Introduction
Ovarian cancer is considered to have one of the
highest mortality rates of gynecological malignancies across the
globe, which is due to late diagnosis and is associated with poor
prognosis. This malignancy is the fourth leading cause of
cancer-associated deaths in women worldwide, and ~70–75% of
patients with ovarian cancer are diagnosed at an advanced stage of
the disease, which makes its treatment very difficult (1,2).
Ovarian cancer is classified into two groups that are termed type I
and type II; type I tumors are slow growing and are usually present
at an early stage, while type II tumors are highly aggressive, fast
growing and are present at an advanced stage (3). Treatment of ovarian cancer usually
involves surgical resection to reduce the number of cancerous
cells, which is followed by adjuvant chemotherapy using
taxol/platinum-based drugs. This type of treatment is associated
with 75–80% response rates. However, ultimately, after a period of
1–2 years a large number of patients experience disease recurrence
and >50% of treated patients eventually relapse (3,4).
Certain ovarian tumors may become resistant to platinum or
taxol-based chemotherapy, and the next treatment regimen for these
patients involves the use of various other potent chemotherapeutic
agents, including topotecan, doxorubicin and gemcitabine (4–6). It
has been reported that a large fraction of the ovarian cancer cell
population is in a dormant and non-proliferating stage, which
results in the failure of the majority of chemotherapeutic agents
as cytotoxic chemotherapeutic agents kill fast proliferating cells
and spare non-proliferating cells, which subsequently acquire
resistance. These non-proliferating cells have the capacity to
replace a tumor following therapy and lead to disease recurrence
(7,8). Therefore, based on the failure of
chemotherapy in these cases, and the high recurrence and acquired
drug resistance of ovarian tumors, novel, effective and relatively
non-toxic anticancer agents that target ovarian cancer cells are
required. The primary aim of the present study was to determine the
antiproliferative effects of ferruginol, a naturally occurring
phenolic meroterpene (abietane diterpene), on OVCAR-3 human ovary
cancer cells. The effects of ferruginol on apoptosis induction,
cancer cell migration, and cell cycle phase distribution were also
investigated.
Materials and methods
Chemicals and other reagents
Ferruginol (>95% purity by high-performance
liquid chromatography) and MTT reagent were purchased from
Sigma-Aldrich (Merck KGaA; Darmstadt, Germany). Dulbecco's modified
Eagle's medium (DMEM), RPMI-1640 medium, acridine orange (AO),
ethidium bromide (EB) and propidium iodide (PI) were purchased from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Fetal
bovine serum (FBS), penicillin and streptomycin were purchased from
Tianjin Haoyang Biological Products Co., Ltd. (Tianjin, China). All
other chemical reagents used were of analytical grade.
Cell lines, culture conditions and MTT
cell proliferation assay
OVCAR-3 human ovary cancer cells were purchased from
the Cell Bank of the Basic Medical College of Huazhong University
of Science and Technology (Wuhan, China). Cells were cultured in
DMEM containing 10% FBS with 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere with 5%
CO2. The cell cytotoxicity induced by ferruginol in
these cancer cells was evaluated by an MTT cell viability assay
using various doses and incubation durations. Briefly, OVCAR-3
cells were plated at a density of 1×106 cells per well
in 96-well plates for 12 h at 37°C. The cells were subsequently
treated with 20, 40, 80, 160, 300 and 400 µM ferruginol for 24 and
48 h at 37°C, with vehicle control cells being treated with
dimethyl sulfoxide instead of ferruginol. MTT solution (10 µl) was
added to each well at 37°C for 2 h. The medium was completely
removed and 500 µl dimethyl sulfoxide was added to solubilize MTT
formazan crystals. The optical density was determined at 570 nm
using an ELISA plate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Fluorescence microscopy assay for
apoptosis evaluation
OVCAR-3 human ovary cancer cells were seeded at a
density of 2×106 cells per well and exposed to various
treatment doses (0, 20, 80 and 300 µM) of ferruginol and incubated
for 48 h at 37°C. Cells treated only with pure dimethyl sulfoxide
served as vehicle control. Following trypsinization and washing
with PBS, cells were stained with acridine orange/ethidium bromide
(AO/EB) double stain (1 µl of each 5 mg/ml AO and 3 mg/ml EB stock
solution). The cells were then washed with PBS, fixed in
formaldehyde (10%) and the again washed with PBS prior to analysis
using a fluorescence microscope at ×400 magnification (Nikon
Corporation, Tokyo, Japan).
