Introduction
Obesity is a common public health problem and is a
significant risk factor for a number of metabolic disorders,
including type 2 diabetes, hypertension and cardiovascular disease
(1). The development of obesity is
characterized by an increase in the number and size of mature
adipocytes that are produced by differentiation and mitogenesis
(2). Adipogenesis is controlled by
many transcription factors, including peroxisome
proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer
binding proteins (C/EBPs) (3–5). At
the initial stage of adipogenesis, C/EBPβ is activated, followed by
the sequential activation of C/EBPα and PPARγ, two major
late-adipogenic transcription factors. Activation of C/EBPα and
PPARγ subsequently leads to the expression of a number of genes
involved in adipocyte differentiation, including genes encoding
lipid metabolizing enzymes [such as fatty acid binding protein
(FABP)-4 and fatty acid synthase (FAS)] and adipokines (such as
adiponectin and leptin) (6).
Adenosine monophosphate-activated protein kinase
(AMPK) is an important cellular energy sensor, and its activation
is closely related to the balance between lipid accumulation and
carbohydrate metabolism (7). AMPK
phosphorylation inhibits metabolic enzymes involved in fatty acid
synthesis, leading to suppressed adipogenesis (8,9).
Adiponectin is an adipokine that is mainly produced
by adipocytes. Unlike other adipokines, plasma adiponectin levels
are reduced in obese subjects (10). Adiponectin binds to the adiponectin
receptors, AdipoR1 and AdipoR2, and reduces obesity-related insulin
resistance (11). AdipoRon, a
newly discovered, orally active small molecule, is an adiponectin
receptor agonist that binds to and activates both AdipoR1 and
AdipoR2, and has been previously reported to improve
obesity-related insulin resistance and type 2 diabetes by
activating AMPK and PPARα pathways (12). However, the effects of adiponectin
on obesity itself and on adipogenesis are controversial.
Adiponectin may promote adipogenesis induced by weight gain
(11,13). By contrast, elevated adiponectin
levels were recently reported to reduce lipid content and lipid
metabolism in 3T3-L1 cells (14).
However, the effects of AdipoRon on adipogenesis have not yet been
investigated. The present study investigated the effects of
AdipoRon on adipogenesis and explored the underlying mechanism in
C3H10T1/2 cells.
Materials and methods
Reagents
AdipoRon,
5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR),
dexamethasone (Dex), 3-isobutyl-1-methylxanthine (IBMX),
indomethacin, insulin and Oil Red O were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Minimum essential
Eagle's medium with Earle's Balanced Salts (MEM-EBSS) was purchased
from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). Fetal
bovine serum (FBS) was purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cell Counting Kit-8 (CCK-8)
was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). Monoclonal antibodies, including rabbit anti-PPARγ (cat no.
2443), rabbit anti-FABP4 (cat no. 3544), rabbit anti-C/EBPβ (cat
no. 3087), rabbit anti-C/EBPα (cat no. 2295), rabbit anti-AMPKα
(cat no. 5831), rabbit anti-phosphorylated-acetyl-CoA carboxylase
(p-ACC; cat no. 11818), rabbit anti-adiponectin (cat no. 2789) and
rabbit anti-GAPDH (cat no. 5174) were purchased from Cell Signaling
Technology (Danvers, MA, USA). Rabbit polyclonal anti-p-AMPKα1/2
antibody (cat no. sc-33524) and goat polyclonal anti-AdipoR1
antibody (cat no. sc-46748) and anti-AdipoR2 antibody (cat no.
sc-46751) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Cell culture and adipocyte
differentiation
The C3H10T1/2 mouse embryonic mesenchymal stem cell
line was purchased from the Chinese Academy of Medical Sciences
(Beijing, China). Cells were cultured in MEM-EBSS supplemented with
10% FBS at 37°C in a 5% CO2 atmosphere until adipocyte
differentiation. Two days after reaching confluence (day 0), the
C3H10T1/2 cells were cultured in differentiation medium (DM;
MEM/EBSS containing 10% FBS, 1 µM Dex, 0.5 mM IBMX, 50 mM
indomethacin and 1 µg/ml insulin) for 2 days to induce
differentiation. Following incubation, MEM/EBSS supplemented with
10% FBS and 1 µg/ml insulin was added for 2 days as a
differentiation medium and was changed every 2 days.
Cell viability assay
Cell viability was detected by using a CCK-8 kit,
according to the manufacturer's instructions. Briefly, C3H10T1/2
cells were seeded in 96-well plates at a density of
5×104 cells/ml at 37°C in a humidified 5% CO2
atmosphere and treated with increasing doses of AdipoRon (1, 5, 10,
20 and 40 µM) for 24, 48 or 72 h. Subsequently, 10 µl of kit
reagent was added to each well and incubated at 37°C for 2 h. The
plates were scanned with a microplate reader (Benchmark; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 450 nm.
