Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disease characterized by articular inflammation and leads to joint
destruction (1,2). RA pathogenesis involves a complex
humoral and cellular immune response, including the infiltration of
lymphocytes and monocytes into the synovium. These infiltrating
cells and synoviocytes release numerous proinflammatory cytokine
and chemokines, including interleukin IL-6, IL-1 and tumor necrosis
factor-α (TNF-α), which may serve a significant role in RA
pathogenesis (3,4), and may determine the activation and
proliferation of the synovial lining and the recruitment of
inflammatory cells that induce inflammation and promote
destruction. Elevated levels of proinflammatory cytokines have been
observed in the sera and synovial fluids of patients with RA, such
as IL-6, IL-8, TNF-α, IL-20, IL-17 and IL-33 (5–9).
Neutralizing these cytokines with monoclonal antibodies or soluble
receptors has previously been developed as a novel treatment for RA
(10). The TNF-α-inducible protein
8 (TNFAIP8) family consists of TNFAIP8, TNF-α-induced protein
8-like 1 (TIPE1, also termed TNFAIP8L1), TIPE2 (also termed
TNFAIP8L2) and TIPE3 (also termed TNFAIP8L3) (11). These are recently identified
proteins that share considerable sequence homology for the
regulation of cellular and immune homeostasis (12). TIPE2 is preferentially expressed in
lymphoid tissues and hematopoietic cells, and negatively regulates
immunity (12,13). TIPE2 deletion in mice leads to
multi-organ inflammation, splenomegaly and premature death
(14–16). TIPE2-deficient cells hyperactivate
toll-like receptors (TLRs) and T cell receptors (11,12).
TIPE2 is significantly downregulated in during infection or
autoimmune disease (17,18). TIPE2-deficient cells lead to the
production of various kinds of proinflammatory cytokines and
activation of the phosphoinositide 3-kinase (PI3K)-Ras-related C3
botulinum toxin substrate (Rac) signaling pathway, which enhances
protein kinase B (AKT), Rac and interferon (IFN) regulatory factor
3 activities (19–21).
In the present study, TIPE2 regulated
lipopolysaccharide (LPS)-induced RA immune responses by targeting
Rac GTPases in a PI3K-dependent manner. Tipe2 protein has the
potential to be used to treated RA (19,21–25).
Materials and methods
Cell lines and plasmids
The induction of adjuvant arthritis in rats, the
preparation of rheumatoid arthritis (RA) synovial fibroblasts (RSF)
and identification techniques were performed according to previous
publications (10,26). Normal synovial fibroblasts (NSFs)
and RSFs were grown in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China), penicillin and streptomycin.
To generate stable cell lines, 5×105 adjuvant arthritis
fibroblast-like synoviocytes were transduced with
MIGR1/TIPE2-overexpression recombinant lentiviral vectors and a
MIGR1 lentiviral vector was used as the negative control (gift from
Dr Chen Youhai, University of Pennsylvania School of Medicine,
Philadelphia, PA, USA). The lentiviral vectors contained human and
rat inserts for the gene encoding TIPE2 and the sequences are at:
http://www.addgene.org/27490/sequences/#addgene-partial.
The cells were cultured at 37°C and the culture medium was replaced
24 h after transduction. After an additional 48 h culture at 37°C,
transduced cells were observed visually under a fluorescent
microscope, 12 fields were counted and infected cells constituted
>90% of the total cell count. The infected cells were selected
by flow cytometry and identified by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis (11,26).
RT-qPCR
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) from NSFs and RSFs and
purified with RNeasy Mini kits (Qiagen, Inc., Valencia, CA, USA)
according to the manufacturer's protocol (11,26).
After processing with RNase-free DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.), RNA samples were
reverse transcribed with oligo (dT) and SuperScript II
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). PCR was
performed using an Applied Biosystems 7500 system and Power
SYBR-Green PCR Master mix (Applied Biosystems, Thermo Fisher
Scientific, Inc.). Relative gene expression levels were determined
using GAPDH as the control. The PCR products were run in an agarose
gel and were in all cases confined to a single band of the expected
size. A melting-curve analysis was also performed to ensure
specificity of the products (11,24).
Analysis of relative gene expression data was performed using the
2−ΔΔCq method (27).
TLR2, TLR3 and TLR4 mRNA
expression levels were analyzed by RT-qPCR. The cycling conditions
used were as follows: An initial predenaturation step at 94°C for 3
min, followed by 30 cycles of denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec, extension at 72°C for 1 min and a
final extension step at 72°C for 5 min. Each sample was normalized
to the expression levels of β-actin or GAPDH. (11,26).
TNFAIP8L2, IL-1, and GAPDH
primers were purchased from Qiagen, Inc. Other primers were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc., and the
sequences were as follows: 5′-GTTGACAGCCACTGCCTTCC-3′ (forward) and
5′-CTGACAGTGCATCATCGCTG-3′ (reverse) for IL-6;
5′-CCTCTCCATCGACTACAAGC-3′ (forward) and 5′-CTCTTCTCCATCTGTGACGG-3′
(reverse) for IFNG; 5′-CCAGGAGAAAGTCAGCCTCC-3′ (forward) and
5′-GTTGACCTCAGCGCTGAGC-3′ (reverse) for TNF;
5′-CTGTGATAGGCCTCTCAAGG-3′ (forward) and 5′-CACCATGGCCAATGTAGGTG-3′
(reverse) for TLR2; 5′-CCAGCTGTTAGCAACCAGC-3′ (forward) and
5′-CGAGGGACAGATACTTCAGG-3′ (reverse) for TLR3;
5′-GTAGCCGCTCTGGCATCATC-3′ (forward) and 5′-CTCCCCAGAGCATTGTCCTC-3′
(reverse) for TLR4; 5′-TGCGTGACATCAAAGAGAAG-3′ (forward) and
5′-TCCATACCCAAGAAGGAAGG-3′ (reverse) for β-actin
5′-TGATTCTACCCACGGCAAGTT-3′ (forward) and
5′-TGATGGGTTTCCCATTGATGA-3′ (reverse) for GAPDH;
5′-CCTTGTGCAAGTGTCTGAAGC-3′ (forward) and
5′-CCCAAGTCAAGGGCTTGGAA-3′ (reverse) for IL-1; and,
5′-GGGAACATCCAAGGCAAG-3′ (forward) and 5′-AGCTCATCTAGCACCTCACT-3′
(reverse) for TNFAIP8L2.