Transmission electron microscopy (TEM)
for ultrastructural cell analysis
OVCAR-3 human ovary cancer cells were seeded in a
flask at the density of 2×106 cells per well and
subsequently treated with various doses (0, 20, 80 and 300 µM) of
ferruginol for 48 h at 37°C. Cells were then harvested and washed
with PBS twice prior to the addition of 2.5% glutaraldehyde and
fixation for 3 h at 37°C. The cells were embedded in an LR White
resin for 30 min (Sigma-Aldrich;Merck KGaA) at 37°C. Following
embedding, the resin block was sectioned using an ultramicrotome
(JEOL, Ltd., Tokyo, Japan) and sections of 50–70 nm thickness were
collected. TEM was performed using a transmission electron
microscope (JEOL, Ltd.) at ×400 magnification. Apoptosis evaluation
was performed by examining the ultrastructural cell changes in
ferruginol-treated cells.
In vitro wound healing assay
The wound healing assay was performed as described
previously (9). Briefly, OVCAR-3
cells at a density of 2×105 cells/ml were seeded in a
6-well plate and incubated at 37°C to attain a 100% monolayer of
confluent cells. After starving cells for 24 h, a 50 ml pipette tip
was used to create a straight cell-free wound in the wells.
Subsequent to washing with PBS three times, the cells were treated
with varying doses (0, 20, 80 and 300 µM) of ferruginol for 48 h at
37°C. Cells were subsequently fixed and stained with 3.5% ethanol
containing 1.5% crystal violet dye for 20 min. Using an inverted
light microscope (Nikon Corporation), 10 randomly selected fields
were photographed and the fraction of cells that migrated into the
scratched area was determined visually.
Cell cycle analysis
OVCAR-3 human ovary cancer cells were seeded at
2×105 cells per well in 60-mm plates and treated with 0,
