Introduction
Oral cancer is currently the 11th most common cancer
in the world, with the highest incidence and mortality rates in
developing countries (1). Oral
tongue squamous cell carcinoma (OTSCC) is the most common type of
oral tumor, accounting for 94% of all oral malignancies (2,3).
OTSCC is characterized by local infiltrating growth in the oral
cavity, expanding invasion into the lymph node and uterine
metastasis (4,5). Clinically, the significance of
primary tumor thickness identified direction and partial
glossectomy as primary treatments. These are usually only suitable
for OTSCC patients of clinical stage I and II (6). However, OTSCC has been traditionally
believed to be associated with easy recurrence, metastasis and poor
prognosis due to rapid migration and invasion (6). Local migration to perienchymas, and
long distance metastasis to organs, are the main reasons leading to
mortality and poor survival rate among patients with OTSCC
(7). Previous studies have
suggested that patients diagnosed with early-stage OTSCC (T1-2, N0)
have improved 5-year survival rates of between 75 and 89% (8,9). In
addition, a previous study indicated that prognosis of patients
with OTSCC can be improved by early detection and appropriate
treatment (10). Therefore,
efficient diagnoses and treatments for patients with OTSCC are
required to improve the survival rate for patients.
A previous study indicated that CXC chemokine
receptor-7 (CXCR-7) promotes progression and metastasis of tumor
cells, representing a potential target molecule for cancer therapy
(11). CXCR-7 has been
characterized as a novel receptor for CXC-7 (12). A previous study demonstrated that
the CXCR-7 gene encodes members of the G protein-coupled receptor
family, which was identified as an orphan receptor for vasoactive
intestinal peptides (13). CXCR-7
is now classified as a novel receptor for the CXC motif chemokine
12/stromal cell-derived factor 1-alpha, which may serve a role in
the progression, metastasis and angiogenesis of
otorhinolaryngologic tumors (14).
In addition, CXCR-7 has been considered to serve an essential role
in the pathogenesis of tumor angiogenesis and metastasis (11). Furthermore, Yates et al
(11) demonstrated that CXCR-7
forms a functional complex associated with epidermal growth factor
(EGF)-receptor, extracellular signal-regulated kinase (ERK),
phosphoinositide 3-kinase/protein kinase B (AKT) and proto-oncogene
tyrosine-protein kinase Src, which subsequently induced the
phosphorylation and upregulation of B-cell lymphoma 2 (Bcl-2) and
cyclin-D1. These studies indicated that suppression of CXCR-7
expression by CXC-7 reversed these effects and resulted in
suppressed growth, inhibition of migration and induction of
apoptosis. Therefore, it was hypothesized that CXC-7 inhibits OTSCC
cell migration and invasion via regulation of the ERK/AKT signaling
pathway.
The epithelial-to-mesenchymal transition (EMT)
signaling pathway is a key process in tumor cell progression and
metastasis stimulated by EGF/Ras and transforming growth factor-β
(TGF-β) signaling pathways, which lead to a complex biochemical
reaction processes in tumor cells (15,16).
A previous study indicated that suppressing the progress of EMT may
be clinically helpful for cancer therapy (17). EMT was reported as a fundamental
process in cancer cell progression, during which epithelial cells
disassemble, fibroblastic-mesenchymal phenotype acquire, basement
membranes digest, and tosurrounding tissues transmigrate (18). EMT is involved in pathological
situations of tumor cells such as the acquisition of an invasive,
metastatic phenotype in tumors of epithelial origin (19). A previous study examined the
molecular mechanisms of the EMT signaling pathway by Ras-induce
signaling, which regulates EMT process in human tumor cells
(20). In process of
carcinogenesis, EMT promotes epithelial cells to dislodge
epithelial polarity, digest the basement membrane and invade
adjacent tissues, and even enter the bloodstream and colonize new
palingenetic base (21). These
references suggested that EMT signaling pathway was a potential
tumor-therapy process during progression of neoplasm, and indicated
that inhibition of EMT signaling pathway may suppress migration,
invasion and metastasis through loss of inhibitory signaling in EMT
signaling pathway.
The present study investigated CXCR-7 expression and
the function of CXC-7 in the growth and migration of OTSCC cells.
The CXCR-7 signaling pathway in relation to migration, invasion and
protein expression in OTSCC cells was examined. It was demonstrated
that inhibition of CXCR-7 expression suppressed OTSCC cell growth,
migration and invasion both in vitro and in vivo.
These results suggested that CXCR-7 is a potential target for OTSCC
therapy.
Materials and methods
Cell lines
The Tca8113 and SCC25 oral squamous carcinoma cell
lines were purchased from the American Type Culture Collection
(Manassas, MA, USA) and cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen, Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (Invitrogen,
Carlsbad, CA, USA). The hNOE human normal oral epithelial cell line
was obtained from the School of Stomatology of Shandong University
(Jinan, China). All cells were cultured in a humidified incubator
with 2 mM penicillin/streptomycin (37°C, 5% CO2).
MTT assay
Tca8113 and SCC25 cells (1×106) were
treated with CXC-7 (2 mg/ml) or PBS in 6-well plates for 48 h in
triplicate at 37°C. After culturing in DMEM, 20 µl MTT (5 mg/ml)
was added to the cells. Then the cells were further incubated for 4
h at 37°C. The medium was removed and 100 µl dimethylsulfoxide was
added into the wells to solubilize the crystals. The results were
measured using an ELISA reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) reader at a wavelength of 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from Tca8113 and SCC25 cells
using an RNA Easy Mini Extract kit (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 4°C. The expression levels of CXCR-7 in
SCC25 and Tca8113 cells were calculated by RT-qPCR using iQ SYBR
Green Supermix (Bio-Rad Laboratories, Inc.) with β-actin expression
as an endogenous control. Primer sequences were as follows:
Forward, 5′-CACTGAAGGAGCCTGCAGC-3′ and reverse,
5′-CATCTTCTTCCTCGCATGCA-3′ for CXCR-7; forward,
5′-GTGGGCGCCCAGGCACCA-3′ and reverse 5′-CTCCTTAATGTCACGCACGATTT-3′
for β-actin. Cycling conditions were as follows: 45 cycles of
denaturation at 95°C for 2 min, annealing at 66°C for 30 sec with
touchdown to 56°C for 30 sec and extension at 72°C for 10 min. All
procedures were performed according to the manufacturer's protocol.
