Introduction
Accounting for 2–3% of all human malignancies, renal
cell carcinoma (RCC) is the most common kidney malignancy
worldwide, with clear-cell RCC (ccRCC) being the most common
subtype (1). The incidence of RCC
continues to increase and in 2012, there were >338,000 novel
cases and 143,000 individuals succumbed to kidney cancer worldwide
(2). Despite advances in diagnosis
and treatment, particularly improved imaging techniques, patients
with RCC have a poor prognosis, making RCC a serious problem for
oncological healthcare around the world (3). The 5-year cancer-specific survival
rate of metastatic ccRCC is <27.1%, decreased compared with that
of nonmetastatic ccRCC, which is 70%, and ccRCC is prone to
metastasis (4). The mechanism of
cancer metastasis and the cause of resistance to treatment are
currently poorly understood, and deserve more attention and
study.
The epithelial-mesenchymal transition (EMT), a
process that transforms epithelial cell phenotypes into mesenchymal
ones, has been demonstrated to serve a pivotal role in numerous
steps of metastatic progression in tumors. In EMT, epithelial cells
lose polarity, disassemble cell-cell junctions and gain more
mesenchymal and motile properties (5), which provides cancer cells with a
greater capacity to invade and disseminate to distant sites. This
phenomenon is triggered by a series of complex and multi-layered
growth factors recruited from the tumor microenvironments (6).
Transforming growth factor-β (TGF-β) is a
pluripotent cytokine with divergent roles in cancer progression
(7). The factor that determines
whether TGF-β acts as a tumor suppressor or promoter has been the
subject of study. Several transcription factors, including
zinc-finger proteins SNAI1 and SNAI2, zinc finger E-box-binding
homeobox 1 and 2, and Twist-related protein 1 have been defined as
initiators of EMT (8). TGF-β has
also been reported to serve a crucial role in initiating EMT in
various types of cancer (5).
However, the understanding of the function of early response
transcription factors remains unclear. The Mothers against
decapentaplegic homolog 4 (SMAD4) protein is recognized as a
central mediator of TGF-β and/or bone morphogenetic protein
signaling pathways (9). A recent
study has demonstrated that the loss of SMAD4 leads to the
dysfunction of the canonical TGF-β signaling pathway. However, in
numerous types of cancer TGF-β switches from tumor suppressor to
tumor promoter, thereby driving invasion and metastasis (10). By activating SMAD-dependent and
independent pathways, TGFβ acts as an inducer of EMT (11), and SMAD4 has been considered to be
an independent prognostic factor in ccRCC (12).
Epigenetic modification including histone
acetylation is one mechanism for controlling gene expression.
Histone acetylation is mediated by the counteracting activity of
histone acetyltransferases and histone deacetylases (HDACs). The
reversible acetylation and deacetylation of histones is always
accompanied by the activation and silencing of gene expression
(13). Histone post-translational
modifications (PTMs) serve a fundamental role in the control of
processes involving the DNA template within the cell. Although a
number of PTMs mediate their effects through histone-histone or
histone-DNA interaction, a large fraction function through the
recruitment of chromatin-associated proteins that harbor conserved
‘reader’ domains (14).
Valproic acid (VPA), a classic anticonvulsant drug
used for decades, has previously been demonstrated to be a potent
class I HDAC inhibitor (15). VPA
may induce several anticancer effects, particularly the inhibition
of cancer cell proliferation, growth and differentiation (16). Cell cycle, growth and apoptosis
were also influenced by VPA in RCC (17).
The present study presents the evidence that VPA
negatively regulates SMAD4 expression in RCC cells, thereby
inhibiting cancer cell metastasis and invasion. The aim was to
identify the role served by SMAD4 in RCC progression and metastasis
by administering VPA to RCC cell lines, and analyzing the
association between VPA, SMAD4 and EMT regulation.
Materials and methods
Cell culture and reagents
Caki-1, 786-O and HK-2 cells were used in the
present study and were purchased from the China Center for Type
Culture Collection (Wuhan, China). According to the American Type
Culture Collection, Caki-1 is a metastatic RCC cell line that
demonstrates highly invasive behavior, 786-O is a non-metastatic
RCC cell line and HK-2 is a normal renal cell line. Caki-1 cells
were cultured in Mac5a media (GIBCO; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 786-O cells were cultured in RPMI-1640
media (GIBCO; Thermo Fisher Scientific, Inc.), HK-2 cells were
cultured in DMEM/F12 media (GIBCO; Thermo Fisher Scientific, Inc.).
All cell lines were cultured in medium supplemented with 10% fetal
bovine serum (FBS; Biological Industries, Kibbutz Beit Haemek,
Israel) and 1% penicillin/streptomycin at 37°C under 5%
CO2. VPA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was diluted to a concentration of 50 mmol/l and stored at −20°C.
VPA was dissolved in medium at concentrations of 1.2, 2.4 and 5
mmol/l. Caki-1 and 786-O cells were treated in a dose-(1.2, 2.4 and
5 mmol/l for 48 h) and time-dependent manner (12, 24, 48 h at 2.4
mmol/l), and cells in the control groups were incubated with medium
alone.
Plasmids and transfection
Genechem-GV230 and Genechem-GV248 plasmids, which
express full-length human SMAD4 and short hairpin RNA (shRNA)
against SMAD4, respectively, were provided by Shanghai GeneChem
Co., Ltd., (Shanghai, China). Negative control plasmids (cat. no.
BCON0831407400) were also purchased from Shanghai GeneChem Co., Ltd
(Shanghai, China). Transfection of 786-O cells with these plasmids
was performed using Lipofectamine® 2000 in vitro
transfection reagent (Thermo Fisher Scientific, Inc.); 1 µg plasmid
was added per well of 6-well plate (1×104 cells/well).
After 10 h transfection, the medium was changed to complete
RPMI-1640 media. Then, following culture for 48 h, the cells were
treated by VPA for 48 h. The transfection efficiency was detected
by fluorescence microscopy.
Antibodies
Mouse anti-SMAD4 (cat. no. sc-7966; 1:500) and mouse
anti-β-actin (cat. no. sc-130301; 1:1,000) were obtained from Santa
Cruz Biotechnology, Inc., (Dallas, TX, USA). Mouse anti-epithelial
(E)-cadherin (cat. no. 610181; 1:1,000), mouse anti-neural
(N)-cadherin (cat. no. 610920, 1:500), and mouse anti-vimentin
(cat. no. 550513; 1:1,000 dilution) antibodies were purchased from
BD Biosciences (Franklin lakes, NJ, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-mouse immunoglobulin G (heavy and light
chains) secondary antibodies (cat. no. A0216) were acquired from
Beyotime Institute of Biotechnology (Haimen, China).
Western blot analysis
Cell lysates were prepared from transfected cells
and from VPA-treated cells following lysis in RIPA Lysis and
Extraction Buffer (Thermo Fisher Scientific, Inc.). The protein
concentrations were detected using the BCA Protein Assay kit
(23235; Thermo Fisher Scientific, Inc.) Then, 40 µg of total
proteins were separated by 10% SDS-PAGE and transferred onto a PVDF
membrane (EMD Millipore, Billerica, MA, USA). Following blocking
with 5% milk in TBST for 60 min at room temperature and probing
with protein-specific antibodies at 4°C overnight, the membranes
were incubated with secondary antibodies diluted in TBS-Tween-20
(0.075%) for 1 h at room temperature. The blots were detected with
Chemiluminescent HRP Substrate (EMD Millipore) and visualized using
an LAS-4000 Luminescent Image Analyzer (Fujifim Corporation, Tokyo,
Japan). The densitometry was assessed using ImageJ version 1.49 V
(National Institutes of Health, Bethesda, MD, USA) All experiments
were performed in duplicate and repeated three times.
Immunohistochemical staining
A total of 39 cancer tissue samples were obtained
from patients with RCC who underwent partial or radical nephrectomy
at the Shandong Provincial Hospital (Shandong, China) between
January 2004 and May 2015. Of those patients, 29 were male (age,
59.2±8.7), and 10 were female (age, 61.5±6.9). Samples were cut
into 5 µm paraffin sections, which were then deparaffinized and
rehydrated. Antigen retrieval was performed by heating the sections
for 2 min in citrate buffer at a pH 6.0. Endogenous peroxidase
activity was blocked with 3% H2O2 for 30 min.