Oil Red O (ORO) staining
Cells were cultured in 24-well plates with a seeding
density of 5×104 cells/ml and differentiated as
described above. On day 8, the cells were stained with ORO as
previously described (2). Briefly,
the cells were fixed with 10% formalin (Solarbio, Beijing, China)
at 4°C for 30 min and stained with ORO at room temperature for 30
min following washes with phosphate-buffered saline. Oil droplets
in the cells were observed under an inverted microscope
(Olympus-CKX41; Olympus Corporation, Tokyo, Japan). The ORO-stained
plates were then washed and treated with 100% isopropanol (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China), and
lipid accumulation was detected by measuring the absorbance at 490
nm using a Benchmark microplate reader (Bio-Rad Laboratories,
Inc.).
Western blot analysis
Cells were cultured in 6-well plates with a seeding
density of 5×104 cells/ml and differentiated as
described above. Cells were lysed in radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Shanghai, China)
supplemented with a protease inhibitor cocktail and phosphatase
inhibitors (Roche Molecular Diagnostics, Pleasanton, CA, USA) for
20 min and centrifuged at 12,000 × g for 20 min at 4°C. Protein
concentration was determined using the Bicinchoninic Acid Protein
Assay reagent kit (Beijing Solarbio Science & Technology Co.,
Ltd.). A total of 30 µg of protein lysates from each sample were
separated on 10 or 12% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). The
membrane was incubated with 5% bovine serum albumin in
Tris-buffered saline with 0.1% Tween-20 (TBST) (both from Beijing
Solarbio Science & Technology Co., Ltd.) for 1 h at room
temperature, followed by hybridization with primary antibodies
against PPARγ (1:1,000), C/EBPβ (1:1,000), C/EBPα (1:1,000), FABP4
(1:2,000), adiponectin (1:500), AMPKα (1:1,000), p-ACC (1:500),
GAPDH (1:1,000), p-AMPKα1/2 (1:200), AdipoR1 (1:200) and AdipoR2
(1:200) overnight at 4°C. Following three washes with TBST, the
membrane was incubated with a horseradish peroxidase-conjugated
goat anti-rabbit IgG secondary antibody (cat no. sc2004; 1:10,000;
Santa Cruz Biotechnology, Inc.) or a horseradish
peroxidase-conjugated rabbit anti-goat IgG secondary antibody (cat
no. ZB-2306; 1:10,000; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China) for 1 h at room temperature. Following three
washes with TBST, the bands were visualized with an Enhanced
Chemiluminescence Substrate (Thermo Fisher Scientific, Inc.).
Densitometry of the western blot bands was performed using ImageJ
software version 1.31 (National Institutes of Health, Bethesda, ML,
USA).
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were cultured in 6-well plates with a seeding
density of 5×104 cells/ml and differentiated as
described above. RNA was extracted from C3H10T1/2 cells on day 8
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
A High Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to synthesize the cDNA,
according to the manufacturer's protocol. mRNA expression levels
were determined using an ABI 7500 Fast real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and a SYBR
Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Primers were obtained from Genotech (Shanghai, China) and
the sequences are provided in Table
I. GAPDH was used as the internal control. qPCR was performed
using the following previous described cycling conditions (15): Initial denaturation at 95°C for 10
min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Each experiment was performed in triplicate. Gene transcript levels
were calculated using the 2−ΔΔCq method (16).
 | Table I.Primers used for reverse transcription
quantitative polymerase chain reaction. |
Table I.
Primers used for reverse transcription
quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| C/EBPα | F:
CGGGAACGCAACAACATCGC |
| C/EBPα | R:
CGGTCATTGTCACTGGTCAACTC |
| C/EBPβ | F:
GTTTCGGGAGTTGATGCAATC |
| C/EBPβ | R:
AACAACCCCGCAGGAACAT |
| PPARγ | F:
CATTCGCATTCCTTTGAC |
| PPARγ | R:
CGCACTTTGGTATTCTTGGAG |
| FAS | F:
CAAGTGTCCACCAACAAGCG |
| FAS | R:
GGAGCGCAGGATAGACTCAC |
| FABP4 | F:
TGTGCGAAACTGAATTTCCTGC |
| FABP4 | R:
GAGATCGGTCCTGAGCCAGC |
| Adiponectin | F:
GCCGCTTATGTGTATCGCTCAG |
| Adiponectin | R:
GCCAGTGCTGCCGTCATAATG |
| Leptin | F:
TCGCCTTTCTCCTGATGACG |
| Leptin | R:
GCAATCACACGGATGGCTTC |
| SCD-1 | F:
CGCTGGCACATCAACTTCAC |
| SCD-1 | R:
AGGAACTCAGAAGCCCAAAGC |
| GAPDH | F:
TCAATGACAACTTTGTCAAGCTCA |
| GAPDH | R:
GTGGGTGGTCCAGGGTTTCTTACT |
Statistical analysis
All experiments were performed at least three times.