All primers were synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. Relative CXCR-7 expression level was determined by
the 2−ΔΔCq method (22). The final results were presented as
the n-fold manner compared to β-actin.
Cell migration and invasion
assays
Tca8113 cells were incubated with CXC-7 in
serum-free DMEM media. For the migration assay, CXC-7-treated cells
were suspended at a density of 5×105 in serum-free DMEM
and then transferred to the tops of BD BioCoat Matrigel Migration
Chambers (BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. For the invasion assay, Tca8113 and SCC25
cells were treated with CXC-7 or PBS for 24 h using a control
insert (BD Biosciences) instead of a Matrigel Migration Chamber.
The tumor cells invasion and migration were observed in at least
three random fields under a photomicroscope (Olympus Corporation,
Tokyo, Japan).
Flow cytometric analysis of
apoptosis
Tca8113 and SCC25 cells were cultured in DMEM
supplemented with 10% fetal bovine serum for 48 h. Tca8113 and
SCC25 cells were treated with CXC-7, CXCR-7 or PBS as a control for
48 h. All cells were subsequently treated with cisplatin for 12 h.
The apoptosis of suspended cells were analyzed by flow cytometry
(BD Biosciences) using FACSDiva software version 10.0.7 (Flowjo
LLC, BD Biosciences, Franklin Lakes, NJ, USA) as described
previously (23).
Animal study
Specific pathogen-free male BALB/c mice (age, 6–8
weeks; n=40, body weight, 32–35 g) were purchased from Slack
Experimental Animals Co., Ltd. (Shanghai, China). All animals had
free access to food and water and were housed in a
temperature-controlled facility at 23±1°C and relative humidity of
50±5% with a 12 h light/dark cycle. Tca8113 cells at a density of
1×107 suspended in 100 µl PBS were subcutaneously
injected into BALB/c mice. When tumor diameters reached 5–6 mm on
day 7 after tumor inoculation, Tca8113-bearing mice were randomly
divided into 2 groups (n=20/group), which received CXC-7 (80 mg/kg)
or the same volume of PBS. The total treatments were 6 times at
2-day intervals. The mice were observed for 25 days and were
sacrificed using sodium pentobarbital anesthesia (50 mg/kg,
Invitrogen; Thermo Fisher Scientific, Inc.) when the tumor diameter
reached 12 mm. Tumor diameters were recorded every 2 days and tumor
volume was calculated by using the formula: 0.52 × smallest
diameter2 x largest diameter, as described previously
(24). Animal experiments were
approved by the Animal Care and Welcome Committee of Shandong
University (Jinan, China).
Western blotting
Tca8113 cells were cultured to 90% monolayer cell
formation. The cells were lysed with radioimmunoprecipitation assay
lysate buffer containing protease-inhibitor (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and were centrifuged at 6,500 × g
at 4°C for 10 min. The supernatant of mixture was analyzed using
western blotting for ERK, TGF-β, Vimentin (Vim), collagen type I
(CT-I) and Slug protein expression, as described previously
(25). For western blotting, the
following rabbit anti-human primary antibodies were used: CXC-7
(1:1,000; catalog no. ab89256; Abcam, Cambridge, UK), EGF (1:500;
catalog no. ab9695; Abcam) and TGFβ (1:500; catalog no. ab92486;
Abcam) and (1:500; catalog no. ab8226; Abcam). The horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (Bio-Rad
Laboratories, Inc.) was used at a 1:5,000 dilution and detected
using an Enhanced Chemiluminescence substrate solution (GE
Healthcare, Chicago, IL, USA) according to the manufacturer's
protocol.
Histological analysis
Tumors from experimental mice were fixed in 10%
formaldehyde and were then embedded in paraffin. Tumor samples were
sectioned (4 µm) using a microtome and subjected to
deparrafinization in a series of alcohols and antigen retrieval.
Tumor sections were incubated with the following primary
antibodies: CXC-7 (1:1,000; catalog no. ab89256; Abcam), EGF
(1:500; catalog no. ab9695; Abcam) and TGFβ (1:500; catalog no.
ab92486; Abcam) and (1:500; catalog no. ab8226; Abcam) for 12 h at
4°C. Horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (Bio-Rad Laboratories, Inc.) was used at a 1:5,000
dilution for 1 h at 37°C. Tumor sections were captured with an
inverted fluorescence microscope (Olympus Corporation) at ×400
magnification and Leica Application Suite X software (version
3.0.2).
Immunofluorescence
Tca8113 and SCC25 cells were cultured until 90%
monolayer cells were formatted. The cells subsequently incubated
with a mouse anti-human CXCR-7 primary antibody (1:1,000; catalog
no. ab89256; Abcam). Following this, a goat anti-mouse CXCR-7
secondary antibody (1:1,000; catalog no. ab89251; Abcam) was
applied for immobilized cells. Finally, the cells were washed with
PBS to completely remove the residual antibody. The Ventana
Benchmark automated staining system was used for observation of
CXCR-7 expression by confocal microscopy.
