Introduction
Hypercholesterolemia is implicated as a risk factor
for various health problems (1).
To date, the association between cholesterol and dementia,
cognitive decline or impairment is not fully understood (2). In the last decade, there is growing
evidence that hypercholesterolemia and dietary cholesterol are
linked to an increased risk of cognitive decline or impairment that
do not meet diagnostic criteria and dementias with different
etiologies (3,4). Studies examining the potential
mechanisms linking cholesterol to neurotoxicity have identified
amyloidogenic proteins to be involved in neurodegenerative diseases
(5–7). However, clinical studies using
statins to lower cholesterol for therapeutic management of
neurodegeneration revealed contradictory results (8). In addition, the role of cholesterol
on the regulation of amyloid-β (Aβ) generation and aggregation
remains elusive. To gain insight into the association between
cholesterol and cognitive decline, the present study focused on the
oxidized derivatives of cholesterol, including
27-hydroxycholesterol (OHC), 24S-OHC, 7α-OHC and 7β-OHC. It has
been reported that elevated oxysterols in the plasma have cytotoxic
and pro-apoptotic effects on neurons (9,10).
Furthermore, oxysterols can pass through the blood brain barrier
into the central nervous system (11) and increase Aβ production via
upregulation of the amyloidogenic pathway (12). A previous study from our group has
also demonstrated that 27-OHC serves a negative role on learning
and memory abilities (13).
However, it remains unknown whether cholesterol affects the
learning and memory ability as well as related biomarkers via
oxysterols. The aim of the present study was, therefore, to
investigate the effects of dietary cholesterol on plasma levels of
cholesterol and oxysterols and their potential effect on Aβ
production in rats, to further elucidate the role of dietary and
blood cholesterol in learning and memory abilities and to provide
scientific evidence and new ideas for therapeutic and preventive
strategies of cognitive disorders.
Materials and methods
Animals and experimental design
A total of 35 10-month old male Sprague-Dawley rats
(SPF class, 450–600 g) were provided by the Academy of Military
Medical Sciences (Beijing, China) and housed 1 per cage in a room
of controlled illumination (12-h light/dark cycle), humidity
(30–50%), and temperature (18–22°C). A standard rodent diet and
water were accessed ad libitum. Following 1 week's
acclimation, the rats were randomly divided into 5 groups (n=7 in
each group) which were respectively fed 0.015 (control diet group),
0.05, 0.2, 0.5 and 1.6% cholesterol-containing diets for 8 weeks.
The detailed composition of the diets is listed in Table I. Experiments were designed and
conducted in accordance with the Chinese Committee of Experimental
Animal Supervision and the guidelines of Animal Ethics Committee of
Capital Medical University (Beijing, China).
 | Table I.Ingredients of the experimental
diets. |
Table I.
Ingredients of the experimental
diets.
|
| Dietary
cholesterol |
|---|
|
|
|
|---|
| Ingredient (g) | 0.02% | 0.05% | 0.20% | 0.50% | 1.60% |
|---|
| Sucrose | 530 | 530 | 530 | 530 | 530 |
| Skimmed milk | 40 | 40 | 40 | 40 | 40 |
| Casein | 230 | 230 | 230 | 230 | 230 |
| Cystine | 2 | 2 | 2 | 2 | 2 |
| Lard | 92 | 92 | 92 | 92 | 92 |
| Nut oils | 8 | 8 | 8 | 8 | 8 |
| Salt-mixture | 50 | 50 | 50 | 50 | 50 |
| Vitamin mix | 2 | 2 | 2 | 2 | 2 |
| Cellulose | 23 | 23 | 23 | 23 | 23 |
| Yeast | 23 | 23 | 23 | 23 | 23 |
| Cholesterol | 0.08 | 0.43 | 1.93 | 4.93 | 15.93 |
| Total | 1,000.08 | 1,000.43 | 1,001.93 | 1,004.93 | 1,015.93 |
Plasma and tissue collection
Body weight was measured and tail vein blood was
collected once every two weeks to evaluate levels of cholesterol,
Aβ1-40, Aβ1-42 and oxysterols (27-OHC, 24S-OHC, 7α-OHC and 7β-OHC).
