Introduction
Enhanced activity of the sympathetic nervous system
in the myocardium is an important feature of heart failure
(1). Notably, a significant loss
of cardiomyocytes by apoptosis is a major pathogenic feature of
various cardiovascular diseases, including heart failure,
myocardial ischemia and infarction (2). Norepinephrine (NE), which is the
primary transmitter in the sympathetic nervous system, has been
demonstrated to induce apoptosis of adult and neonatal rat
cardiomyocytes (3). Enhancement of
cardiac sympathetic nerve activity in myocardial ischemia elicits
the release of NE from nerve endings (4). NE-induced apoptosis of cardiomyocytes
is closely associated with heart failure (5,6). Our
previous study indicated that transport stress resulted in an
increase in NE and mitochondrial apoptosis (7). Therefore, in the present study, NE
was used to stimulate H9c2 cells to explore the underlying
mechanism of apoptosis.
Spina date seed (SZS), which is the mature seed of
Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H.F.
Chow, is traditionally used as a folk medicine, due to its
anti-anxiety activity (8).
Jujuboside A (JUA) is a classic natural product extracted from SZS,
which has been demonstrated to be the most effective
pharmacological active component of SZS (9). Previous studies have reported that
JUA exerts antioxidant, anti-anxiety, anti-inflammatory and
hypnotic-sedative activities, and is able to reduce cell apoptosis
(10,11). Our previous study indicated that
JUA could markedly reduce the damage caused by isoproterenol via
activating the phosphoinositide 3-kinase/AKT/mammalian target of
rapamycin pathway (12). However,
to the best of our knowledge, no previous studies have focused on
the protective effects of JUA on NE-induced cardiomyocyte
apoptosis.
The present study aimed to examine the protective
effects of JUA on H9c2 cardiomyocytes against NE-induced apoptosis
in vitro, in order to elucidate the underlying mechanism of
JUA. The present study investigated JUA-mediated protection against
NE-induced apoptosis, and the involvement of the mitogen-activated
protein kinase (MAPK) and AKT signaling pathways.
Materials and methods
Reagents and antibodies
JUA (>98% purity, as determined by
high-performance liquid chromatography) was purchased from the
National Institutes for Food and Drug Control (Beijing, China). NE
and MTT were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). High-glucose Dulbecco's modified Eagle's (DMEM), fetal
bovine serum (FBS) and antibiotic-antimycotic were purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Bicinchoninic acid (BCA) protein assay kit was purchased from
Pierce (Thermo Fisher Scientific, Inc.). Antibodies against GAPDH
(cat. no. 5174S), c-Jun N-terminal kinase (JNK; cat. no. 9258),
phosphorylated (p)-JNK (cat. no. 4668), p38 (cat. no. 9212), p-p38
(cat. no. 9215), extracellular signal-regulated kinase (ERK; cat.
no. 4695), p-ERK (cat. no. 4370), AKT (cat. no. 4685), p-AKT (cat.
no. 4060), cleaved caspase-3 (cat. no. 9661), cleaved caspase-8
(cat. no. 8592), cleaved caspase-9 (cat. no. 7237) and cleaved
caspase-12 (cat. no. 2202) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit secondary
antibody was obtained from LI-COR Biotechnology (Lincoln, NE, USA;
cat. no. 925–32211).
Cell culture and treatment
H9c2 cells were purchased from the Cell Resource
Center of Chinese Academy of Medical Sciences (Peking Union Medical
College, Beijing, China). Cells were cultured in DMEM supplemented
with 10% FBS and antibiotics (100 U/ml penicillin and 100 U/ml
streptomycin) at 37°C in a humidified atmosphere containing 5%
CO2. The cells were subcultured with 0.05% trypsin
(Gibco; Thermo Fisher Scientific, Inc.). JUA and NE were diluted in
DMEM. H9c2 cells were washed with phosphate-buffered saline (PBS)
twice and serum-starved for 2 h prior to incubation with JUA or
NE.
Cell viability assay
Cell viability was determined using the MTT
reduction assay as previously described (12). Briefly, H9c2 cells were
preincubated with DMEM containing 10% FBS overnight in 96-well
plates at a density of 5×104 cells/well. After reaching
85% confluence, the cells were washed twice with PBS and incubated
with medium containing various concentrations of JUA (0, 2, 5, 10,
20, 50 and 100 µM) for 3, 24, 48 and 72 h prior to treatment with
or without NE (5 µM) for 6, 12 and 18 h at 37°C in a humidified
atmosphere containing 5% CO2. The medium was removed,
and 100 µl DMEM containing 10% MTT (0.5 mg/ml) was added to each
well for 4 h. The formazan crystals that formed in intact cells
were dissolved in 200 µl dimethyl sulfoxide. Absorbance was
recorded at a wavelength of 490 nm, and at a reference wavelength
of 630 nm, using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Morphological observations
Cells were treated with or without NE (0 and 5 µM)
for 12 h at 37°C in a humidified atmosphere containing 5%
CO2. Following treatment, cell morphology was observed
under a phase-contrast inverted biological microscope (IX71/IX2;
Olympus Corporation, Tokyo, Japan), and by scanning electron
microscopy (SEM; S-3400 N, Hitachi, Ltd., Tokyo, Japan) and
transmission electron microscopy (TEM; 1230, JEOL, Ltd., Tokyo,
Japan). For ultrastructural studies, H9c2 cells were harvested and
fixed with 3.0% glutaraldehyde and 1.5% paraldehyde, washed three
times in PBS, and post-fixed in cold 1% osmium tetroxide. Following
dehydration with a graded series of alcohol, all samples were
freeze-dried, coated with a layer of gold using a sputter-coater
and embedded in epoxy resin (EPON812; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). Subsequently, the
cells were examined by SEM using an Hitachi S-3400 N operated at 15
kV. Following dehydration with a graded series of alcohol, samples
were embedded in 50/50 LR White Embedding Resin (Electron
Microscopy Sciences, Hatfield, PA, USA) and treated with pure
ethanol solution for 15 min at 37°C, then with a pure resin
solution overnight at 4°C followed by incubation at 60°C for 1 h
for polymerization. Thin slices (80 nm) were produced using an
Ultracut Microtome (Leica EM UC7 Ultramicrotome; Leica Microsystems
GmbH, Wetzlar, Germany). Ultra-thin sections were stained with
saturated uranyl acetate in 50% ethanol and lead citrate, and H9c2
cardiomyocyte ultrastructure was examined by TEM.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptotic analysis
Cellular apoptosis was determined using the Annexin
V-FITC/PI cell apoptosis detection kit (Thermo Fisher Scientific,
Inc.). Following treatment, the cells were harvested by
trypsinization, washed twice with cold PBS and were centrifuged at
1,200 × g for 10 min at 4°C. Cells were resuspended in 100 µl 1X
binding buffer and were transferred to a sterile flow cytometry
glass tube. Subsequently, 5 µl Annexin V-FITC and 5 µl propidium
iodide were added and incubated at room temperature (25°C) in the
dark for 15 min. Finally, detection was performed by flow cytometry
(Beckman Coulter, Inc., Brea, CA, USA) according to the
manufacturer's protocol, and data was analyzed using FCS Express
software (version 3.0; De Novo Software, Glendale, CA, USA).
