Introduction
Obstructive sleep apnea syndrome (OSAS), which
affects 2–4% of the population worldwide, is a common sleep
disorder and potential clinical risk factor. OSAS has been widely
considered as a severe problem associated with a range of
pathological consequences, including hypoxia (1), hypercapnia (2), imbalance of nerve regulation function
(3), activation of the rennin
angiotensin aldosterone system, catecholamine and endothelin
secretion, endocrine dysfunction and hemodynamic changes (4). Of all the problems reported in
patients with OSAS, the most important consequence is the risk of
cardiovascular and cerebrovascular disease (5). OSAS has long been confirmed as one of
the most important independent risk factors for atherosclerosis
(AS) and hypertension (6). Tissue
hypoxia followed by chronic inflammation damage and the involvement
of a variety of inflammatory cytokines leads to AS (7).
Continuous positive airway pressure (CPAP) therapy
is the most effective method for OSAS (8). Adequate CPAP treatment can increase
pulmonary ventilation and ameliorate the inflammation of arteries
in patients with OSAS (9).
Patients show improvements in blood oxygen concentration on the
first day following CPAP treatment, and further benefits are
observed during an extended course of treatment (10). The molecular and immunological
mechanisms underlying this type of therapy are a major concern when
selecting CPAP in patients with OSAS.
According to clinical observations and previous
investigations, the present study hypothesized that, in patients
with OSAS without clinical interventions, the plasma levels of
interleukin (IL)-18, tumor necrosis factor (TNF)-α (11), C-reactive protein (CRP),
intercellular cell adhesion molecule 1 (ICAM-1), vascular cell
adhesion molecule 1 (VCAM-1), E-selectin and P-selectin are
significantly altered. The present study investigated these factors
and compared changes following CPAP (12). On the basis of these observations,
alterations in the clinical characteristics of OSAS following
treatment were observed. In order to evaluate the effect of CPAP
(5) on inflammatory factors and
AS, the present study also examined the carotid intima-media
thickness (IMT) and brachial-ankle pulse wave velocity (Ba-PWV)
(13). The aim of the present
study was to evaluate the effects of a longer (3-month) period of
CPAP treatment on oxygen ventilation in patients with OSAS.
Subjects and methods
Subjects and healthy donors
In the present study, a total of 100 patients were
recruited from The Affiliated Jiangning Hospital of Nanjing Medical
University (Nanjing, China) upon diagnosis on interpretation of
clinical guidelines on the treatment of OSAS (American College of
Physicians, 2013) (14). The
patients included 82 men and 18 women, aged between 43 and 69 years
old (mean age, 55.28±7.12 years). These patients were prospectively
recruited on preparing to attend the Sleep Clinic for overnight
polysomnography (PSG) between March 2014 and March 2016, who met
the standard definition of an apnea-hypopnea index (AHI) ≥5. None
of the patients were suffering from serious disease requiring
treatment. As a healthy control group, 50 individuals undergoing
physical examination at The Affiliated Jiangning Hospital of
Nanjing Medical University were invited to join in this study
sequence. There were no differences in age, gender or body mass
index (BMI) between the healthy control group and the OSAS
group.
The exclusion criteria included patients or healthy
donors with coronary heart disease, valvular heart disease,
cardiomyopathy, tumors, severe liver and kidney dysfunction, severe
lung disease, hyperlipidemia, diabetes, hypertension, infection or
trauma, or had undergone surgery in the previous 2 weeks. The
present study was approved by the Ethics Committee of The
Affiliated Jiangning Hospital of Nanjing Medical University.
Written informed consent was obtained from each patient and healthy
donor.
Specimen collection and detection of
inflammatory factors
Blood samples were collected from the study subjects
in the early morning (6:00 a.m.). Fasting blood was collected again
from the patients with OSAS following CPAP treatment for 3 months
(again at 6:00 a.m.). All blood samples were 10 ml in volume and
obtained from the elbow vein. The samples underwent 300 × g
centrifugation at 10°C for 10 min, and the upper plasma was stored
at −80°C. IL-18 and TNF-α, CRP, ICAM-1, VCAM-1, E-selectin and
P-selectin were detected using an enzyme-linked immunosorbent assay
(ELISA). The concentrations of the inflammatory factors were
measured using human ELISA kits (Amersham; GE Healthcare Life
Sciences, Chalfont, UK).
CPAP treatment
The recording and comparing of sleep monitoring
parameters during the 3-month treatment period were performed using
a compumedics PSG monitoring system (ResMed, Sydney, Australia).
The AHI and transcutaneous oxygen saturation (SpO2) were
detected and recorded prior to and following the 3-month treatment
period.
Ultrasound measurement of carotid
IMT
The carotid artery IMT was detected using the Mylab
90 ultrasonic instrument (Esaote, Genoa, Italy) as the quality of
IMT.
Detection of Ba-PWV
The Ba-PWV was detected using an automatic AS
testing equipment (BP-203RPEII; Omron Colin, Tokyo, Japan).
