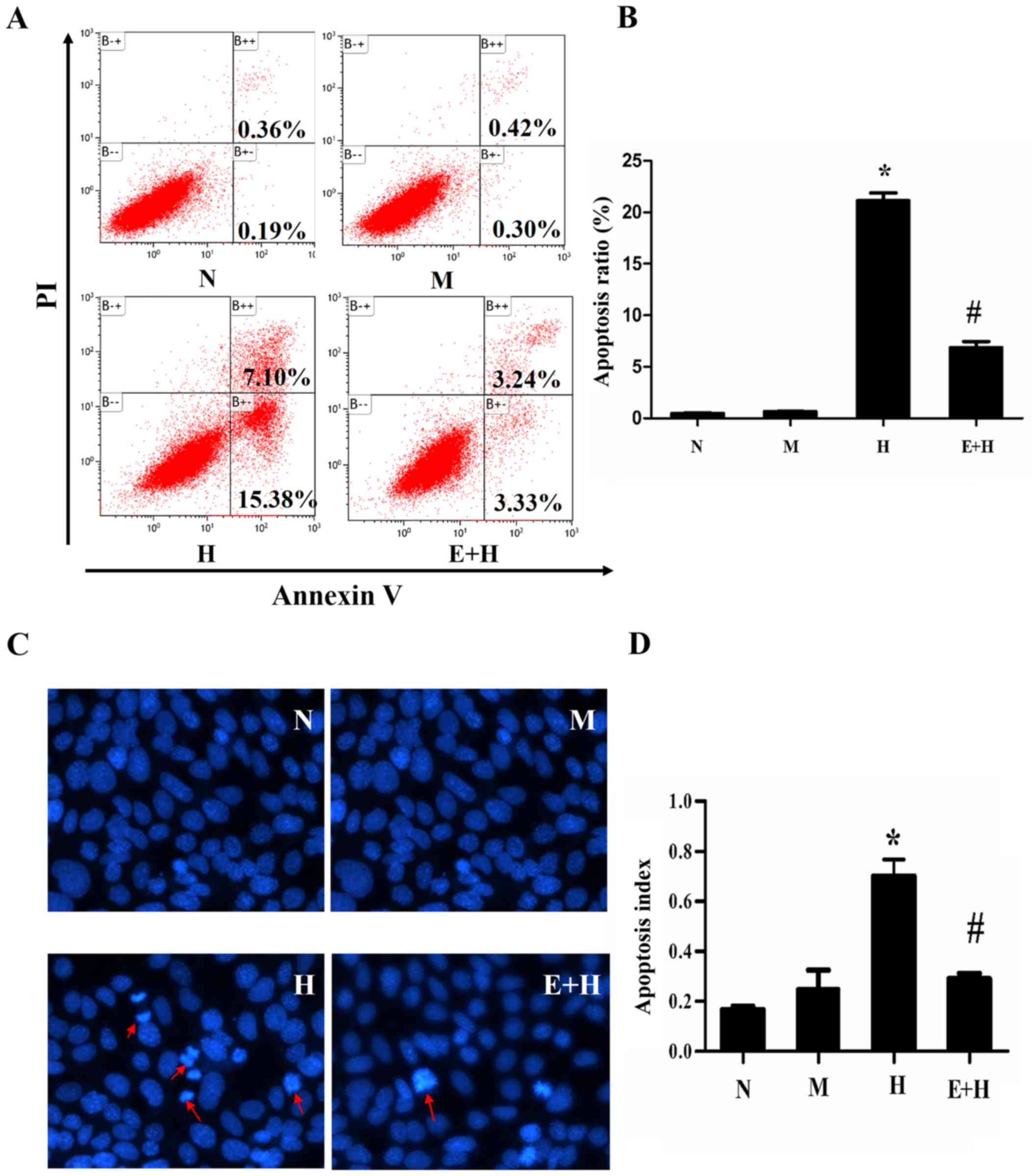
EGCG reduces podocyte apoptosis
induced by high glucose
Cells under different conditions were cultured for
72 h and cell apoptosis was analyzed by flow cytometry. There was
little population of apoptotic cells in either the N (0.36%) and M
(0.42%) groups, but the apoptotic population significantly
increased in the high glucose group (7.1%) compared with the normal
and mannitol groups (P<0.05, Fig.