Acridine orange (AO)/ethidium bromide
(EB) staining
The apoptotic morphology of H9c2 cells was detected
by staining the cells with a combination of the fluorescent
DNA-binding dyes AO and EB (100 µg/ml each in PBS; Sigma-Aldrich;
Merck KGaA) for 15 min at room temperature. Subsequently, the cells
were observed under a fluorescence microscope (IX71/IX2; Olympus
Corporation).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from H9c2 cells using the
phenol and guanidine isothiocyanate-based TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA concentration and
purity were determined using a spectrophotometer (SmartSpec Plus;
Bio-Rad Laboratories, Inc.) based on the ratios of optical density
(OD)260/OD280 and
OD260/OD230. Total mRNA was reverse
transcribed using the iScript cDNA synthesis kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. The expression levels of Bax and Bcl-2 were determined by
qPCR analysis using the DNA Engine Mx3000P® fluorescence
detection system (Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA). The designed paired primers were as follows:
β-actin, forward 5′-CCTGCGGCATTCACGAAACTAC-3′ and reverse
5′-ACTCCTGCTTGCTGATCCACATC-3′; B-cell lymphoma 2(Bcl-2)-associated
X protein (Bax), forward 5′-CAGGACGCATCCACCAAGAA-3′, reverse
5′-GGGTCCCGAAGTAGGAAAGG-3′; and Bcl-2, forward
5′-CTGGGAGAACAGGGTATG-3′ and reverse 5′-CGTAGAAGAGGAGGGTC-3′.
RT-qPCR analysis was performed using the SYBR PrimeScript™ RT-PCR
kit (Takara Biotechnology Co., Ltd., Dalian, China). The
thermocycling conditions were as follows: 94°C for 5 min, followed
by 40 cycles of 94°C for 10 sec, 60°C for 20 sec and 72°C for 60
sec. RNA expression was quantified using the 2−ΔΔCq
relative quantification method (13).
Western blot analysis
Protein was extracted from the cells using a total
protein extraction kit (Biochain Institute, Inc., Newark, CA, USA),
and was quantified using a BCA protein assay kit according to the
manufacturer's protocol. Proteins (20 µg) were separated by 12%
SDS-PAGE and were transferred to nitrocellulose membranes (Pierce;
Thermo Fisher Scientific, Inc.). Subsequently, membranes were
blocked with SuperBlock T20 (TBS) blocking buffer (cat. no. 37536,
Pierce, USA) for 2.5 h at room temperature, and were then incubated
overnight at 4°C with the specific primary antibodies to GAPDH
(1:1,000), JNK (1:1,000), p-JNK (1:1,000), p38 (1:1,000), p-p38
(1:1,000), ERK (1:1,000), p-ERK (1:2,000), AKT (1:1,000), p-AKT
(1:2,000), cleaved caspase-3 (1:1,000), cleaved caspase-8
(1:1,000), cleaved caspase-9 (1:1,000) and cleaved caspase-12
(1:1,000). The membranes were then incubated with the secondary
antibody (1:15,000) for 1 h at room temperature. Subsequently, the
blots were visualized and analyzed using the Odyssey Infrared
Imaging system (LI-COR Biotechnology). Blots were normalized to
GAPDH and were semi-quantified using ImageJ version 2.1.4.7
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of at least three independent experiments. The results
were analyzed by one-way analysis of variance followed by Duncan's
test for multiple comparisons using SPSS 19.0 (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
NE inhibits cell viability, enhances
apoptosis and induces morphological alterations in H9c2 cells
H9c2 cells were treated with various concentrations
of NE for 0, 6, 12, and 18 h. The results of an MTT assay
demonstrated that the number of viable cells decreased in response
to the increased concentration and duration of NE treatment.
(Fig. 1A). Following stimulation
with 20 or 50 µM NE for 6 h, cell viability was significantly
inhibited (P<0.05; Fig. 1A). A
more marked decrease (P<0.01; Fig.
1A) in cell viability occurred following treatment with NE for
12 or 18 h. Furthermore, flow cytometric analysis demonstrated that
the apoptotic rate of H9c2 cells was increased following treatment
with 5 µM NE for 12 h (Fig. 1B).
As observed under SEM, NE-treated cells exhibited an abnormal
cellular microstructure, which was characterized by
irregular-arranged cell shape, sparser cell surface and increased
nuclear gap (Fig. 1C). Electron
microscopy analysis of H9c2 cells revealed marked alterations in
the structure of cardiomyocytes and the cellular architecture.
Ultrastructural images of H9c2 cells using TEM showed obvious
nuclear chromatin margination, aggregation, condensation, and
mitochondrial vacuolization in NE-treated cells (Fig. 1D). According to these results,
treatment with 5 µM NE for 12 h was selected for the generation of
an in vivo model of H9c2 cell damage.
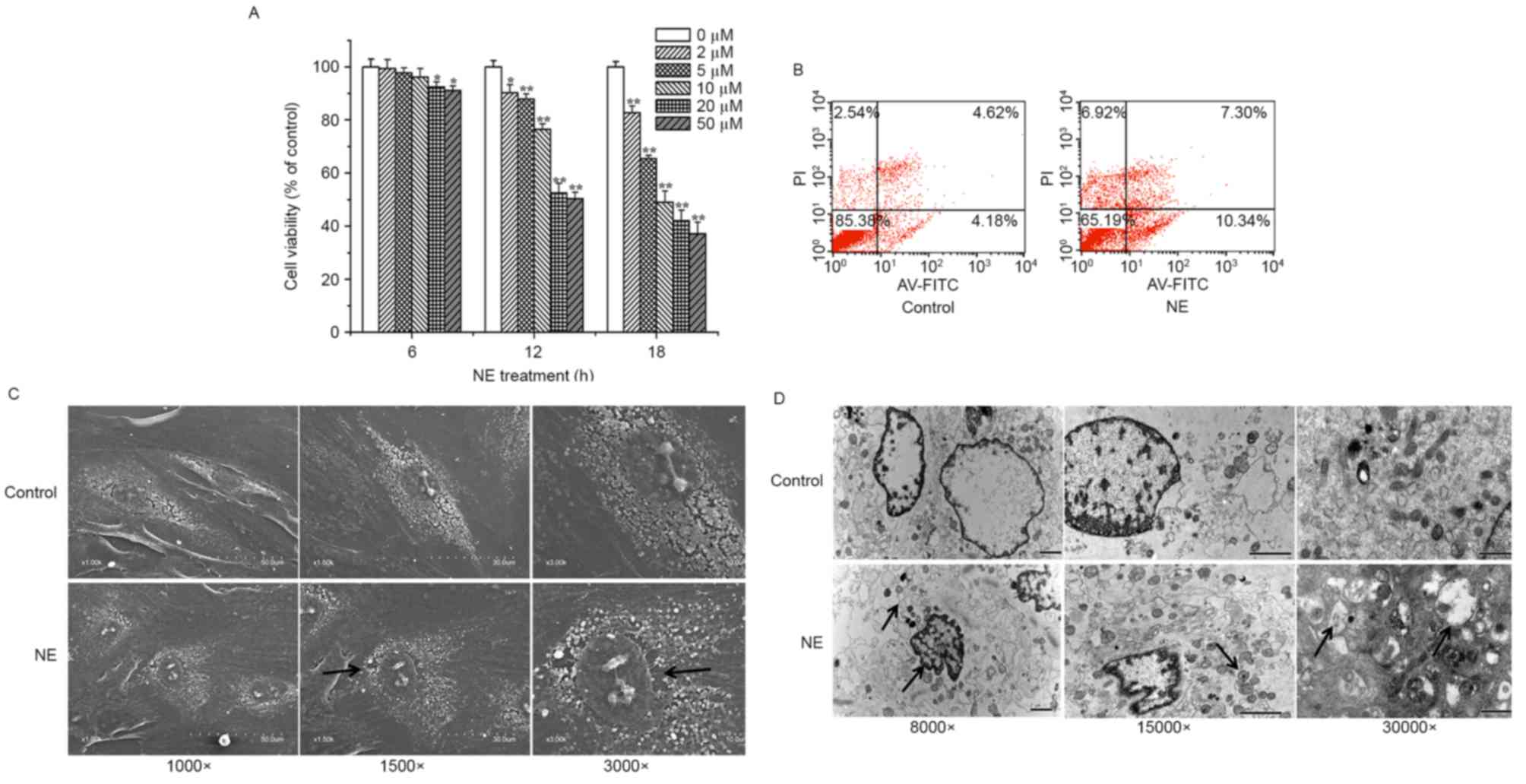 | Figure 1.Effects of NE on cell viability,
ultrastructural alterations and apoptosis of H9c2 cells. (A) Cell
viability was measured using an MTT assay following treatment with
various concentrations (0, 2, 5, 10, 20 and 50 µM) of NE for 6, 12
and 18 h. (B-D) Cells were treated with or without NE (5 µM) for 12
h. (B) Apoptosis was measured by flow cytometry, following AV-FITC
(FL 1 channels) and PI (FL 2 channels) double staining.