The upper arm to the ankle propagation distance (L)
and the pulse wave transit time (T) were automatically measured
according to the height of the body. The formula Ba-PWV=L/T was
calculated from Ba-Pwv on both sides, and the average value of both
sides was recorded.
Statistical analysis
The statistical analyses performed included a
matched t-test of measurement data and a rank-sum test using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). All data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
General characteristics of study
subjects
All 100 patients and 50 healthy controls were
included in the study. Table I
lists the characteristics of the subjects. No differences in
gender, age or BMI were found between the two groups (P>0.05).
The baseline clinical data of the subjects are also listed in
Table I.
 | Table I.General characteristics of the study
participants. |
Table I.
General characteristics of the study
participants.
| Variable | OSAS | Healthy control | P-value |
|---|
| Number | 100 | 50 | ND |
| Male/female | 82/18 | 37/13 | 0.288 |
| Age (years) | 55.284±7.128 | 56.131±6.210 | 0.193 |
| BMI
(kg/m2) | 26.746±3.500 | 25.196±2.449 | 0.528 |
| SBP (mmHg) | 162.549±7.315 | 128.124±8.341 | 0.021 |
| DBP (mmHg) | 95.328±5.515 | 83.425±4.216 | 0.007 |
| TC (mmol/l) | 6.718±2.998 | 4.624±2.378 | 0.022 |
| AHI (/h) | 38.011±8.040 | 3.623±1.537 | 0.001 |
| SPO2
(%) | 89.183±7.234 | 95.271±2.490 | 0.018 |
| Hypertension duration
(years) | 6.227±2.887 | ND | ND |
| Classes of
hypotensor used (no.) | 2.6±0.4 | ND | ND |
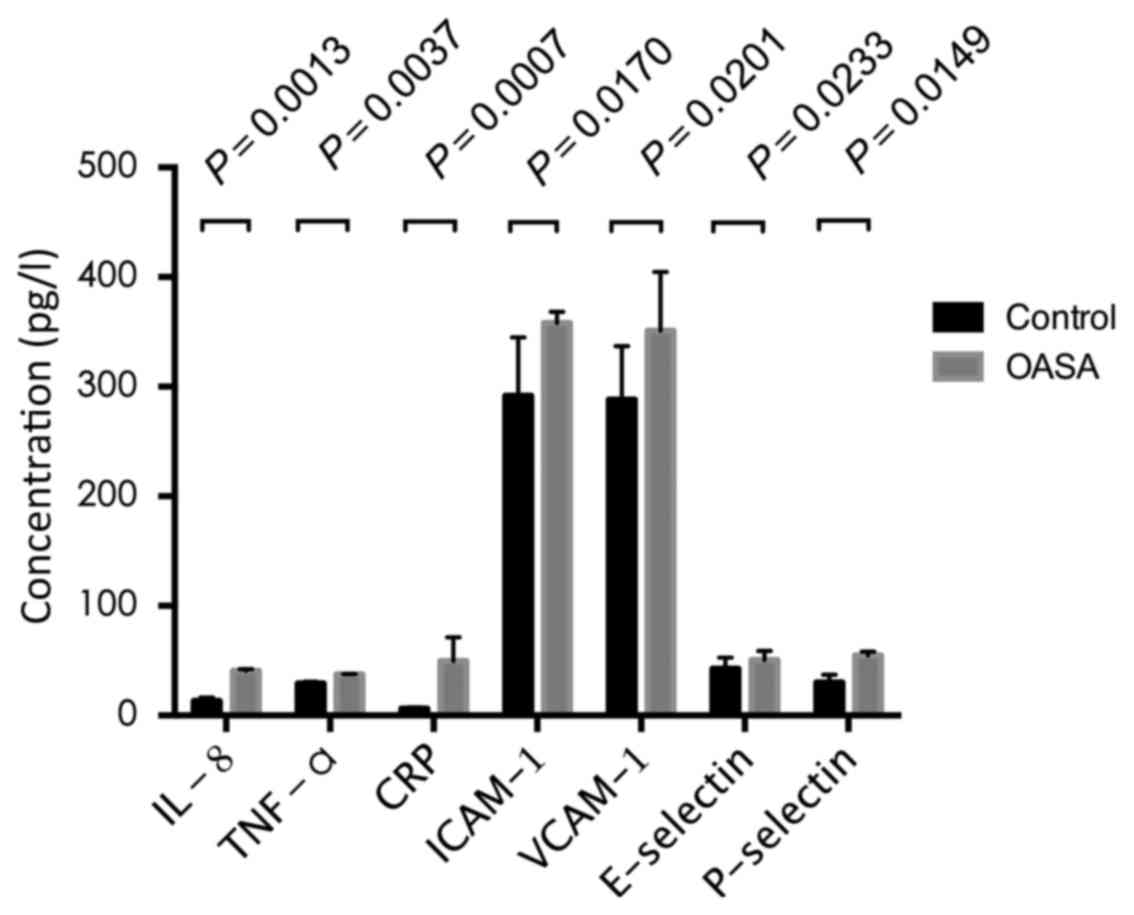
Inflammatory factors in OSAS
Compared with the healthy control group, the
expression levels of IL-8, TNF-α, CRP, ICAM-1, VCAM-1, E-selectin
and P-selectin in the patients with OSAS were significantly
increased. The respective statistical comparisons of expression in
the healthy controls, vs. patients with OSAS were as follows: IL-8,
13.28±3.15, vs. 40.72±1.60 pg/ml (P=0.0013); TNF-α, 29.15±1.74, vs.