Following subsequent blocking with 5% bovine serum albumin (BSA;
A8020; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 30 min, the sections were incubated overnight
at 4°C with primary antibodies against Smad4 (cat. no.SC-7966;
1:200; Santa Cruz Biotechnology, Inc.), TIF1-γ (cat. no. SC-101179;
1:250; Santa Cruz Biotechnology, Inc.) in phosphate-buffered saline
(PBS) and TGF-β (cat. no. RAB-023B; Fuzhou Maxim (Maixin) Biotech
Co., Ltd., Fuzhou, China), and with PBS alone as a negative
control. The sections were then incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (SE13; 1:5,000;
Beijing Solarbio Science & Technology Co., Ltd.) for 20 min at
37°C. HRP activity was detected using 3′3-diaminobenzidine for 1
min. The slides were stained with haematoxylin for 5 min at room
temperature and then dehydrated and mounted using neutral balsam.,
and three independent observers blindly performed the measurements
(magnification, ×400) using an Olympus microscope (X31-32C02;
Olympus Corporation, Tokyo, Japan). The present study was approved
by the Provincial Hospital Affiliated to Shandong University ethics
committee (Jinan, China) and all participants provided written
informed consent.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from 786-O and Caki-1 cells was analyzed
using RT-qPCR via two kits purchased from Takara Bio, Inc., Otsu,
Japan: MiniBEST Universal RNA Extraction kit (cat.no. 9767) and
PrimeScriptTM RT reagent kit (cat.no. RR037A). qPCR was performed
as follows: 42°C for 30 min, 95°C for 10 min and followed by 40
cycles of amplification at 95°C for 20 sec, 62°C for 30 sec, 72°C
for 30 sec. The following primer sequences were used: SMAD4
forward, 5′-CTTTCCCAACATTCCTGTGG-3′; reverse,
5′-ATCCATTCTGCTGCTGTCCT-3′; E-cadherin forward
5′-AGAATGACAACAAGCCCGAAT-3′; reverse, 5′-CGGCATTGTAGGTGTTCACA3′;
N-cadherin forward GGACAGTTCCTGAGGGATCA-3′; reverse,
GGATTGCCTTCCATGTCTGT, β-actin forward, 5′-AATCCCATCACCATCTTCCA-3′;
and reverse, 5′-TGGACTCCACGACGTACTCA-3′. The relative expression of
SMAD4 mRNA was determined using the expression of β-actin as a
reference. Relative mRNA expression change was determined using
2−ΔΔCt method (18).
All experiments were performed in duplicate and repeated three
times.
Migration and invasion assays
Cellular migration and invasion assays were
performed using a Boyden chamber containing 24-well Transwell
plates (Corning Incorporated, Corning, NY, USA) with 8-mm pores in
the membrane. For migration assays, ~7.5×104 cells in
200 ml of FBS-free medium were transferred into the upper chamber
and the lower chamber was filled with complete medium (containing
10% FBS) as a chemoattractant. Following 24 h of incubation at 37°C
in a 5% CO2 atmosphere, the membranes containing the
cells were fixed with 95% alcohol in 30 min at room temperature and
stained with 0.1% crystal violet Images were captured of the lower
surfaces of the membranes at 100× magnification. Images were
captured of five random fields in each chamber to determine
migration. For invasion assays, the membrane was coated with 50 ml
of diluted Matrigel® (1:7; BD Biosciences). Following
the solidification of the Matrigel at 37°C, 1.0×105
cells in 200 ml of culture medium supplemented with 1% FBS were
seeded into the upper chamber, whereas the lower chamber was filled
with complete medium. Then, the Boyden chamber was incubated at
37°C with a 5% CO2 atmosphere for 24 h. The subsequent
staining and observation procedures were identical to those of the
migration assays. Quantification was performed by counting
migratory cells using light microscopy in three individual fields
per insert. All experiments were performed in duplicate and
repeated three times.
Statistical analysis
All experiments were performed in triplicate as a
minimum. The data were statistically analyzed using SPSS for
Windows Statistics version 20 (IBM Corp., Armonk, NY, USA). The
Kruskal-Wallis H test was used to analyze
immunohistochemical-staining scores. One-way analysis of variance
was used to analyze the differences of the grey level data of the
protein bands, the relative mRNA expression levels, and the number
of invasive and migratory cells between four groups, following that
Fisher's least significant difference method was used for post hoc
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Profile of SMAD4 status and EMT
expression in renal cells of different characteristics
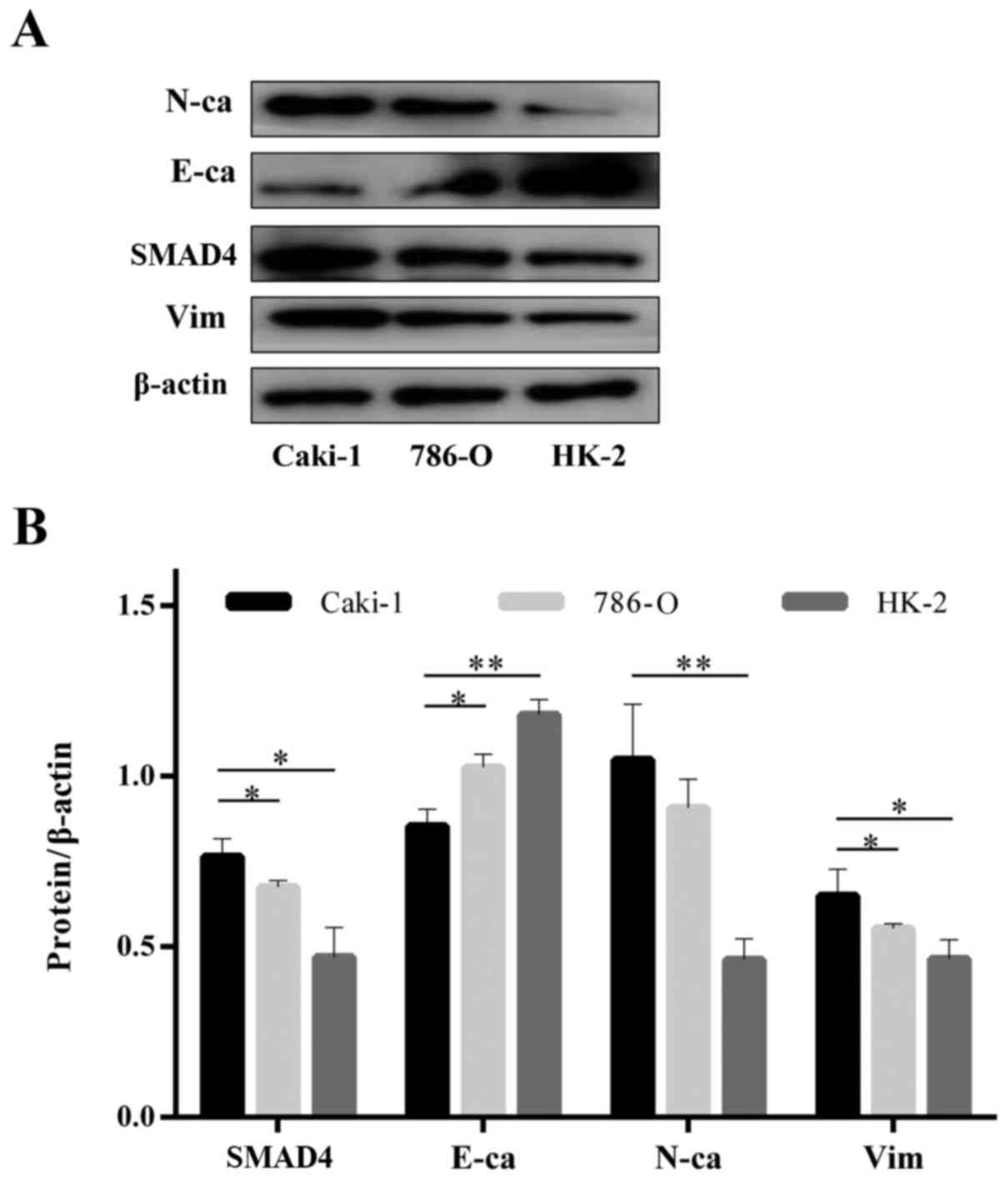
Different expression patterns of SMAD4 and EMT
markers were observed in Caki-1 and 786-O cell lines and therefore
all three of the cell lines (Caki-1, 786-O, HK-2) of varying
invasive capacities were screened for SMAD4 and EMT marker
expression. SMAD4 protein was highly expressed in Caki-1 cells,
which exhibited greater invasive behavior, whereas 786-O and HK-2
cells had a lower expression level of SMAD4 and mesenchymal markers
(N-cadherin and vimentin) and a lower invasive ability (Fig. 1A and B). Together, these results
suggest a potential correlation between EMT characteristics, SMAD4
expression and cell invasion in RCC cells.