Morphological characteristics of H9c2 cells were observed using a
(C) scanning electron microscope and a (D) transmission electron
microscope. Black arrows indicate the following: (C) sparser cell
surface (×1,500 image) and increased nuclear gap (×3,000 image);
(D) nuclear chromatin margination and swelling of endoplasmic
reticulum (×8,000 image), mitochondrial (×15,000 image) and
mitochondrial vacuolization (×30,000 image). Data are presented as
the mean ± standard error of the mean (n=6). *P<0.05,
**P<0.01 compared with the control group. AV-FITC, Annexin
V-fluorescein isothiocyanate; NE, norepinephrine; PI, propidium
iodide. |
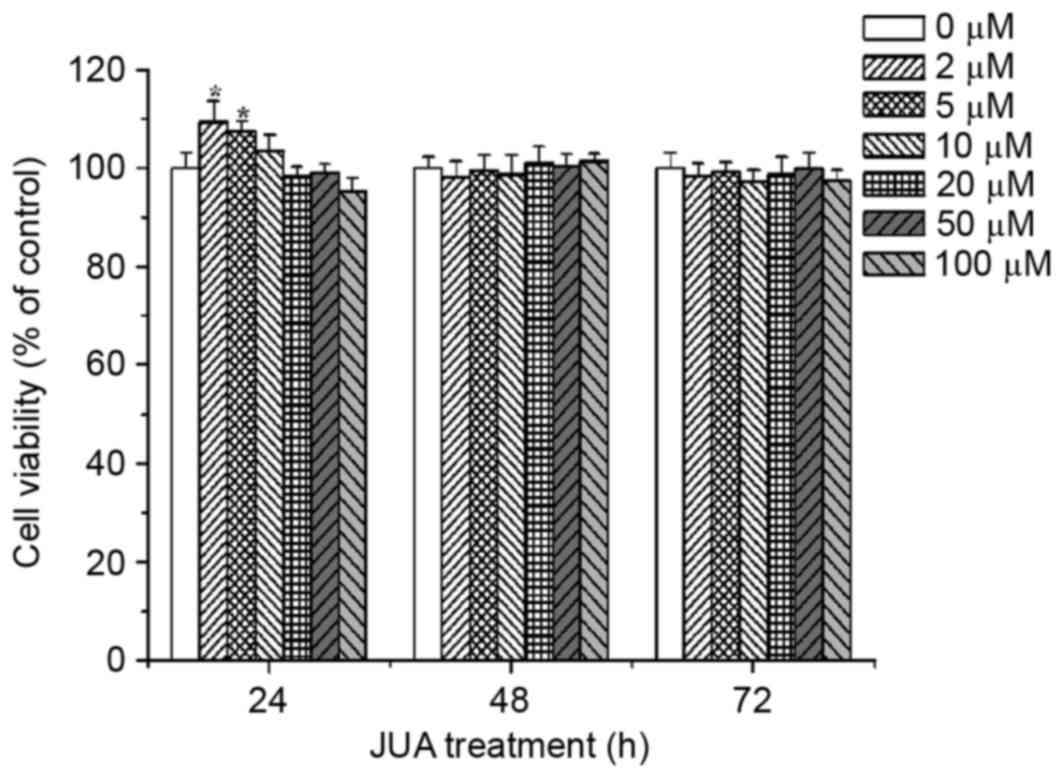
Cytotoxic effects of JUA on H9c2
cells
H9c2 cells were treated with various concentrations
of JUA (0, 2, 5, 10, 20, 50 and 100 µM) for 24, 48 and 72 h. Cell
viability was determined using an MTT assay. The results
demonstrated that treatment with between 0 and 100 µM JUA had no
cytotoxic effect on H9c2 cells (Fig.
2).
JUA improves cell viability and
reduces H9c2 apoptosis following NE exposure
The results of an MTT assay demonstrated that JUA
significantly enhanced the survival rate of the cells following
exposure to NE (Fig. 3A). In
addition, flow cytometry indicated that JUA reduced apoptosis in a
dose-dependent manner at 12 h following NE exposure (Fig. 3B and C). Pretreatment with JUA was
also revealed to alleviate the apoptotic response in H9c2 cells
following treatment with NE, as determined by AO and EB staining
(Fig. 3D).