3A). As predicted, EGCG (20 µmol/l) treatment in high glucose
incubated cells significantly reduced the apoptotic cell population
percentage (3.24%; P<0.05; Fig. 3A
and B).
In order to identify apoptotic cells, Hoechst 33258
was used for staining cell nuclear to detect cell apoptosis. In the
H group, there were significantly more typical apoptotic cells, in
which the nuclear shape became petals or nucleolus pyknosised,
compared with the N and M groups (Fig.
3C). EGCG (20 µmol/l) greatly decreased the number of apoptotic
cells treated with high glucose (P<0.05; Fig. 3D).
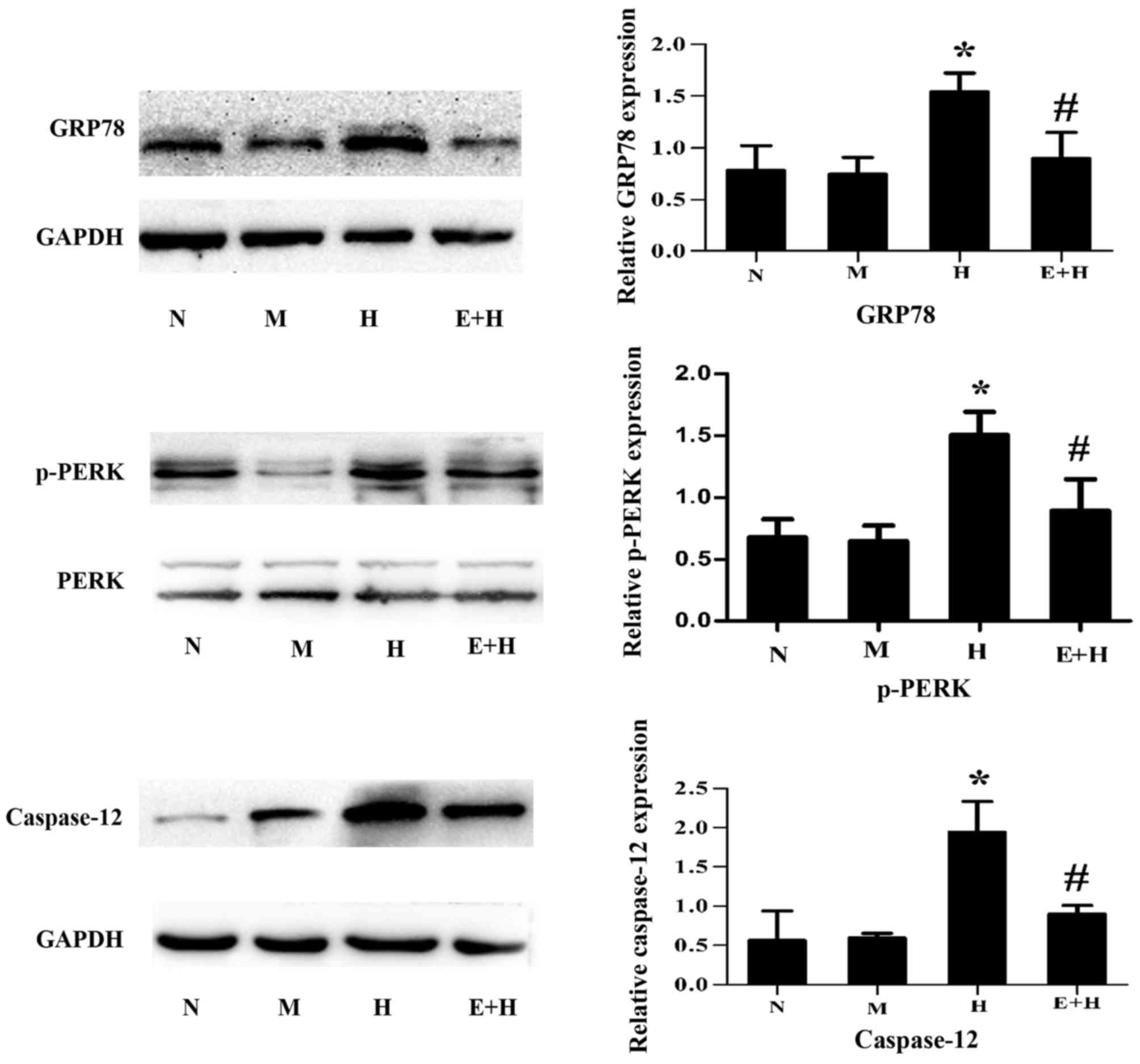
EGCG attenuates GRP78, p-PERK and
caspase-12 expression after high glucose stimulation
To investigate whether EGCG protects against high
glucose-induced podocyte injury via ER stress signaling, the
expression of ER stress-associated markers, GRP78, p-PERK and
caspase-12, were assessed. After high glucose stimulation, GRP78,
p-PERK and caspase-12 were significantly increased compared with
the normal glucose and mannitol groups (P<0.05; Fig. 4). However, these protein expression
levels were markedly attenuated after EGCG (20 µmol/l) treatment
(P<0.05; Fig. 4).
Discussion
Podocytes are a type of highly differentiated and
non-renewable glomerular epithelial cell (22). It is generally accepted that the
loss and apoptosis of podocytes which occur at an early stage of DN
are closely associated with its progression, and is a main cause of
proteinuria and glomerulosclerosis development (23). Previous studies have demonstrated
that EGCG treatment can attenuate ischemia/reperfusion-induced