37.67±0.21 pg/ml (P=0.0037); CRP, 6.37±1.30, vs. 49.64±21.66 mg/l
(P=0.0007); ICAM-1, 291.68±53.29, vs. 357.92±10.52 µg/l (P=0.0170);
VCAM-1, 288.16±48.81, vs. 351.06±53.61 µg/l (P=0.0201); E-selectin,
42.57±10.4, vs. 50.65±8.29 µg/l (P=0.0233); P-selectin 30.26±6.80
and 54.79±3.34 µg/l (P=0.0149). The results are shown in Fig. 1.
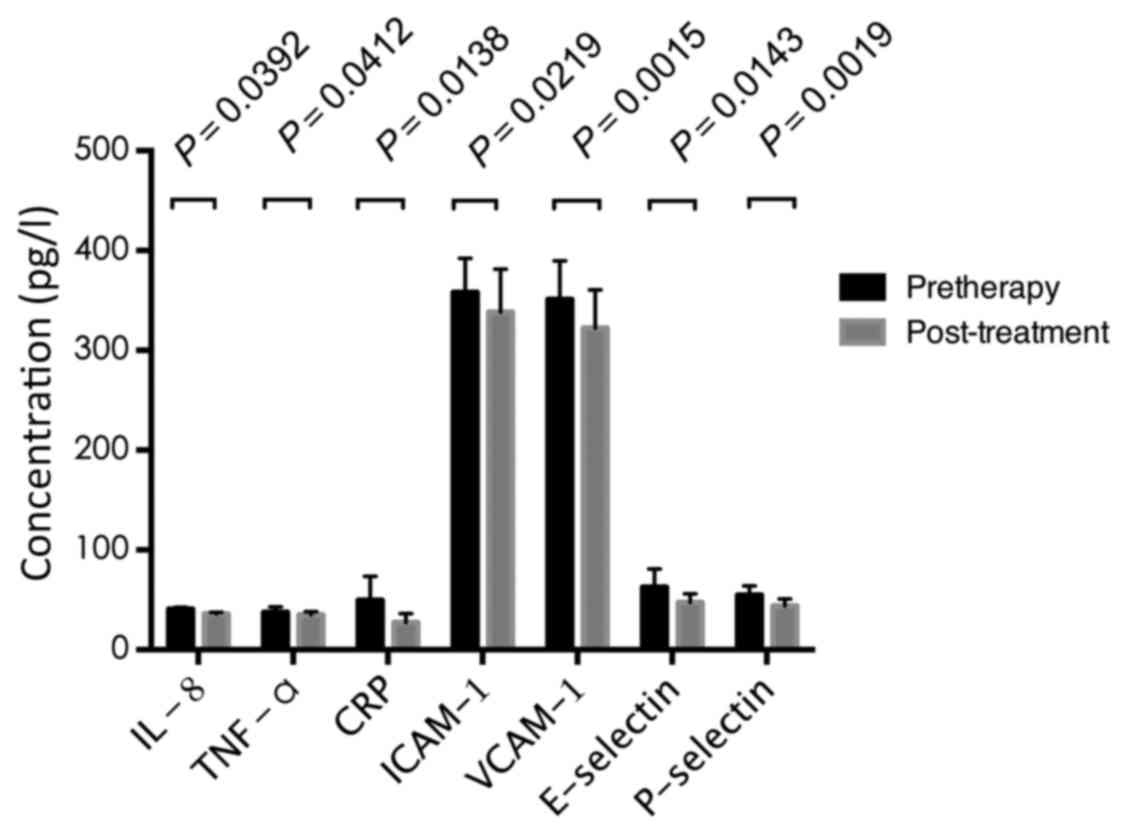
Changes of inflammatory cytokines
following treatment
PSG was performed on patients treated with CPAP
using an automatically programmed PSG system following 3 months of
treatment. Compared with the pre-therapy data, the expression
levels of IL-8, TNF-α, CRP, ICAM-1, VCAM-1, E-selectin and
P-selectin were significantly decreased following treatment. The
statistical comparisons of post-treatment, vs. pre-treatment data
were as follows: IL-8, 35.79±1.63 and 40.72±1.60 pg/ml (P=0.0392);
TNF-α, 34.97±3.18 vs. 37.67±0.21 pg/ml (P=0.0412); CRP, 27.41±8.86
vs. 49.64±21.66 mg/l (P=0.0138); ICAM-1, 338.29±43.03 vs.