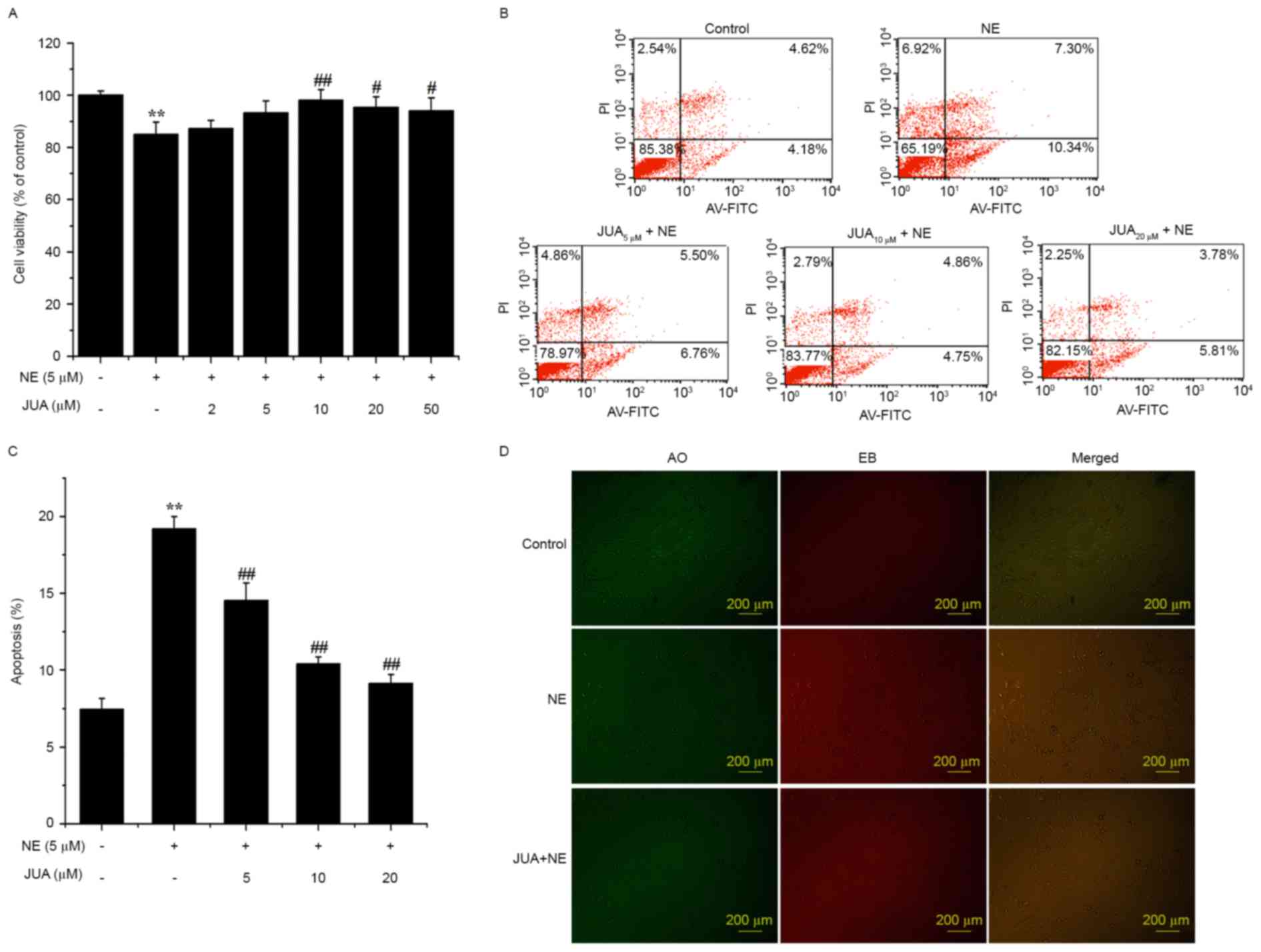 | Figure 3.Effects of JUA on NE-induced
cytotoxicity and apoptosis of H9c2 cells. Cells were treated with
or without JUA at the indicated concentrations for 3 h and then
incubated with NE (5 µM) for a further 12 h. (A) Cell viability was
determined using an MTT assay. (B-D) Apoptosis was detected by flow
cytometry and AO/EB staining. Data are presented as the mean ±
standard error of the mean (n=6), **P<0.01, compared with the
control group; #P<0.05, ##P<0.01,
compared with the NE-treated group. AO, acridine orange; AV-FITC,
Annexin V-fluorescein isothiocyanate; EB, ethidium bromide; JUA,
jujuboside A; NE, norepinephrine; PI, propidium iodide. |
JUA initially upregulates the
Bax/Bcl-2 ratio and then downregulates the ratio following
treatment of H9c2 cells with NE
The results of an RT-qPCR demonstrated that the mRNA
expression levels of Bax were upregulated following exposure to NE
for 3 and 6 h; this effect was markedly inhibited by JUA (Fig. 4A). In addition, pretreatment with
JUA was able to downregulate the mRNA expression levels of Bcl-2 at
3 and 6 h following NE exposure, and upregulate the mRNA expression
levels of Bcl-2 at 9 and 12 h following NE exposure (Fig. 4B). Correspondingly, the ratio of
Bax/Bcl-2 in JUA-pretreated cells was increased following exposure
to NE for 3 and 6 h (with all JUA pretreatments), and was decreased
after exposure to NE for 9 h (with all JUA pretreatments) and 12 h
(5 and 20 µM JUA pretreatments only). However, the Bax/Bcl-2 ratio
increased following JUA (10 µM) pretreatment and NE incubation for
12 h (Fig. 4C). With the prolonged
incubation time with NE, the mRNA expression levels of the
anti-apoptotic gene Bcl-2 were downregulated, whereas the
expression levels of the proapoptotic gene Bax were upregulated,
thus suggesting that JUA may exert its anti-apoptotic effect by
regulating the ratio of Bax/Bcl-2.