renal dysfunction though inhibition of proinflammatory cytokine
cell apoptosis (24), and protect
against cisplatin-induced nephrotoxicity via suppression of ER
stress-induced (25) and
mitochondrial dependent apoptotic pathways (26). The results of the present study
demonstrated that EGCG is able to promote high-glucose induced
podocyte proliferation and can also suppress high-glucose induced
cell apoptosis of podocytes.
The ER serves an important role in adaptation of
cell alterations under different conditions. It has been
demonstrated that numerous factors, including free oxygen,
immoderate nutrients, high glucose and free fatty acids, all can
initiate apoptosis and generate ER stress and tissue damage
(27). Podocyte loss in DN may be
associated with ER stress-induced apoptosis (28,29).
High glucose can lead to cell injury and trigger ER stress in
podocytes (28). GRP78, the first
response protein and one of the main modulators of the UPR, has
been generally implicated as a marker for the initiation of ER
stress (30,31). GRP78 combines with the N-termini of
transmembrane ER proteins to prevent protein aggregation under
normal conditions. When unfolded proteins amass, GRP78 is released
and the vital transmembrane ER signaling proteins, including PERK,
IRE1 and ATF6, are triggered to launch ER stress (31,32).
PERK cleaves from GRP78, inducing its autophosphorylation through
oligomerization to further phosphorylate the α subunit of
translation initiation factor 2 (eIF2α) to trigger ER stress
(27). Thus, GRP78 serves a
fundamental role in identification of unfolded proteins.