357.92±10.52 µg/l (P=0.0219); VCAM-1, 322.36±38.25 vs. 351.06±53.61
µg/l (P=0.0015); E-selectin, 47.46±8.58 vs. 50.65±8.29 µg/l
(P=0.0143); P-selectin, 44.05±6.97 vs. 54.79±3.34 µg/l (P=0.0019).
The results are shown in Fig.
2.
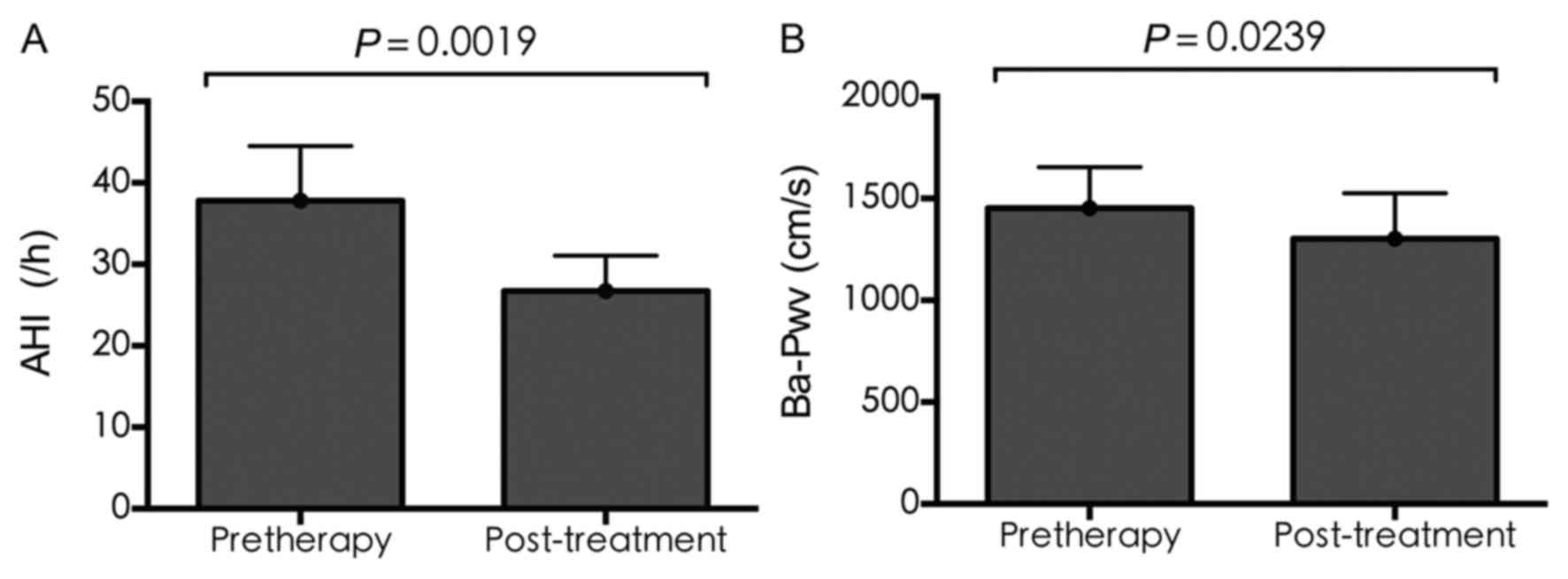
AHI and Ba-Pwv
Following CPAP treatment, the AHI and Ba-Pwv
improved significantly. As shown in Fig. 3A, the average value of AHI
decreased significantly (37.80±6.70 vs. 26.73±4.34; P=0.0019), as
did that of Ba-Pwv (1,418.86±199.58 vs. 1,265.31±219.36; P=0.0239;
Fig. 3B).