VPA alters the expression of EMT
markers and suppresses migration and invasion in RCC cells
The expression of SMAD4 and EMT markers in RCC cell
lines was analyzed using western blotting. In Caki-1 and 786-O
cells, treatment with VPA decreased expression of SMAD4 and the
mesenchymal markers N-cadherin and vimentin but increased
E-cadherin expression (Figs. 2 and
3), providing more evidence of the
influence of VPA on EMT. Further experiments demonstrated that the
SMAD4 mRNA level was suppressed in a dose (VPA doses of 1.2, 2.4
and 5 mmol/l) and a time-dependent manner (0, 12, 24, 48 h) in the
two cell lines (Fig. 4). In
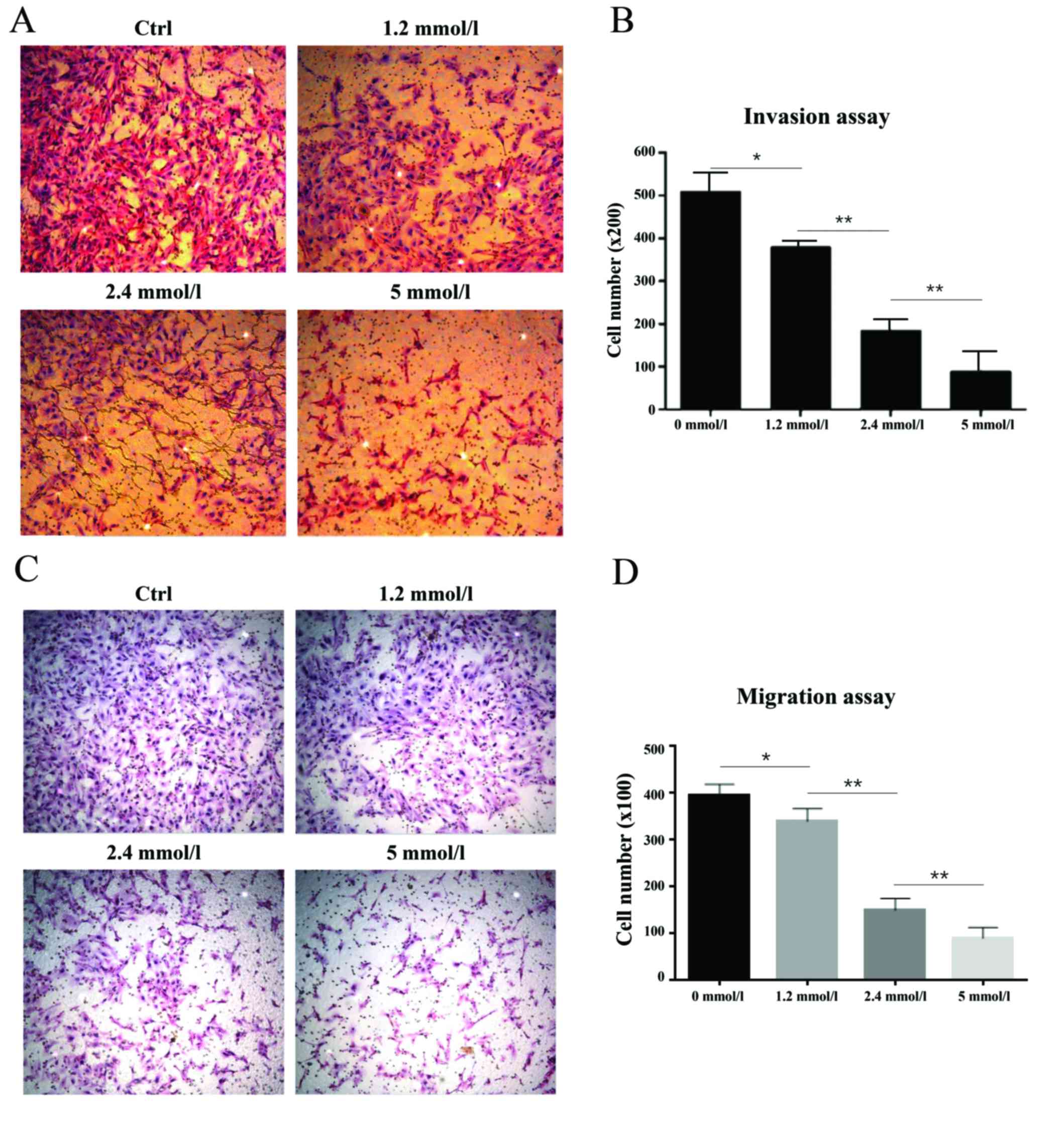
vitro cell invasion and migration assays were performed to
study the invasive and migratory ability of 786-O cells treated
with different VPA concentrations (Fig. 5). It was demonstrated that the
lower and higher concentrations of VPA significantly diminished
cell invasive and migratory ability in 786-O cells (P<0.05).
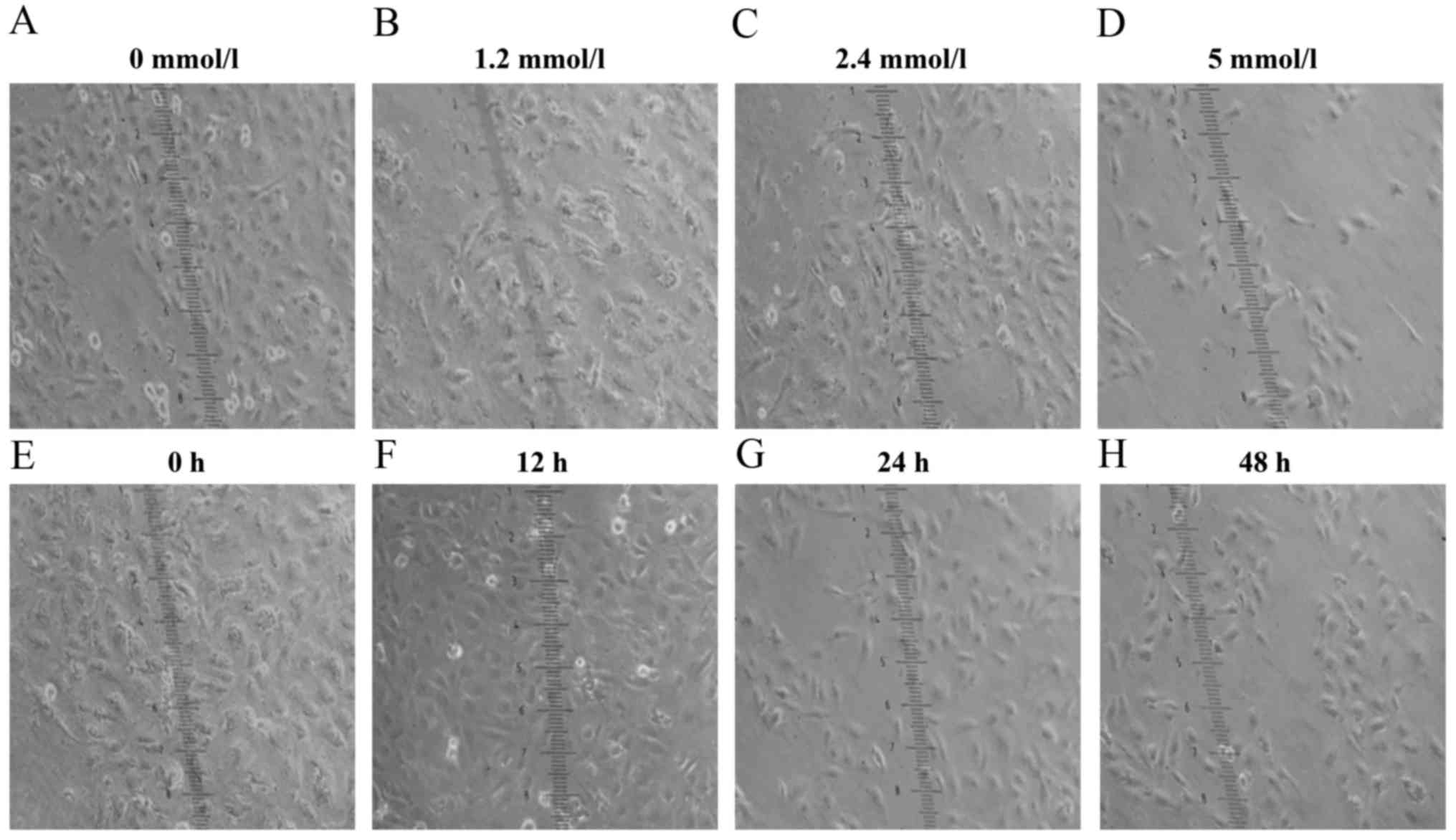
Tumor cell invasion and migration ability were suppressed by VPA
treatment. VPA treatment induced a transition from round-like to
long-shaped morphology (Fig.