The results are expressed as the mean ± standard deviations (SD).
The data were analyzed using Student's t-test or one-way analysis
of variance followed by least significance difference test. All
analyses were performed with GraphPad Prism version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
AdipoRon suppresses lipid accumulation
in C3H10T1/2 cells without cytotoxicity
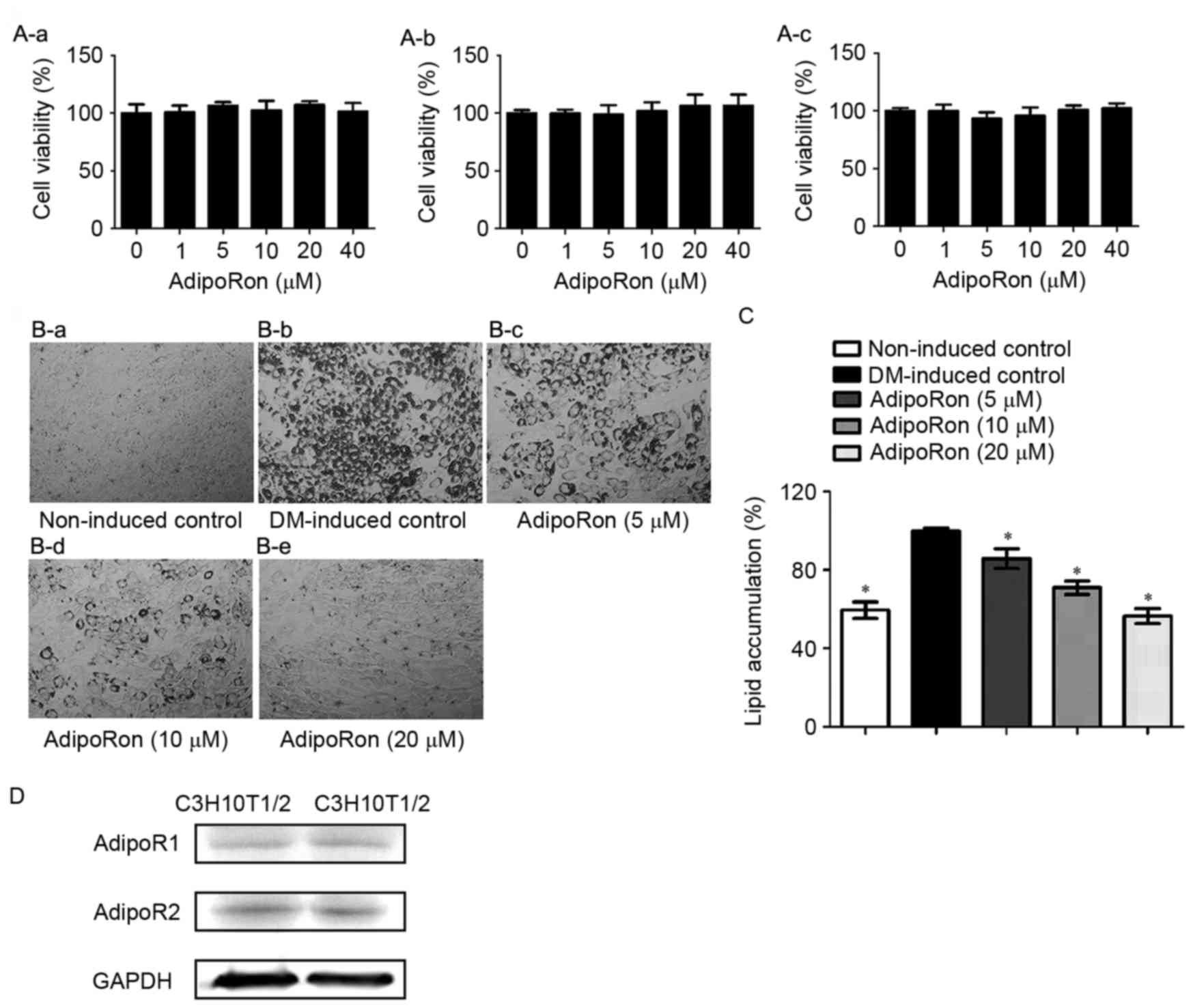
Following treatment for 24, 48 or 72 h, AdipoRon
exhibited no significant effects on C3H10T1/2 cell viability at
concentrations ≤40 µM (Fig. 1A).