Statistical analysis
All data in this study are presented as the mean ±
standard error of triplicate experiments. Data was analyzed using
SPSS Statistics version 19.0 software (IBM Corp., Armonk, NY, USA),
Graphpad Prism version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) and Microsoft Excel (Microsoft Corporation,
Redmond, WA, USA). Data was analyzed by one-way analysis of
variance followed by Fisher's exact test, or a Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
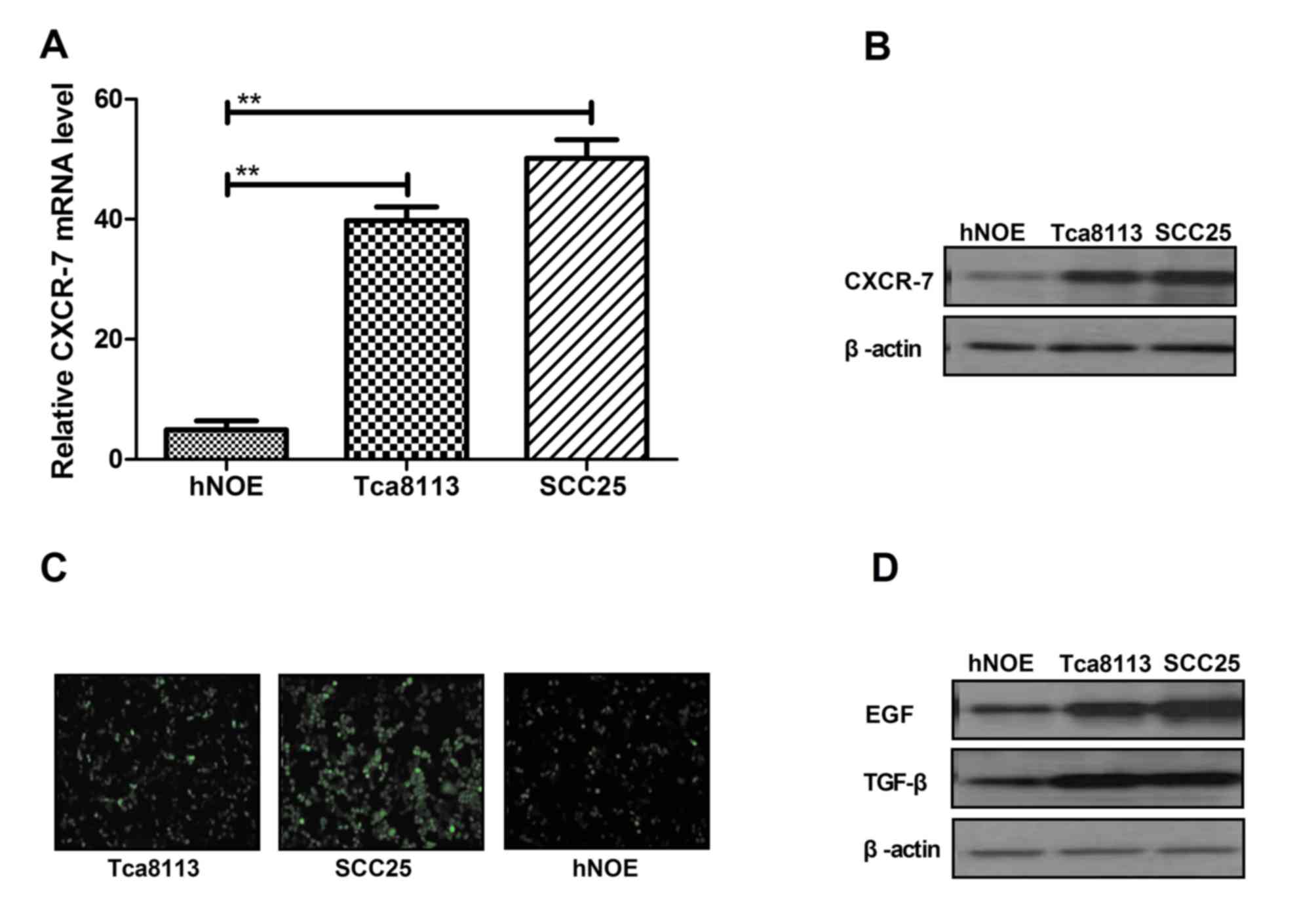
Expression of CXCR-7 in cultured OTSCC
lines
To elucidate the role of CXCR-7 in the migration and
progress of Tca8113, SCC25 and hNOE cells were cultured and
harvested for RT-qPCR analysis. CXCR-7 mRNA expression levels were
upregulated in Tca8113 and SCC25 cells compared with hNOE cells
(Fig. 1A). Western blotting
revealed that the protein expression levels were higher in Tca8113
and SCC25 cells compared with hNOE cells (Fig. 1B). Immunofluorescence staining
demonstrated that CXCR-7 expression was lower in hNOE cells
compared with the other two cell lines (Fig. 1C). Furthermore, EGF and TGF-β
expression levels were increased in Tca8113 and SCC25 cells
compared with hNOE cells (Fig.
1D). These data indicated that CXCR-7 expression was
upregulated in Tca8113 and SCC25 cells compared with hNOE cells,
suggesting that these factors are upregulated in OTSCC.