6).
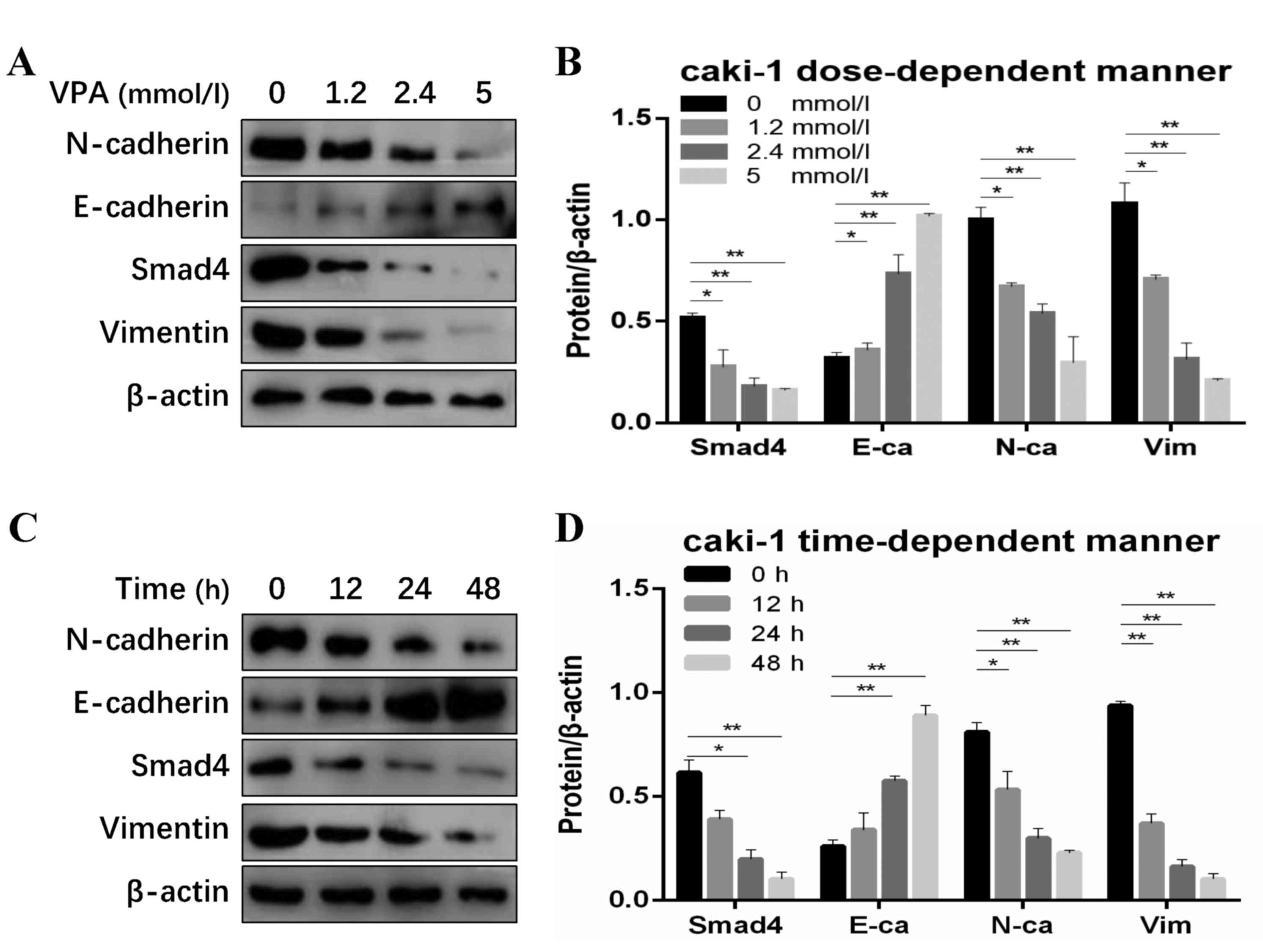 | Figure 2.SMAD4 and EMT markers were inhibited
by VPA in renal cell carcinoma cells. (A) Western blot and (B)
quantified protein expression levels of SMAD4 and EMT makers
treated with VPA in a dose-dependent manner (0, 1.2, 2.4 and 5
mmol/l) in Caki-1 cells. (C) Western blot and (D) quantified
protein expression levels of SMAD4 and EMT markers treated with VPA
in a time-dependent (0, 12, 24 and 48 h) manner in Caki-1 cells.
Data is demonstrated as the mean ± standard deviation from three
independent experiments. *P<0.05 vs. control group, **P<0.01
vs. control group. N-ca, neural cadherin; E-ca, epithelial
cadherin; EMT, epithelial-mesenchymal transition; VPA, valproic
acid; Vim, vimentin; SMAD 4, Mothers against decapentaplegic
homolog 4. |
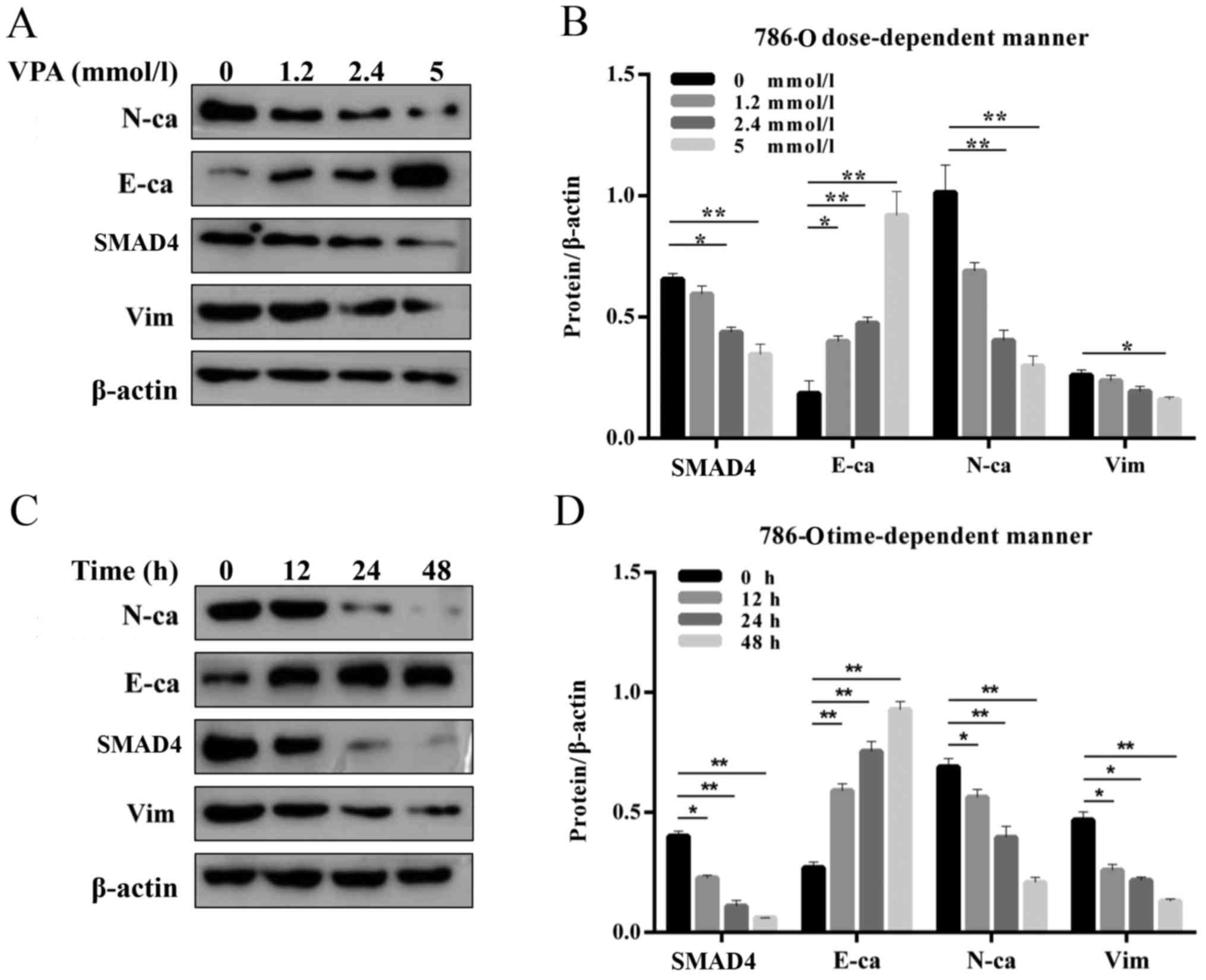 | Figure 3.SMAD4 and EMT makers were inhibited by
VPA in renal cell carcinoma cells. (A) Western blot and (B)
quantified protein expression levels of SMAD4 and EMT makers
treated with VPA in a dose-dependent manner (0, 1.2, 2.4 and 5
mmol/l) in 786-O cells. (C) Western blot and (D) quantified protein
expression levels of SMAD4 and EMT makers treated with VPA in a
time-dependent (0, 12, 24 and 48 h) manner in 786-O cells. Data is
demonstrated as the mean ± standard deviation from three
independent experiments. *P<0.05 vs. control group, **P<0.01
vs. control group. N-ca, neural cadherin; E-ca, epithelial
cadherin; EMT, epithelial-mesenchymal transition; VPA, valproic
acid; Vim, vimentin; SMAD4, Mothers against decapentaplegic homolog
4. |
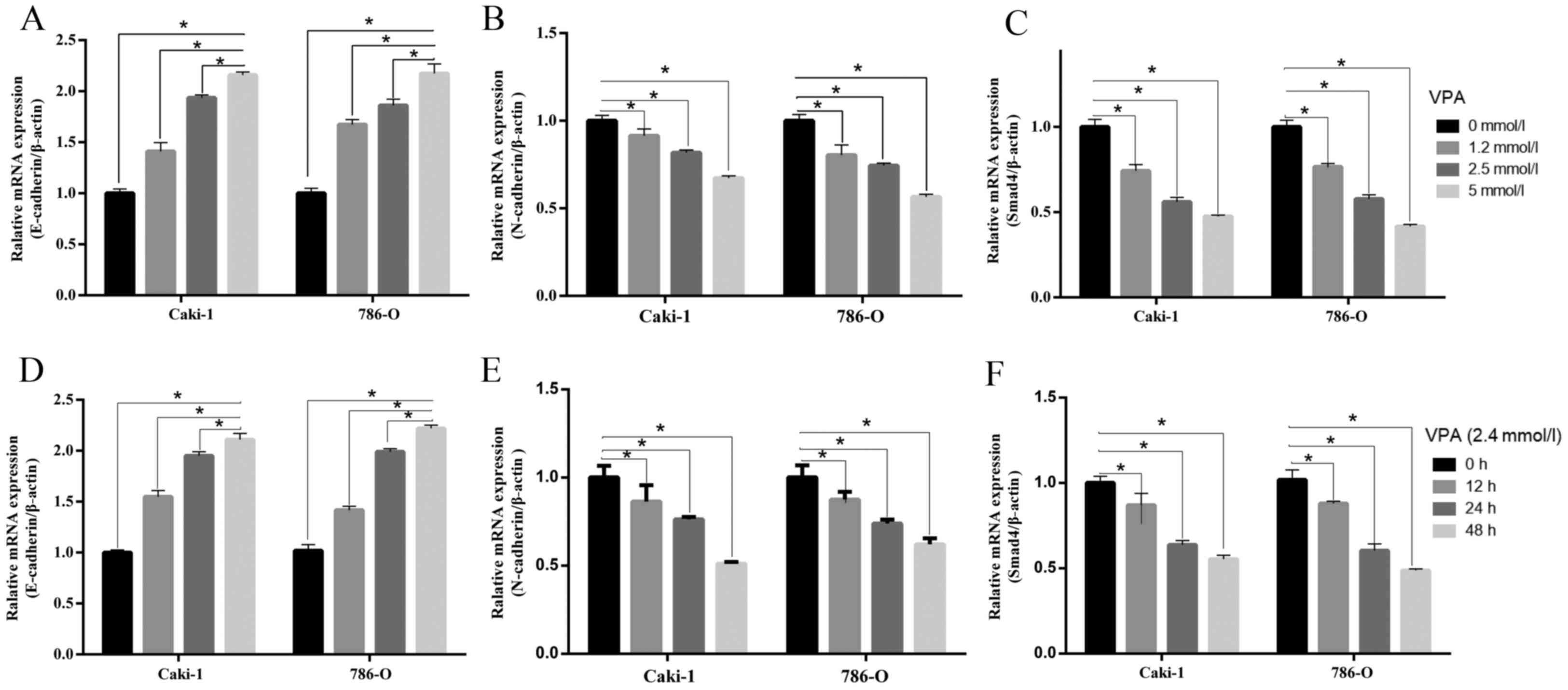 | Figure 4.mRNA levels in SMAD4 and