LPS stimulation assay
Control RFSs and TIPE2-overexpressed RSFs were
treated by LPS (100 ng/ml; 9001-62-1; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 3 time-points (0 min, 2, 8 h). After LPS
treatment the cells were collected and Total RNA was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Analysis of relative gene expression data was performed using the
2−ΔΔCq method (27).
PI3K and Rac inhibition assay
Control RSFs were treated with or without the
indicated concentrations of LY294002 or NSC23766 inhibitors for 20
min prior to stimulation with LPS (100 ng/ml) for 2 h. Cytokine
expression was determined using RT-qPCR (19,26).
Western blot analysis
The protein was isolated from RSFs and NSFs using
the ProteoPrep Total Extraction Sample kit (PROTTOT-1KT;
Sigma-Aldrich; Merck KGaA), and the protein concentration was
determined by BCA assay (p0012 BCA; Beyotime Institute of
Biotechnology; Shanghai, China). Then, 0.6 µg aliquots of synovial
cell lysates were loaded and separated by 12% SDS-PAGE, transferred
onto a polyvinylidene difluoride membrane, blocked by 5% FBS
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) for
1 h at room temperature and probed with the following primary
antibodies for 75 min at room temperature: rabbit anti-β-actin
(m1210-5; 1:1,000; Hangzhou Huaan Biotechnology Co., Ltd.,
Hangzhou, China), rabbit anti-TIPE2 (ab110389; 1:1,000; Abcam,
Cambridge, UK), rabbit anti-caspase-8 (p18; ab25901; 1:1,000;
Abcam), rabbit anti-p21 activated kinase 1 protein (PAK1; ab40852;
1:1,000 Abcam), rabbit anti-phosphorylated pPAK1 (phospho; ab40795;
1:1,000; Abcam), rabbit anti-pTANK-binding kinase 1 (phospho; NAK;
ab40676; 1:1,000; Abcam), rabbit anti-pAKT (phospho; ab81283;
1:1,000; Abcam) and rabbit anti-Rac (Cell Signaling Technology,
Inc., Danvers, MA, USA). The second antibodies (goat anti rabbit;
A32732; 1;1,000; Thermo Fisher Scientific, Inc.) were added for 1 h
at room temperature. The protein bands were visualized with an
Enhanced Chemiluminescence substrate (NCI4106; Thermo Fisher
Scientific, Inc.). Immunoblot analysis was performed as previously
described (19,26). Densitometric analysis was performed
with ImageJ software (version 1.50; National Institutes of Health,
Bethesda, MD, USA).
ELISA analysis
The levels of inflammatory cytokines in supernatants
were measured with commercially available ELISA kits for IL-6
(BMS625TWO), IL-1 (BMS627TWO), IFN-γ (BMS621) and TNF-α (BMS622TWO;
all from eBioscience; Thermo Fisher Scientific, Inc.), in
accordance with the manufacturers' protocols. The absorbance was
measured at a wavelength of 450 nm using a 680XR microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). All samples were
analyzed in duplicate. The standard curve for interpolating the
protein concentration in each sample was generated using linear
regression analysis, and was performed as previously described
(11,28).
Rac pulldown assay
To assess Rac activation, cells
(2×108/ml) were incubated with
PAK-glutathione-S-transferase fusion protein beads (Cytoskeleton,
Inc., Denver, CO, USA) at 4°C for 60 min. The collected beads were
then washed three times and resuspended in SDS protein sample
buffer. Bound proteins and total cell lysates were analyzed by
western blotting using an anti-Rac antibody (Cell Signaling
Technology, Inc.). The Rac pulldown assay was performed as
previously described (26,29).
Statistical analysis
Statistical analysis was performed using SPSS
version 11.5 software (SPSS, Inc., Chicago, IL, USA). Data are
expressed as mean ± standard deviation of three experiments. The
significance of the differences in the mean values between or
within multiple groups was determined with a Student's t-test test
and two-way analysis of variance followed by Tukey's post hoc test,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inverse association between TIPE2 and
cytokine gene expression in RSFs following TLR stimulation with
LPS
RSFs serve important roles in immunity to RA
pathogens. To explore the roles of TIPE2 in RSFs-mediated
proinflammatory cytokine secretion, TNFAIP8L2 and TIPE2
expression was examined in rat RSFs by RT-qPCR and western
blotting, respectively. The results demonstrated that
TNFAIP8L2 mRNA (Fig. 1A)
and TIPE2 protein expression (Fig.
1B) was lower rat RSFs than in NSFs. Upon stimulation with LPS
(the TLR4 ligand), the mRNA expression levels of the cytokines
IL-6, IL-1, TNF and IFNG (Fig. 1C) and their protein products (IL-6,
IL-1, TNF-α and IFN-γ, respectively; Fig. 1D), were significantly increased in
RSFs. This inverse association between TIPE2 and cytokine levels in
RSFs treated with the TLR ligand LPS, suggested a role for TIPE2 in
regulating RA inflammatory responses.