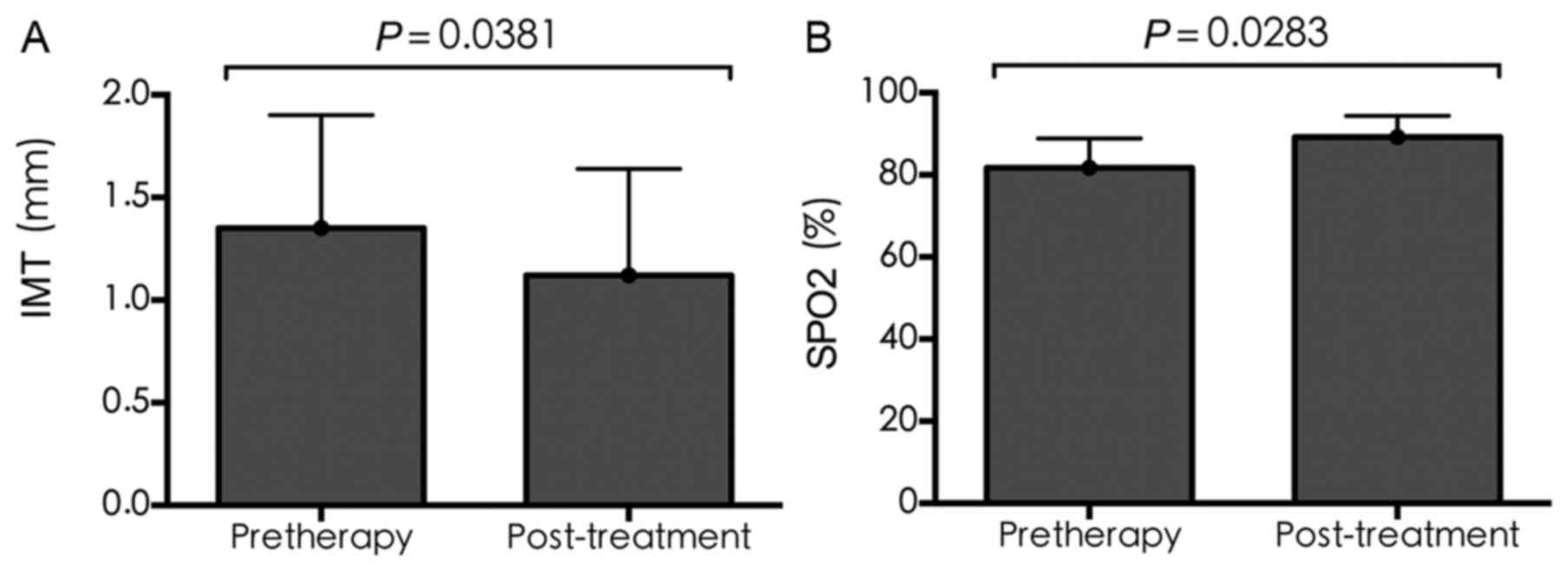
IMT and SPO2
Compared with pre-treatment, the IMT following
treatment was significantly reduced (1.35±0.55 vs. 1.12±0.52,
respectively; P=0.0381), whereas SPO2 was significantly
increased (81.67±7.23 vs. 89.18±5.19, respectively; P=0.0283), as
shown in Fig. 4A and B.
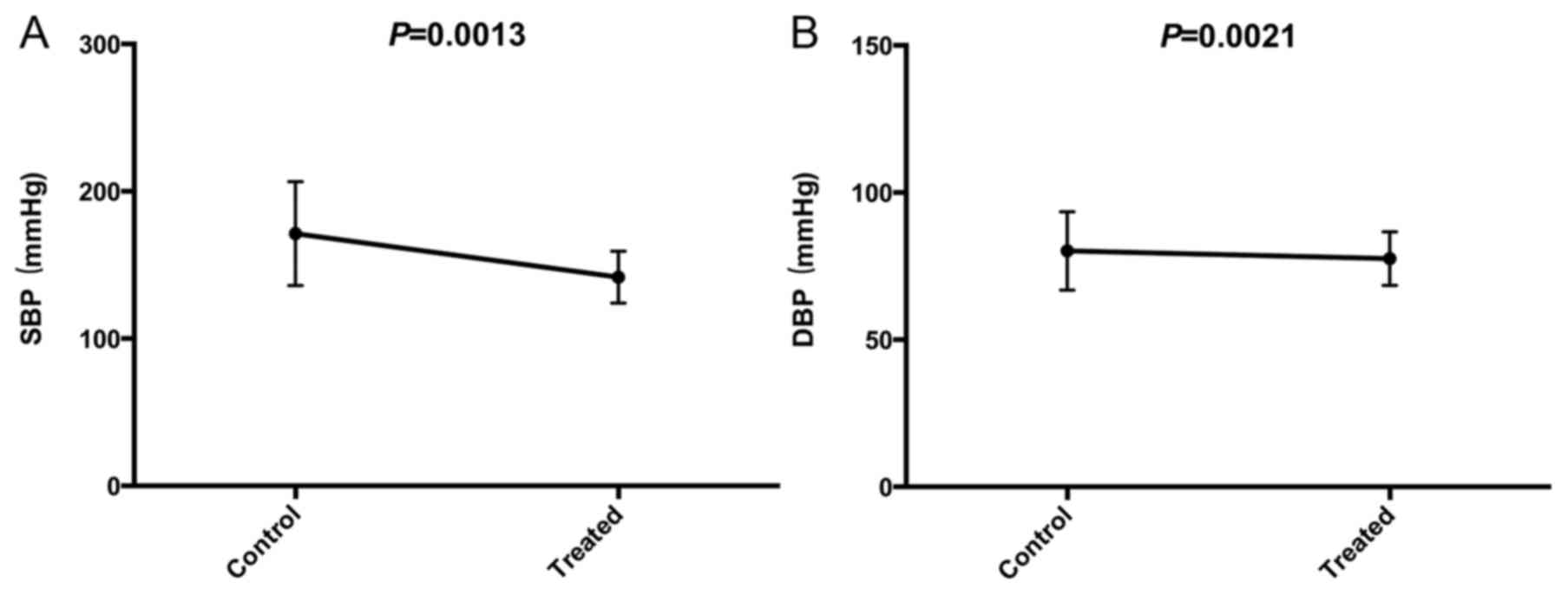
SBP and DBP
In the patients whose blood pressure remained high
despite medication (n=78), the blood pressure was improved
following treatment. As is shown in Fig. 5A and B, the decreases in SBP
(168.84±32.57 vs. 144.29±17.85; P=0.0013) and DBP (84.21±11.85 vs.