epithelial-mesenchymal transition markers. (A) E-cadherin, (B)
N-cadherin and (C) SMAD4 mRNAs were measured by reverse
transcription-quantitative polymerase chain reaction assay
following cells treated with VPA in a dose-dependent (0, 1.2, 2.4
and 5 mmol/l) manner in Caki-1 and 786-O cells. (D) E-cadherin, (E)
N-cadherin and (F) SMAD4 expression following treatment with VPA in
a time-dependent (0, 12, 24 and 48 h) manner in Caki-1 and 786-O
cells. Data is demonstrated as the mean ± standard deviation from
three independent experiments. *P<0.05 vs. control group,
**P<0.01 vs. control group. N-cadherin, neural cadherin;
E-cadherin, epithelial cadherin; VPA, valproic acid; SMAD4, Mothers
against decapentaplegic homolog 4. |
SMAD4 expression regulates EMT status
with or without VPA treatment
To confirm the interaction between SMAD4 and EMT
status, 786-O cells were transfected with a plasmid to transiently
knock down SMAD4 expression. Western blot analysis confirmed the
efficiency of transfection and demonstrated near absence of SMAD4
in the knockdown 786-O cells, accompanied with significantly
upregulated E-cadherin, and downregulated N-cadherin and vimentin
expression (Fig. 7). Following
treatment with VPA, SMAD4-knockdown cells exhibited little
alteration in the expression levels of E-cadherin and N-cadherin.
Additionally, 786-O cells were transfected with a plasmid that
upregulated SMAD4 and were then treated by VPA. Compared with the
control, E-cadherin protein levels were significantly decreased,
and N-cadherin and vimentin expression were increased, which
indicated that SMAD4 overexpression negated the inhibitory effect
of VPA on EMT (Fig. 7).