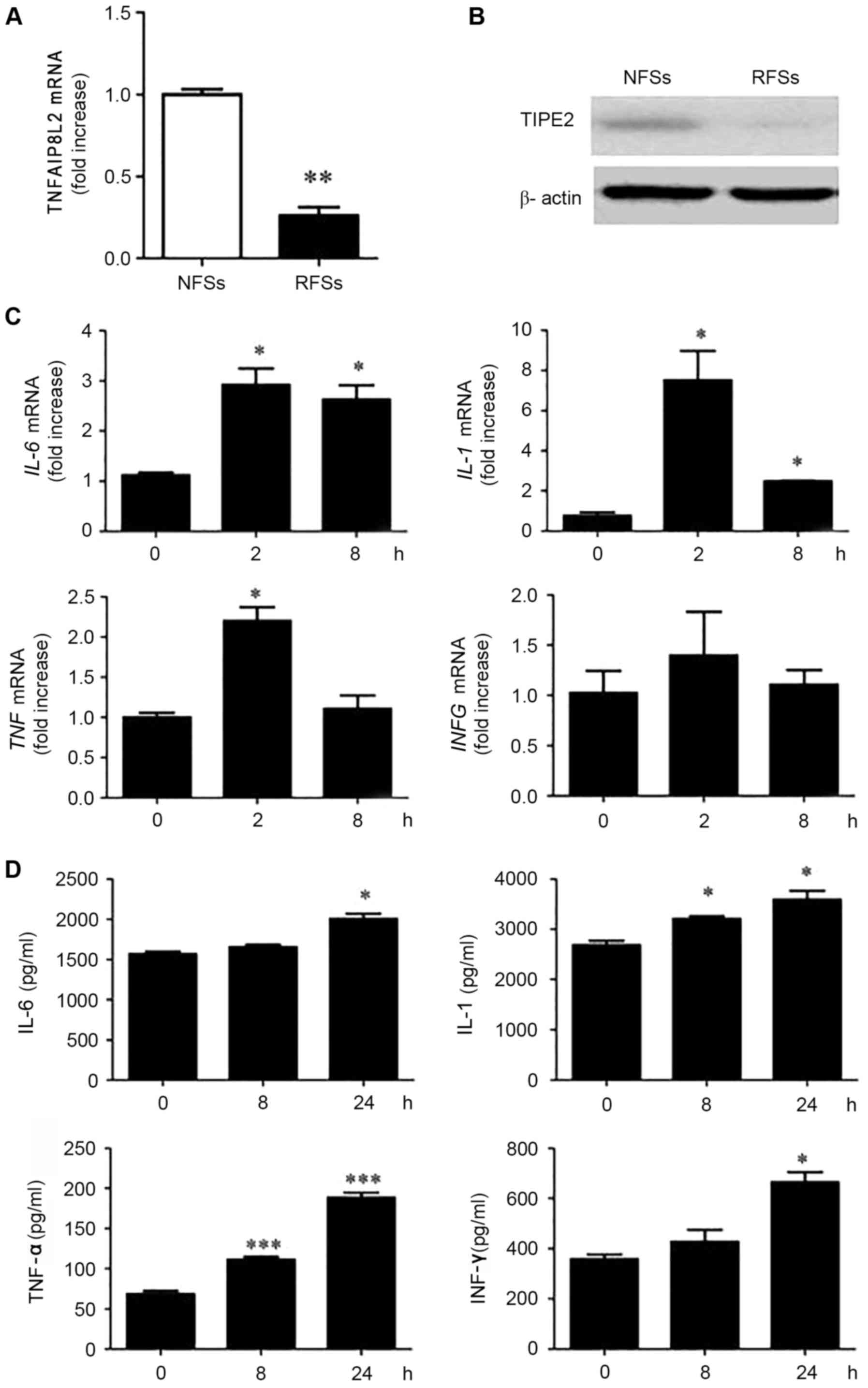 | Figure 1.Inverse association between the gene
encoding TIPE2 and cytokine expression levels in RSFs following TLR
stimulation with LPS. (A) TNFAIP8L2 expression levels, as
measured by RT-qPCR. **P<0.01 vs. NSFs. (B) TIPE2 protein
expression by western blot analysis, in freshly harvested NSFs and
RSFs. (C) RSFs were stimulated with LPS (100 ng/ml) for 2 or 8 h
and IL-6, IL-1, TNF and IFNG mRNA
expression levels were determined by RT-qPCR. (D) RSFs were
stimulated with LPS (100 ng/ml) for 2 or 8 h and IL-6, IL-1, TNF-α
and IFN-γ concentrations were determined by ELISA. *P<0.05,
**P<0.01 ***P<0.001 vs. 0 h. Data are presented as the mean ±
standard deviation (n=3). RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; TIPE2, tumor
necrosis factor-α-induced protein-8 like 2; TNFAIP8L2, tumor
necrosis factor-α-induced protein-8 like 2 gene; RSFs, rheumatoid
arthritis synovial fibroblasts; NSFs, normal synovial fibroblasts;
LPS, lipopolysaccharide; IL-6, interleukin 6 gene;
IL-1, interleukin 1 gene; TNF, tumor necrosis
factor-α gene; IFNG, interferon-γ gene; IL-6, interleukin-6;
IL-1, interleukin-1; TNF-α, tumor necrosis factor-α; IFN-γ,
interferon-γ. |
Enhanced TIPE2 expression in RSFs
decreases cytokine expression
To determine whether TIPE2 expression affected the
production of IL-1, IL-6 and TNF-α by RSFs, a TIPE2 overexpression
plasmid was transfected with MIGRI retrovirus which contained the
lentiviral constructs or with MIGRI retrovirus negative control.
TIPE2 overexpression in rat RSFs was termed TIPE2-overexpressed
RSFs, whereas RSFs transfected with the MIGRI retrovirus were named
control RSFs. Subsequently, expression levels of TNFAIP8L2
and its TIPE2 protein product were measured by RT-qPCR and western
blotting, respectively. The results revealed that TNFAIP8L
and TIPE2 expression levels were elevated in the
TIPE2-overexpressed group compared with the control (Fig. 2A). By contrast, mRNA expression
levels of the cytokines IL-6, IL-1, TNF and
IFNG (Fig. 2B) and their
protein products (IL-6, IL-1, TNF-α and IFN-γ, respectively;
Fig. 2C), were significantly
increased in the control group compared with the
TIPE2-overexpressed group.