20, 80 and 300 µM ferruginol for 48 h at 37°C. Subsequent to drug
treatment, cells were subjected to trypsinization and washed twice
with PBS. Cells were fixed with 70% cold ethanol overnight and
treated with 20 µg/ml RNase A at 37°C, which was followed by
staining with 10 µg/ml PI at 37°C. The DNA content and cell cycle
distribution was analyzed by flow cytometry using a FACSCalibur
instrument (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of at least three independent experiments. The differences
between groups were analyzed by Student t test and one-way analysis
of variance (in case of comparisons between >2 groups) using
GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA,
USA). *P<0.05 and **P<0.01 were considered to indicate a
statistically significant difference.
Results
Ferruginol induces potent cytotoxicity
in OVCAR-3 human ovary cancer cells
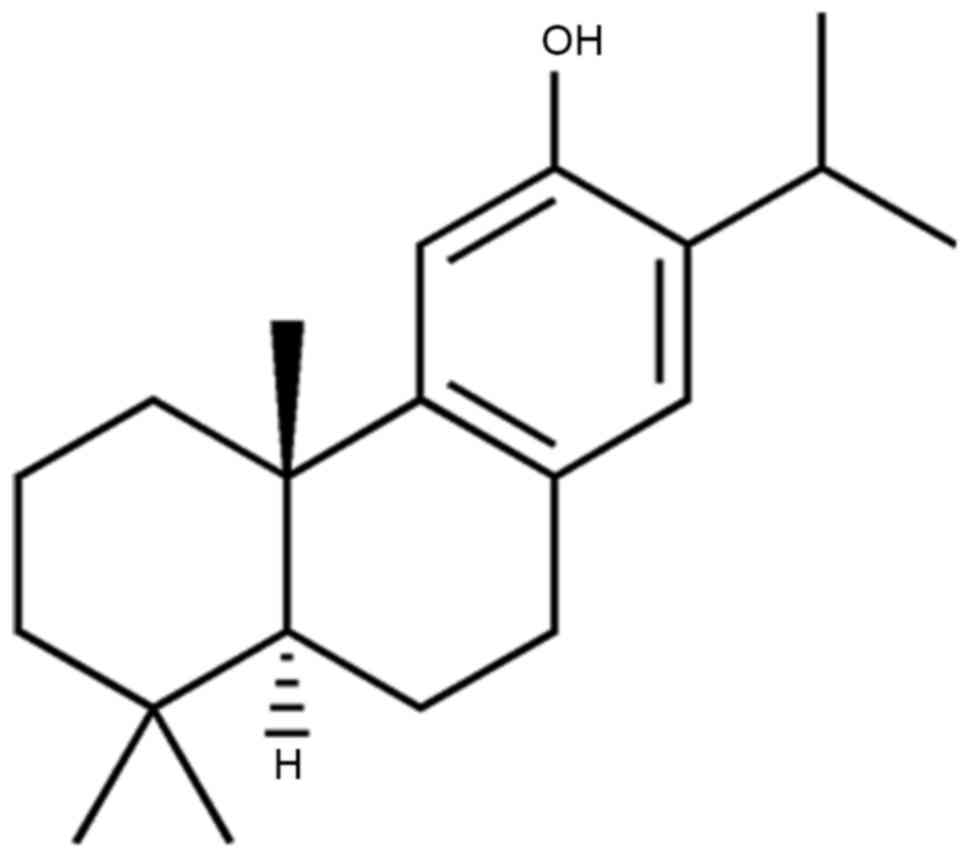
Ferruginol belongs to the meroterpene class of
natural products and has a phenolic structure (Fig. 1). The cytotoxic effects of
ferruginol against OVCAR-3 human ovary cancer cells were evaluated
by an MTT cell viability assay at various doses and incubation
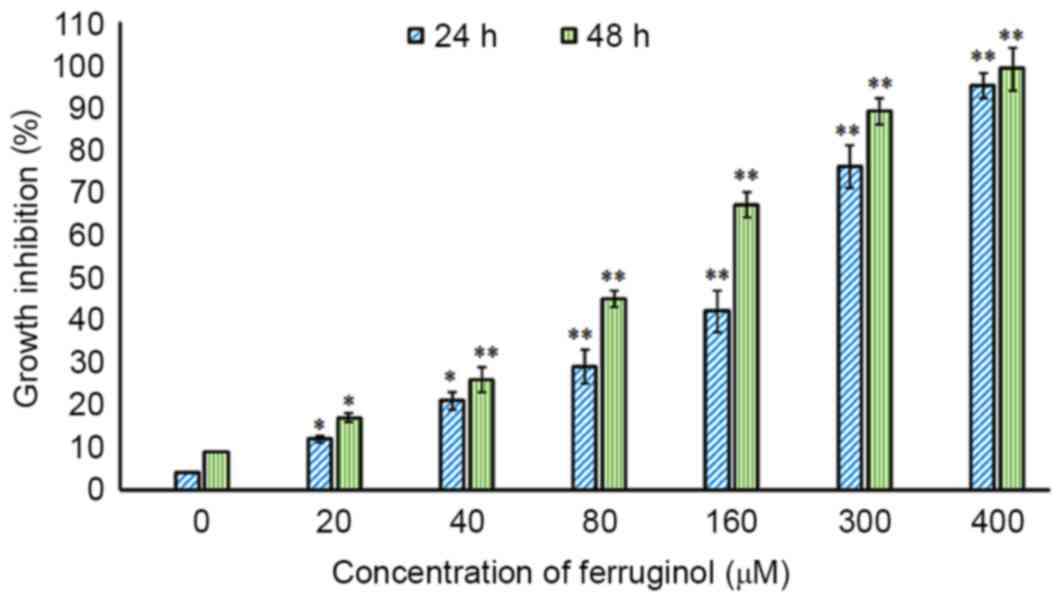
durations. The results presented in Fig. 2 indicate that ferruginol inhibited
the growth rate of OVACR-3 cells in a dose- and time-dependent
manner. Cells that were exposed to higher doses and higher
treatment durations exhibited lower cell viability (Fig. 2). The IC50 (half maximal
inhibitory concentration) provides an indication regarding the
effectiveness of a substance in inhibiting a specific biological or
biochemical function, and IC50 values for 24 and 48 h
treatment durations were determined to be 175.2 and 84.6 µM,
respectively.
