Introduction
Angiotensin II (Ang II), a primary effector in the
renin-angiotensin system (RAS), regulates cell growth and
differentiation, blood pressure, fluid and electrolyte homeostasis,
and cytokine production (1). Ang
II is crucial for renal and cardiovascular function (2), and previous studies have demonstrated
that Ang II induces receptor activator of nuclear factor-κB ligand
(RANKL) expression in osteoblasts, leading to osteoclast activation
and accelerated osteoporosis (3–5).
During bone metabolism, monocyte chemoattractant protein-1 (MCP-1)
secreted by osteoblast is important for osteoclast nactivation
(6). However, whether Ang II
upregulates MCP-1 expression in osteoblasts remains to be
investigated.
Ang II stimulates MCP-1 expression in endothelia
cells, vascular smooth muscle cells (VSMCs) and monocytes. Ang II
upregulates MCP-1 expression in rat glomerular endothelial cells by
activating the NAD(P)H oxidase-dependent nuclear factor (NF)-κB
signaling pathway (7).
Furthermore, Ang II enhances MCP-1 expression in proximal tubular
cells by activating reactive oxygen species (ROS)-mediated
signaling (8). In addition, Ang II
through its Ang II receptor type 1 (AT1R) promotes ROS generation
in hepatocytes (9). Previous
studies have indicated that ROS activates NF-κB signaling (10). However, whether Ang II also induces
ROS production which activates the NF-κB signaling pathway,
resulting in upregulated MCP-1 expression in osteoblasts, remains
to be clarified.
The present study used the MC3T3-E1 mouse
osteoblastic cell line to investigate the hypothesis that Ang II
induces ROS production to activate the NF-κB signaling pathway, and
upregulates MCP-1 expression in osteoblasts.
Materials and methods
Materials
α-Modified minimum essential medium (α-MEM), fetal
bovine serum (FBS), streptomycin and penicillin were purchased from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Ang II,
PD123319, N-acetylcysteine (NAC), 2′,7′-dichlorodihydrofluorescein
diacetate (DCFH-DA) and ammonium pyrrolidine thiocarbamate (PDTC)
were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
A rabbit anti-mouse AT1R (ab18801), anti-mouse IκB kinase (IKK)β
(ab32135) and anti-mouse phosphorylated (p)-IKKβ antibodies
(ab59195) were purchased from Abcam (Cambridge, MA, USA). In
addition, rabbit polyclonal anti-mouse NF-κBp65 (ab16502) and
p-NF-κBp65 (ab86299) antibodies were obtained from Abcam. Rabbit
polyclonal anti-mouse IκBα (sc371), and mouse monoclonal anti-mouse
β-actin antibody (sc47778), were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
All MC3T3-E1 cells (American Type Culture
Collection, Manassas, VA, USA) were grown to ~80% confluence and
were routinely grown overnight in 10% FBS supplemented with α-MEM
containing 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C
in 5% CO2 and 95% air. In order to determine the effects
of Ang II on MCP-1 expression in the MC3T3-E1 cells, the cells were
treated with 10−9~-5 M Ang II or without Ang II (control
group) for 24 h at 37°C and were treated with 10−6 M Ang
II for varying durations (0, 3, 6, 12, 18 and 24 h)at 37°C.
Furthermore, cells were in the presence of 10−6 M Ang II
or absence of Ang II which served as the control group; cells were
then separated into groups that were pretreated with
10−5 M olmesartan (AT1R blocker), 10−5 M
PD123319 (AT2R blocker), 10−3 M N-acetylcysteine (NAC, a
scavenger of free radicals), or 5×10−6 M PDTC (the NF-κB
inhibitor) for 24 h at 37°C. The cells were harvested for
subsequent analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from different groups of
MC3T3-E1 cells by TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and the cDNA was generated using the Revert
Aid™ First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.) according to manufacturer's protocol. The
relative levels of MCP-1 and AT1R gene mRNA transcripts to control
β-actin in different groups of cells were determined using the cDNA
as the template, the SYBR Green 1 PCR master mix (Takara Bio, Inc.,
Otsu, Japan), under the following conditions: 95°C for 30 sec, 95°C
for 5 sec, and 60°C for 30 sec for 40 cycles and 95°C for 15 sec in
an ABI 7300 Real-time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The primer sequence are presented in Table I. The data were normalized to the
control β-actin expression and analyzed by 2−ΔΔCq method
(11). All assays were performed
in quintuplicate.
 | Table I.Primers sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers sequences for reverse
transcription-quantitative polymerase chain reaction.
| Target | Primer sequence
(5′-3′) | GenBank no. |
|---|
| MCP-1 | F:
TCACCTGCTGCTACTCATTC | NM_011333.3 |
|
| R:
ATGTCTGGACCCATTCCTTC |
|
| AT1R | F:
CTCTTGCTGCCTCGTCTACC | NM_009642.4 |
|
| R:
TGCAGCAGCGTCTGATGATG |
|
| β-actin | F:
AGGCCAACCGTGAAAAGATG | NM_007393.3 |
|
| R:
TGGCGTGAGGGAGAGCATAG |
|
ELISA
The levels of MCP-1 in the supernatants of cultured
MC3T3-E1 cells were determined by ELISA using a specific kit
(ab100721), according to the manufacturer's protocol (Abcam). The
limitation of MCP-1 detection was 10 pg/ml.