79.47±8.98; P=0.0021) were statistically significant.
Discussion
In previous years, OSAS has been widely accepted as
a mechanism for hypertension, and specific treatment for this
change has also been considered. In the present study, CAPA was
used for the treatment of patients with OSAS and hypertension, and
the efficacy was observed in order to examine the novel treatment
approach for patients with OSAS and hypertension.
The pro-inflammatory cytokine IL-18 is important in
the occurrence and development of atherosclerotic plaque rupture
(15). An epidemiological
follow-up study revealed that IL-18 is an independent risk factor
for coronary events, and is also a predictor of life-threatening
cardiac events in patients with acute coronary syndrome (10). TNF-α is critical in the
inflammatory cascade reaction of AS. Although CRP cannot predict
disease specificity, it is an important inflammatory response
factor in the majority of types of chronic inflammation,
particularly in AS. In addition, inflammatory factors, including
ICAM-1, VCAM-1 (16), E-selectin
and P-selectin, have been found to be important in predicting the
occurrence and development of AS (17). The pathological basis of AS
involves chronic inflammation of the vascular wall (18), however, whether inflammation is
also involved in the pathogenesis of cardiovascular disease in
patients with OSAS remains to be fully elucidated. The present
study hypothesized that hypoxia causes chronic inflammation in
patients with OSAS, and chronic inflammation leads to AS and
hypertension, whereas mechanical ventilation improves the condition
of hypoxia and the inflammatory response in patients.
The results of the present study showed that IL-18,
TNF-α, high-sensitivity CRP, and serum levels of ICAM-1, VCAM-1,
E-selectin and P-selectin in the OSAS group were significantly
increased, compared with those in the healthy control group. It was
indirectly confirmed that these inflammatory factors may be
involved in hypoxia and in vascular sclerosis. In order to directly
confirm this mechanism, CPAP treatment and follow-up investigations
were performed on patients with OSAS. Following CPAP treatment,
significant differences were found in these inflammatory markers,
compared with those at the pre-treatment stage.
In patients with OSAS with significant
characteristics of upper airway collapse (19) and effects on the circulatory
system, the primary mechanism involves the excitement of
sympathetic nerves (3), increase
of endothelin levels (20),
vascular endothelial function, abnormalities in vascular active
substances (21), vascular tension
(22) and excessive renin
secretion causing sustained hypertension (23). In previous studies, plasma levels
of CRP and other inflammatory cytokines in patients with OSAS
caused by inflammation have been reported to promote cardiovascular
and cerebrovascular diseases (24), including AS. The overexpression of
IL-18, TNF-α, E-selectin and P-selectin in OSAS is an important
factor in the occurrence and development of hypertension (25).
It has been reported that OSAS is closely associated
with the occurrence and development of hypertension (22). Under the condition of low oxygen,
substantial cellular metabolic waste accumulation leads to the
generation of oxygen free radicals (26). Free radicals react with the
unsaturated fatty acids in cells (27), in a process called lipid
peroxidation, generating cytotoxic effects of peroxide (28). Oxyradicals and subsequent lipid
peroxidation lead to various types of damage in the body. In the
electron transport chain of the redox reaction, coenzyme Q in
complex III can produce high activity free radical intermediates in
the process of reduction (29).
The reactive oxygen species generated in these cells include
hypochlorite, hydrogen peroxide, and free radicals, including
superoxide anions and hydroxyl radicals (30). The chemical properties of hydroxyl
radical are unstable and can be divided into specific biological
molecules. These biological macromolecules are predominantly
produced by the catalytic reduction of hydrogen peroxide by metal
enzymes. The oxidant can trigger a chain reaction, for example,
lipid peroxidation or oxidation of DNA and protein, resulting in
cell damage. Damaged DNA can cause mutations and induce several
diseases if not repaired. The damage causes the degradation of
protein and inhibits the activity of the enzyme. CPAP can improve
patient ventilation status, and subsequently improve in the
long-term chronic hypoxia status, with reductions in the redox
reaction and generation of free radicals (31).
In the present study, in addition to the changes of
inflammatory factors and clinical indices of the patients, AHI,
IMT, Ba-Pwv (32) and blood
pressure were detected. Patients with OSAS and hypertension
exhibited significantly higher IMT and Ba-Pwv, compared with the
normal population. Following treatment of the patients with OSAS,
blood pressure (SBP and DBP) and PSO2 was decreased.
This showed that CAPA alleviated the changes in OSAS patients with
hypertension.