Fluorescence microscopic evaluation of
ferruginol-induced apoptosis in OVCAR-3 cells
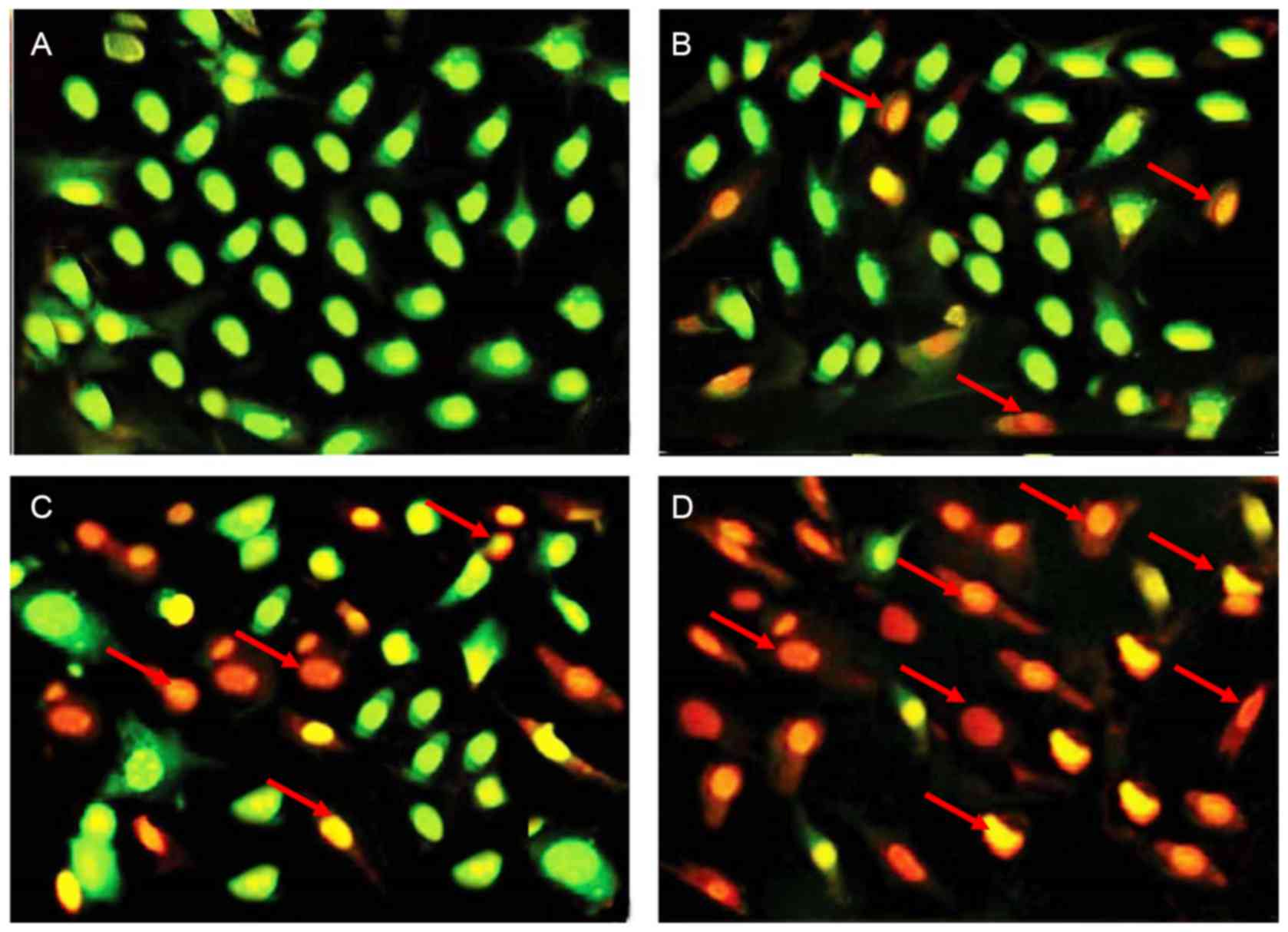
In this assay, fluorescence microscopy using AO/EB
staining was employed to investigate apoptotic effects induced by
ferruginol in OVCAR-3 human ovary cancer cells. Results presented
in Fig. 3 demonstrated that
untreated control cells did not exhibit any red/yellow
fluorescence, which indicated no signs of apoptosis. However, cells
that were treated with 20, 80 and 300 µM ferruginol began to
exhibit red/yellow fluorescence, which indicated the onset of the
apoptotic process. Furthermore, it was observed that the amount of
these apoptotic cells increased with increasing concentrations of
ferruginol.