Western blot analysis
The different groups of MC3T3-E1 cells were washed
with ice-cold PBS, and lysed in radioimmunoprecipitation assay
buffer (Thermo Fisher Scientific, Inc.). The cell lysates were
centrifuged at 10,000 × g for 20 min at 4°C. The concentration of
protein was quantified by the Bicinchoninic Acid protein assay
reagent (Pierce; Thermo Fisher Scientific, Inc.). The lysates (20
µg/lane) were subjected to 5% and 10% SDS-PAGE, which was performed
using a constant voltage of 90 V for 120 min. Following this,
proteins were transferred onto polyvinyl difluoride membranes. The
membranes were blocked with 5% fat-free milk and probed with
anti-AT1R (1:600), anti-NF-κBp65 (1:1,000), anti-p-NF-κBp65
(1:1,000), anti-IκBα (1:1,000), anti-IKKβ (1:1,000), anti-p-IKKβ
(1:500) and anti-β-actin (1:100) primary antibodies respectively,
and incubated overnight at 4°C. Following three washes with PBS,
the bound antibodies were detected with horseradish
peroxidase-conjugated mouse anti-rabbit immunoglobulin G (IgG;
1:3,000, sc2357) secondary antibody, which was obtained from Santa
Cruz Biotechnology, Inc., at room temperature for 2 h. The bands
were visualized by an Enhanced ECL Chemiluminescent Substrate kit
(JC-PC001, Jingcai Biotechnology Co. Ltd., Xi'an, China). The
relative levels of each target protein to the control β-actin were
analyzed using Quantity One v4.52 software (Bio-Rad Laboratories,
Inc.).
Immunostaining
The cells were pre-treated with 10−5 M
olmesartan (an AT1R blocker) for 30 min, and exposed to Ang II
(10−6 M) for 12 h. The cells were fixed with 10%
methanol at room temperature, blocked with rabbit serum in PBS for
60 min at room temperature, and subsequently stained with an
anti-AT1R antibody (1:500) overnight at 4°C. Following three washes
with PBS, the cells were incubated with goat anti-rabbit IgG
secondary antibody for 2 h at 37°C (1:200, cw0159), which was
obtained from ComWin Biotech Co., Ltd. (Beijing, China). The cells
were washed with PBS for three times prior to staining with with
phycoerythrin-streptavidin and DAPI (Sigma-Aldrich; KGaA). Images
were obtained under a fluorescent microscope at excitation
wavelengths of 550 and 340 nm (BX50; Olympus Corporation, Tokyo,
Japan).
Flow cytometry and fluorescent
microscopy
Intracellular ROS was evaluated by flow cytometry
and fluorescent microscopy. Briefly, the cells (~80% confluence)
were washed with PBS buffer for two times at room temperature and
then were pre-treated at 37°C with or without olmesartan (AT1R
blocker), PD123319 (AT2R blocker), NAC (scavenger of free radicals)
or PDTC (NF-κB inhibitor) for 30 min, and then exposed to Ang II
(10−6 M) for 12 h in 10% FBS medium (Gibco; Thermo
Fisher Scientific, Inc.). The cells were washed with fetal calf
serum-free medium for two times at room temperature and cultured in
a-MEM containing 10 µM DCFH-DA (s0033, Beyotime Institute of
Biotechnology, Jiangsu, China) for 30 min at 37°C in 5%
CO2 and 95% air. The cells were then digested with 0.25%
pancreatic enzyme (Gibco; Thermo Fisher Scientific, Inc.) and were
resuspended in PBS buffer. The contents of intracellular ROS which
were detected by dichlorodihydrofluorescein diacetate (s0033;
Beyotime Institute of Biotechnology) were analyzed by flow
cytometry using FACSort flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). In addition, the intracellular ROS were analyzed
via fluorescent microscopy at an excitation wavelength of 488 nm
prior to digestion with 0.25% pancreatic enzyme.