Caspase-12, unlike other caspases, is specifically located in the
cytoplasm of the ER and can be activated during ER stress but not
through mitochondrial signals and other death stimuli (14). Upon caspase-12 activation, it can
directly enter cytosol and process with downstream caspase family
mainly including caspase-3, which leads to cell apoptosis (31). GRP78 is an inductor of ER stress
and caspase-12 is an executor of ER stress-induced apoptosis. The
current study indicates that ER stress is activated by high
glucose, and protein expression of GRP78, p-PERK and caspase-12 are
upregulated. EGCG treatment can reduce high glucose-induced ER
stress. All the results suggest that EGCG may protect from podocyte
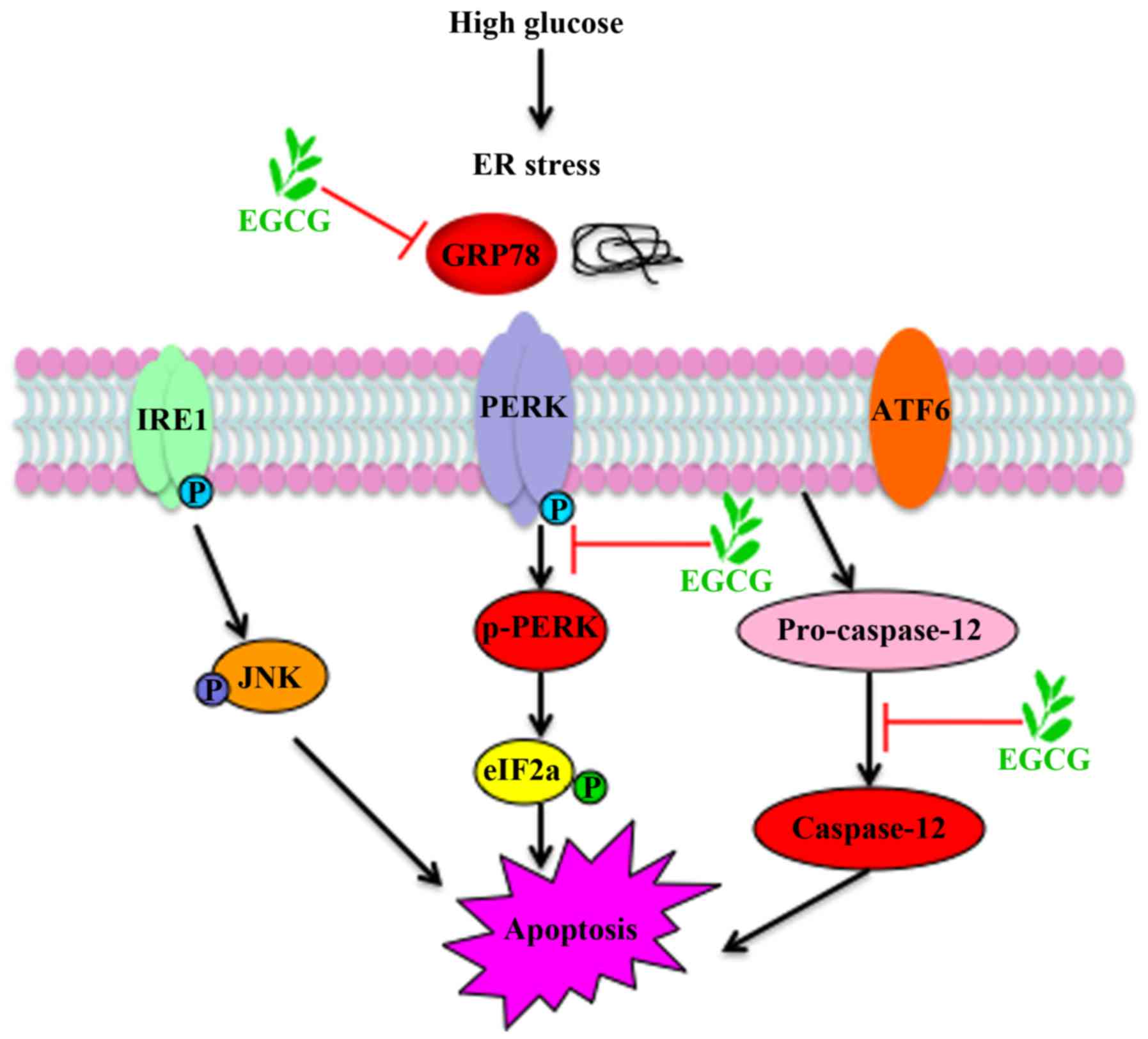
apoptosis via suppression of ER stress pathway. A schematic
representation of EGCG protection against podocyte apoptosis from
high glucose stimulation, via ER stress signaling, is presented in
Fig. 5.