In conclusion, CPAP increases oxygen supply,
improves chronic inflammation in OSAS, and inhibits the expression
of inflammatory factors and other factors promoting cardiovascular
and cerebrovascular diseases, in addition significantly improving
to pulmonary function in patients (33). In the present study, it was
confirmed that CPAP inhibited the inflammatory response of patients
with OSAS and hypertension, and inhibited the pathological basis of
OSAS. Therefore, the patients with OSAS exhibited hypertension and
hypoxia, leading to inflammation. CPAP indirectly improved AS and
high blood pressure in the patients. However, due to the complexity
of clinical investigation and confounding factors, there are still
some deficiencies in the present study. More cases and longer
follow-up are needed to investigate the association between OSAS,
chronic inflammation and hypertension.
References
|
1
|
Ulrich S, Nussbaumer-Ochsner Y, Vasic I,
Hasler E, Latshang TD, Kohler M, Muehlemann T, Wolf M and Bloch KE:
Cerebral oxygenation in patients with OSA: Effects of hypoxia at
altitude and impact of acetazolamide. Chest. 146:299–308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hollier CA, Harmer AR, Maxwell LJ, Menadue
C, Willson GN, Unger G, Flunt D, Black DA and Piper AJ: Moderate
concentrations of supplemental oxygen worsen hypercapnia in obesity
hypoventilation syndrome: A randomised crossover study. Thorax.
69:346–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi K, Inoue Y, Ohki N, Satoya N,
Inoue F, Maeda Y, Sekiguchi H, Suzuki M, Tsuji T, Aoshiba K and
Nagai A: Gender-specific impacts of apnea, age, and BMI on
parasympathetic nerve dysfunction during sleep in patients with
obstructive sleep apnea. PLoS One. 9:e928082014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cha J, Zea-Hernandez JA, Sin S,
Graw-Panzer K, Shifteh K, Isasi CR, Wagshul ME, Moran EE, Posner J,
Zimmerman ME and Arens R: The effects of obstructive sleep apnea
syndrome on the dentate gyrus and learning and memory in children.
J Neurosci. 37:4280–4288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dal-Fabbro C, Garbuio S, D'Almeida V,
Cintra FD, Tufik S and Bittencourt L: Mandibular advancement device
and CPAP upon cardiovascular parameters in OSA. Sleep Breath.
18:749–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marin JM, Artal J, Martin T, Carrizo SJ,
Andres M, Martin-Burriel I, Bolea R, Sanz A, Varona L, Godino J, et
al: Epigenetics modifications and subclinical atherosclerosis in
obstructive sleep apnea: The EPIOSA study. BMC Pulm Med.
14:1142014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mutlu M, Vuralkan E, Akaydin S Yardim,
Akin I and Miser E: Effects of adenoid/tonsillectomy on
inflammatory response in snoring children with witnessed apnoea.
Clin Otolaryngol. 39:266–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parra O, Sánchez-Armengol Á, Capote F,
Bonnin M, Arboix A, Campos-Rodríguez F, Pérez-Ronchel J,
Durán-Cantolla J, Martínez-Null C, de la Peña M, et al: Efficacy of
continuous positive airway pressure treatment on 5-year survival in
patients with ischaemic stroke and obstructive sleep apnea: A
randomized controlled trial. J Sleep Res. 24:47–53. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Batool-Anwar S, Goodwin JL, Drescher AA,
Baldwin CM, Simon RD, Smith TW and Quan SF: Impact of CPAP on
activity patterns and diet in patients with obstructive sleep apnea
(OSA). J Clin Sleep Med. 10:465–472. 2014.PubMed/NCBI
|
|
10
|
Shahid ML, Chitiboi T, Ivanovska T,
Molchanov V, Völzke H and Linsen L: Automatic MRI segmentation of
para-pharyngeal fat pads using interactive visual feature space
analysis for classification. BMC Med Imaging. 17:152017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Augelli DM and Krieger AC: Social and
economic impacts of managing sleep hypoventilation syndromes. Sleep
Med Clin. 12:87–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin CC, Liaw SF, Chiu CH, Chen WJ, Lin MW
and Chang FT: Effects of nasal CPAP on exhaled SIRT1 and tumor
necrosis factor-α in patients with obstructive sleep apnea. Respir
Physiol Neurobiol. 228:39–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yilmaz YF, Kum RO, Ozcan M, Gungor V and
Unal A: Drug-induced sleep endoscopy versus Muller maneuver in
patients with retropalatal. Laryngoscope. 125:2220–2225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hui DS, Shang Q, Ko FW, Ng SS, Szeto CC,
Ngai J, Tung AH, To KW, Chan TO and Yu CM: A prospective cohort
study of the long-term effects of CPAP on carotid artery
intima-media thickness in obstructive sleep apnea syndrome. Respir
Res. 13:222012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qaseem A, Holty JE, Owens DK, Dallas P,
Starkey M and Shekelle P: Clinical Guidelines Committee of the
American College of Physicians: Management of obstructive sleep
apnea in adults: A clinical practice guideline from the American
College of Physicians. Ann Intern Med. 159:471–483. 2013.PubMed/NCBI
|
|
16
|
Niżankowska-Jędrzejczyk A, Almeida FR,
Lowe AA, Kania A, Nastałek P, Mejza F, Foley JH,
Niżankowska-Mogilnicka E and Undas A: Modulation of inflammatory
and hemostatic markers in obstructive sleep apnea patients treated
with mandibular advancement splints: A parallel, controlled trial.