Following 8-week dietary intervention and 24 h of fasting from the
last feeding, all of the rats were weighed, deeply anesthetized
with 5% chloral hydrate (400 mg/kg) and dissected. Blood samples
were collected and fresh tissue, including brain, was removed,
weighed and subsequently frozen at −80°C until use. Serum and
plasma were obtained by centrifugation of blood samples at 3,000 ×
g for 10 min at 4°C and stored at −80°C until use.
Biochemical analysis of blood and
brain samples
Cholesterol, Aβ1-40 and Aβ1-42 levels in blood and
brain samples were determined using commercial kits (Kexin Biotech
Co., Ltd. Shanghai, China) on a Hitachi 7250 automatic clinical
analyzer (Hitachi, Ltd., Tokyo, Japan), following the
manufacturers' instructions. All samples were analyzed in
duplicate.
Measurement of oxysterols in blood and
brain samples
Plasma and brain levels of oxysterols were measured
using High Performance Liquid Chromatography-Mass Spectrometry
(HPLC-MS) as described by Burkard et al (14), with slight modifications. The C-7
position of cholesterol is liable to autoxidize. In order to
prevent potential autoxidation during sample preparation, 50 µg
butylated hydroxytoluene (BHT) was added per ml plasma. During the
collection and preparation of blood samples, standard procedures
were conducted to avoid repeated freeze-thaw cycles to minimize the
impact of the plasma concentration of 7α-OHC and 7β-OHC. Briefly,
0.1 ml of plasma sample was transferred to a screw-capped vial.
Then, 100 ng 19-OHC and 1.5 ml of 1 M ethanolic sodium hydroxide
were added to the vial, serving as internal standard and alkaline
hydrolysis, respectively. Alkaline hydrolysis was performed in a
water bath at 50°C for 2 h. Phosphoric acid (50%) and 1 ml of
phosphate buffer were added to the samples for pH adjustment to 7.
The supernatant was harvested following centrifugation at 1,000 × g
for 5 min at 4°C and then applied to the C18 cartridges for
solid-phase extraction. The eluted substances were dried at 30°C
and dissolved in 100 µl of methanol for future testing. Total lipid
was extracted from approximately 20–70 mg of the brain on ice by
homogenation in 2 ml of ice-cold chloroform:methanol (2:1 v/v),
containing 0.005% (v/v) BHT as an antioxidant and 19-OHC as an
internal standard. The extract was ultrasonicated for 15 min at
room temperature, centrifuged at 5,000 × g for 5 min at 4°C and the
supernatant collected. The supernatant was evaporated under
nitrogen and the residue was redissolved in 1 ml of hydrolyzate
(10% KOH and methanol) overnight. The hydrolyzate was extracted
with 0.5 ml of water and 2 ml of ether and then washed twice with
distilled water. The extracts were taken to dryness under nitrogen
at room temperature and then 1 ml of methanol:water (9:1 v/v) was
added. The mixture was centrifuged at 2,400 × g at 4°C for 15 min,
and the supernatant was collected (15). A total of 40 µl sample was injected
into the HPLC-MS system. HPLC with an Agilent G1312B HPLC pump and
an Agilent C18 column (0.35 µm bead size; 4.6×250 mm; Agilent
Technologies, Inc., Santa Clara, CA, USA) was used for the
measurement of oxysterols. For the first 10 min, the mobile phase
consisted of water:acetonitrile (90:10 v/v) with a corresponding
flow rate of 0.25 ml/min. The eluents were then linearly changed in
a gradient system to water:acetonitrile (10:90 v/v) within 5 min.
Afterwards, the eluents were again changed to the previous ratio
for 2 min. Quantification of oxysterols was performed using the
multiple reaction monitoring mode. The ionization mode was positive
atmospheric pressure chemical ionization. The gas temperature and
the nebulizer were maintained at 325°C and 4,000 pounds per square
inch (psi) respectively. The discharge current was fixed at 5 µA,
and the capillary voltage was set at 4,000 V. The vaporizer
temperature was held at 450°C, and the sheath gas pressure was
maintained at 35 psi.