Therefore, 5–20 µM AdipoRon was used in all subsequent experiments.
The antiadipogenic effects of AdipoRon treatment were not due to
cytotoxicity. C3H10T1/2 cells were induced into adipocytes for 8
days in the presence or absence of various doses of AdipoRon (0–20
µM) to investigate the effects of AdipoRon on adipocyte
differentiation. Based on the results of ORO staining, lipid
accumulation in C3H10T1/2 cells was dose-dependently decreased by
AdipoRon (Fig. 1B and C). Protein
expression of the adiponectin receptors, AdipoR1 and AdipoR2, were
detected in C3H10T1/2 cells (Fig.
1D).
Effects of AdipoRon on adipogenic
transcription factors expression in C3H10T1/2 cells
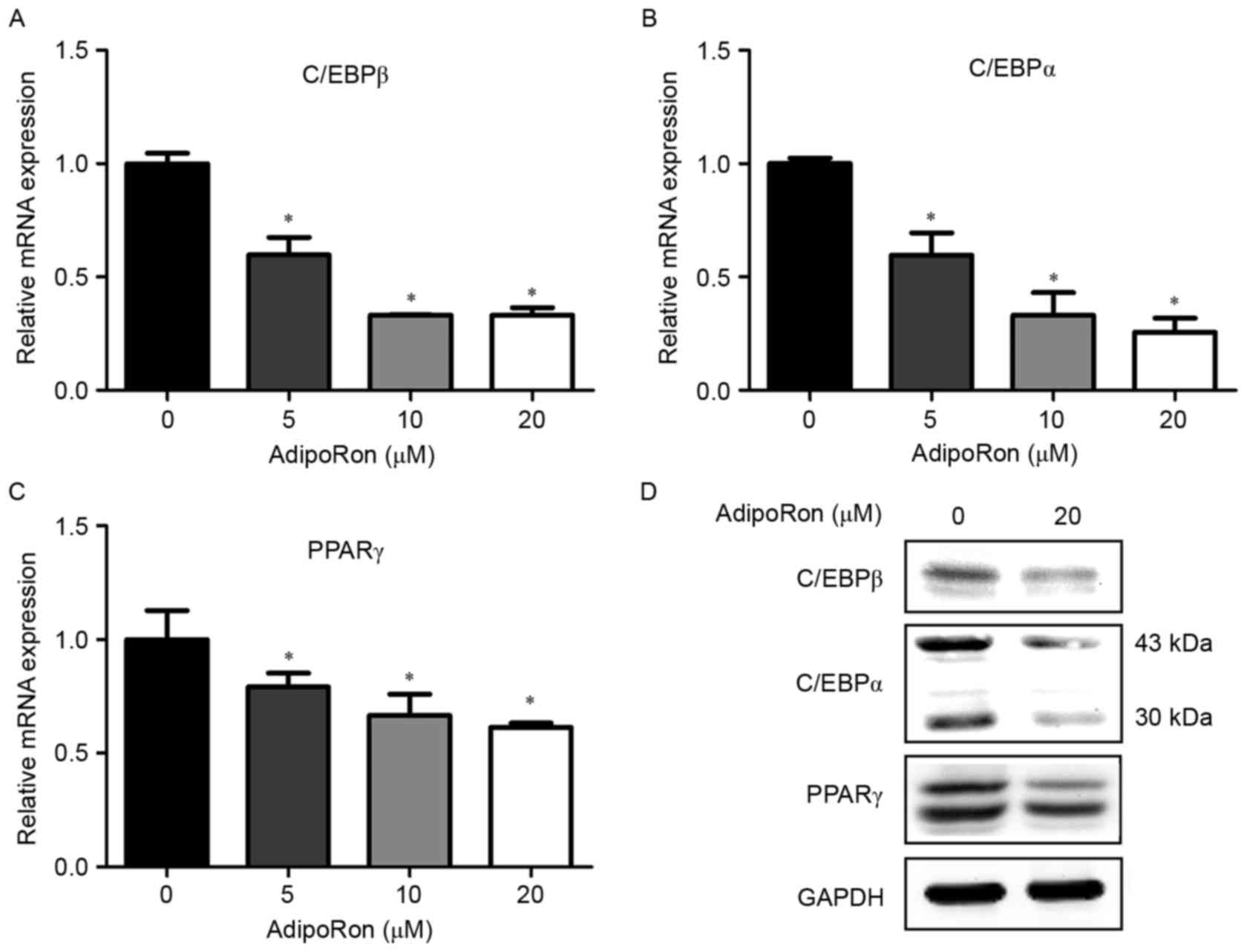
Adipogenesis is controlled by numerous transcription
factors; the present study examined the effects of various
concentrations of AdipoRon (0–20 µM) on the mRNA expression levels
C/EBPβ, C/EBPα and PPARγ, to investigate whether AdipoRon inhibits
adipogenesis by downregulating the expression of these adipogenic
transcription factors. AdipoRon treatment significantly reduced the
expression levels of the C/EBPβ, C/EBPα and PPARγ mRNAs in a
dose-dependent manner (Fig. 2A-C).