TEM evaluation of apoptosis induction
by ferruginol
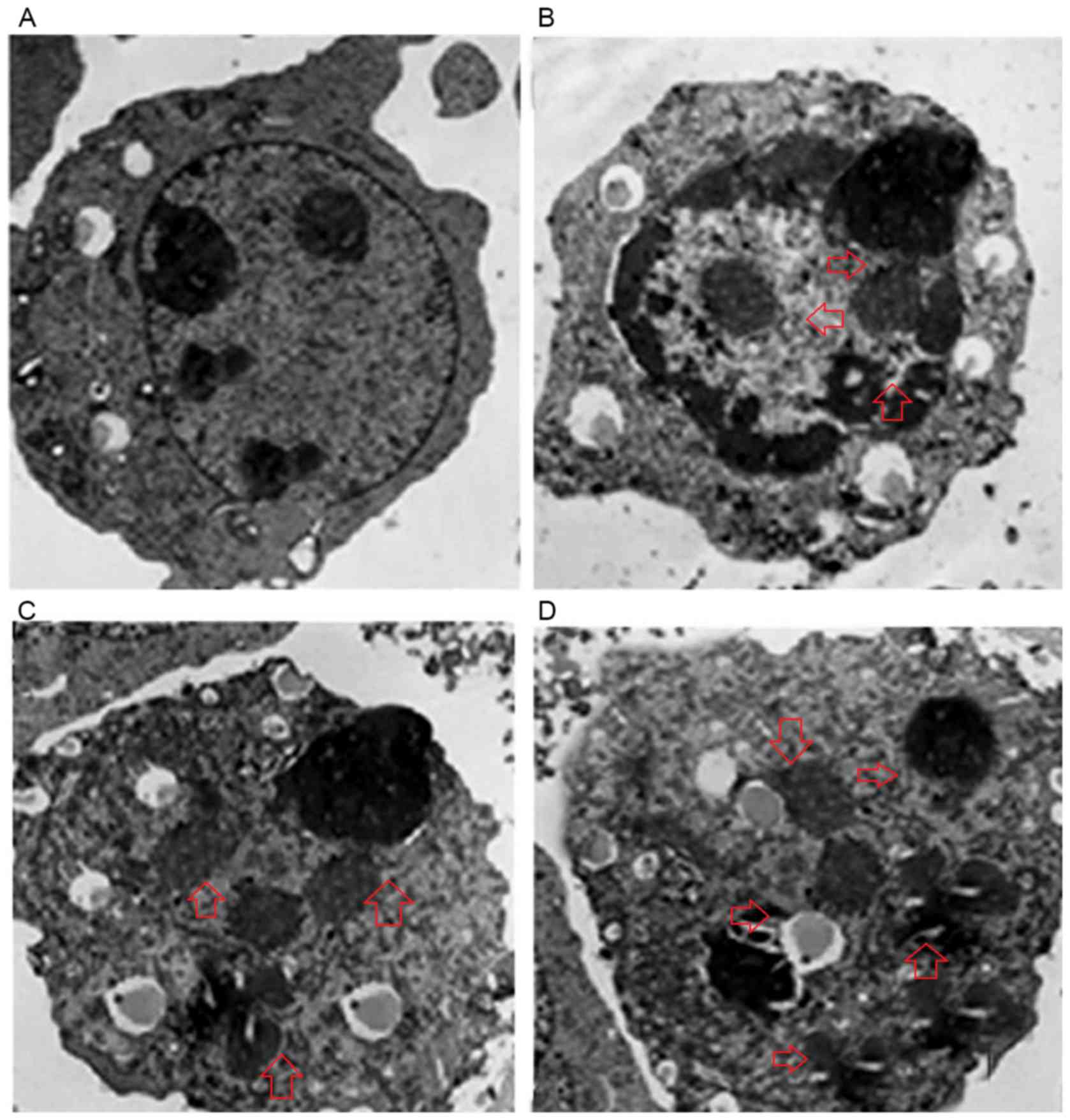
TEM was also used to investigate the apoptotic
effects of ferruginol in OVCAR-3 cells. The results demonstrate
that untreated control cells exhibited intact cellular nuclei with
undamaged nucleolus (Fig. 4A).
However, cells that were treated with increasing concentrations
(20, 80 and 300 µM) of ferruginol exhibited chromatin condensation
and disappearance of the nuclear envelope. At higher doses of
ferruginol, apoptotic body formation was observed (Fig. 4B-D). Therefore, TEM results
indicate that ferruginol induced apoptosis-associated morphological
changes in OVCAR-3 human ovary cancer cells.