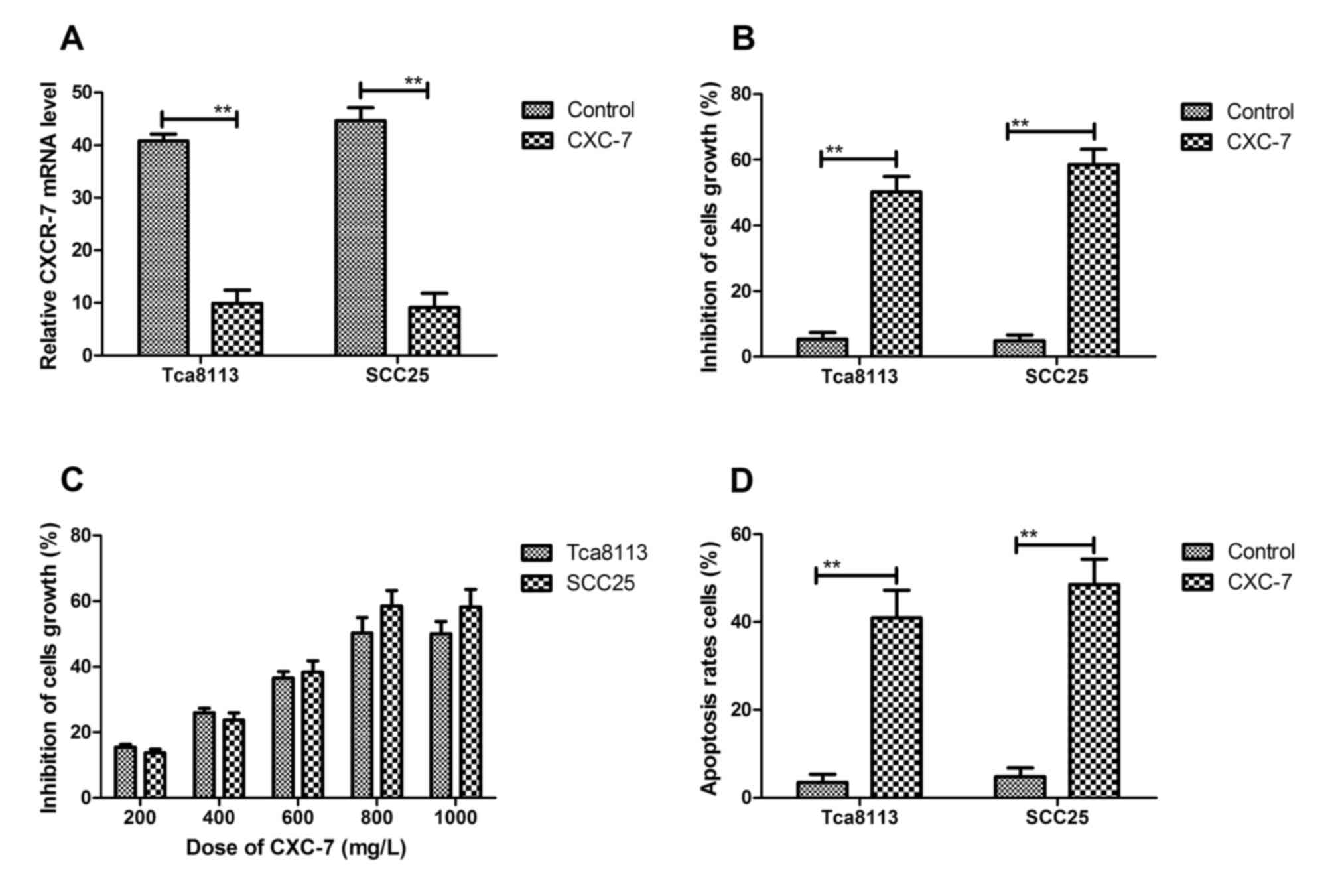
In vitro effects of CXC-7 on OTSCC
cells
The effects of CXC-7 on growth induction of OTSCC
cells were examined in vitro. The results in Fig. 2A showed that CXC-7 significantly
decreased CXCR-7 expression in cultured Tca8113 and SCC25 cells.
Tca8113 and SCC25 cell growth was inhibited by CXC-7 via
suppression of CXCR-7 expression (Fig.
2B). The results in Fig. 2C
demonstrated the inhibitory effect on cell growth in a
dose-dependent manner in Tca8113 and SCC25 cells. The inhibition
arrived at maximum degree for tumor cell growth when the
concentration reached 800 mg/ml. Therefore, 800 mg/ml CXCR-7 was
chosen for further analysis. As shown in Fig. 2D, the apoptosis rate was
significantly promoted in Tca8113 and SCC25 cells after CXCR-7
treatment for 48 h. Collectively, these results suggested that
inhibition of CXCR-7 expression by CXC-7 significantly suppressed
growth and promoted apoptosis in Tca8113 and SCC25 cell lines.
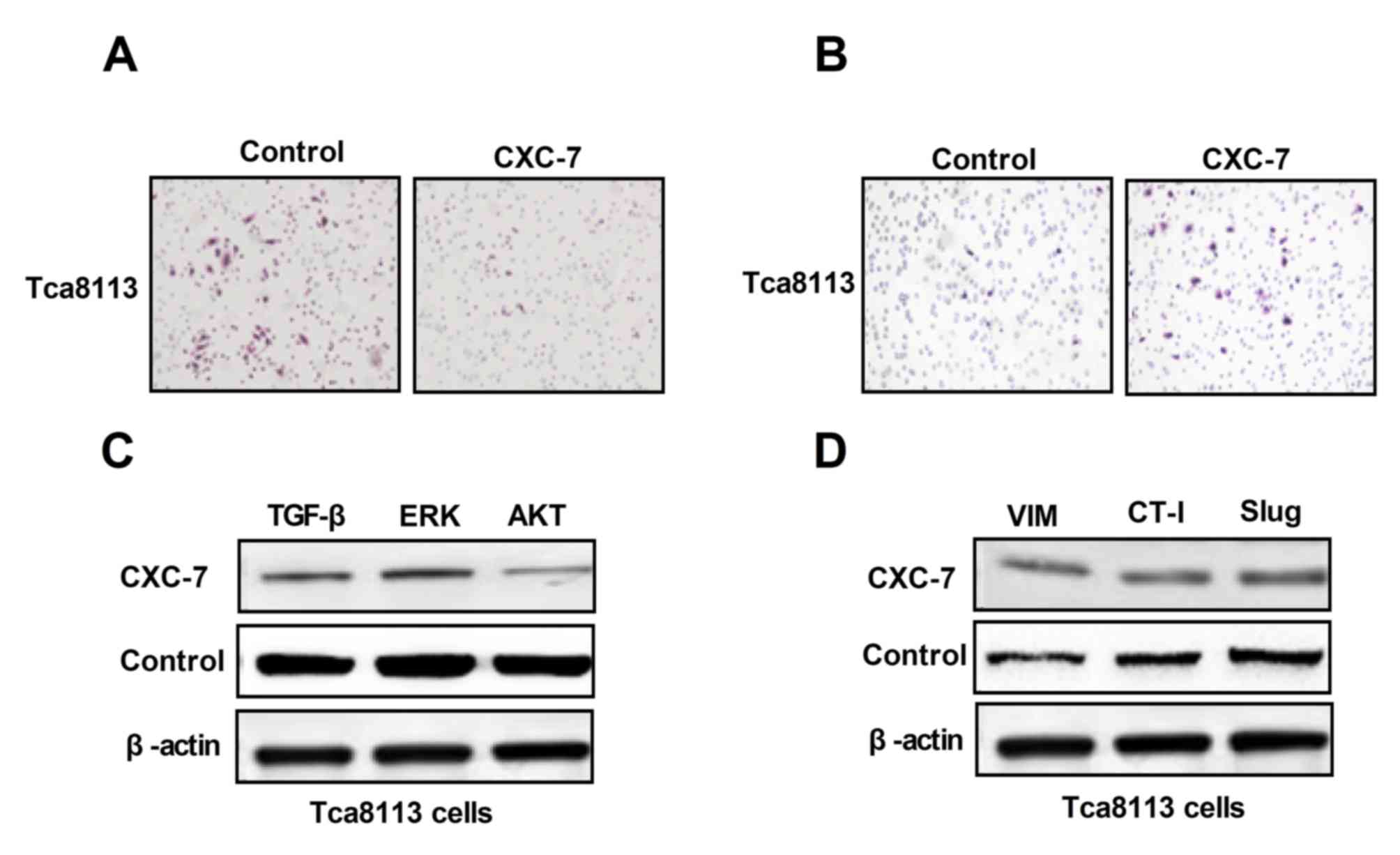
CXC-7 inhibits migration and invasion
via regulating EMT pathways in OTSCC cells
To investigate whether EMT may be associated with
OTSCC cell migration and invasion, the effect of CXC-7 on
EMT-associated proteins and aggressive behavior in Tca8113 cells
was investigated in vitro. Migration and invasion abilities
of Tca8113 cells after treatment with CXC-7 were analyzed. As shown