In addition, treatment with 20 µM AdipoRon was used to examine the
expression levels of the C/EBPβ, C/EBPα and PPARγ proteins. The
expression levels of the C/EBPβ, C/EBPα and PPARγ proteins were
decreased by 20 µM AdipoRon compared with those of the controls
(Fig. 2D).
Effects of AdipoRon on the expression
levels of adipogenesis-related genes in C3H10T1/2 cells
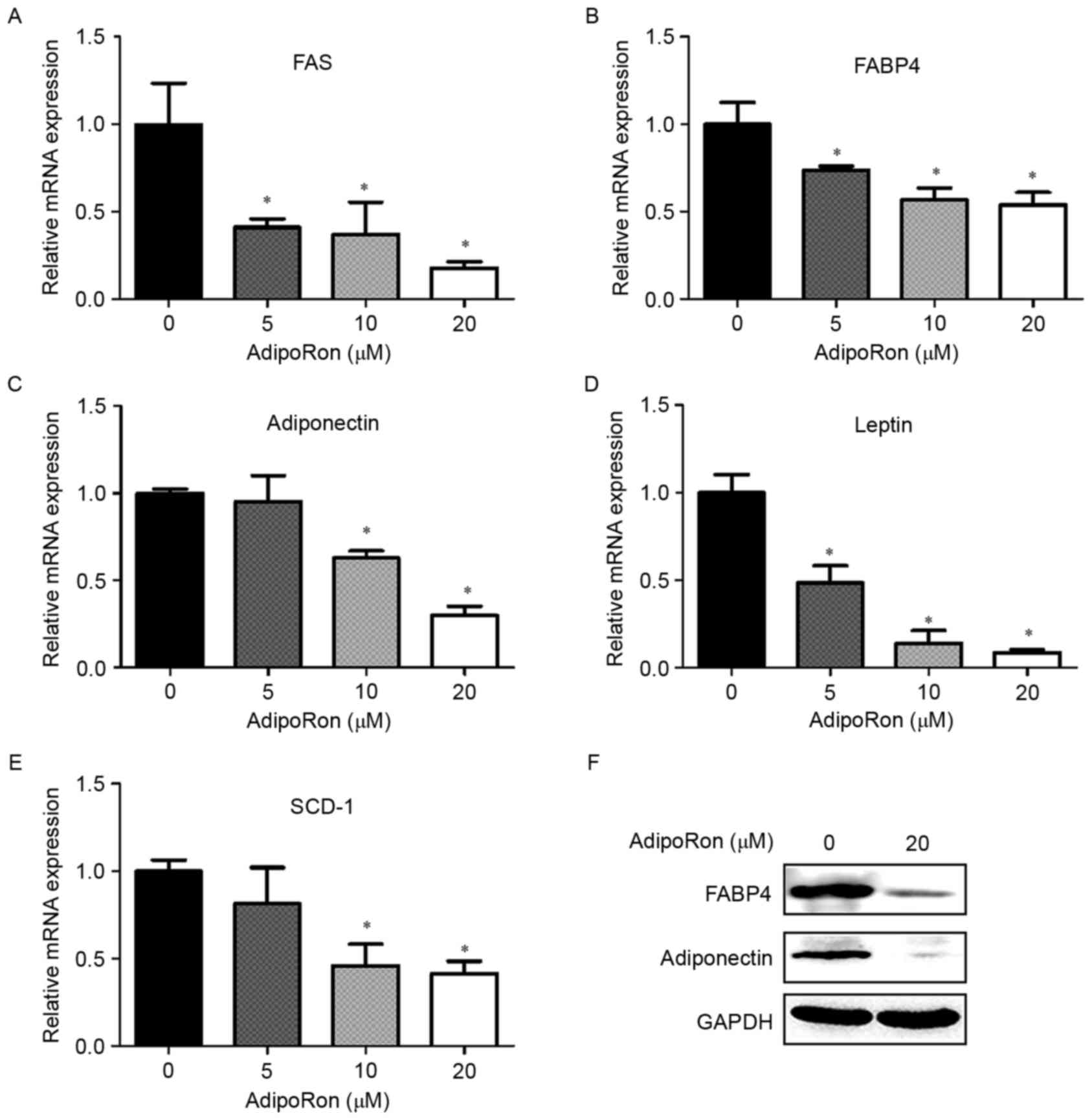
As AdipoRon treatment significantly downregulated
the expression of adipogenic transcription factors, the effects of
various concentrations of AdipoRon on the expression levels of
adipogenesis-related genes were also examined. AdipoRon exposure
dose-dependently suppressed the mRNA expression of FAS, leptin,
stearoyl-CoA desaturase (SCD)-1, adiponectin and FABP4 (Fig. 3A-E). Consistent with the changes in
mRNA expression levels, 20 µM AdipoRon decreased the expression
levels of the adiponectin and FABP4 proteins (Fig. 3F).