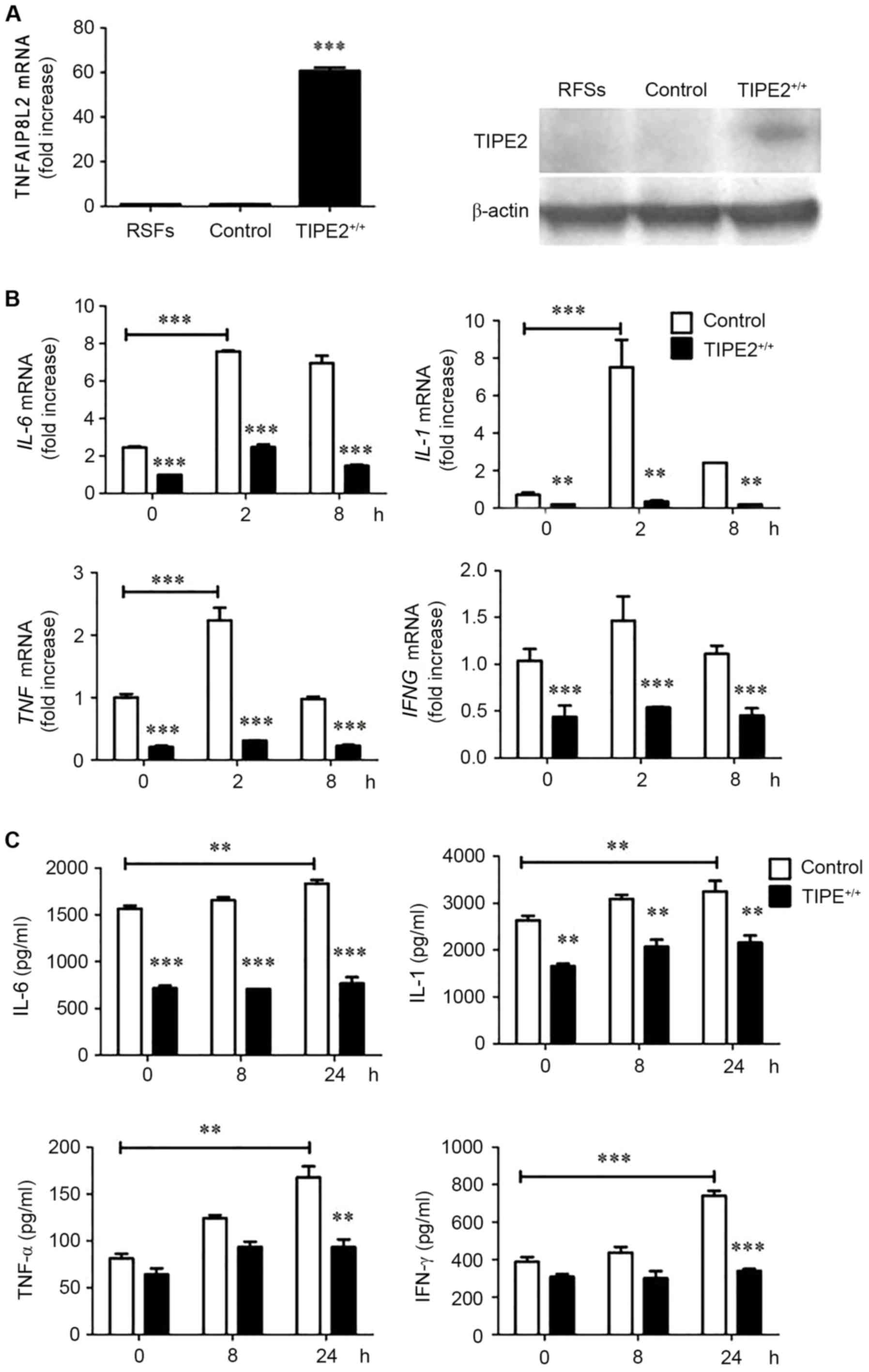 | Figure 2.Enhanced TNFAIP8L2 expression
levels in RSFs associates with decreased cytokine expression. (A)
RT-qPCR analysis of TNFAIP8L2 expression and western blot
analysis of TIPE2 expression, in freshly harvested control RSFs and
TIPE+/+ (TIPE2-overexpressed) RSFs stimulated with LPS
(100 ng/ml) for the indicated times. (B) IL-6, IL-1,
TNF and IFNG mRNA expression levels and (C) IL-6,
IL-1, TNF-α, and IFN-γ expression levels, as determined by ELISA.
Data are presented as the mean ± standard deviation (n=3).
**P<0.01 and ***P<0.001 vs. control at each time point,
unless otherwise indicated. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; TIPE2, tumor
necrosis factor-α-induced protein-8 like 2; TNFAIP8L2, tumor
necrosis factor-α-induced protein-8 like 2 gene; RSFs, rheumatoid
arthritis synovial fibroblasts; LPS, lipopolysaccharide;
IL-6, interleukin 6 gene; IL-1, interleukin 1 gene;
TNF, tumor necrosis factor-α gene; IFNG, interferon-γ
gene; IL-6, interleukin-6; IL-1, interleukin-1; TNF-α, tumor
necrosis factor-α; IFN-γ, interferon-γ. |
Enhanced TIPE2 expression in RSFs
decreases Rac activation
To explore the potential underlying mechanism of
TIPE2 in LPS-induced cytokine expression, TLR mRNA expression
levels between the TIPE2-overexpressed RSFs and control RSFs were
compared (Fig. 3A), and results
suggested that decreased cytokine expression in TIPE2-overexpressed
cells was not due to the decreased expression of the TLR2,
TLR3 or TLR4 (16).
Endogenous TIPE2 may constitutively bind to the small GTPase, Rac,
in immune cells. Therefore, Rac activation between LPS-treated
TIPE2-overexpressed RSFs and control RSFs was compared. Elevated
constitutive Rac activation was observed in the control RSFs
(Fig. 3B), suggesting that Rac was
activated and involved in LPS-mediated cytokine expression. PAK and
AKT are downstream effectors of Rac and PI3K, respectively. As
demonstrated in Fig. 3C, following
LPS-treatment (30, 60 and 120 min), the levels of phosphorylation
and activation in control RSFs were higher compared with
TIPE2+/+ RSFs at corresponding time points. Rac1 and
PAK1 have been reported to act upstream of TBK1/inhibitor of κ B
(IκB) kinase-ε in the viral activation of interferon regulatory
factor 3 (IRF3) (17). Decreased
phosphorylation and activation of NAK was also observed in the
control RSFs at 60 min LPS treatment compared with the
TIPE2-overexpressed RSFs (Fig.
3D), suggesting that TIPE2 regulates NAK via the Rac/PAK
signaling pathway.