in Fig. 3A and 3B, CXC-7
suppressed migration and invasion of Tca8113 cells. Next,
migration-associated protein expression in Tca8113 cells prior and
post treatment of CXC-7 was investigated. As demonstrated in in
Fig. 3C, CXC-7 downregulated key
functional proteins including TGF-β, ERK and AKT in Tca8113 cells
compared to control group. In addition, migration-associated
proteins, Vim, CT-I and Slug were analyzed after CXC-7 treatment.
Results showed that CXC-7 downregulated the expression levels of
these migration-promoting proteins in OTSCC cells compared to
control group (Fig. 3D). These
data suggested that CXC-7 inhibited migration and invasion via the
TGF-β-mediated EMT signaling pathway.
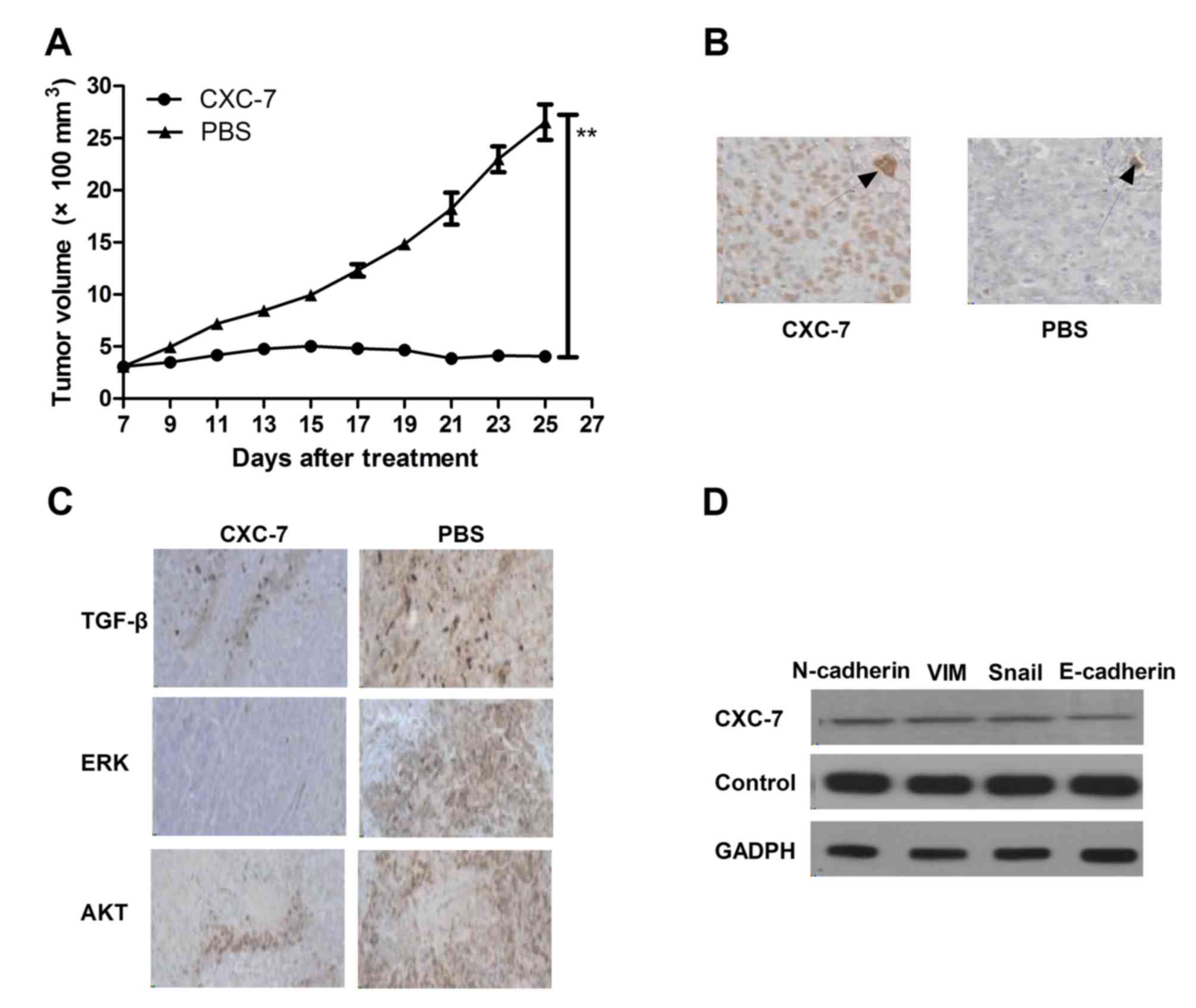
Inhibition of CXCR-7 expression
exhibits beneficial effects on OTSCC-bearing mice
The in vivo effects of CXC-7 was analyzed in
Tca8113-bearing mice in vivo. Tca8113 (1×106)
cells were injected subcutaneously into BALB/c nude mice. The mice
received CXC-7 or the same volume of PBS as a control when tumor
diameter reached 5–6 mm. CXC-7 significantly suppressed tumor
growth compared with PBS-treated mice in a 25 day observation
(Fig. 4A). After 25 days CXC-7
treatment, CXCR-7 expression in tumors was detected in each
experimental mouse. The results in Fig. 4B demonstrated that apoptosis was
increased after CXC-7 treatment compared to the PBS-treated group.