Effects of AdipoRon on AMPK
phosphorylation in C3H10T1/2 cells
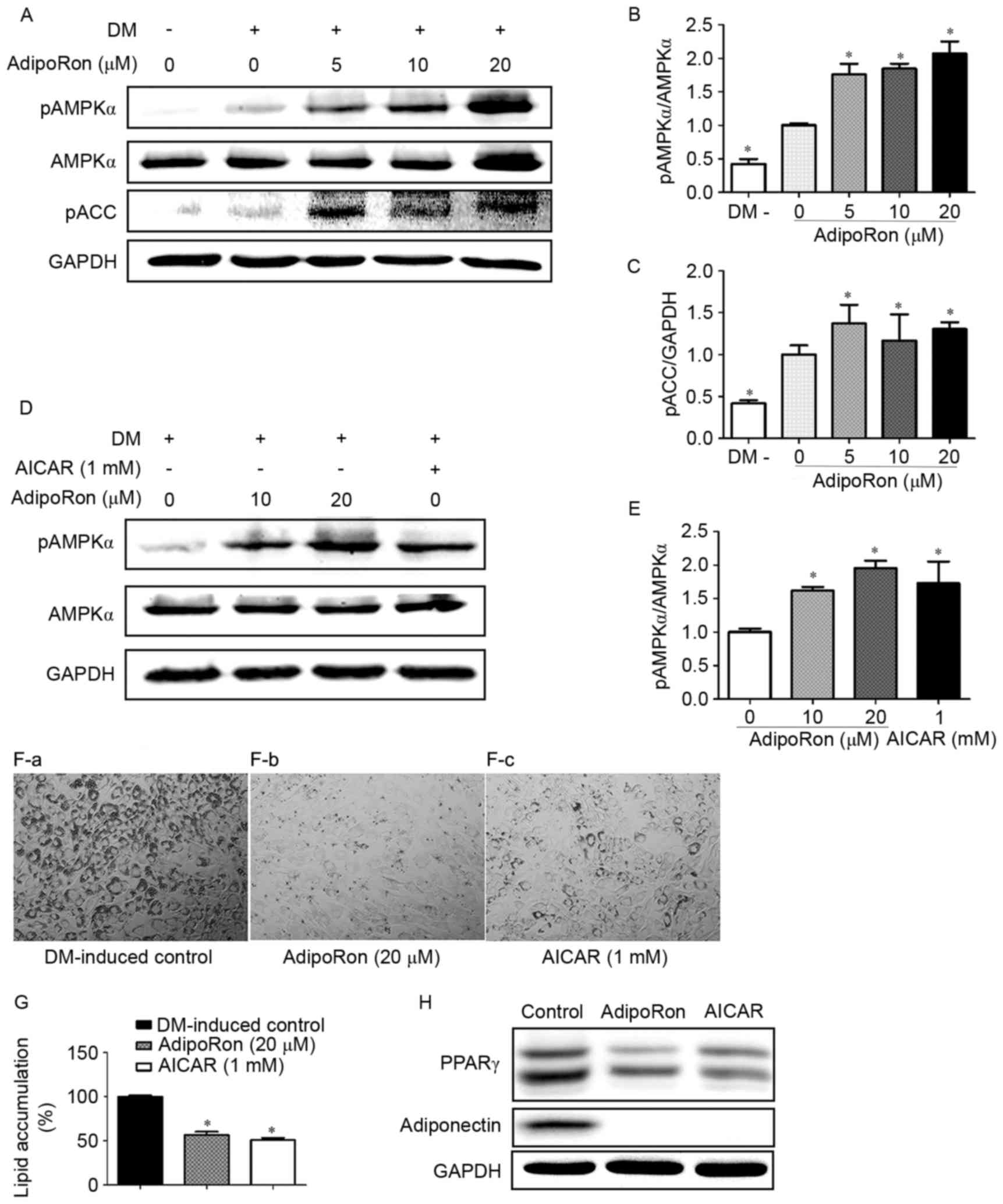
AdipoRon treatment significantly increased the
levels of p-AMPK and p-ACC, which is the downstream target of AMPK
(Fig. 4A-C). The AMPK activator
AICAR was used as a positive control to further explore the
association between the activation of AMPK signaling and the
antiadipogenic effects mediated by AdipoRon. As shown in Fig. 4D and E, both AICAR (1 mM) and
AdipoRon (10 and 20 µM) were able to activate the AMPK signaling
pathway. Both AICAR and AdipoRon treatments were also able to
significantly reduce lipid accumulation and the expression of
adipogenesis-related genes, PPARγ and adiponectin, in C3H10T1/2
cells (Fig. 4F-H).