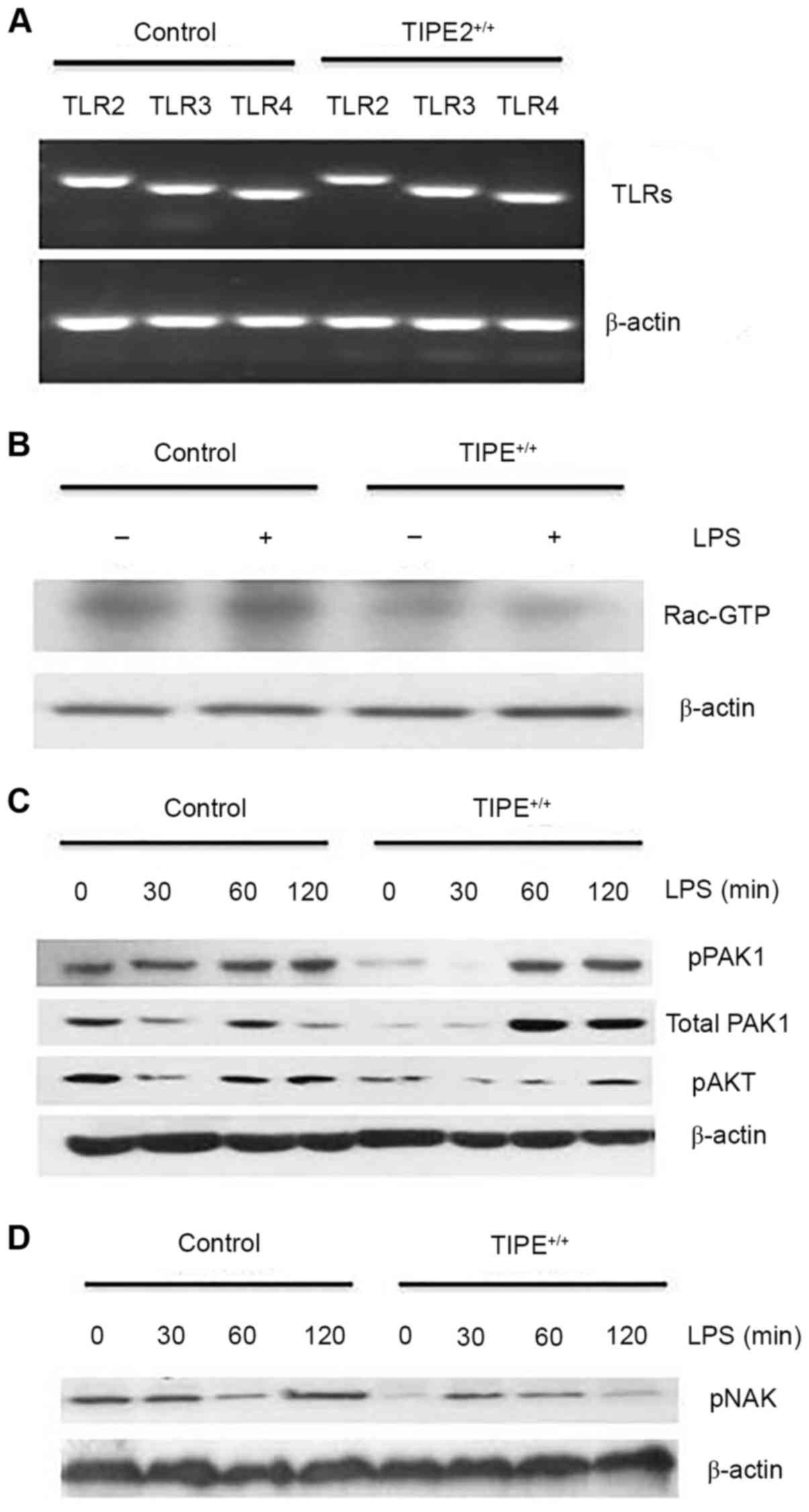 | Figure 3.Enhanced TIPE2 expression in RSFs
decreases Rac activation. (A) TLR2, TLR3 and
TLR4 mRNA expression levels in freshly harvested in control
RSFs and TIPE+/+ (TIPE2-overexpressed) RSFs, as
determined by reverse transcription-quantitative polymerase chain
reaction. (B) Rac is activated in control RSFs. Cells were lysed
and incubated with PAK-glutathione-S-transferase fusion protein
beads and activated Rac was detected by western blotting. (C and D)
Increased pNAK, pPAK and pAKT in control RSFs. Control RSFs and
TIPE+/+ RSFs were treated with LPS (100 ng/ml) for the
indicated times. The levels of the total proteins and
phosphorylated proteins were determined by western blotting. Data
presented in this figure are representative of at least three
independent experiments. TIPE2, tumor necrosis factor-α-induced
protein-8 like 2; TLR2, toll-like receptor 2; TLR3,
toll-like receptor 3; TLR4, toll-like receptor 4; RSFs,
rheumatoid arthritis synovial fibroblasts; PAK, p21 activated
kinase 1 protein; pNAK, phosphorylated TANK-binding kinase 1; pPAK,
phosphorylated p21 activated kinase 1 protein; pAKT, phosphorylated
protein kinase B; LPS, lipopolysaccharide. |
PI3K and Rac inhibition significantly
diminishes LPS-induced cytokine gene expression
TIPE2 is a negative regulator in immune response and
TIPE2-overexpression cells have low LPS-induced cytokine gene
expression. To further dissect the pathways involved in
LPS-mediated cytokine production in RSFs, inhibitors of various
signaling pathways were used. As presented in Fig. 4A, the PI3K inhibitor LY294002 and
the Rac inhibitor NSC 23,766 effectively inhibited TNF and
IL-6 expression levels in a dose-dependent manner. Next, it
was examined whether LY294002 may also affect Rac activation. As
demonstrated in Fig. 4B, LY294002
partially blocked Rac activation. PAK, AKT, and NAK expression
levels were also compared in the control RSFs with and without PI3K
inhibitor LY294002 and Rac inhibitor NSC 23,766 added to cells 30
min prior to LPS stimulation. The expression level of pAKT was
reduced in the control RSFs with PI3K inhibitor LY294002 compared
with those without the inhibitor present. The expression levels of
pPAK and pNAK were also diminished. (Fig. 4C) Reduced expression levels of
pPAK, pAKT and pNAK were observed in the control RSFs with Rac
inhibitor NSC 23,766 compared with those without, (Fig. 4D), suggesting that Rac was
activated significantly and involved in LPS-mediated cytokine
expression. PI3K was segmental activated and involved in
LPS-mediated cytokine expression. These results suggested that Rac
activation is directly involved in LPS-mediated cytokine
expression.