In addition, TGF-β, ERK and AKT expression was detected in tumors
by histological staining in the different treatment groups. The
results in Fig. 4C demonstrated
that CXC-7 downregulated TGF-β, ERK and AKT expression compared
with the PBS group. Furthermore, CXC-7 treatment significantly
downregulated E-cadherin, Snail, N-cadherin and Vim compared with
the control group, as determined by western blotting (Fig. 4D). These results suggested that
CXC-7 may be inhibit tumor growth, angiogenesis and apoptosis in
OTSCC.
Discussion
The current treatments for the majority of patients
with OTSCC are partial glossectomy performed by surgery, with
generally promising outcomes (24,25).
However, the rapid growth of OTSCC cells, local migration toward to
adjacent tissue, and fast metastasis to cervical, lymphatic and
other organs shortens the 5-year survival period (26). Therefore, cell migration and
invasion is the most important characteristic of OTSCC, and limits
drug tumor therapy in clinics, which has contributed to adjacent
migration and long distance metastasis to other organs or tissues
(27,28). A previous study has suggested that
early diagnosis is beneficial for patients with OTSCC in
eradication of tumor cells (29).
The results of the present study indicated that inhibition of OTSCC
adjacent migration and long distance metastasis may be efficient to
improve the efficacy and clinical outcomes of the drug.
Previous studies have reported that patients with
OTSCC overexpress levels of CXCR-7, leading to a poorer prognosis.
Overexpression of CXCR-7 significantly increased cell growth,
migration and invasion, while inhibition of CXCR-7 expression
markedly suppresses the invasive properties of OTSCC cell lines
(2,30,31).
Therefore, the mechanism of CXCR-7-associated signaling pathways on
the invasive properties of tumor cells requires further study.
Previous studies have indicated that CXCR-7 associates with
EGF-receptor to form a complex that recruits downstream signaling
molecules and activates phosphorylation of ERK/AKT, as determined
by co-localization and co-immunoprecipitation experiments (32,33).
In addition, CXCR-7 enhances apoptotic resistance and strengthens
cyclin D1 expression levels through modulating AKT and ERK
expression, and regulation of B-cell lymphoma 2 expression levels
(34). The present study suggested
that CXCR-7 expression regulates apoptosis, proliferation,
migration, invasion, survival and motility in tumor cells via
signaling events such as EGF-receptor activation and the AKT/ERK
signaling pathway.
OTSCC is characterized by rapid growth in tumors,
local migration toward to adjacent tissue, and rapid metastasis to
organs (35,36). In addition, the incidence of OTSCC
in young patients is increasing in the world (37). Therefore, more efficient anti-tumor
agents for the treatment of OTSCC are urgently required. The
present study aimed to investigate the expression of CXCR-7 in
OTSCC cells and tissue samples, and to determine the role of CXC-7
in the development of OTSCC. It was demonstrated that CXCR-7 served
an essential role in the progression, metastasis and tumor
angiogenesis of OTSCC via regulation of the EMT signaling pathway.
Immunofluorescence analysis demonstrated that CXCR-7 and
migration-associated protein expression levels were significantly
upregulated in OTSCC cells. Therefore, CXCR-7 expression may
associate with the clinical features, degree of tumor spread, poor
overall survival of OTSCC patients and the prognosis of patients
receiving oncotherapy. The results of the present study
demonstrated that treatment with CXC-7 significantly inhibited
TGF-β, ERK and AKT expression and phosphorylation levels both in
OTSCC tumors and cell lines, which augmented the
anchorage-independent growth, migration and invasive abilities of
OTSCC cells. In addition, the results indicated that CXC-7
downregulated Vim, CT-I and Slug expression levels and provoked the
ability of OTSCC carcinoma to suppress neovascularization of tumor
angiogenesis that enhanced OTSCC carcinoma cell apoptosis, induced
by the chemotherapeutic agent cisplatin. These findings provided
novel insights into the potential roles of CXCR-7 deregulation in
promoting tumor cell metastasis, angiogenesis and progression,
which may be a molecular target in the treatment of OTSCC.
The clinical value of identifying OTSCC target
molecules not only helps determine the degree of aggressive tumor
behaviors, but also provides target therapeutic agents for patients
with OTSCC, which allows for the early diagnosis and efficient
treatment of patients in earlier stages (29,38,39).