Discussion
Obesity and its associated complications have become
common problems worldwide. AdipoRon, an adiponectin receptor
agonist, is a promising new orally administered drug for the
treatment of obesity-related diabetes (12). However, its effects on obesity
itself and adipogenesis have not yet been investigated. Results
from the present study demonstrated that AdipoRon treatment
inhibited the differentiation of C3H10T1/2 cells into adipocytes by
downregulating the expression of adipogenic transcription factors
and regulating the AMPK signaling pathway.
Abnormal adipogenesis and lipid accumulation have
previously been associated with the development of obesity
(2). A potential treatment
strategy for obesity is to inhibit adipogenesis. The presents study
results indicated that AdipoRon dose-dependently inhibited lipid
accumulation in C3H10T1/2 cells without cytotoxicity. Adipogenesis
is coordinated by many transcription factors, such as C/EBPs and
PPARs; AdipoRon exposure downregulated the expression levels of the
C/EBPβ mRNA, which subsequently suppressed the expression of the
C/EBPα and PPARγ mRNAs. PPARγ and C/EBPα are major regulators of
adipogenesis by activating the transcription of terminal adipocyte
differentiation marker genes, including FABP4, FAS, leptin, SCD-1
and adiponectin (3–5); the expression levels of these mRNAs
were demonstrated to be significantly decreased by AdipoRon in the
present study. Therefore, AdipoRon treatment suppressed the
expression of transcription factors during adipogenesis, which then
inhibited adipocyte differentiation of C3H10T1/2 cells.
Obesity occurs when total energy intake exceeds
total energy expenditure. AMPK is a metabolic energy sensor that
senses nutritional stress for the purpose of regulating glucose and
lipid metabolism and may be a potential target for the treatment of
obesity (17). AMPK has been
reported to serve an important role in inhibiting adipogenesis in
3T3-L1 cells that have been induced by a number of different
natural compounds (2,6,8,18–20).
Activated AMPK inhibits the differentiation of adipocytes and the
expression of pro-adipogenesis transcription factors PPARγ and
C/EBPα (21). AMPK phosphorylation
induces the phosphorylation of its substrate, ACC, which functions
to strictly regulate the enzymes that are involved in the fatty
acid synthesis pathway that produces malonyl-CoA. Notably, p-ACC
lacks the ability to synthesize fatty acids (22). AdipoRon was previously reported to
activate AMPK signaling in muscle and liver of obese mice (12). To elucidate whether AdipoRon
inhibited adipogenesis through the AMPK signaling pathway, the
present study measured the levels of p-AMPK and p-ACC; AdipoRon
treatment significantly increased the phosphorylation of AMPK and
ACC. In addition, the AMPK activator AICAR was used as a positive
control to further elucidate the association between the
anti-adipogenesis ability of AdipoRon and the activation of the
AMPK signaling pathway. AICAR was previously reported to inhibit
adipogenesis in 3T3-L1 adipocytes and restore metabolic alterations
in a diet-induced mouse model of obesity (23–25).
Results from the present study revealed that both AICAR and
AdipoRon were able to activate the AMPK signaling pathway and
markedly reduce lipid accumulation in C3H10T1/2 cells, which
indicated that the anti-adipogenic effects of AdipoRon may be
related to the activation of the AMPK signaling pathway. As
C3H10T1/2 is a mouse embryonic mesenchymal stem cell line, the
anti-adipogenesis effects of AdipoRon require further validation in
human-sourced mesenchymal stem cell line.
In conclusion, AdipoRon, an adiponectin receptor
agonist, suppressed adipocyte differentiation of C3H10T1/2 cells,
probably by activating the AMPK signaling pathway and
downregulating the expression of key regulators of adipogenesis.
Therefore, AdipoRon may be a drug with the potential to prevent and
treat obesity.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81670098 and
81270572) and the National Basic Research 973 Program (grant no.