In conclusion, the present study demonstrated that
EGCG promotes cell proliferation and apoptosis of high
glucose-induced podocytes. Notably, EGCG was observed to suppress
high glucose-induced ER stress-induced upregulation of GRP78,
p-PERK and caspase-12 protein expression levels. Further study is
required to clarify the efficacy of EGCG in the treatment of
disease in vivo; however, the results of the present study
indicated that it may have potential for the development of a
therapeutic drug for DN.
Acknowledgements
The present study was supported by a General
Financial Grant from the China Postdoctoral Science Foundation
(grant no. 2015M572048), the Natural Science Fund of Shandong
Province (grant no. ZR2013HM100 and ZR2014HM037) and the Science
& Technology Development Program of Shandong Province (grant
no. 201401241).
References
|
1
|
Ilatovskaya DV, Levchenko V, Lowing A,
Shuyskiy LS, Palygin O and Staruschenko A: Podocyte injury in
diabetic nephropathy: Implications of angiotensin II-dependent
activation of TRPC channels. Sci Rep. 5:176372015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khazim K, Gorin Y, Cavaglieri RC, Abboud
HE and Fanti P: The antioxidant silybin prevents high
glucose-induced oxidative stress and podocyte injury in vitro and
in vivo. Am J Physiol Renal Physiol. 305:F691–F700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma J, Wu H, Zhao CY, Panchapakesan U,
Pollock C and Chadban SJ: Requirement for TLR2 in the development
of albuminuria, inflammation and fibrosis in experimental diabetic
nephropathy. Int J Clin Exp Pathol. 7:481–495. 2014.PubMed/NCBI
|
|
4
|
Liu BC, Song X, Lu XY, Li DT, Eaton DC,
Shen BZ, Li XQ and Ma HP: High glucose induces podocyte apoptosis
by stimulating TRPC6 via elevation of reactive oxygen species.
Biochim Biophys Acta. 1833:1434–1442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brunskill EW, Georgas K, Rumballe B,
Little MH and Potter SS: Defining the molecular character of the
developing and adult kidney podocyte. PLoS one. 6:e246402011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao AL, Wang L, Chen X, Wang YM, Guo HJ,
Chu S, Liu C, Zhang XM and Peng W: Ursodeoxycholic acid and
4-phenylbutyrate prevent endoplasmic reticulum stress-induced
podocyte apoptosis in diabetic nephropathy. Lab Invest. 96:610–622.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Madhusudhan T, Wang H, Dong W, Ghosh S,
Bock F, Thangapandi VR, Ranjan S, Wolter J, Kohli S, Shahzad K, et
al: Defective podocyte insulin signalling through p85-XBP1 promotes
ATF6-dependent maladaptive ER-stress response in diabetic
nephropathy. Nat Commun. 6:64962015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown MK and Naidoo N: The endoplasmic
reticulum stress response in aging and age-related diseases. Front
Physiol. 3:2632012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo B and Lee AS: The critical roles of
endoplasmic reticulum chaperones and unfolded protein response in
tumorigenesis and anticancer therapies. Oncogene. 32:805–818. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okada T, Yoshida H, Akazawa R, Negishi M
and Mori K: Distinct roles of activating transcription factor 6
(ATF6) and double-stranded RNA-activated protein kinase-like
endoplasmic reticulum kinase (PERK) in transcription during the
mammalian unfolded protein response. Biochem J. 366:585–594. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koumenis C, Naczki C, Koritzinsky M,
Rastani S, Diehl A, Sonenberg N, Koromilas A and Wouters BG:
Regulation of protein synthesis by hypoxia via activation of the
endoplasmic reticulum kinase PERK and phosphorylation of the
translation initiation factor eIF2alpha. Mol Cell Biol.