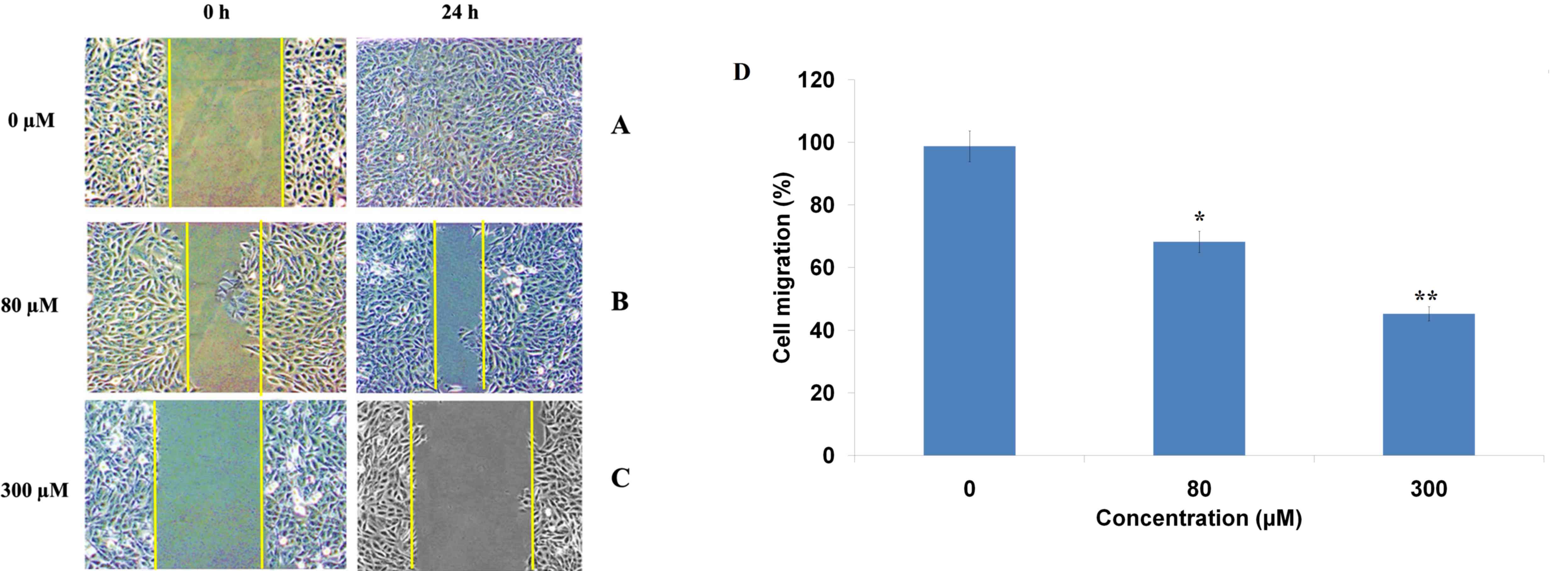
Ferruginol inhibits cancer cell
migration
An in vitro wound healing assay was performed
to investigate the effects ferruginol on cancer cell migration. The
results indicated that the untreated cells did not exhibit any
inhibition of cell migration after 24 h incubation with ferruginol.
However, after 24 h treatment with 80 and 300 µM ferruginol, a
dose-dependent inhibition of OVCAR-3 cancer cell migration was
observed (Fig. 5). The percentage
of migrated cells decreased from 98.7% in control to 68.2 and 45.3%
in 80 and 300 µM ferruginol-treated cells, respectively. The number
of cells that migrated into the scratched area were photographed
before and after drug treatment, at 0 and 24 h.