2013CB733701).
References
|
1
|
Despres JP and Lemieux I: Abdominal
obesity and metabolic syndrome. Nature. 444:881–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ko SC, Lee M, Lee JH, Lee SH, Lim Y and
Jeon YJ: Dieckol, a phlorotannin isolated from a brown seaweed,
Ecklonia cava, inhibits adipogenesis through AMP-activated protein
kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ Toxicol
Pharmacol. 36:1253–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White UA and Stephens JM: Transcriptional
factors that promote formation of white adipose tissue. Mol Cell
Endocrinol. 318:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosen ED and Spiegelman BM: PPARgamma: A
nuclear regulator of metabolism, differentiation, and cell growth.
J Biol Chem. 276:37731–37734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Z, Rosen ED, Brun R, Hauser S, Adelmant
G, Troy AE, McKeon C, Darlington GJ and Spiegelman BM:
Cross-regulation of C/EBP alpha and PPAR gamma controls the
transcriptional pathway of adipogenesis and insulin sensitivity.
Mol Cell. 3:151–158. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Kim T, Lee JJ, Lee KJ, Kim HK, Yun
B, Jeon J, Kim SK and Ma JY: The herbal medicine KBH-1 inhibits fat
accumulation in 3T3-L1 adipocytes and reduces high fat diet-induced
obesity through regulation of the AMPK pathway. PLoS One.
10:e01420412015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin JT, Chen HM, Chiu CH and Liang YJ:
AMP-activated protein kinase activators in diabetic ulcers: From
animal studies to phase II drugs under investigation. Expert Opin
Investig Drugs. 23:1253–1265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SK and Kong CS: Anti-adipogenic effect
of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes.
Chem Biol Interact. 186:24–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ceddia RB: The role of AMP-activated
protein kinase in regulating white adipose tissue metabolism. Mol
Cell Endocrinol. 366:194–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotta K, Funahashi T, Arita Y, Takahashi
M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K,
et al: Plasma concentrations of a novel, adipose-specific protein,
adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc
Biol. 20:1595–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma
T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T,
Shirouzu M, et al: A small-molecule AdipoR agonist for type 2
diabetes and short life in obesity. Nature. 503:493–499. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holland WL and Scherer PE: Cell Biology.
Ronning after the adiponectin receptors. Science. 342:1460–1461.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lazra Y, Falach A, Frenkel L, Rozenberg K,
Sampson S and Rosenzweig T: Autocrine/paracrine function of
globular adiponectin: Inhibition of lipid metabolism and
inflammatory response in 3T3-L1 adipocytes. J Cell Biochem.
116:754–766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poudel B, Nepali S, Xin M, Ki HH, Kim YH,
Kim DK and Lee YM: Flavonoids from Triticum aestivum inhibit
adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol
Med Rep. 12:3139–3145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cantó C, Gerhart-Hines Z, Feige JN,
Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P and Auwerx
J: AMPK regulates energy expenditure by modulating NAD+
metabolism and SIRT1 activity. Nature. 458:1056–1060. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liou CJ, Lai XY, Chen YL, Wang CL, Wei CH
and Huang WC: Ginkgolide C suppresses adipogenesis in 3T3-L1
adipocytes via the AMPK signaling pathway. Evid Based Complement
Alternat Med. 2015:2986352015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ
and Park OJ: Curcumin exerts antidifferentiation effect through
AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory
effect through AMPKalpha-COX-2 in cancer cells. J Agric Food Chem.
57:305–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn J, Lee H, Kim S, Park J and Ha T: The
anti-obesity effect of quercetin is mediated by the AMPK and MAPK
signaling pathways. Biochem Biophys Res Commun. 373:545–549. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Li Z, Li W, Shan Z and Zhu W:
Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via
activation of AMPK. Can J Physiol Pharmacol. 89:793–799.
2011.PubMed/NCBI
|
|
22
|
Daval M, Foufelle F and Ferré P: Functions
of AMP-activated protein kinase in adipose tissue. J Physiol.
574:55–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giri S, Rattan R, Haq E, Khan M, Yasmin R,
Won JS, Key L, Singh AK and Singh I: AICAR inhibits adipocyte
differentiation in 3T3L1 and restores metabolic alterations in
diet-induced obesity mice model. Nutr Metab (Lond). 3:312006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Habinowski SA and Witters LA: The effects
of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem
Biophys Res Commun. 286:852–856. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee H, Kang R, Bae S and Yoon Y: AICAR, an
activator of AMPK, inhibits adipogenesis via the WNT/β-catenin
pathway in 3T3-L1 adipocytes. Int J Mol Med. 28:65–71.
2011.PubMed/NCBI
|