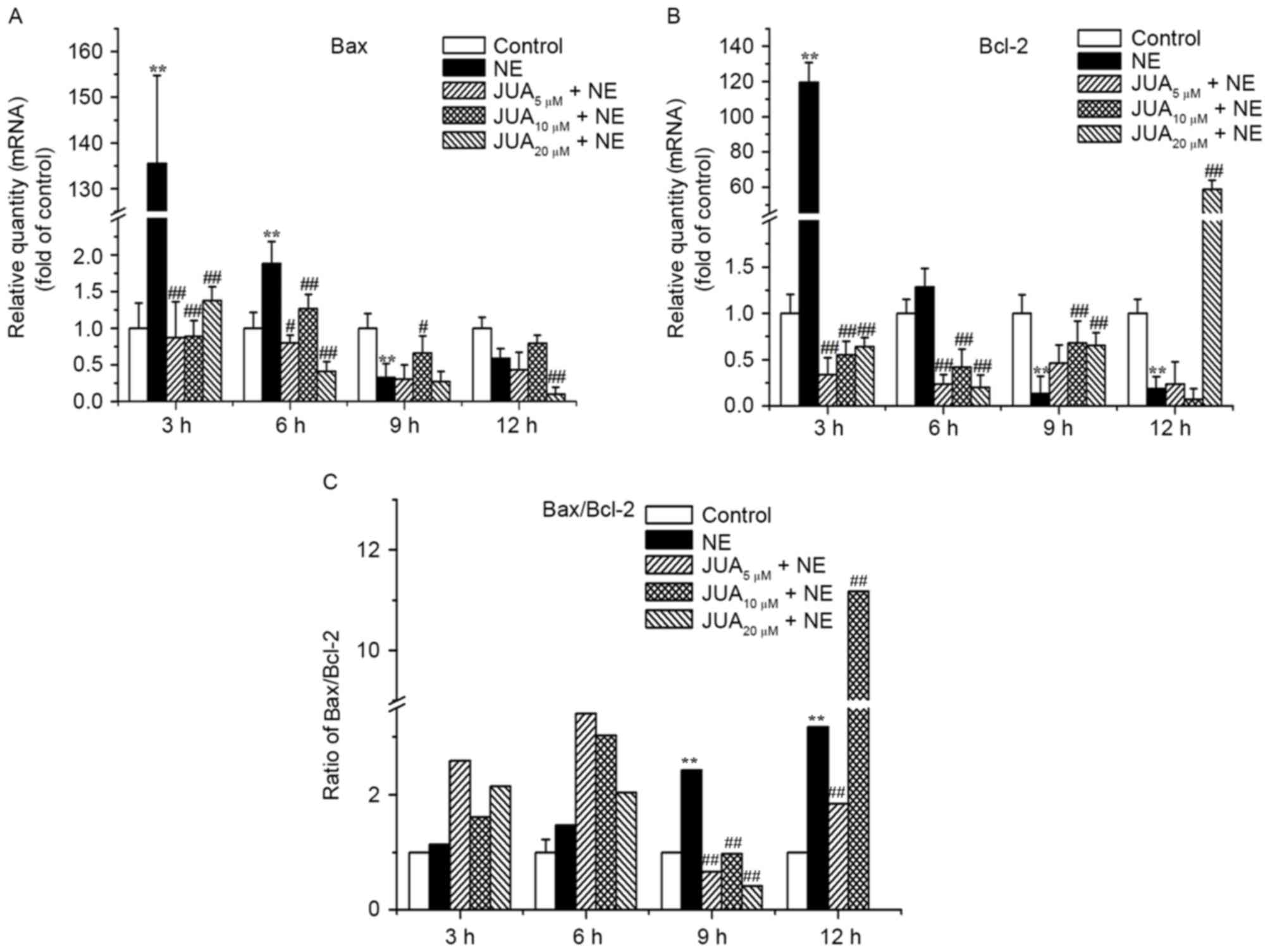 | Figure 4.Effects of JUA on NE-induced Bax and
Bcl-2 mRNA expression in H9c2 cells. Cells were pretreated with or
without JUA at the indicated concentrations for 3 h and were then
incubated with NE (5 µM) for 3, 6, 9 and 12 h. Total RNA was
isolated at the indicated time points and analyzed by RT-qPCR.
RT-qPCR analysis was used to detect the mRNA expression levels of
(A) Bax and (B) Bcl-2. (C) Ratio of Bax/Bcl-2 was calculated
according to the difference in mRNA expression. Data are presented
as the mean ± standard error of the mean (n=3). **P<0.01,
compared with the control group; #P<0.05,
##P<0.01, compared with the NE-treated group. Bax,
B-cell lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma 2;
JUA, jujuboside A; NE, norepinephrine; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
JUA inhibits NE-induced cleaved
caspase-3 and cleaved caspase-9 protein expression in H9c2
cells
Western blotting revealed that the protein
expression levels of cleaved caspase-3 and cleaved caspase-9 were
significantly increased in cells treated with NE for 6 and 12 h,
whereas pretreatment with JUA significantly decreased the protein
expression levels of cleaved caspase-3 and cleaved caspase-9 in the
cells (Fig. 5A-C). However, the
protein expression levels of cleaved caspase-12 and cleaved
caspase-8 were not significantly altered following exposure to NE
or JUA pretreatment (Fig. 5A, D and
E), thus indicating that JUA may alleviate NE-induced apoptosis
via the mitochondrial-dependent pathway in H9c2 cardiomyocytes.
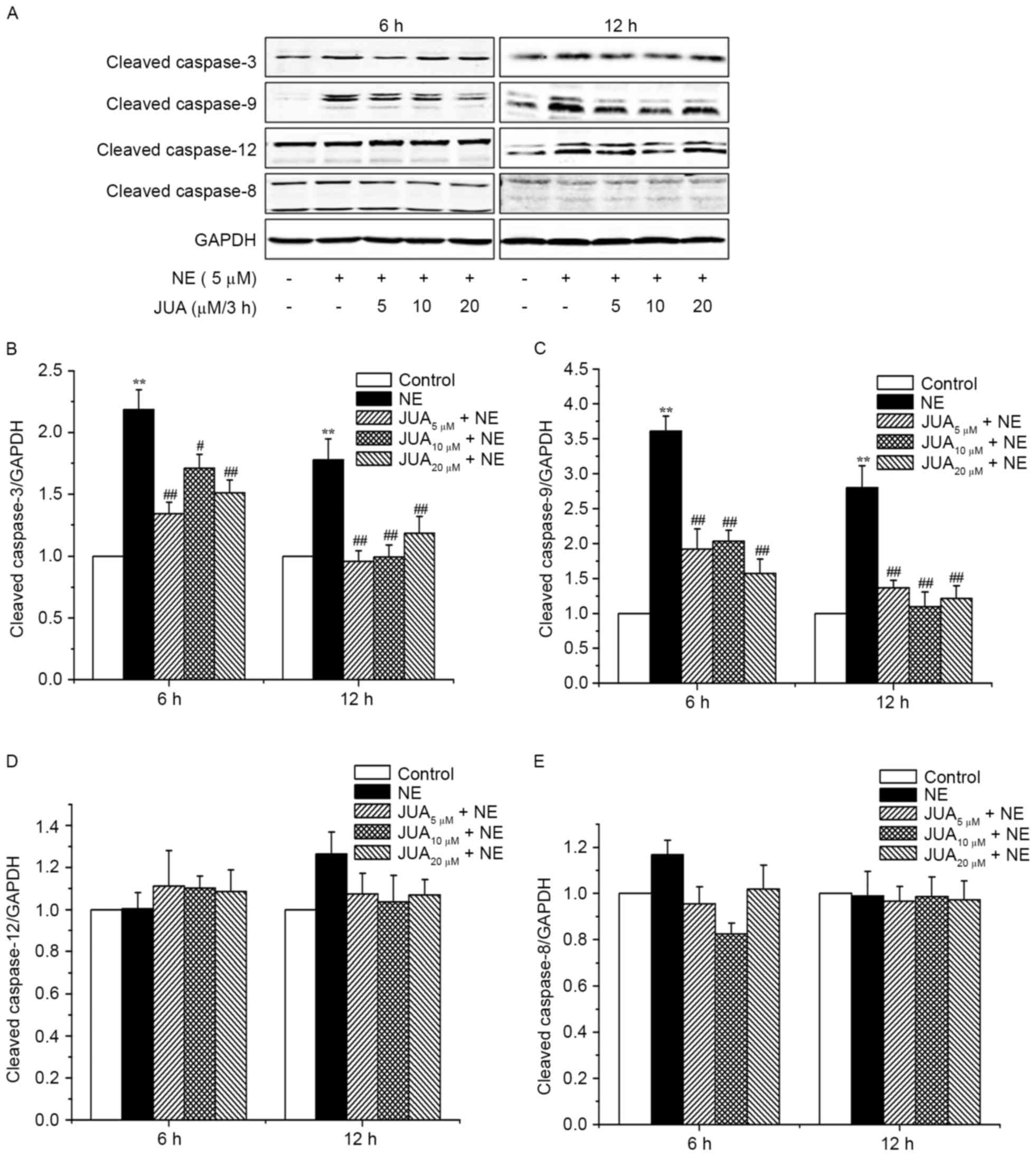 | Figure 5.Effects of JUA on NE-induced cleaved
caspase-3, cleaved caspase-9, cleaved caspase-12 and cleaved
caspase-8 protein expression in H9c2 cells. Cells were pretreated
with or without JUA at the indicated concentrations for 3 h and
were then incubated with NE (5 µM) for 6 and 12 h. Total proteins
were extracted at the indicated time points and analyzed by western
blotting. (A) Proteins were subjected to western blotting with
antibodies against cleaved caspase-3, cleaved caspase-9, cleaved
caspase-12 and cleaved caspase-8 (B-E). Relative density of the
bands was normalized to GAPDH and semi-quantification of the blots,
conducted by densitometry, are shown in (B-E). Data are presented
as the mean ± standard error of the mean (n=3), **P<0.01,
compared with the control group; #P<0.05,
##P<0.01, compared with the NE-treated group. JUA,
jujuboside A; NE, norepinephrine. |
JUA reduces NE-induced apoptosis via
influencing MAPK and AKT signals in H9c2 cells
To further elucidate the signaling pathways by which
JUA exerts its anti-apoptotic effects, the activation of MAPK and
AKT were examined. Western blotting results demonstrated that the
expression levels of p-JNK and p-p38 were significantly increased
following exposure to NE for 6 and 12 h. Conversely, pretreatment
with JUA was able to reduce the expression levels of p-JNK and
p-p38 at 6 or 12 h following NE exposure (Fig. 6A-C). In addition, the expression
levels of p-ERK were significantly increased following treatment
with NE. Pretreatment with JUA significantly reduced the expression
levels of p-ERK at 6 h following NE exposure. However, in cells
pretreated with 20 µM JUA, the expression levels of p-ERK were
significantly increased at 12 h following NE exposure (Fig. 6A and D). Furthermore, in the groups
receiving 10 and 20 µM JUA, the expression levels of p-AKT were
significantly increased at 6 or 12 h following NE exposure
(Fig. 6A and E). The total
expression levels of JNK, p38, ERK and AKT were not significantly
altered following exposure to NE or JUA pretreatment.