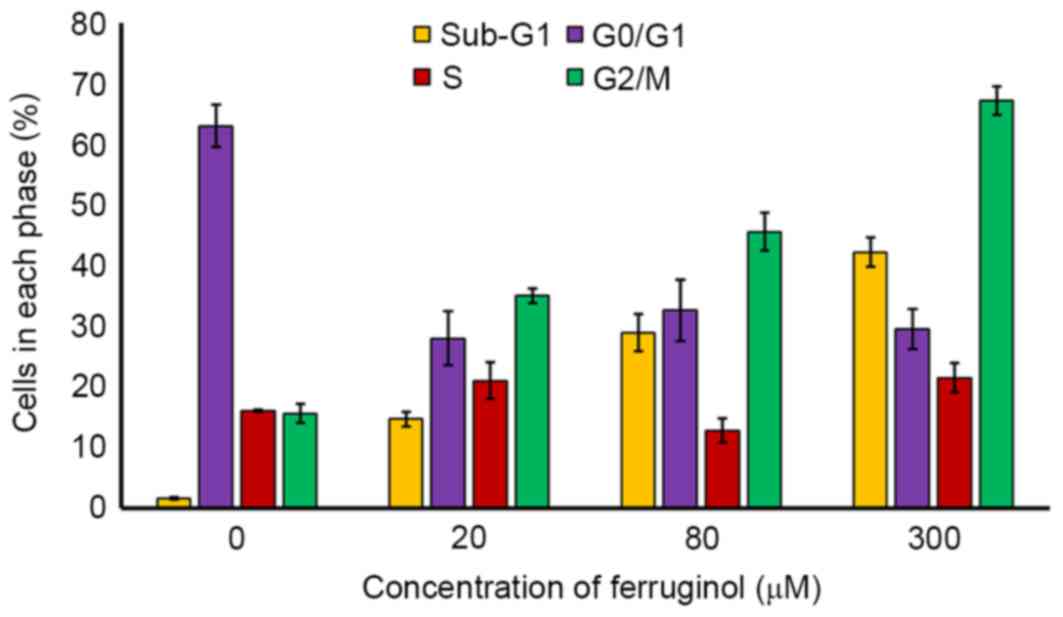
Ferruginol causes G2/M cell cycle
arrest in OVCAR-3 cells
The effects of ferruginol on cell cycle phase
distribution were observed by flow cytometry using PI as a probe.
The results presented in Fig. 6
demonstrated that the number of cells in the G2/M phase of the cell
cycle increased in a dose-dependent manner between 0 and 300 µM.
The percentage of cells in the sub-G1 phase also increased in a
dose-dependent manner. However, the percentage of cells in the
G0/G1 phase was decreased in ferruginol-treated groups compared
with the control group.
Discussion
Cancer is an established global health problem and
accounts for ~7.6 million deaths (~13% of all deaths) globally,
which is expected to increase to 13.1 million by 2030 (1). Apoptosis involves the programmed cell
death of unwanted cells, which subsequently leads to the
elimination of these cells from the body and maintains body
homeostasis. Apoptosis has an important function in multicellular
organisms as it aids the elimination of damaged and nonfunctional
cells from the body. The process involves several biochemical and
morphological changes that eventually lead to cell death. The
series of events in cell apoptosis include cell shrinkage,
condensation of chromatin material, membrane blebbing,
fragmentation of the nuclear material and DNA fragmentation. In
contrast to necrotic cell death, apoptosis is a highly controlled
and regulated biochemical process (10–12).
Apoptotic cell bodies are produced during apoptosis, which are
engulfed by phagocytes and rapidly removed before the cell releases
its toxic substances and causes damage to surrounding cells. There
are two different pathways by which apoptosis is initiated, which
are termed intrinsic and extrinsic pathways. Current anticancer
therapies, including chemotherapeutic agents and radiotherapy,
exhibit their effects by inducing apoptosis in cancer cells. It has
been reported that the common initial event in the majority of
apoptotic processes involves DNA damage or damage to various other
critical molecules (13,14). Plant-based natural products have
been recognized for their role in anticancer drug discovery. A vast
range of plant species have been identified that synthesize various
classes of chemical compounds with the ability to target cancer
cells, and the majority of these compounds function by targeting
fast-proliferating tumor cells with limited damage to normal cells
(15–17).
The primary aims of the current study were to
investigate the anticancer effects of ferruginol against OVCAR-3
human ovary cancer cells and its effects on apoptosis induction,
cancer cell migration and cell cycle arrest. Ferruginol belongs to
the meroterpene class of natural compounds with a phenolic moiety
and the compound has been naturally isolated from the needles of
the Sequoia sempervirens. Previous studies involving this
compound have reported that ferruginol exhibited potent in
vitro anticancer properties in human lung, colon and breast
cancer cells (18–20). Ferruginol has also been
demonstrated to exert inhibitory effects in non-small cell lung
cancer cells by inducing caspase-associated apoptosis (19). An additional study indicated that
ferruginol exhibited gastroprotective effects in mice and rats by
affecting gastric secretion and endogenous prostaglandins (20). The results of the present study
demonstrated that ferruginol inhibited the growth rate of OVACR-3
cells in a dose- and time-dependent manner. Cells that were exposed
to higher doses of ferruginol and longer treatment durations
exhibited lower cell viability. Fluorescence microscopy revealed
that treatment with increasing doses of ferruginol led to increases
in the levels of red/yellow fluorescence, which indicates the onset
of the apoptotic process. TEM results demonstrated that, in
contrast with control cells, ferruginol-treated cells exhibited
loss of nuclear envelope and the presence of apoptotic bodies.