Statistical analysis
All data are presented as the mean ± standard
deviation. Multiple comparisons were assessed by analysis of
variance followed by post hoc least significant difference test
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Ang II induces MCP-1 expression in
osteoblasts
To determine the effect of Ang II on MCP-1
expression in osteoblasts, MC3T3-E1 cells were treated with or
without various concentrations of Ang II
(10−9−10−5 M) for 24 h. The results showed
that treatment with 10−9−10−6 M Ang II
increased the levels of MCP-1 in MC3T3-E1 cells in a dose-dependent
manner, but treatment with 10−5 M Ang II slightly
reduced the levels of MCP-1, relative to that of 10−6 M
Ang II (Fig. 1A). Furthermore,
treatment with 10−6 M Ang II enhanced MCP-1 expression
in MC3T3-E1 cells in a time-dependent manner (Fig. 1B). The enhanced effect of Ang II on
MCP-1 mRNA (Fig. 1C) and serum
(Fig. 1D) expression levels was
completely abrogated by pre-treatment with olmesartan (an AT1R
blocker), NAC (a scavenger of free radicals) or PDTC (a NF-κB
inhibitor), but not by PD123319 (an AT2R antagonist). Therefore,
AT1R-associated ROS production and associated NF-κB signaling
pathways may be important for Ang II-induced MCP-1 expression in
osteoblasts.
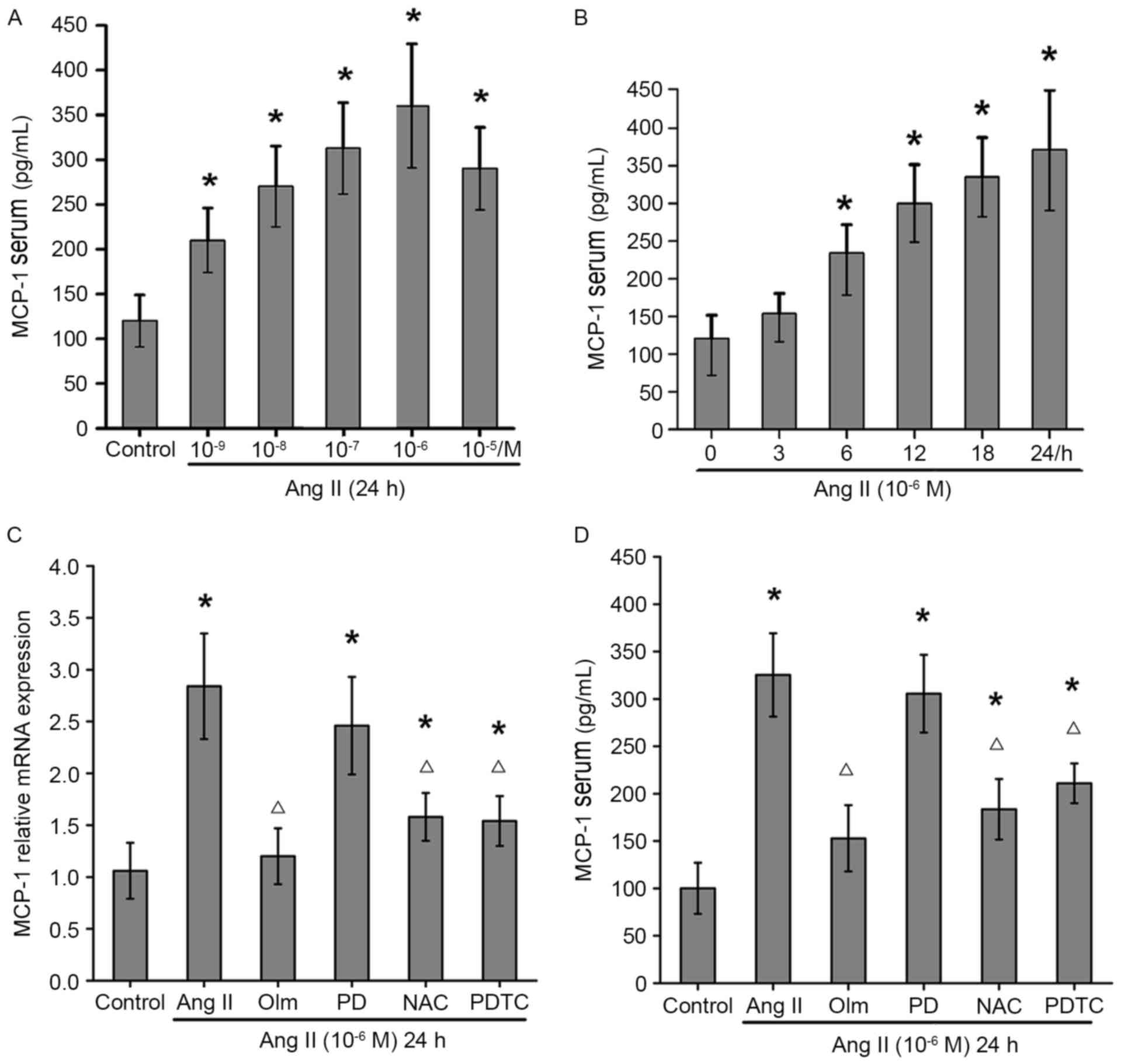 | Figure 1.Effects of Ang II on MCP-1 expression
in osteoblasts. MCP-1 expression levels following (A) various doses
of Ang II, and (B) treatment with 10−6 M Ang II for 0,
3, 6, 12, 18 and 24 h. (C) mRNA expression and (D) serum levels of
MCP-1 following treatment with Ang II and various inhibitors, as
assessed by reverse transcription-quantitative polymerase chain
reaction and ELISA, respectively. Data are expressed as the mean ±
standard deviation of five independent experiments. *P<0.05 vs.