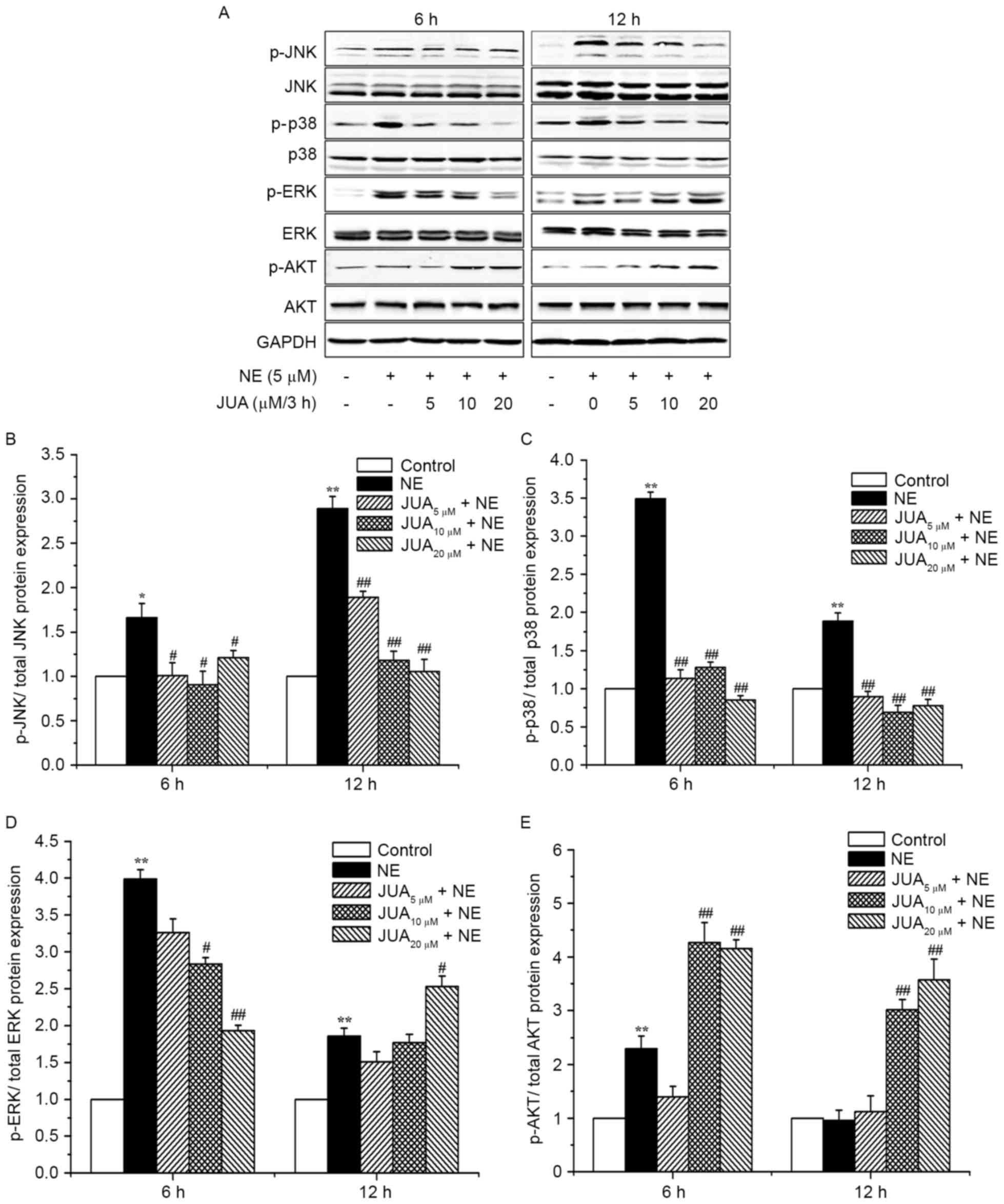 | Figure 6.Effects of JUA on NE-induced JNK,
p38-MAPK, ERK and AKT protein expression in H9c2 cells. Cells were
pretreated with or without JUA at the indicated concentrations for
3 h and were then incubated with NE (5 µM) for a further 6 and 12
h. (A) Total proteins were prepared at the indicated time points
and were then subjected to western blotting with antibodies against
the total and phosphorylated forms of JNK, p38, ERK and AKT. (B-E)
Semi-quantitative results of western blot analysis used to
determine p-JNK, p-p38, p-ERK and p-AKT expression. Data are
presented as the mean ± standard error of the mean (n=3),
*P<0.05, **P<0.01, compared with the control group;
#P<0.05, ##P<0.01, compared with the
NE-treated group. ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; JUA, jujuboside A; NE, norepinephrine; p-,
phosphorylated. |
Discussion
The majority of in vitro and in vivo
studies have demonstrated that NE-induced cardiotoxicity is a major
mediator of the biochemical alterations leading to cardiomyocyte
apoptosis and necrosis (14–17).