Ferruginol also led to inhibition of cancer cell migration in a
dose-dependent manner. The percentage of migrated cells decreased
from 98.7% in control to 68.2 and 45.3% in 80 and 300 µM
ferruginol-treated cells, respectively. Furthermore, flow cytometry
results indicated that ferruginol led to G2/M cell cycle arrest in
OVCAR-3 human ovary cancer cells.
In conclusion, the present study indicates that
ferruginol may exhibit anticancer effects in OVCAR-3 human ovary
cancer cells by inducing apoptosis, inhibiting cancer cell
migration and inducing G2/M cell cycle arrest. Taken together, it
is concluded that ferruginol may prove beneficial in the treatment
of ovarian cancer. However, further in vivo evaluation is
urgently required.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tingulstad S, Skjeldestad FE, Halvorsen TB
and Hagen B: Survival and prognostic factors in patients with
ovarian cancer. Obstet Gynecol. 101:885–891. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozols RF, Bookman MA, Connolly DC, Daly
MB, Godwin AK, Schilder RJ, Xu X and Hamilton TC: Focus on
epithelial ovarian cancer. Cancer Cell. 5:19–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hille S, Rein DT, Riffelmann M, Neumann R,
Sartorius J, Pfützner A, Kurbacher CM, Schöndorf T and Breidenbach
M: Anticancer drugs induce mdr1 gene expression in recurrent
ovarian cancer. Anticancer Drugs. 17:1041–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson RC: The problem of the quiescent
cancer cell. Adv Enzyme Regul. 29:27–46. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mellor HR, Ferguson DJ and Callaghan R: A
model of quiescent tumour microregions for evaluating multicellular
resistance to chemotherapeutic drugs. Br J Cancer. 93:302–309.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green DR: Means to an End: Apoptosis and
Other Cell Death Mechanisms. Cold Spring Harbor Laboratory Press;
Long Island, NY: 2011
|
|
11
|
Jose A. Karam and Jer-Tsong Hsieh:
Anti-cancer strategy of transitional cell carcinoma of bladder
basedon induction of different types of programmed cell deaths:
Apoptosis in Carcinogenesis and Chemotherapy. Springer;
Netherlands: pp. 25–50. 2009
|
|
12
|
D'Amico AV and McKenna WG: Apoptosis and a
re-investigation of the biologic basis forcancer therapy. Radiother
Oncol. 33:3–10. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makin G and Dive C: Apoptosis and cancer
chemotherapy. Trends Cell Biol. 11 Suppl:S22–S26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fulda S and Debatin KM: Targeting
apoptosis pathways in cancer therapy. Curr Cancer Drug Targets.
4:569–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J
NatProd. 75:311–335. 2012.
|
|
17
|
Chin YW, Yoon KD and Kim J: Cytotoxic
anticancer candidates from terrestrial plants. Anticancer Agents
Med Chem. 9:913–942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Son KH, Oh HM, Choi SK, Han DC and Kwon
BM: Anti-tumor abietane diterpenes from the cones of Sequoia
sempervirens. Bioorg Med Chem Lett. 15:2019–2021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho ST, Tung YT, Kuo YH, Lin CC and Wu JH:
Ferruginol inhibits non-small cell lung cancer growth by inducing
caspase-associated apoptosis. Integr Cancer Ther. 14:86–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Areche C, Theoduloz C, Yáñez T,
Souza-Brito AR, Barbastefano V, de Paula D, Ferreira AL,
Schmeda-Hirschmann G and Rodríguez JA: Gastroprotective activity of
ferruginol in mice and rats: Effects on gastric secretion,
endogenous prostaglandins and non-protein sulfhydryls. J Pharm
Pharmacol. 60:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|