control; ΔP<0.05 vs. Ang II. Ang II, angiotensin II;
MCP-1, monocyte chemoattractant protein-1; Olm, olmesartan; PD,
PD123319; NAC, N-acetylcysteine; PDTC, ammonium pyrrolidine
thiocarbamate. |
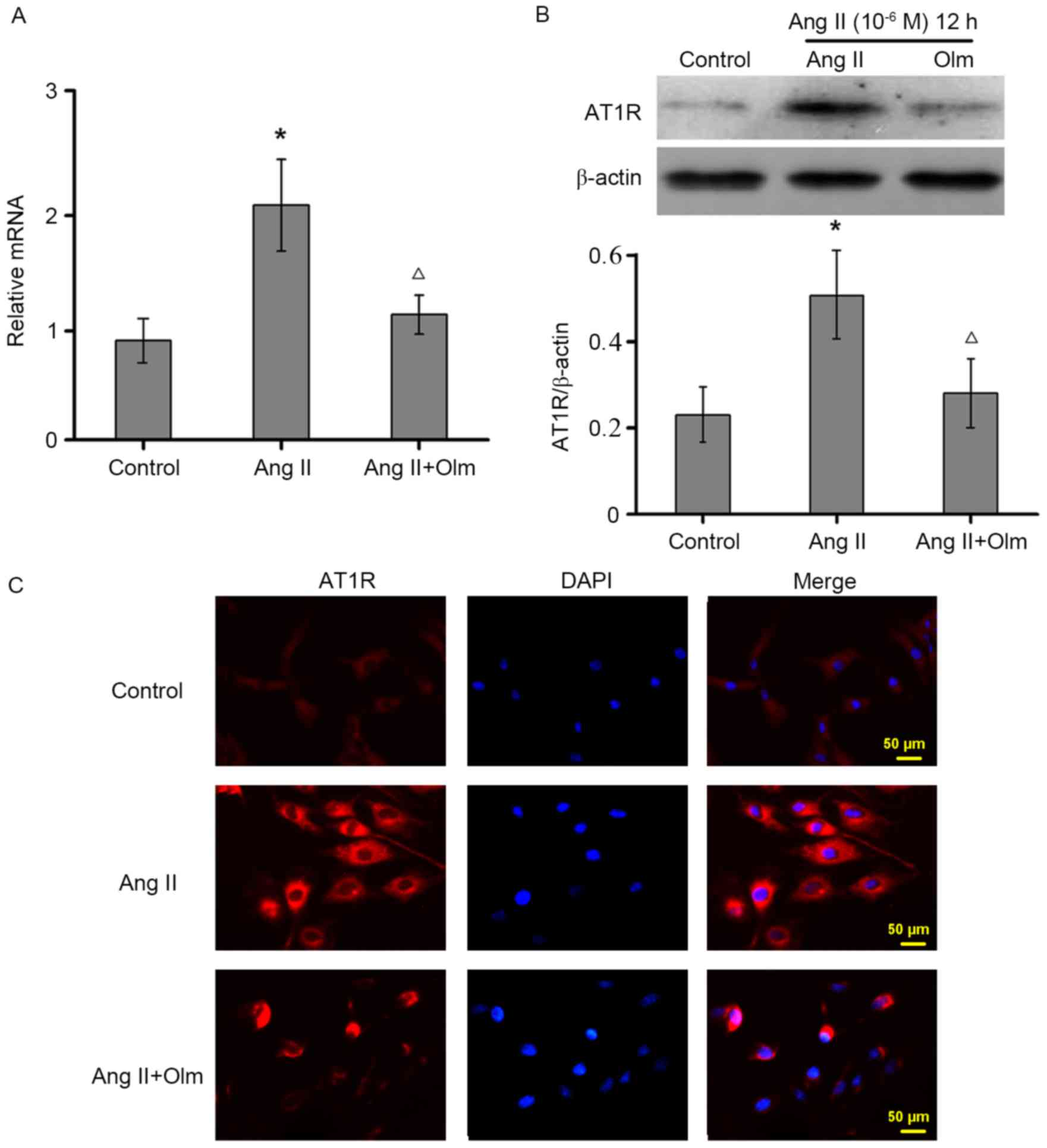
Ang II enhances AT1R expression in
osteoblasts
To determine whether Ang II induces AT1R expression
in osteoblasts, the levels of AT1R expression were measured by
RT-qPCR and western blot assays. Treatment with Ang II upregulated
AT1R mRNA (Fig. 2A) and protein
(Fig. 2B) expression levels in
osteoblasts, which was completely blocked by the selective AT1R
antagonist of olmesartan. A similar pattern of AT1R expression was
detected by immunofluorescent assay (Fig. 2C). Thus, Ang II upregulates AT1R
expression in osteoblasts in vitro.