Our previous study revealed that increased plasma NE and
epinephrine levels in stress-induced rats promoted myocardial
damage and cardiac dysfunction (6). Therefore, the present study exposed
H9c2 cells to NE, and subsequently assessed cell viability,
apoptosis and the morphological alterations of H9c2 cell
microstructure. The results demonstrated that the survival rate of
H9c2 cells was decreased in a time- and dose-dependent manner
following exposure to NE. Furthermore, marked NE-induced cell
damage and apoptosis were detected in H9c2 cells. These findings
verified that NE induced cell damage and apoptosis of H9c2
cells.
JUA is a natural product isolated from the seeds of
Zizyphus jujuba, which possesses numerous biological effects
(8). Previous studies have
reported that JUA exerts anti-injury effects, and neuroprotective
and cardioprotective activity via antioxidative and
anti-inflammatory effects in animal models of dementia (10,12,18,19).
In the present study, the results of an MTT assay indicated that
JUA (0–100 µM) did not exhibit a cytotoxic effect on H9c2 cells.
Furthermore, JUA effectively reversed the decreased cell viability
caused by NE and reduced NE-induced H9c2 cell apoptosis.
It has previously been reported that NE may induce
cell apoptosis through activating the Bax/Bcl-extra large/caspase-3
pathway, the Bcl-2/caspase-2 pathway, the β-adrenergic pathway and
the p38 MAPK pathway (3,20). The present study confirmed that NE
activated the p38 MAPK pathway, which was consistent with previous
research (21). In addition, the
Bax and Bcl-2 mRNA expression levels were detected in the present
study. Bax is a proapoptotic member of the Bcl-2 protein family
that resides in the outer mitochondrial membrane (22). A change in the Bax/Bcl-2 ratio may
contribute to an increase in caspase-3 activity via the release of
cytochrome c from the mitochondria (23). The release of cytochrome c
initiates the deoxyadenosine triphosphate-dependent oligomerization
of apoptotic protease activating factor 1 and caspase-9 to form the
apoptosome, which can further activate effector caspase-3,
resulting in cell apoptosis (24).
The present study revealed that the Bax/Bcl-2 ratio was not
significantly altered at 3 and 6 h following NE exposure, however,
it was upregulated at 9 and 12 h following NE exposure. This
regulation was attenuated by JUA, with exception to cells
pretreated with 10 µM JUA and NE for 12 h, which produced a
significant increase in the Bax/Bcl-2 ratio when compared with the
NE group. The increase in the Bax/Bcl-2 ratio observed in cells
treated with NE for 9 and 12 h indicated that H9c2 cell apoptosis
may occur over a long period time. In addition, JUA reversed
NE-induced increases in the protein expression levels of cleaved
caspase-3 and cleaved caspase-9, whereas the protein expression
levels of cleaved caspase-8 and cleaved caspase-12 were not
significantly altered. Bax/Bcl-2, cleaved caspase-3 and cleaved
caspase-9 have been reported to serve important roles in the
mitochondrial apoptotic pathway; however, cleaved caspase-8 and
cleaved caspase-12 are key proteins in the death receptor signaling
pathway and endoplasmic reticulum apoptotic pathway, respectively
(25–28). The results of the present study
suggested that the anti-apoptotic effects of JUA may be mediated
through the mitochondrial-dependent apoptotic pathway.
MAPKs regulate various cellular responses to
environmental stimuli, including cell survival, transformation and
apoptosis, and serve important roles in cardiomyocyte apoptosis
(29–31). ERK protects cardiomyocytes from
apoptosis, whereas p38 MAPK is involved in the induction of
cardiomyocyte apoptosis (32,33).
Furthermore, JNK signaling exerts pro- and anti-apoptotic effects;
however, the proapoptotic effects appear to be most dominant in the
majority of experimental models (34,35).
AKT exerts direct effects on apoptosis, and AKT activation is able
to suppress the apoptotic effects of activated JNK and P38 MAPK
signaling pathways (36). In
addition, AKT has been reported to be involved in apoptosis and
endoplasmic reticulum stress in the rat small intestine (37). Furthermore, JNK has been reported
to negatively regulate AKT activation (38). The present study indicated that NE
upregulated p-JNK and p-p38 expression in H9c2 cells, and that this
regulation was attenuated by JUA. Treatment with NE for 6 h also
upregulated p-AKT expression, however, JUA pretreatment further
increased expression. Conversely, ERK was inhibited in
JUA-pretreated cells at 6 h following NE exposure, whereas it was
activated at 12 h following NE exposure, which suggested that JUA
may attenuate the upregulation of ERK induced by NE during the
early stages and ERK may adaptively respond to NE treatment in
these short time periods. These results indicated that JUA may
reduce NE-induced apoptosis by inhibiting the JNK and p38 signaling
pathways, regulating the ERK pathway and activating the AKT pathway
in H9c2 cells.
In conclusion, the present study provided a feasible
molecular explanation regarding the inhibitory effects of JUA on
NE-induced apoptosis of H9c2 cells, which may offer a therapeutic
option for preventing NE-induced heart failure. Further
investigations are required to elucidate the precise mechanisms
underlying the protective effects of JUA on cardiomyocyte
apoptosis.
Acknowledgements
The present study was supported by grants from the
Ministry of Agriculture, Public Service Sectors Agriculture
Research Projects (grant no. 201403051-07) and the National Natural
Science Foundation of China (grant nos. 31572584 and 31272478).
References
|
1
|
Ahuja P, Sdek P and MacLellan WR: Cardiac
myocyte cell cycle control in development, disease, and
regeneration. Physiol Rev. 87:521–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu B, Che W, Xue J, Zheng C, Tang K,
Zhang J, Wen J and Xu Y: SIRT4 prevents hypoxia-induced apoptosis
in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 32:655–662.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai KB, Sanderson JE and Yu CM: High dose
norepinephrine-induced apoptosis in cultured rat cardiac
fibroblast. Int J Cardiol. 136:33–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lameris TW, de Zeeuw S, Alberts G, Boomsma
F, Duncker DJ, Verdouw PD, Veld AJ and van Den Meiracker AH: Time
course and mechanism of myocardial catecholamine release during
transient ischemia in vivo. Circulation. 101:2645–2650. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT
and Hsu SL: Norepinephrine induces apoptosis in neonatal rat
cardiomyocytes through a reactive oxygen species-TNF alpha-caspase
signaling pathway. Cardiovasc Res. 62:558–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thakur A, Alam MJ, Ajayakumar MR,
Ghaskadbi S, Sharma M and Goswami SK: Norepinephrinr-induced
apoptotic and hypertrophic responses in H9c2 cardiac myoblasts are
characterized by different repertoire of reactive oxygen species
generation. Redox Bio. 5:243–252. 2015. View Article : Google Scholar
|
|
7
|
Wan C, Chen Y, Yin P, Han D, Xu X, He S,
Liu M, Hou X, Liu F and Xu J: Transport stress induces apoptosis in
rat myocardial tissue via activation of the mitogen-activated
protein kinase signaling pathways. Heart Vessels. 31:212–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You ZL, Xia Q, Liang FR, Tang YJ, Xu CL,
Huang J, Zhao L, Zhang WZ and He JJ: Effects on the expression of
GABAA receptor subunits by jujuboside A treatment in rat
hippocampal neurons. J Ethnopharmacol. 128:419–423. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Zhao X, Liu B, Liu AJ, Li H, Mao X,
Wu B, Bi KS and Jia Y: Jujuboside A, a neuroprotective agent from
semen Ziziphi Spinosae ameliorates behavioral disorders of the
dementia mouse model induced by Aβ 1–42. Eur J Pharmacol.
738:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao JX, Zhang QY, Cui SY, Cui XY, Zhang J,
Zhang YH, Bai YJ and Zhao YY: Hypnotic effect of jujubosides from
Semen Ziziphi Spinosae. J Ethnopharmacol. 130:163–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XX, Ma GI, Xie JB and Pang GC:
Influence of JuA in evoking communication changes between the small
intestines and brain tissues of rats and the GABAA and GABAB
receptor transcription levels of hippocampal neurons. J
Ethnopharmacol. 159:215–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han D, Wan C, Liu F, Xu X, Jiang L and Xu
J: Jujuboside A protects H9C2 cells from isoproterenol-induced
injury via activating PI3K/Akt/mTOR signaling pathway. Evid Based
Complement Alternat Med. 2016:95937162016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT
and Hsu SL: Norepinephrine induces apoptosis in neonatal rat
endothelial cells via down-regulation of Bcl-2 and activation of
beta-adrenergic and caspase-2 pathways. Cardiovasc Res. 61:143–151.
2014. View Article : Google Scholar
|
|
15
|
Kohli S, Chhabra A, Jaiswal A, Rustagi Y,
Sharma M and Rani V: Curcumin suppresses gelatinase B mediated
norepinephrine induced stress in H9c2 cardiomyocytes. PLoS One.
8:e765192013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang C, Shan XL, Liao YL, Zhao P, Guo W,
Wei HC and Lu R: Effects of stachydrine on norepinephrine-induced
neonatal rat cardiac myocytes hypertrophy and intracellular calcium
transients. BMC Complement Altern Med. 14:4742014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jain A, Atale N, Kohli S, Bhattacharya S,
Sharma M and Rani V: An assessment of norepinephrine mediated
hypertrophy to apoptosis transition in cardiac cells: A signal for
cell death. Chem Biol Interact. 225:54–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao QH, Wu CS and Wang M: The jujube
(Ziziphus jujuba Mill.) fruit: A review of current knowledge
of fruit composition and health benefits. J Agr Food Chem.
61:3351–3363. 2013. View Article : Google Scholar
|
|
19
|
Shou CH, Wang J, Zheng XX and Guo DW:
Inhibitory effect of jujuboside A on penicillin sodium induced
hyperactivity in rat hippocampal CA1 area in vitro. Acta Pharmacol
Sin. 22:986–990. 2001.PubMed/NCBI
|
|
20
|
Communal C, Singh K, Pimentel DR and
Colucci WS: Norepinephrine stimulates apoptosis in adult rat
ventricular myocytes by activation of the beta-adrenergic pathway.
Circulation. 98:1329–1334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lajevic MD, Suleiman S, Cohen RL and
Chambers DA: Activation of p38 mitogen-activated protein kinase by
norepinephrine in T-lineage cells. J Immunol. 132:197–208. 2011.
View Article : Google Scholar
|
|
22
|
Jürgensmeier JM, Xie Z, Deveraux Q,
Ellerby L, Bredesen D and Reed JC: Bax directly induces release of
cytochrome c from isolated mitochondria. P Natl Acad Sci USA.
95:4997–5002. 1998. View Article : Google Scholar
|
|
23
|
McDonald TE, Grinman MN, Carthy CM and
Walley KR: Endotoxin infusion in rats induces apoptotic and
survival pathways in hearts. Am J Physiol Heart Circ Physiol.
279:H2053–H2061. 2000.PubMed/NCBI
|
|
24
|
Bratton SB, Walker G, Srinivasula SM, Sun
XM, Butterworth M, Alnemri ES and Cohen GM: Recruitment, activation
and retention of caspases-9 and −3 by Apaf-1 apoptosome and
associated XIAP complexes. Embo J. 20:998–1009. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agata N, Ahmad R, Kawano T, Raina D,
Kharbanda S and Kufe D: MUC1 oncoprotein blocks death
receptor-mediated apoptosis by inhibiting recruitment of caspase-8.
Cancer Res. 68:6136–6144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Funakoshi T, Aki T, Nakayama H, Watanuki
Y, Imori S and Uemura K: Reactive oxygen species-independent rapid
initiation of mitochondrial apoptotic pathway by chelerythrine.
Toxicol In vitro. 25:1581–1587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Zhang Z and Xing D: Cell death via
mitochondrial apoptotic pathway due to activation of Bax by
lysosomal photodamage. Free Radical Bio Med. 51:53–68. 2011.
View Article : Google Scholar
|
|
28
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu W, Zou Y, Aikawa R, Harada K, Kudoh S,
Uozumi H, Hayashi D, Gu Y, Yamazaki T, Nagai R, et al: MAPK
superfamily plays an important role in daunomycin-induced apoptosis
of cardiac myocytes. Circulation. 100:2100–2107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang F, Liu J, Fu H, Wang J, Li F, Yue H,
Li W, Zhao J and Yin D: GSK-3β promotes PA-induced apoptosis
througu changing β-arrestin 2 nucleus location in H9c2
cardiomyocytes. Apoptosis. 21:1045–1055. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang G, Cui J, Guo Y, Wang Y, Kang L and
Liu L: Cyclosporin A protectes H9c2 cells against chemical
hypoxia-induced injury via inhibition of MAPK signaling pathway.
Int Heart J. 57:483–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Zhang S, Su D, Liu J, Cheng Y, Zou
L, Li W and Jiang Y: Inhibiting (pro)renin receptor-mediated p38
MAPK signaling decreases hypoxia/reoxygenation-induced apoptosis in
H9c2 cells. Mol Cell Biochem. 403:267–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song YH, Cai H, Zhao ZM, Chang WJ, Gu N,
Cao SP and Wu ML: Icariin attenuated oxidative stress
induced-cardiac apoptosis by mitochondria protection and ERK
activation. Biomed Pharmacother. 83:1089–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baines CP and Molkentin JD: STRESS
signaling pathways that modulate cardiac myocyte apoptosis. J Mol
Cell Cardiol. 38:47–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma L, Liu H, Xie Z, Yang S, Xu W, Hou J
and Yu B: Ginsenoside Rb3 protects cardiomyocytes against
ischemia-reperfusion injury via the inhibition of JNK-mediated
NF-κB pathway: A mouse cardiomyocyte model. PLoS One.
9:e1036282014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Downward J: PI3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin P, Xu J, He S, Liu F, Yin J, Wan C,
Mei C, Yin Y, Xu X and Xia Z: Endoplasmic reticulum stress in heat-
and shake-induced injury in the rat small intestine. PLoS One.
10:e01439222015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Chen WR and Xing D: A pathway from
JNK through decreased ERK and Akt activities for FOXO3a nuclear
translocationin response to UV irradiation. J Cell Physiol.
227:1168–1178. 2011. View Article : Google Scholar
|