The present study demonstrated that CXCR-7 regulated progression,
metastasis and tumor angiogenesis of OTSCC cells via regulation of
the EMT signaling pathway. The EMT signaling pathway has been
indicated as a developmental process with a function in tumor
progression and metastasis, and has been extensively studied
previously (20,40,41).
TGF-β and Ras are essential for the EMT signaling pathway; however,
their expression levels often depend on tumor cell type, which
hinders comprehensive analysis of key underlying mechanisms in EMT
(20). The present study suggested
that CXC-7 significantly inhibits TGF-β-induced EMT signaling in
OTSCC cell lines. In addition, levels of the epithelial marker
E-cadherin and the mesenchymal marker fibronectin were
downregulated in OTSCC cells. Furthermore, Vim, CT-I and Slug
expression levels were also downregulated after CXC-7 treatment.
Therefore, the identification of CXCR-7 can define the stage of
OTSCC, and achieve more efficient target therapy of CXC-7 for
patients with OTSCC.
In conclusion, the present study identified that the
CXCR-7 gene is upregulated in OTSCC cells and tumors, and the
ability of CXC-7 to repress CXCR-7 may inhibit OTSCC cell growth,
migration and invasion through TGF-β-induced EMT signaling.
Additionally, CXC-7 significantly inhibited OTSCC cell growth,
migration and invasion, and the expression of epithelial markers
was retained and the blood vessel density was suppressed in OTSCC.
These results suggested that CXC-7 regulates CXCR-7 gene expression
via TGF-β-induced EMT signaling, leading to decreased apoptosis
resistance, tumor progression and metastasis. These results provide
novel therapeutic agents for patients with OTSCC.
References
|
1
|
Dik EA, Willems SM, Ipenburg NA, Rosenberg
AJ, Van Cann EM and van Es RJ: Watchful waiting of the neck in
early stage oral cancer is unfavourable for patients with occult
nodal disease. Int J Oral Maxillofac Surg. 45:945–950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kolokythas A, Park S, Schlieve T, Pytynia
K and Cox D: Squamous cell carcinoma of the oral tongue:
Histopathological parameters associated with outcome. Int J Oral
Maxillofac Surg. 44:1069–1074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhartiya D, Kumar A, Singh H, Sharma A,
Kaushik A, Kumari S and Mehrotra R: OrCanome: A comprehensive
resource for oral cancer. Asian Pac J Cancer Prev. 17:1333–1336.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong WM, Parvathaneni U, Jewell PD,
Martins RG, Futran ND, Laramore GE and Liao JJ: Squamous cell
carcinoma of the oral tongue in a patient with Fanconi anemia
treated with radiotherapy and concurrent cetuximab: A case report
and review of the literature. Head Neck. 35:E292–298.
2013.PubMed/NCBI
|
|
5
|
Amichetti M: Squamous cell carcinoma of
the oral tongue in patients less than fifteen years of age. Report
of a case and review of the literature. J Craniomaxillofac Surg.
17:75–77. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martínez C, Hernández M, Martínez B and
Adorno D: Frequency of oral squamous cell carcinoma and oral
epithelial dysplasia in oral and oropharyngeal mucosa in Chile. Rev
Med Chil. 144:169–174. 2016.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shrestha A, Marla V, Shrestha S and
Agrawal D: Awareness of undergraduate dental and medical students
towards oral cancer. J Cancer Educ. Mar 28–2016.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumoto K, Sasaki T, Shioyama Y,
Nakamura K, Atsumi K, Nonoshita T, Ooga S, Yoshitake T, Uehara S,
Hirata H and Honda H: Treatment outcome of high-dose-rate
interstitial radiation therapy for patients with stage I and II
mobile tongue cancer. Jpn J Clin Oncol. 43:1012–1017. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirota K, Wakisaka N, Sawada-Kitamura S,
Kondo S, Endo K, Tsuji A, Murono S and Yoshizaki T:
Lymphangiogenesis in regional lymph nodes predicts nodal recurrence
in pathological N0 squamous cell carcinoma of the tongue.
Histopathology. 61:1065–1071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehrotra R and Yadav S: Oral squamous cell
carcinoma: Etiology, pathogenesis and prognostic value of genomic
alterations. Indian J Cancer. 43:60–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yates TJ, Knapp J, Gosalbez M, Lokeshwar
SD, Gomez CS, Benitez A, Ekwenna OO, Young EE, Manoharan M and
Lokeshwar VB: C-X-C chemokine receptor 7: A functionally associated
molecular marker for bladder cancer. Cancer. 119:61–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D
and Xu T: CXCR-7 receptor promotes SDF-1α-induced migration of bone
marrow mesenchymal stem cells in the transient cerebral
ischemia/reperfusion rat hippocampus. Brain Res. 1575:78–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Shiozawa Y, Wang J, Wang Y, Jung
Y, Pienta KJ, Mehra R, Loberg R and Taichman RS: The role of
CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate
cancer. J Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang T, Xia QJ, Qiao X and Xi M:
Expression of C-X-C chemokine receptor type 7 in
otorhinolaryngologic neoplasms. Singapore Med J. 57:157–160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Liu H, Zhang H and Shao RG: The
TGF-β signaling pathway induced EMT in breast cancer. Yao Xue Xue
Bao. 50:385–392. 2015.(In Chinese). PubMed/NCBI
|
|
16
|
Elsum IA, Martin C and Humbert PO:
Scribble regulates an EMT polarity pathway through modulation of
MAPK-ERK signaling to mediate junction formation. J Cell Sci.
126:3990–3999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S
and Yu Y: Interleukin-17-induced EMT promotes lung cancer cell
migration and invasion via NF-κB/ZEB1 signal pathway. Am J Cancer
Res. 5:1169–1179. 2015.PubMed/NCBI
|
|
18
|
David CJ, Huang YH, Chen M, Su J, Zou Y,
Bardeesy N, Iacobuzio-Donahue CA and Massagué J: TGF-β tumor
suppression through a lethal EMT. Cell. 164:1015–1030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attramadal CG, Kumar S, Boysen ME, Dhakal
HP, Nesland JM and Bryne M: Tumor budding, EMT and cancer stem
cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res.