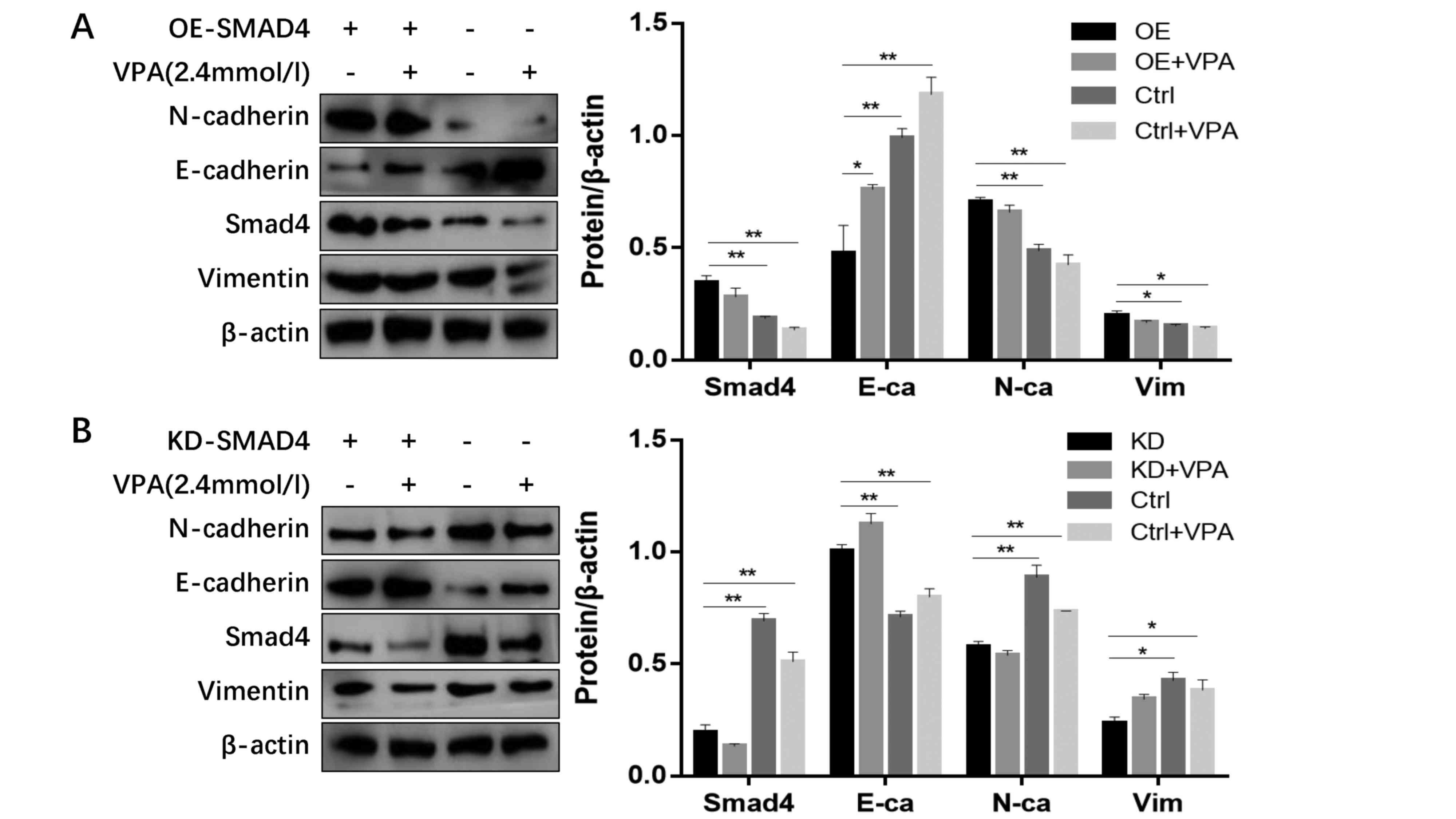 | Figure 7.Western blot analysis of 786-O cells
that received various treatments. (A) Western blot analysis and
quantified relative protein expression levels of E-cadherin,
N-cadherin, vimentin and SMAD4 expression in OE-SMAD4 786-O cells
treated with 2.4 mmol/l VPA. (B) Western blot analysis and
quantified relative protein expression levels of E-cadherin,
N-cadherin, vimentin and SMAD4 expression in KD-SMAD4 786-O cells
treated with 2.4 mmol/l VPA. *P<0.05 vs. control group,
**P<0.01 vs. control group. OE, over-expression; KD, knockdown;
VPA, valproic acid; E-ca/E-cadherin, epithelial-cadherin;
N-ca/N-cadherin, neural-cadherin; Ctrl, control; SMAD4, Mothers
against decapentaplegic homolog 4. |
SMAD4, TIF1-γ and TGF-β expression in
vivo
To further investigate the role of SMAD4 in the
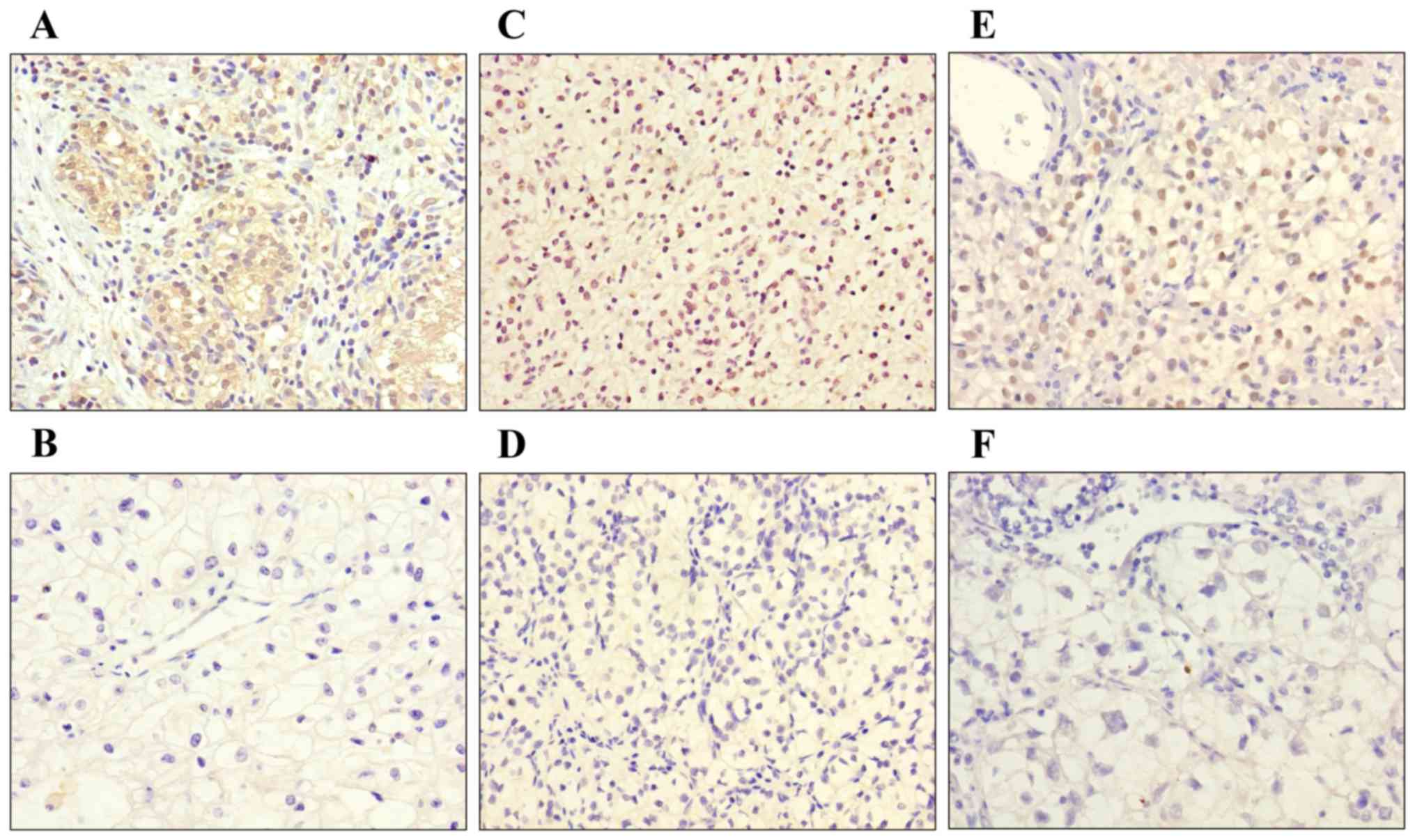
TGF-β signaling pathway, immunohistochemical staining was performed
on 39 specimens to detect the expression levels of SMAD4, TGF-β and
TIF1-γ in RCC. According to pathology results, 8 cases (20.5%) were
assessed as Fuhrman grade I, 24 (61.5%) as grade II and 7 (18%) as
grade III. It was observed that TGF-β expression was high in 5
cases (12.8%; Fig. 8A), while 21
cases (53.8%) demonstrated low expression (Fig. 8B). Nuclear and cytoplasmic SMAD4
expression was high in 6 cases (15.4%; Fig. 8C) and low in 20 cases (51.3%;
Fig. 8D). TIF1-γ expression was
high in 5 cases (12.8%; Fig. 8E),
while 19 cases (48.7%) demonstrated low expression (Fig. 8F). Immunohistochemical staining
demonstrated that SMAD4 expression was associated with higher
Fuhrman grade and low expression of TIF1-γ was associated with
lower tumor Fuhrman grade (P<0.05). The expression levels in the
present study are listed in Table
I. However, the associations between SMAD4, TIF1-γ and TGF-β
expression and patient age, tumor size, pathological tumor, node
and metastasis (pTNM) stage, cancer-specific survival or
progression-free survival were not analyzed.
 | Table I.SMAD4 and TIF1-γ expression in 39
clear renal cell carcinoma (RCC) patients with correlation with
Fuhrman grade. |
Table I.