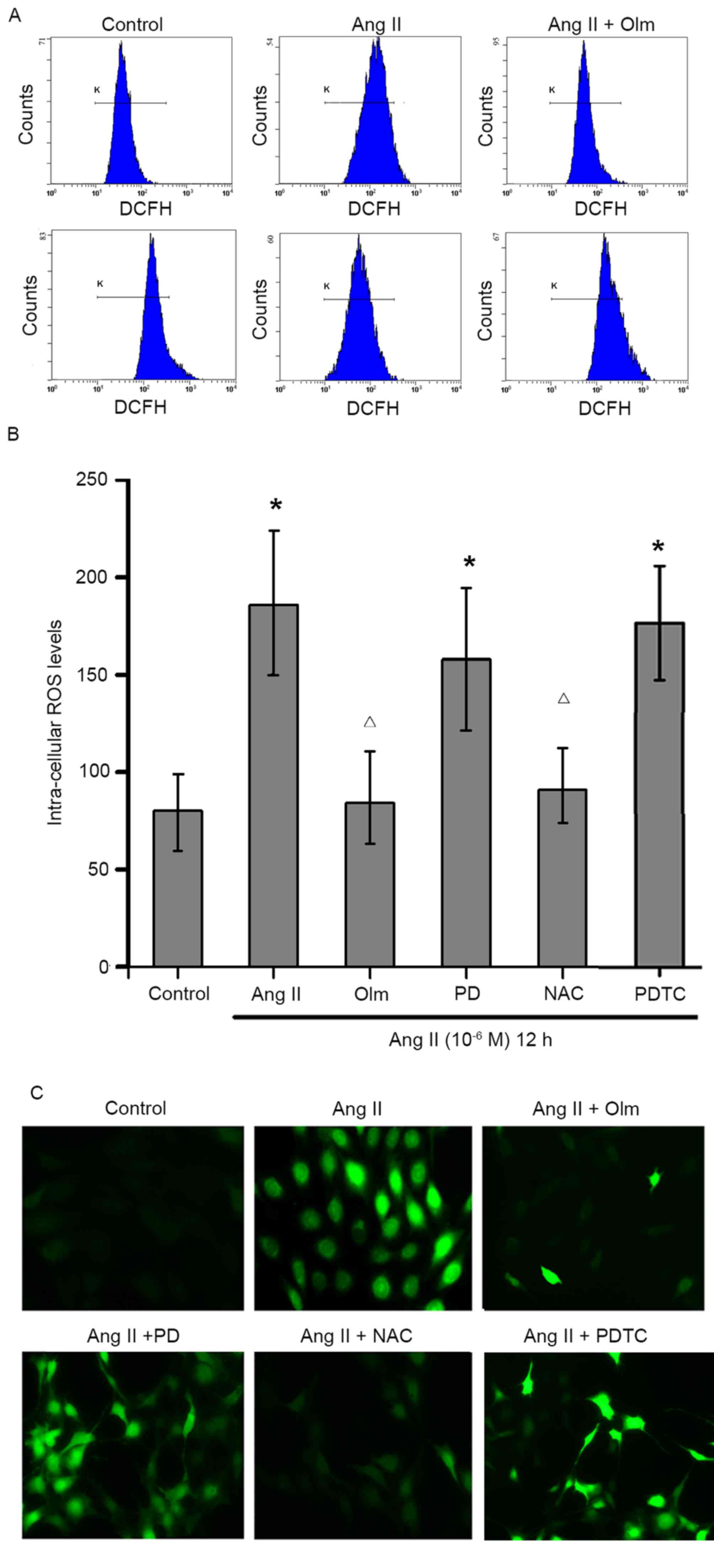
Ang II induces ROS production via AT1R
in osteoblasts
To examine the effects of Ang II on ROS production
in osteoblasts, MC3T3-E1 cells were pre-treated with or without
olmesartan, PD123319, NAC or PDTC, and then exposed to Ang II
(10−6 M) for 12 h. The contents of intracellular ROS
were determined by flow cytometry (Fig. 3A and B) and immunofluorescent
assays (Fig. 3C). The results
indicated that treatment with Ang II significantly increased the
levels of intracellular ROS in osteoblasts, which was abrogated by
pre-treatment with olmesartan or NAC, but not PD123319 or PDTC
(Fig. 3). These data suggested
that Ang II increased ROS production by activating AT1R, but is
independent of NF-κB signaling in osteoblasts.
Ang II induces NF-κB activation in
osteoblasts
As NF-κB activation is important for Ang II to
enhance MCP-1 expression, the levels of the NF-κB activation in the
different groups of cells were examined by western blot assays.
Treatment with Ang II significantly increased NF-κB p65 and IKKβ
phosphorylation (Fig. 4A and B,
respectively), but decreased the levels of IκBα protein expression
(Fig. 4C) in MC3T3-E1 cells.