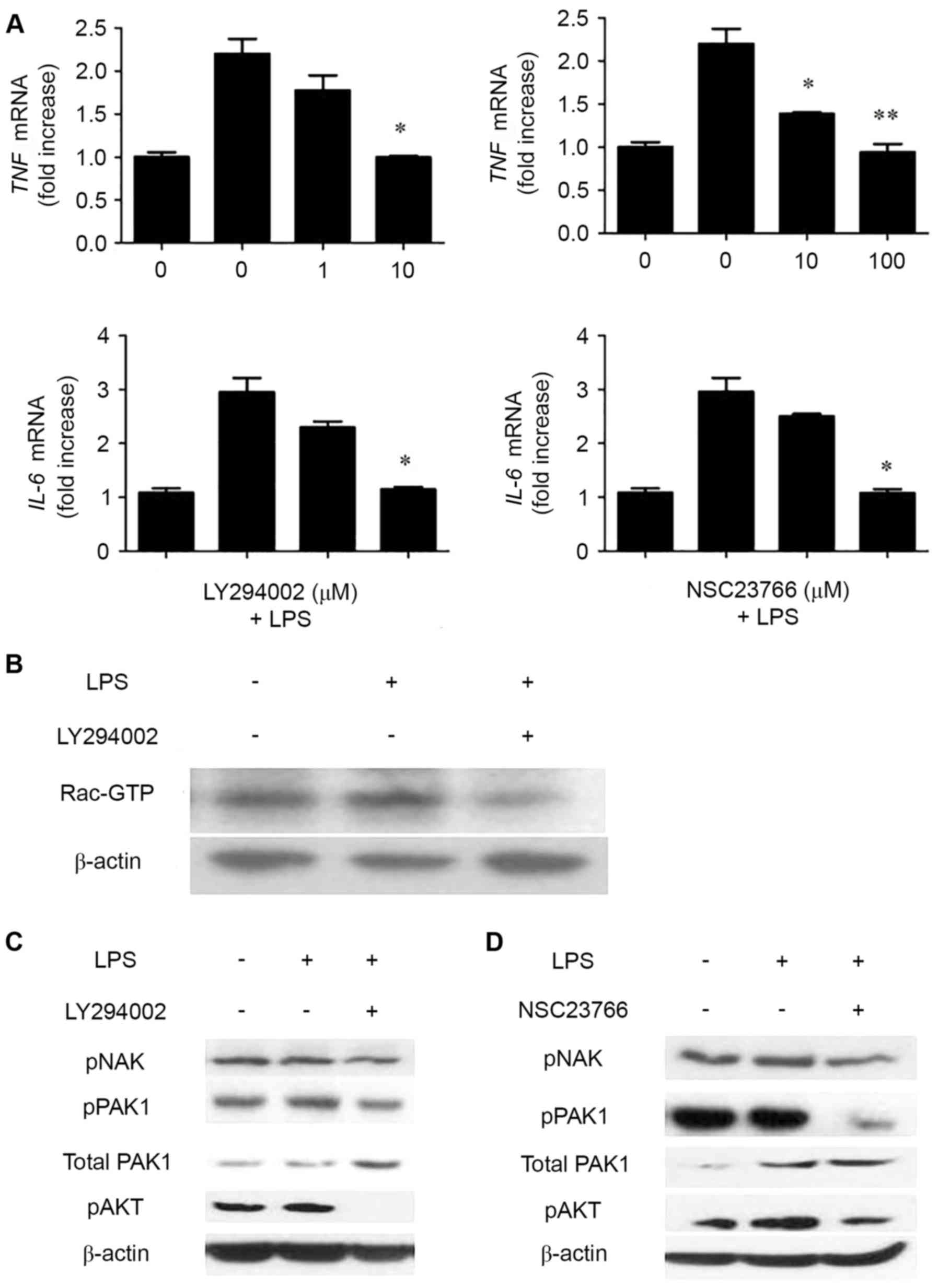 | Figure 4.PI3K and Rac inhibition significantly
diminishes LPS-induced cytokine gene expression in control RSFs.
(A) Control RSFs were treated with or without the indicated
concentrations of LY294002 (left) or NSC23766 (right) inhibitors
for 20 min prior to stimulation with LPS (100 ng/ml) for 2 h.
Cytokine expression was determined with reverse
transcriptase-quantitative polymerase chain reaction. Data are
presented as the mean ± standard deviation (n=3). *P<0.05 and
**P<0.01 vs. LPS treated NSFs. (B) Control RSFs were treated
with or without LY294002 (10 mM) for 30 min prior to stimulation
with LPS (100 ng/ml) for 2 h. Cells were lysed, and activated
Rac-GTP was detected via the pulldown assay and western blotting.
(C) Control RSFs were treated with or without LY294002 (10 µM) and
(D) NSC23766 (100 µM) for 30 min prior to stimulation with LPS (100
ng/ml) for 2 h. Cells were lysed, and pNAK, pPAK and pAKT were
detected. PI3K, phosphoinositide 3-kinase; LPS, lipopolysaccharide;
RSFs, rheumatoid arthritis synovial fibroblasts; GTP, guanosine
triphosphate; pNAK, phosphorylated TANK-binding kinase 1; pPAK,
phosphorylated p21 activated kinase 1 protein; pAKT, phosphorylated
protein kinase B. |
Discussion
TLRs recognize pathogen-associated molecular
patterns, which are microbial and viral products that induce cell
activation (30,31). Exogenous TLR ligands include
lipoteichoic acid, LPS, CpG motifs of bacterial DNA and viral RNA
(32,33). TLR2, TLR3, TLR4, TLR7 and various
other ligands were demonstrated to be highly expressed in synovial
tissue from RA patients compared with that from healthy donors
(22,23). TLR-mediated activation of RSFs from
patients with RA leads to significantly higher levels of key
proinflammatory cytokines, including IL-8, IL-6 and IL-15, compared
with fibroblast-like synoviocytes from healthy counterparts
(5,11,31).