SMAD4 and TIF1-γ expression in 39
clear renal cell carcinoma (RCC) patients with correlation with
Fuhrman grade.
|
| SMAD4
expression | TIF1-γ
expression |
|---|
|
|
|
|
|---|
| No. | Low | Moderate | High | P-value | Low | Moderate | High | P-value |
|---|
| Grade |
|
|
| 0.048 |
|
|
| 0.014 |
| G
I | 3 | 4 | 1 |
| 5 | 3 | 0 |
|
| G
II | 15 | 8 | 1 |
| 13 | 10 | 1 |
|
| G
III | 2 | 1 | 4 |
| 1 | 2 | 4 |
|
|
| 20 | 13 | 6 |
| 19 | 15 | 5 |
|
Discussion
Previous studies have demonstrated that EMT, a
process by which epithelial cells lose their polarity and acquire a
more aggressive tumor behavior, is a crucial process in the
induction of tumor invasion and metastasis (19). The loss of E-cadherin expression,
which is associated with the epithelial phenotype, is an event in
EMT and in tumor progression, and facilitates cell invasion and
metastasis, a crucial step in RCC progression (20). Early metastasis remains a challenge
to the treatment of RCC. HDAC inhibitors (HDACi) have been
recognized as anti-cancer agents. However, numerous other
applications of HDACi have been discussed, including in infection,
inflammation and innate immunity (21). HDACi have been used in renal
disease, including injury by vorinostat (22) and have been used in combination
with other drugs to treat RCC (23). An epidemiological phenomenon was
that alterations in histone status and DNA methylation occurring in
RCC induced cell proliferation and inhibited tumor growth,
differentiation and apoptosis (24). Additionally, the HDACi Trichostatin
A and sodium butyrate potently inhibited the development of a
cancer stem cell-like phenotype in squamous cell carcinoma
(25), and MS-275 and vorinostat
inhibited the metastatic capacity of lung and breast cancer cells
(26).
The authors previously demonstrated that VPA
inhibited metastasis in prostate cancer (27). The expression of E-cadherin
increased following treatment with VPA in ovarian cancer (28) and this effect was further
demonstrated in thyroid cancer cells (29). VPA has been used increasingly in
clinical practice (30) in a novel
role for the well-known drug. VPA suppresses tumor growth and
metastasis (31), and also induces
tumor differentiation and apoptosis in vitro and in
vivo, in hematopoietic and solid malignant diseases. However,
little is known about how VPA influences the invasion and
metastasis of tumors. An additional study reported that HDACi could
inhibit EMT (32) Based on the
present study, VPA has a role in reversing EMT in RCC; VPA
significantly changed EMT markers in both 786-O and Caki-1 cells.
These results could provide targeted treatment in RCC.
The major control point of EMT is the TGF-β
signaling pathway and SMAD4 serves a central role in this pathway.
SMAD proteins are divided into the following three subclasses: The
receptor-regulated SMADs (R-SMADs), the common-partner SMADs
(Co-SMAD) and the inhibitory SMADs. R-SMADs (SMAD2 and 3) bind to
the Co-SMAD, SMAD4, to form complexes that modulate downstream gene
expression in the nucleus (33).
Previous studies have uncovered a connection between canonical
SMAD4 signaling and EMT (34,35).
Therefore, it is necessary to test the role of SMAD4 in EMT in
renal carcinoma. The present study identifies novel molecular
mechanisms that may contribute to the invasion and/or metastasis of
cancer cells. In the present study, 786-O cells were transfected
with a plasmid knocking down SMAD4 expression. In vitro
knock-down of SMAD4 resulted in increased E-cadherin levels,
decreased N-cadherin expression, whereas overexpression of SMAD4
lowered E-cadherin and increased N-cadherin expression. Invasion
and metastasis are largely mediated by the loss of E-cadherin
protein or functionality, as it is vital for maintaining signal
transduction and preserves physical junctions in epithelial cells.
E-cadherin is associated with ccRCC staging and grading as well as
with lymph node involvement and the presence of distant metastasis.
The epithelial or mesenchymal phenotype of cells is characterized
by N-cadherin, E-cadherin and vimentin expression. These could be
targets for EMT, but to the best of the authors' knowledge no
studies have been conducted into the underlying mechanism of
VPA-regulation of the EMT process. In the present study, it was
demonstrated that in RCC cells knockdown of SMAD4 produced the same
effect as treatment with VPA: Inhibiting tumor cell invasion and
migration in RCC cell lines. It was demonstrated that SMAD4 could
alter N-cadherin and E-cadherin expression, indicating a
TGF-β-SMAD4-N-cadherin/E-cadherin transcription pathway. These
results highlight a broad role for SMAD4 in TGF-β induced EMT.
Despite greater understanding and identification of
SMAD4 protein, it remains unclear as to what controls the switch of
TGF-β from tumor suppressor to tumor promoter. The exact function
of SMAD4 has not been investigated extensively. A previous study
reported that SMAD4 serves as a novel prognostic marker in patients
with RCC; nuclear expression of SMAD4 was correlated with smaller
tumor size, lower nuclear grade, pTNM stage and reduced tumor
progression (12). Paradoxically,
another study presented opposite results (33). Tumor cells usually secrete abundant
TGF-β, which promotes tumor progression and the nuclear SMAD3/SMAD4
complex promotes breast cancer metastasis (36). Kang et al (33) identified that SMAD4 is critical for
the TGF-β-driven upregulation of N-cadherin and increases migration
and invasion of human pancreatic ductal epithelial cells. Notably,
elevation of SMAD4 is associated with poor patient outcome
following surgery. Knockdown of SMAD4 reduced the efficiency of
colony formation and the migratory capacity of HCC cells in
vivo (37).
The inhibitory effect of VPA on the EMT process was
confirmed and the role of SMAD4 in this process was tested.
However, certain problems remain to be solved. Other mechanisms may
also exist that mediate the VPA-regulated EMT process. Noguchi
et al (38) revealed that
histone modification correlated with EMT. It is reasonable to make
a connection between different media proteins and EMT regulation.
TIF1-γ may serve a potential role in cancer, targeting SMAD4
expression and cellular localization. As VPA can selectively
regulate TIF1-g expression, future work should focus on TIF1-γ and
ubiquitinated SMAD4 protein, or combinational therapy.
In conclusion, the results of the present study
suggest that further studies are warranted to dissect the roles of
SMAD4 in tumor progression. The present study establishes the
diverse mechanisms underlying cancer metastasis that involve SMAD4
regulation of EMT via the TGF-β signaling pathway and points to
potential applications of SMAD4 as an indicator of innovative,
clinically effective therapies for RCC.
References
|
1
|
Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y,
Ai Q, Zhang P, Song EL, Huang QB, et al: Downregulation of FOXO3a
promotes tumor metastasis and is associated with metastasis-free
survival of patients with clear cell renal cell carcinoma. Clin
Cancer Res. 20:1779–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
King SC, Pollack LA, Li J, King JB and
Master VA: Continued increase in incidence of renal cell carcinoma,
especially in young and high-grade disease: United States 2001 to
2010. J Urol. 191:1665–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ljungberg B, Campbell SC, Choi HY, Jacqmin
D, Lee JE, Weikert S and Kiemeney LA: The epidemiology of renal
cell carcinoma. Eur Urol. 60:615–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novara G, Ficarra V, Antonelli A, Artibani
W, Bertini R, Carini M, Cunico S Cosciani, Imbimbo C, Longo N,
Martignoni G, et al: Validation of the 2009 TNM version in a large
multi-institutional cohort of patients treated for renal cell
carcinoma: Are further improvements needed? Eur Urol. 58:588–595.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertran E, Crosas-Molist E, Sancho P, Caja
L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E
and Fabregat I: Overactivation of the TGF-α pathway confers a
mesenchymal-like phenotype and CXCR4-dependent migratory properties
to liver tumor cells. Hepatology. 58:2032–2044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kotiyal S and Bhattacharya S: Breast
cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res
Commun. 453:112–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isogaya K, Koinuma D, Tsutsumi S, Saito
RA, Miyazawa K, Aburatani H and Miyazono K: A Smad3 and
TTF-1/NKX2-1 complex regulates Smad4-independent gene expression.