However, the enhanced effect of Ang II on the NF-κB activation was
completely abrogated by pre-treatment with olmesartan, NAC, or
PDTC, but not with PD123319 in osteoblasts (Fig. 4). Therefore, Ang II may promote ROS
production via its AT1R, and subsequently activate the NF-κB
signaling pathway, leading to enhanced MCP-1 expression in
osteoblasts.
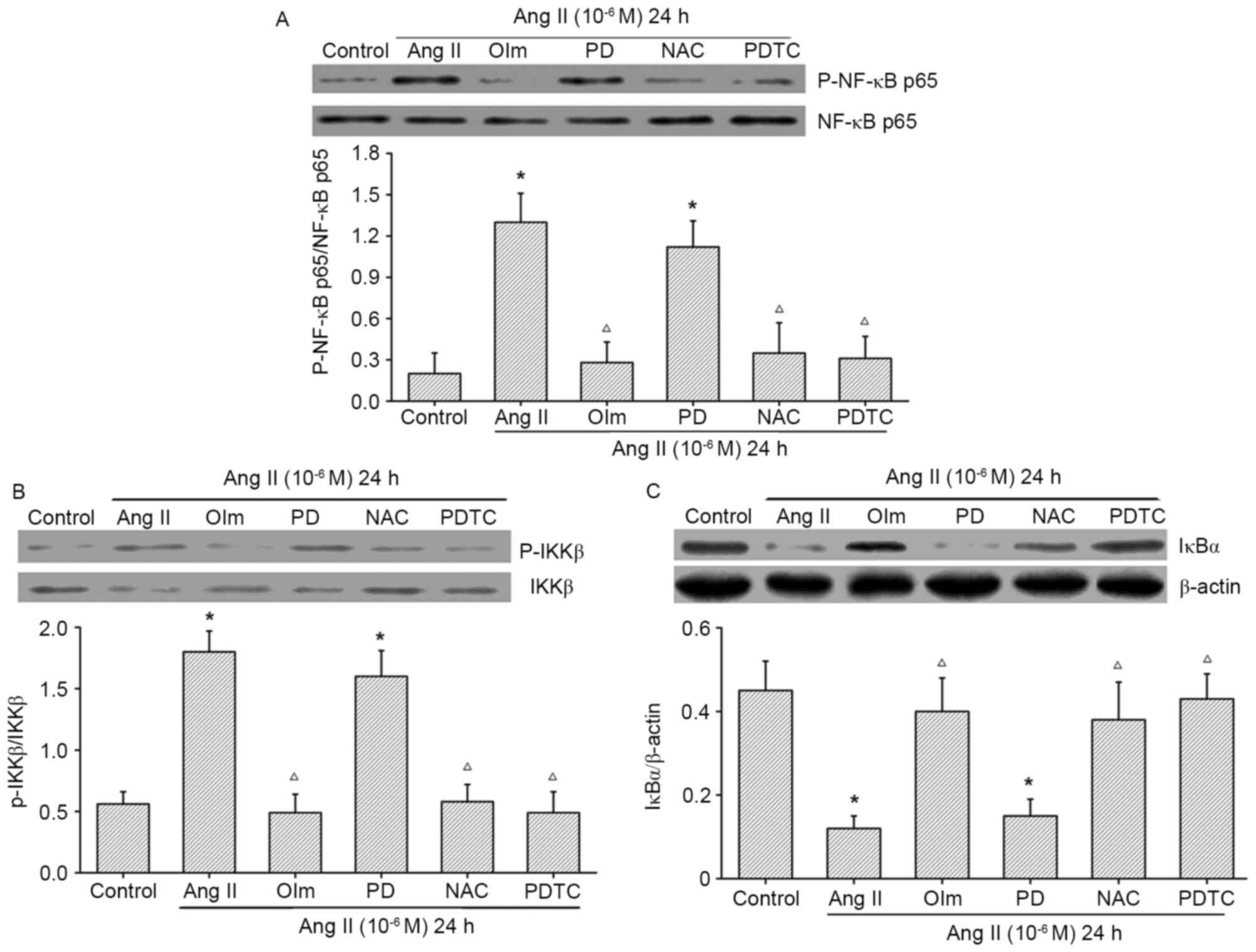 | Figure 4.Effect of Ang II on NF-κB activation
in osteoblasts. Osteoblasts were pre-treated with Olm (10 µM), PD
(10 µM), NAC (1 mM) or PDTC (50 µM) for 30 min, and exposed to Ang
II (10−6 M) for 24 h. Representative western blot images
and quantification of (A) NF-κBp65 phosphorylation, (B) IKKβ
phosphorylation and (C) IκBα expression, as assessed by western
blotting. Data are presented as the mean ± standard deviation.