TIPE2 is preferentially expressed in lymphoid
tissues such as the thymus and lymph nodes. Although TIPE2 is not
expressed in the NIH 3T3 fibroblast cell line, following
stimulation with TNF-α, NIH 3T3 fibroblasts express detectable mRNA
expression levels of the gene encoding TIPE2, suggesting that TIPE2
may be expressed in other cell types to establish equilibrium
during an inflammatory response (11,34).
Increasing experimental evidence suggests that TIPE2 is closely
associated with the occurrence and development of inflammatory
diseases and autoimmune diseases such as lung injury,
acute-on-chronic hepatitis B liver failure, hepatocellular
carcinoma, colitis, type 2 diabetes and systemic lupus
erythematosus (26,35,36).
However, the present study demonstrated that TIPE2 serves an
inhibitory role in RA. The results revealed that TIPE2 expression
was lower in rat RSFs compared with NSFs, whereas cytokine
expression levels of IL-6, IL-1, IFN-γ and TNF-α were significantly
increased in rat RSFs upon stimulation with LPS. In addition, Rac
activation between TIPE2-overexpressed RSFs and control RSFs were
compared, and elevated Rac activation was observed in control RSFs,
whereas IL-6, IL-1, TNF-α and IFN-γ were significantly increased in
the control group compared with the TIPE2-overexpressed group. The
Rac inhibitor NSC 23,766 effectively inhibited TNF-α and IL-6
production in a dose-dependent manner. Taken together, these
results suggested that TIPE2 regulates cytokines secretion in RSFs
via the Rac signaling pathway.
The TIPE2 protein, which constitutively binds to the
Rac small GTPase in immune cells, serves as a negative regulator of
phagocytosis and oxidative burst during infection in immune cells
(19), which are two fundamental
effector mechanisms of innate immunity. These effector mechanisms
are activated by TLRs and Rac GTPases, that work in unison to
eliminate infectious microbes (24,25).
PAK and AKT are downstream effectors of Rac and PI3K, respectively
(19). Previous studies have
suggested that Rac1 and PAK1 act upstream of NAK (also known as
TBK1/IκB kinase-ε) in the viral activation of IRF3 (20). In addition, Joung et al
(21) reported that AKT
contributes to the activation of the TIR-domain containing
adapter-inducing IFN-β-dependent signaling pathways of TLRs by
interacting with NAK. In the present study, PAK and AKT were also
activated in control RSFs compared with TIPE+/+ RSFs
(37,38). Increased activation of NAK was also
observed in control RSFs compared with TIPE2-overexpressed RSFs,
indicating that TIPE2 regulates NAK via the Rac/PAK pathway
(39,40).
The present study also revealed that the PI3K
inhibitor LY294002 effectively inhibited TNF-α and IL-6 production
in control RSFs in a dose-dependent manner, similar to the Rac
inhibitor NSC 23,766. LY294002 also significantly blocked Rac
activation. In addition, less PAK, AKT and NAK activation was
observed in the control RSFs and partial PAK, AKT and NAK
activation was found when the Rac inhibitor NSC 23,766 and LY294002
were added before LPS stimulation, indicating that Rac and PI3K may
be activated and involved in LPS-mediated cytokine expression.
These results suggested that Rac activation may be directly
involved in LPS-mediated cytokine expression in a PI3K dependent
manner (19–21).
In conclusion, the findings of the present study
demonstrated that TIPE2 serves a negative role in activating the
Rac signaling pathway and in the initiation of the immune response
via the activity of proinflammatory cytokines. These findings
uncovered some of the underlying mechanisms involved in RA with the
large number of inflammatory cytokines produced by RSFs. These
results may be useful in designing novel strategies for preventing
and treating RA.
Acknowledgements
The present study was supported by the Scientific
Research Foundation for the National Nature Science Foundation of
China (grant nos. 81072472 and 81272720) and the Fujian Province
Natural Science Fund (grant no. 2012J01416).
References
|
1
|
Ziff M: Rheumatoid arthritis-its present
and future. J Rheumatol. 17:127–133. 1990.PubMed/NCBI
|
|
2
|
Kim KW, Cho ML, Lee SH, Oh HJ, Kang CM, Ju
JH, Min SY, Cho YG, Park SH and Kim HY: Human rheumatoid synovial
fibroblasts promote osteoclastogenic activity by activating RANKL
via TLR-2 and TLR-4 activation. Immunol Lett. 110:54–64. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bottini N and Firestein G: Duality of
fibroblast-like synoviocytes in RA: Passive responders and
imprinted aggressors. Nat Rev Rheumatol. 9:24–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brenna FM and Mcinnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose-John S, Scheller J, Elson G and Jones
SA: Interleukin-6 biology is coordinated by membrane-bound and
soluble receptors: Role in inflammation and cancer. J Leukoc Biol.
80:227–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu YH, Li HH, Hsieh MY, Liu MF, Huang KY,
Chin LS, Chen PC, Cheng HH and Chang MS: Function of interleukin-20
as a proinflammatory molecule in rheumatoid and experimental
arthritis. Arthritis Rheum. 54:2722–2733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiang EY, Kolumam GA, Yu X, Francesco M,
Ivelja S, Peng I, Gribling P, Shu J, Lee WP, Refino CJ, et al:
Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17
cells inhibits autoimmune disease. Nat Med. 15:766–773. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu F, Shi L, Mu R, Zhu J, Li Y, Ma X, Li
C, Jia R, Yang D, Li Y and Li Z: Hypoxia-inducible factor-1a and
interleukin 33 form a regulatory circuit to perpetuate the
inflammation in rheumatoid arthritis. PLoS One. 8:e726502013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Liu Z, Zhuang G, Yin P, Tao H, Qiu
J, Hu Q and Zhang J: Anti-DR5 mAb ameliorate adjuvant arthritis
rats through inducing synovial cells apoptosis. Exp Biol Med
(Maywood). 234:1468–1476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Fayngerts S, Wang P, Sun H,
Johnson DS, Ruan Q, Guo W and Chen YH: TIPE2 protein serves as a
negative regulator of phagocytosis and oxidative burst during
infection. Proc Natl Acad Sci USA. 109:15413–15418. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MW, Su MX, Wang YH and Qian CY: Effect
of melilotus extract on lung injury via the upregulation of tumor
necrosis factor-a-induced protein-8-like 2 in septic mice. Mol Med
Rep. 11:1675–1684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang LY, Fan YC, Zhao J, Gao S, Sun FK,
Han J, Yang Y and Wang K: Elevated expression of tumour necrosis
factor-α-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral
blood mononuclear cells is associated with disease progression of
acute-on-chronic hepatitis B liver failure. J Viral Hepat.
21:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li D, Song LJ, Fan Y, Li X, Li Y, Chen J,
Zhu F, Guo C, Shi Y and Zhang L: Down-regulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
systemic lupus erythematosus. Clin Immunol. 133:422–427. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Shao Z, Zhang X, Jia X, Xia Y,
Zhang Y, Xin N, et al: TIPE2 Play a Negative Role in TLR-Mediated
Autoimmune T Helper 17 Cell Responses in Patients with Myasthenia
Gravis. J Neuroimmune Pharmacol. 10:635–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun H, Zhuang G, Chai L, Wang Z, Johnson
D, Ma Y and Chen YH: TIPE2 controls innate immunity to RNA by
targeting the phosphatidylinositol 3-kinase-Rac pathway. J Immunol.
189:2768–2773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joung SM, Park ZY, Rani S, Takeuchi O,
Akira S and Lee JY: Akt contributes to activation of the
TRIF-dependent signaling pathways of TLRs by interacting with
TANK-binding kinase 1. J Immunol. 186:499–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goh FG and Midwood KS: Intrinsic danger:
Activation of Toll-like receptors in rheumatoid arthritis.
Rheumatology (Oxford). 51:7–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drexler SK and Foxwell BM: The role of
toll-like receptors in chronic inflammation. Int J Biochem Cell
Biol. 42:506–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diebold BA and Bokoch GM: Rho GTPases and
the control of the oxidative burst in polymorphonuclear leukocytes.
Curr Top Microbiol Immunol. 291:91–111. 2005.PubMed/NCBI
|
|
25
|
Niedergang F and Chavrier P: Regulation of
phagocytosis by Rho GTPases. Curr Top Microbiol Immunol. 291:43–60.
2005.PubMed/NCBI
|
|
26
|
Shi C, Zhang S, Hong S, Pang J, Yesibulati
Y, Yin P and Zhuang G: The pro-apoptotic effects of TIPE2 on AA rat
fibroblast-like synoviocytes via regulation of the
DR5-caspase-NF-κB pathway in vitro. Onco Targets Ther. 9:993–1000.
2016.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arranz A, Gutiérrez-Cañas I, Carrión M,
Juarranz Y, Pablos JL, Martínez C and Gomariz RP: VIP reverses the
expression profiling of TLR4-stimulated signaling pathway in
rheumatoid arthritis synovial fibroblasts. Mol Immunol.
45:3065–3073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YH, Yan HQ, Wang F, Wang YY, Jiang
YN, Wang YN and Gao FG: TIPE2 inhibits TNF-α-induced hepatocellular
carcinoma cell metastasis via Erk1/2 downregulation and NF-kB
activation. Int J Oncol. 46:254–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kyburz D, Rethage J, Seibl R, Lauener R,
Gay RE, Carson DA and Gay S: Bacterial peptidoglycans but not CpG
oligodeoxynucleotides activate synovial fibroblasts by toll-like
receptor signaling. Arthritis Rheum. 48:642–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jung YO, Cho ML, Kang CM, Jhun JY, Park
JS, Oh HJ, Min JK, Park SH and Kim HY: Toll-like receptor 2 and 4
combination engagement upregulate IL-15 synergistically in human
rheumatoid synovial fibroblasts. Immunol Lett. 109:21–27. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakano K, Boyle D and Firestein G:
Regulation of DNA methylation in rheumatoid arthritis synoviocytes.
J Immunol. 190:1297–1303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ospelt C, Brentano F, Jüngel A, Rengel Y,
Kolling C, Michel BA, Gay RE and Gay S: Expression, regulation, and
signaling of the pattern-recognition receptor nucleotide-binding
oligomerization domain 2 in rheumatoid arthritis synovial
fibroblasts. Arthritis Rheum. 60:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Zhao X, Liu X and Liu H: Disruption
of TIM-4 in dendritic cell ameliorates hepatic warm IR injury
through the induction of regulatory T cells. Mol Immunol.
66:117–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Termeer C, Benedix F, Sleeman J, Fieber C,
Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C and Simon JC:
Oligosaccharides of Hyaluronan activate dendritic cells via
toll-like receptor 4. J Exp Med. 195:99–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sacre S, Medghalchi M, Gregory B, Brennan
F and Williams R: Fluoxetine and citalopram exhibit potent
antiinflammatory activity in human and murine models of rheumatoid
arthritis and inhibit toll-like receptors. Arthritis Rheum.
62:683–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ehrhardt C, Kardinal C, Wurzer WJ, Wolff
T, von Eichel-Streiber C, Pleschka S, Planz O and Ludwig S: Rac1
and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral
activation of interferon regulatory factor-3. FEBS Lett.
567:230–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okamura Y, Watari M, Jerud ES, Young DW,
Ishizaka ST, Rose J, Chow JC and Strauss JF III: The extra domain A
of fibronectin activates Toll-like receptor 4. J Biol Chem.
276:10229–10233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hemmi H, Takeuchi O, Kawai T, Kaisho T,
Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K and
Akira S: A Toll-like receptor recognizes bacterial DNA. Nature.
408:740–745. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Ouyang H, Wang D, Shi J, Ouyang
C, Chen H, Xiao S and Fang L: Mycobacterium tuberculosis Rv2185c
contributes to nuclear factor-κB activation. Mol Immunol.
66:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|


