Cell Res. 24:994–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiao Y, Shiue CN, Zhu J, Zhuang T, Jonsson
P, Wright AP, Zhao C and Dahlman-Wright K: AP-1-mediated chromatin
looping regulates ZEB2 transcription: New insights into
TNFα-induced epithelial-mesenchymal transition in triple-negative
breast cancer. Oncotarget. 6:7804–7814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voorneveld PW, Kodach LL, Jacobs RJ, Liv
N, Zonnevylle AC, Hoogenboom JP, Biemond I, Verspaget HW, Hommes
DW, de Rooij K, et al: Loss of SMAD4 alters BMP signaling to
promote colorectal cancer cell metastasis via activation of Rho and
ROCK. Gastroenterology. 147:196–208.e13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Li Y, Yang X, Yuan H, Li X, Qi M,
Chang YW, Wang C, Fu W, Yang M, et al: ERG-SOX4 interaction
promotes epithelial-mesenchymal transition in prostate cancer
cells. Prostate. 74:647–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaudhry P, Fabi F, Singh M, Parent S,
Leblanc V and Asselin E: Prostate apoptosis response-4 mediates
TGF-β-induced epithelial-to-mesenchymal transition. Cell Death Dis.
5:e10442014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JH, Lee C, Suh JH, Chae JY and Moon
KC: Nuclear expression of Smad proteins and its prognostic
significance in clear cell renal cell carcinoma. Hum Pathol.
44:2047–2054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao MJ, Li X, Huang J, Gropp GM, Gjetvaj
B, Lindsay DL, Wei S, Coutu C, Chen Z, Wan XC, et al:
SCARECROW-LIKE15 interacts with HISTONE DEACETYLASE19 and is
essential for repressing the seed maturation programme. Nat Commun.
6:72432015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Musselman CA, Lalonde ME, Côté J and
Kutateladze TG: Perceiving the epigenetic landscape through histone
readers. Nat Struct Mol Biol. 19:1218–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tassara M, Döhner K, Brossart P, Held G,
Götze K, Horst HA, Ringhoffer M, Köhne CH, Kremers S, Raghavachar
A, et al: Valproic acid in combination with all-trans retinoic acid
and intensive therapy for acute myeloid leukemia in older patients.
Blood. 123:4027–4036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouyang DY, Xu LH, He XH, Zhang YT, Zeng
LH, Cai JY and Ren S: Autophagy is differentially induced in
prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing
profiles of ATG5. Autophagy. 9:20–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Zhang X, Huang T, Geng J, Liu M
and Zheng J: Combination of metformin and valproic acid
synergistically induces cell cycle arrest and apoptosis in clear
cell renal cell carcinoma. Int J Clin Exp Pathol. 8:2823–2828.
2015.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HN, Yuan K, Xie N, Wang K, Huang Z,
Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG and Huang C: PDLIM1
stabilizes the e-cadherin/β-catenin complex to prevent
epithelial-mesenchymal transition and metastatic potential of
colorectal cancer cells. Cancer Res. 76:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beier UH, Akimova T, Liu Y, Wang L and
Hancock WW: Histone/protein deacetylases control Foxp3 expression
and the heat shock response of T-regulatory cells. Curr Opin
Immunol. 23:670–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Advani A, Huang Q, Thai K, Advani SL,
White KE, Kelly DJ, Yuen DA, Connelly KA, Marsden PA and Gilbert
RE: Long-term administration of the histone deacetylase inhibitor
vorinostat attenuates renal injury in experimental diabetes through
an endothelial nitric oxide synthase-dependent mechanism. Am J
Pathol. 178:2205–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zibelman M, Wong YN, Devarajan K, Malizzia
L, Corrigan A, Olszanski AJ, Denlinger CS, Roethke SK, Tetzlaff CH
and Plimack ER: Phase I study of the mTOR inhibitor ridaforolimus
and the HDAC inhibitor vorinostat in advanced renal cell carcinoma
and other solid tumors. Invest New Drugs. 33:1040–1047. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramakrishnan S and Pili R: Histone
deacetylase inhibitors and epigenetic modifications as a novel
strategy in renal cell carcinoma. Cancer J. 19:333–340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chikamatsu K, Ishii H, Murata T, Sakakura
K, Shino M, Toyoda M, Takahashi K and Masuyama K: Alteration of
cancer stem cell-like phenotype by histone deacetylase inhibitors
in squamous cell carcinoma of the head and neck. Cancer Sci.
104:1468–1475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bruzzese F, Leone A, Rocco M, Carbone C,
Piro G, Caraglia M, Di Gennaro E and Budillon A: HDAC inhibitor
vorinostat enhances the antitumor effect of gefitinib in squamous
cell carcinoma of head and neck by modulating ErbB receptor
expression and reverting EMT. J Cell Physiol. 226:2378–2390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan X, Lu G, Yuan C, Mao S, Jiang W, Chen
Y, Jin X and Xia Q: Valproic acid (VPA) inhibits the
epithelial-mesenchymal transition in prostate carcinoma via the
dual suppression of SMAD4. J Cancer Res Clin Oncol. 142:177–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shan Z, Feng-Nian R, Jie G and Ting Z:
Effects of valproic acid on proliferation, apoptosis, angiogenesis
and metastasis of ovarian cancer in vitro and in vivo. Asian Pac J
Cancer Prev. 13:3977–3982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Xu D, Zhu SJ, Ye B, Dong JD, Zhang
YL and Zhang Y: Induction of apoptosis and autophagy in metastatic
thyroid cancer cells by valproic acid (VPA). Int J Clin Exp Pathol.
8:8291–8297. 2015.PubMed/NCBI
|
|
30
|
Bilen MA, Fu S, Falchook GS, Ng CS, Wheler
JJ, Abdelrahim M, Erguvan-Dogan B, Hong DS, Tsimberidou AM,
Kurzrock R and Naing A: Phase I trial of valproic acid and
lenalidomide in patients with advanced cancer. Cancer Chemother
Pharmacol. 75:869–874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Byun SS, Kim FJ, Khandrika L, Kumar B,
Koul S, Wilson S and Koul HK: Differential effects of valproic acid
on growth, proliferation and metastasis in HTB5 and HTB9 bladder
cancer cell lines. Cancer Lett. 281:196–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mishra VK, Wegwitz F, Kosinsky RL, Sen M,
Baumgartner R, Wulff T, Siveke JT, Schildhaus HU, Najafova Z, Kari
V, et al: Histone deacetylase class-I inhibition promotes
epithelial gene expression in pancreatic cancer cells in a BRD4-
and MYC-dependent manner. Nucleic Acids Res. Mar 27–2017.
View Article : Google Scholar
|
|
33
|
Kang Y, Ling J, Suzuki R, Roife D,
Chopin-Laly X, Truty MJ, Chatterjee D, Wang H, Thomas RM, Katz MH,
et al: SMAD4 regulates cell motility through transcription of
N-cadherin in human pancreatic ductal epithelium. PLoS One.
9:e1079482014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan Y, Shu X, Sun L, Yu L, Sun L, Yang Z
and Ran Y: miR196a5p modulates gastric cancer stem cell
characteristics by targeting Smad4. Int J Oncol. 50:1965–1976.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu RS, Hong JJ, Wu JF, Yan S, Wu D, Liu N,
Liu QF, Wu QW, Xie YY, Liu YJ, et al: OVOL2 antagonizes TGF-β
signaling to regulate epithelial to mesenchymal transition during
mammary tumor metastasis. Oncotarget. 8:39401–39416.
2017.PubMed/NCBI
|
|
36
|
Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu
M, Feng XH, Sawaya R, Medema RH, Hung MC and Huang S: Sustained
activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer
metastasis. J Clin Invest. 124:564–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hernanda PY, Chen K, Das AM, Sideras K,
Wang W, Li J, Cao W, Bots SJ, Kodach LL, de Man RA, et al: SMAD4
exerts a tumor-promoting role in hepatocellular carcinoma.
Oncogene. 34:5055–5068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Noguchi S, Eitoku M, Moriya S, Kondo S,
Kiyosawa H, Watanabe T and Suganuma N: Regulation of gene
expression by sodium valproate in epithelial-to-mesenchymal
transition. Lung. 193:691–700. 2015. View Article : Google Scholar : PubMed/NCBI
|