*P<0.05 vs. control; ΔP<0.05 vs. Ang II. Olm,
olmesartan; PD, PD123319; NAC, N-acetylcysteine; PDTC, ammonium
pyrrolidine thiocarbamate; Ang II, angiotensin II; p,
phosphorylated; IKK, IκB kinase; NF, nuclear factor. |
Discussion
The present study examined the effect of Ang II on
MCP-1 expression and potential mechanisms underlying the action of
Ang II in osteoblasts. These data indicated that treatment with Ang
II significantly upregulated MCP-1 synthesis in osteoblasts, which
was abrogated by pre-treatment with the AT1R inhibitor olmesartan,
scavenger of free radicals (NAC), and the NF-κB inhibitor PDTC, but
not the AT2R antagonist PD123319. These data demonstrated that Ang
II enhances MCP-1 generation in MC3T3-E1 cells via its AT1R,
dependent on ROS/NF-κB signaling.
Ang II is a potent stimulator of osteoclastic bone
resorption (12,13). Although Ang II does not target
osteoclasts (4), it may stimulate
RANK and interleukin-6 expression in osteoblasts, which promotes
osteoclast maturation, leading to osteoporosis (3,4,14).
The present study demonstrated that Ang II enhances MCP-1
expression in osteoblasts, which previous studies have revealed
should promote osteoclast maturation and activation (15,16).
Therefore, these findings may provide novel insight into regulation
of Ang II on bone metabolism and homeostasis.
Ang II can bind to ATIR and AT2R, which are
expressed by osteoblasts (17,18).
The present study demonstrated that the enhanced effect of Ang II
on MCP-1 expression was completely mitigated by pre-treatment with
the AT1R antagonist olmesartan, but not with the AT2R antagonist
PD123319, indicating that Ang II enhances MCP-1 expression in
osteoblasts via AT1R, but not AT2R. These data were consistent with
a previous study (17), supporting
the notion that Ang II induces MCP-1 expression, dependent on the
AT1R (7). Furthermore, treatment
with Ang II was revealed to upregulate AT1R expression in
osteoblasts, consistent with previous findings (5,19).
Upregulated AT1R may serve as a positive feedback mechanism to
enhance the effect of Ang II on MCP-1 expression and other
bioactivities in osteoblasts.
Ang II may induce ROS production in vascular smooth
muscle cells (20). To understand
the molecular mechanisms underlying the action of Ang II, the
present study investigated the effect of Ang II on ROS production
in osteoblasts. Treatment with Ang II stimulated ROS production in
osteoblasts, which was abrogated by pre-treatment with an AT1R
antagonist and the ROS scavenger NCA. These data suggested that Ang
II stimulated ROS production in osteoblasts via AT1R, which was
similar to the results of our previous experiment (5). As high levels of ROS can activate
NF-κB signaling (21) and
pre-treatment with PDTC mitigates Ang II-induced MCP-1 expression
in osteoblasts, the present study examined the effect of Ang II on
NF-κB activation in osteoblasts. It was demonstrated that treatment
with Ang II enhanced NF-κB activation, evidenced by increased
ratios of NF-κBp65 and IKKβ phosphorylation and decreased levels of
IκBα expression in osteoblasts. The enhanced effect of Ang II on
the NF-κB activation was completely blocked by pre-treatment with
an AT1R antagonist, an NF-κB inhibitor and the ROS scavenger NCA in
osteoblasts. These novel data indicated that Ang II stimulated ROS
production via its AT1R, which activated NF-κB signaling, leading
to upregulated MCP-1 expression in osteoblasts. MCP-1 is important
for regulating osteoclast maturation activation, and the increased
levels of MCP-1 expression by osteoblasts may promote osteoclast
activation and osteoporosis. Therefore, the Ang II/AT1R/MCP-1 axis
may be a novel target for intervention of osteoporosis.
In conclusion, these data indicated that Ang II
stimulated ROS production via ATIR, and activated the NF-κB
signaling pathway, leading to upregulated MCP-1 expression in
osteoblasts. These findings may provide novel insights into
understanding the action of Ang II in regulating bone metabolism
and homeostasis, and may facilitate the development of novel
therapies for osteoporosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81601875, 81472067
and 81772329) and the Shandong Provincial Natural Science
Foundation, China (grant no. ZR2016HB33).
Glossary
Abbreviations
Abbreviations:
|
Ang II
|
angiotensin II
|
|
AT1R
|
angiotensin II type 1 receptor
|
|
AT2R
|
angiotensin II type 2 receptor
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
RAS
|
renin-angiotensin system
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Leung PS: The peptide hormone angiotensin
II: Its new functions in tissues and organs. Curr Protein Pept Sci.
5:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simões E, Silva AC and Teixeira MM: ACE
inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac
inflammation and fibrosis. Pharmacol Res. 107:154–162. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asaba Y, Ito M, Fumoto T, Watanabe K,
Fukuhara R, Takeshita S, Nimura Y, Ishida J, Fukamizu A and Ikeda
K: Activation of renin-angiotensin system induces osteoporosis
independently of hypertension. J Bone Miner Res. 24:241–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimizu H, Nakagami H, Osako MK, Hanayama
R, Kunugiza Y, Kizawa T, Tomita T, Yoshikawa H, Ogihara T and
Morishita R: Angiotensin II accelerates osteoporosis by activating
osteoclasts. FASEB J. 22:2465–2475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Zhang Y, Kou J, Wang C and Wang
K: Role of reactive oxygen species in angiotensin II: Induced
receptor activator of nuclear factor-κB ligand expression in mouse
osteoblastic cells. Mol Cell Biochem. 396:249–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohba T, Cole HA, Cates JM, Slosky DA, Haro
H, Ando T, Schwartz HS and Schoenecker JG: Bisphosphonates inhibit
osteosarcoma-mediated osteolysis via attenuation of tumor
expression of MCP-1 and RANKL. J Bone Miner Res. 29:1431–1445.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan Q, Yang XH and Cheng YX: Angiotensin
II stimulates MCP-1 production in rat glomerular endothelial cells
via NAD(P)H oxidase-dependent nuclear factor-kappa B signaling.
Braz J Med Biol Res. 42:531–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanifuji C, Suzuki Y, Geot WM, Horikoshi
S, Sugaya T, Ruiz-Ortega M, Egido J and Tomino Y: Reactive oxygen
species-mediated signaling pathways in angiotensin II-induced MCP-1
expression of proximal tubular cells. Antioxid Redox Signal.
7:1261–1268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Liu J, Pang X, Wang S, Wu D, Zhang
X and Feng L: Angiotensin II induces C-reactive protein expression
via AT1-ROS-MAPK-NF-κB signal pathway in hepatocytes. Cell Physiol
Biochem. 32:569–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JC, Zhao Y, Chen SJ, Long J, Jia QQ,
Zhai JD, Zhang Q, Chen Y and Long HB: AOPPs induce MCP-1 expression
by increasing ROS-mediated activation of the NF-κB pathway in rat
mesangial cells: Inhibition by sesquiterpene lactones. Cell Physiol
Biochem. 32:1867–1877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatton R, Stimpel M and Chambers TJ:
Angiotensin II is generated from angiotensin I by bone cells and
stimulates osteoclastic bone resorption in vitro. J Endocrinol.
152:5–10. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Grover M, Sibai T, Black J, Rianon
N, Rajagopal A, Munivez E, Bertin T, Dawson B, Chen Y, et al:
Losartan increases bone mass and accelerates chondrocyte
hypertrophy in developing skeleton. Mol Genet Metab. 115:53–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo L, Wang M, Zhang ZY, Hao L, Lou BY, Li
XY, Loo WT, Jin L and Cheung MN: Angiotensin II induces
interleukin-6 synthesis in osteoblasts through ERK1/2 pathway via
AT1 receptor. Arch Oral Biol. 56:205–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Qin L, Bergenstock M, Bevelock LM,
Novack DV and Partridge NC: Parathyroid hormone stimulates
osteoblastic expression of MCP-1 to recruit and increase the fusion
of pre/osteoclasts. J Biol Chem. 282:33098–33106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyamoto K, Ninomiya K, Sonoda KH,
Miyauchi Y, Hoshi H, Iwasaki R, Miyamoto H, Yoshida S, Sato Y,
Morioka H, et al: MCP-1 expressed by osteoclasts stimulates
osteoclastogenesis in an autocrine/paracrine manner. Biochem
Biophys Res Commun. 383:373–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Izu Y, Mizoguchi F, Kawamata A, Hayata T,
Nakamoto T, Nakashima K, Inagami T, Ezura Y and Noda M: Angiotensin
II type 2 receptor blockade increases bone mass. J Biol Chem.
284:4857–4864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakai K, Kawato T, Morita T, Iinuma T,
Kamio N, Zhao N and Maeno M: Angiotensin II induces the production
of MMP-3 and MMP-13 through the MAPK signaling pathways via the
AT(1) receptor in osteoblasts. Biochimie. 95:922–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu JJ, Li DL, Zhou J, Sun L, Zhao M, Kong
SS, Wang YH, Yu XJ, Zhou J and Zang WJ: Acetylcholine prevents
angiotensin II-induced oxidative stress and apoptosis in H9c2
cells. Apoptosis. 16:94–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruder-Nascimento T, Chinnasamy P,
Riascos-Bernal DF, Cau SB, Callera GE, Touyz RM, Tostes RC and
Sibinga NE: Angiotensin II induces Fat1 expression/activation and
vascular smooth muscle cell migration via Nox1-dependent reactive
oxygen species generation. J Mol Cell Cardiol. 66:18–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X, Wang Y and Gao G: High glucose
induces rat mesangial cells proliferation and MCP-1 expression via
ROS-mediated activation of NF-κB pathway, which is inhibited by
eleutheroside E. J Recept Signal Transduct Res. 36:152–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|