35:6111–6120. 2015.PubMed/NCBI
|
|
20
|
Li Y, Ma J, Qian X, Wu Q, Xia J, Miele L,
Sarkar FH and Wang Z: Regulation of EMT by notch signaling pathway
in tumor progression. Curr Cancer Drug Targets. 13:957–962. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawicka D, Chojnacka-Puchta L, Zielinski
M, Plucienniczak G, Plucienniczak A and Bednarczyk M: Flow
cytometric analysis of apoptosis in cryoconserved chicken
primordial germ cells. Cell Mol Biol Lett. 20:143–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pouessel D, Neuzillet Y, Mertens LS, Van
Der Heijden MS, de Jong J, Sanders J, Peters D, Leroy K, Manceau A
and Maille P: Tumor heterogeneity of fibroblast growth factor
receptor 3 (FGFR3) mutations in invasive bladder cancer:
Implications for perioperative anti-FGFR3 treatment. Ann Oncol.
27:1311–1316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allegra M, Zaragkoulias A, Vorgia E,
Ioannou M, Litos G, Beug H and Mavrothalassitis G: Semaphorin-7a
reverses the ERF-induced inhibition of EMT in ras-dependent mouse
mammary epithelial cells. Mol Biol Cell. 23:3873–3881. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goepfert RP, Kezirian EJ and Wang SJ: Oral
tongue squamous cell carcinoma in young women: A matched
comparison-do outcomes justify treatment intensity? ISRN
otolaryngol 2014. 5293952014.
|
|
27
|
Fan KH, Lin CY, Kang CJ, Huang SF, Wang
HM, Chen EY, Chen IH, Liao CT, Cheng AJ and Chang JT:
Combined-modality treatment for advanced oral tongue squamous cell
carcinoma. Int J Radiat Oncol Biol Phys. 67:453–461. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim YC, Lee JS, Koo BS, Kim SH, Kim YH and
Choi EC: Treatment of contralateral N0 neck in early squamous cell
carcinoma of the oral tongue: Elective neck dissection versus
observation. Laryngoscope. 116:461–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiff BA, Roberts DB, El-Naggar A, Garden
AS and Myers JN: Selective vs modified radical neck dissection and
postoperative radiotherapy vs observation in the treatment of
squamous cell carcinoma of the oral tongue. Arch Otolaryngol Head
Neck Surg. 131:874–878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abu-Serriah M, Shah KA, Rajamanohara R,
Fasanmade A, Graystone J, Gerry S and Bond S: Tumour depth of
invasion of pT1 squamous cell carcinoma of the oral tongue and risk
of pathologically detected neck metastases. Head Neck. Jun
3–2015.(Epub ahead of print). PubMed/NCBI
|
|
31
|
Helal HH, Qi CE, Zhao YY, Yao CS and Li de
C: The effects of combination of gefitinib and cisplatin on tongue
squamous cell carcinoma cell lines. J Cancer Res Ther. 11:37–40.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernatoniene J, Zhang Q, Dogan S, Mitchell
TJ, Paton JC and Finn A: Induction of CC and CXC chemokines in
human antigen-presenting dendritic cells by the pneumococcal
proteins pneumolysin and CbpA, and the role played by toll-like
receptor 4, NF-kappaB, and mitogen-activated protein kinases. J
Infect Dis. 198:1823–1833. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maksym RB, Tarnowski M, Grymula K,
Tarnowska J, Wysoczynski M, Liu R, Czerny B, Ratajczak J, Kucia M
and Ratajczak MZ: The role of stromal-derived factor-1-CXCR7 axis
in development and cancer. Eur J Pharmacol. 625:31–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shakeel Uz Zaman, Adeel M and Suhail A:
Squamous cell carcinoma of oral tongue in young patients-A 10 years
tertiary care experience. J Pak Med Assoc. 66:155–158.
2016.PubMed/NCBI
|
|
36
|
Zheng M, Jiang YP, Chen W, Li KD, Liu X,
Gao SY, Feng H, Wang SS, Jiang J and Ma XR: Snail and Slug
collaborate on EMT and tumor metastasis through miR-101-mediated
EZH2 axis in oral tongue squamous cell carcinoma. Oncotarget.
6:6797–6810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ren ZH, Wu HJ, Zhang S, Wang K, Gong ZJ,
He ZJ and Peng J: A new surgical strategy for treatment of tongue
squamous cell carcinoma based on anatomic study with preliminary
clinical evaluation. J Craniomaxillofac Surg. 43:1577–1582. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Keski-Säntti H, Atula T, Törnwall J,
Koivunen P and Mäkitie A: Elective neck treatment versus
observation in patients with T1/T2 N0 squamous cell carcinoma of
oral tongue. Oral Oncol. 42:96–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Umeda M, Komatsubara H, Ojima Y,
Minamikawa T, Shibuya Y, Yokoo S, Ishii J and Komori T: A
comparison of brachytherapy and surgery for the treatment of stage
I–II squamous cell carcinoma of the tongue. Int J Oral Maxillofac
Surg. 34:739–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Li Y, Kong D and Sarkar FH: The
role of Notch signaling pathway in epithelial-mesenchymal
transition (EMT) during development and tumor aggressiveness. Curr
Drug Targets. 11:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Ridder M, Verovski VN, van den Berge
DL, Sermeus AB, Monsaert C, Wauters N and Storme GA: Lipid a
radiosensitizes hypoxic EMT-6 tumor cells: Role of the NF-kappaB
signaling pathway. Int J Radiat Oncol Biol Phys. 57:779–786. 2003.
View Article : Google Scholar : PubMed/NCBI
|