J Clin Sleep Med. 10:255–262. 2014.PubMed/NCBI
|
|
17
|
Ursavas A, Karadağ M, Rodoplu E,
Yilmaztepe A, Oral HB and Gözü RO: Circulating ICAM-1 and VCAM-1
levels in patients with obstructive sleep apnea syndrome.
Respiration. 74:525–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryan S and McNicholas WT: Inflammatory
cardiovascular risk markers in obstructive sleep apnoea syndrome.
Cardiovasc Hematol Agents Med Chem. 7:76–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Utriainen KT, Airaksinen JK, Polo O,
Laitio R, Pietilä MJ, Scheinin H, Vahlberg T, Leino KA, Kentala ES,
Jalonen JR, et al: Sleep apnoea is associated with major cardiac
events in peripheral arterial disease. Eur Respir J. 43:1652–1660.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Archontogeorgis K, Nena E, Papanas N and
Steiropoulos P: Biomarkers to improve diagnosis and monitoring of
obstructive sleep apnea syndrome: Current status and future
perspectives. Pulm Med. 2014:9305352014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tual-Chalot S, Gagnadoux F, Trzepizur W,
Priou P, Andriantsitohaina R and Martinez MC: Circulating
microparticles from obstructive sleep apnea syndrome patients
induce endothelin-mediated angiogenesis. Biochim Biophys Acta.
1842:202–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin CC, Wang YP, Lee KS, Liaw SF and Chiu
CH: Effect of uvulopalatopharyngoplasty on leptin and endothelial
function in sleep apnea. Ann Otol Rhinol Laryngol. 123:40–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buratti L, Viticchi G, Falsetti L,
Cagnetti C, Luzzi S, Bartolini M, Provinciali L and Silvestrini M:
Vascular impairment in Alzheimer's disease: The role of obstructive
sleep apnea. J Alzheimers Dis. 38:445–453. 2014.PubMed/NCBI
|
|
24
|
Deleanu OC, Malaut AE, Nebunoiu AM, Micheu
MM and Mihălţan FD: Obstructive sleep apnea syndrome and arterial
hypertension-a complicated relationship? The role of controlling
blood pressure values in patients with OSAS. Pneumologia. 63:36–43.
2014.PubMed/NCBI
|
|
25
|
Sasaki N, Ozono R, Yamauchi R, Teramen K,
Edahiro Y, Ishii K, Seto A and Kihara Y: The relationship between
morning hypertension and sleep quality in patients with obstructive
sleep apnea syndrome. Clin Exp Hypertens. 35:250–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eda S Alves, Ackel-D'Elia C, Luz GP, Cunha
TC, Carneiro G, Tufik S, Bittencourt LR and de Mello MT: Does
physical exercise reduce excessive daytime sleepiness by improving
inflammatory profiles in obstructive sleep apnea patients? Sleep
Breath. 17:505–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nizam N, Basoglu OK, Tasbakan MS,
Nalbantsoy A and Buduneli N: Salivary cytokines and the association
between obstructive sleep apnea syndrome and periodontal disease. J
Periodontol. 85:e251–e258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Papandreou C: Levels of TBARS are
inversely associated with lowest oxygen saturation in obese
patients with OSAS. Sleep Breath. 17:1319–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Papandreou C: Independent associations
between fatty acids and sleep quality among obese patients with
obstructive sleep apnoea syndrome. J Sleep Res. 22:569–572. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hopps E, Canino B, Calandrino V, Montana
M, Lo Presti R and Caimi G: Lipid peroxidation and protein
oxidation are related to the severity of OSAS. Eur Rev Med
Pharmacol Sci. 18:3773–3778. 2014.PubMed/NCBI
|
|
31
|
Baysal E, Taysi S, Aksoy N, Uyar M, Celenk
F, Karatas ZA, Tarakcioglu M, Bilinç H, Mumbuç S and Kanlikama M:
Serum paraoxonase, arylesterase activity and oxidative status in
patients with obstructive sleep apnea syndrome (OSAS). Eur Rev Med
Pharmacol Sci. 16:770–774. 2012.PubMed/NCBI
|
|
32
|
Basoglu OK, Vardar R, Tasbakan MS, Ucar
ZZ, Ayik S, Kose T and Bor S: Obstructive sleep apnea syndrome and
gastroesophageal reflux disease: The importance of obesity and
gender. Sleep Breath. 19:585–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pulixi EA, Tobaldini E, Battezzati PM,
D'Ingianna P, Borroni V, Fracanzani AL, Maggioni M, Pelusi S,
Bulgheroni M, Zuin M, et al: Risk of obstructive sleep apnea with
daytime sleepiness is associated with liver damage in non-morbidly
obese patients with nonalcoholic fatty liver disease. PLoS One.
9:e963492014. View Article : Google Scholar : PubMed/NCBI
|