22:7405–7416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harding HP, Calfon M, Urano F, Novoa I and
Ron D: Transcriptional and translational control in the Mammalian
unfolded protein response. Annu Rev Cell Dev Biol. 18:575–599.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nowakowska A and Tarasiuk J: Comparative
effects of selected plant polyphenols, gallic acid and
epigallocatechin gallate, on matrix metalloproteinases activity in
multidrug resistant MCF7/DOX breast cancer cells. Acta Biochim Pol.
63:571–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Granja A, Pinheiro M and Reis S:
Epigallocatechin gallate nanodelivery systems for cancer therapy.
Nutrients. 8(pii): E3072016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ortiz-López L, Márquez-Valadez B,
Gómez-Sánchez A, Silva-Lucero MD, Torres-Pérez M,
Téllez-Ballesteros RI, Ichwan M, Meraz-Ríos MA, Kempermann G and
Ramírez-Rodríguez GB: Green tea compound
epigallo-catechin-3-gallate (EGCG) increases neuronal survival in
adult hippocampal neurogenesis in vivo and in vitro. Neuroscience.
322:208–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SM, Chang YH, Chao YC, Lin JA, Wu
CH, Lai CY, Chan KC, Tseng ST and Yen GC: EGCG-rich green tea
extract stimulates sRAGE secretion to inhibit S100A12-RAGE axis
through ADAM10-mediated ectodomain shedding of extracellular RAGE
in type 2 diabetes. Mol Nutr Food Res. 57:2264–2268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SJ, Li M, Jeong CW, Bae HB, Kwak SH,
Lee SH, Lee HJ, Heo BH, Yook KB and Yoo KY:
Epigallocatechin-3-gallate, a green tea catechin, protects the
heart against regional ischemia-reperfusion injuries through
activation of RISK survival pathways in rats. Arch Pharm Res.
37:1079–1085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang EJ, Lee J, Lee SY, Kim EK, Moon YM,
Jung YO, Park SH and Cho ML: EGCG attenuates autoimmune arthritis
by inhibition of STAT3 and HIF-1alpha with Th17/Treg control. PLoS
One. 9:e860622014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leu JG, Lin CY, Jian JH, Shih CY and Liang
YJ: Epigallocatechin-3-gallate combined with alpha lipoic acid
attenuates high glucose-induced receptor for advanced glycation end
products (RAGE) expression in human embryonic kidney cells. An Acad
Bras Cienc. 85:745–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mundel P and Shankland SJ: Podocyte
biology and response to injury. J Am Soc Nephrol. 13:3005–3015.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Butt A and Riaz S: Study of protein
profiling of human urine in diabetic hypertensive nephropathy
versus normal healthy controls. Diabetes Technol Ther. 12:379–386.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv J, Feng M, Zhang L, Wan X, Zeng YC,
Liang PF and Xu AP: Protective effect of epigallocatechin gallate,
a major constituent of green tea, against renal
ischemia-reperfusion injury in rats. Int Urol Nephrol.
47:1429–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen B, Liu G, Zou P, Li X, Hao Q, Jiang
B, Yang X and Hu Z: Epigallocatechin-3-gallate protects against
cisplatin-induced nephrotoxicity by inhibiting endoplasmic
reticulum stress-induced apoptosis. Exp Biol Med (Maywood).
240:1513–1519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou P, Song J, Jiang B, Pei F, Chen B,
Yang X, Liu G and Hu Z: Epigallocatechin-3-gallate protects against
cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int
J Clin Exp Pathol. 7:4607–4616. 2014.PubMed/NCBI
|
|
27
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W
and Duan H: Role of endoplasmic reticulum stress in apoptosis of
differentiated mouse podocytes induced by high glucose. Int J Mol
Med. 33:809–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC,
Zhao DM, Li XN and Sun LK: Valproate attenuates diabetic
nephropathy through inhibition of endoplasmic reticulum
stressinduced apoptosis. Mol Med Rep. 13:661–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Groenendyk J, Sreenivasaiah PK, Kim DH,
Agellon LB and Michalak M: Biology of endoplasmic reticulum stress
in the heart. Circ Res. 107:1185–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schroder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|