Statistical analysis
Results were expressed as means ± standard
deviation. One-way analysis of variance followed by the least
significance difference post hoc test was used to evaluate the
significance of differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Total cholesterol and body weight
Body weight and plasma levels of cholesterol pre-
and post-intervention are summarized in Table II. Dietary intervention for 8
weeks induced a diet-dependent increase in plasma cholesterol
levels (P<0.01; Table II), but
no significant change was observed in body weight (P>0.05;
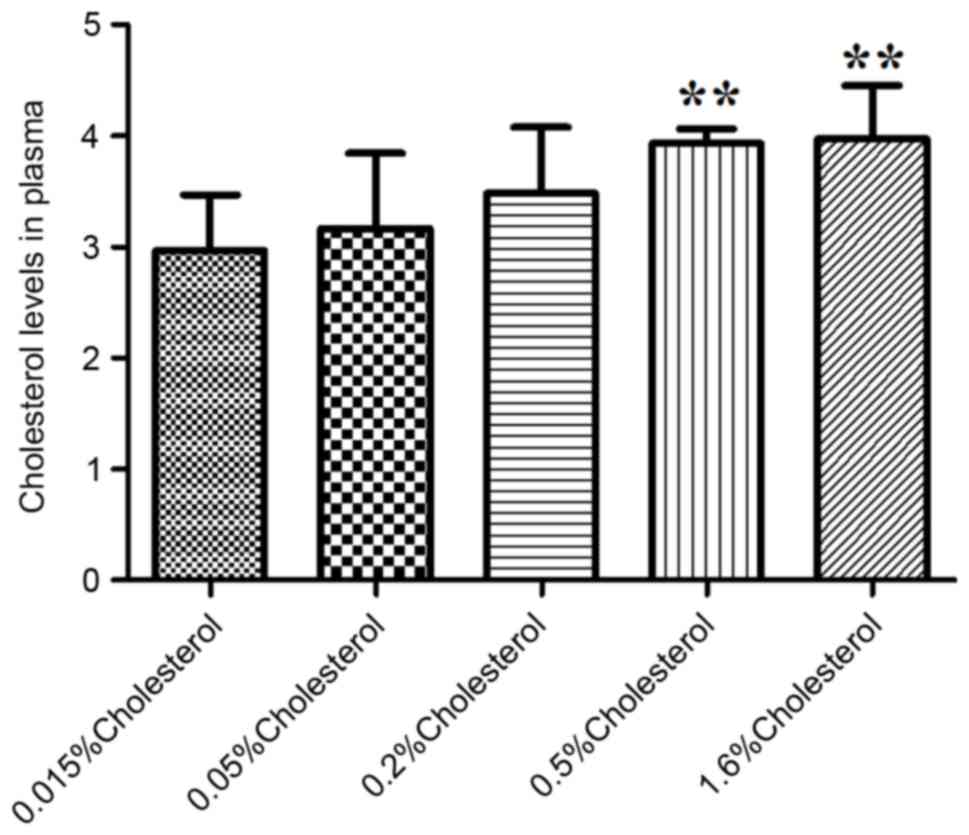
Table II). Plasma cholesterol
levels were significantly elevated following 8 weeks of dietary
intervention in the 0.5 and 1.6% cholesterol-containing diet groups
compared with the control diet group (Fig. 1).
 | Table II.Body weight and plasma cholesterol
levels in rats pre- and post-intervention. |
Table II.
Body weight and plasma cholesterol
levels in rats pre- and post-intervention.
|
| Dietary
cholesterol |
|---|
|
|
|
|---|
| Variable | 0.015% (n=7) | 0.05% (n=7) | 0.20% (n=7) | 0.50% (n=7) | 1.60% (n=7) | P-value |
|---|
| Pre-intervention |
|
|
|
|
|
|
| Body
Weight (g) |
629.0±45.9 |
630.3±34.3 |
631.7±48.8 |
606.9±49.8 |
614.9±43.9 | 0.79 |
|
Cholesterol (mmol/l) |
3.09±0.45 |
3.20±0.69 |
3.21±0.45 |
3.10±0.49 |
3.13±0.22 | 0.98 |
|
Post-intervention |
|
|
|
|
|
|
| Body
Weight (g) |
795.1±45.4 |
772.9±59.7 |
726.3±51.9 |
728.0±44.5 |
734.7±97.4 | 0.18 |
|
Cholesterol (mmol/l) |
2.96±0.51 |
3.17±0.68 |
3.48±0.59 |
3.93±0.14 |
3.97±0.48 | <0.01 |
Oxysterol levels in plasma and
brain
No significant differences were observed in
oxysterol levels among the five diet groups prior to intervention,
as illustrated in Table III.
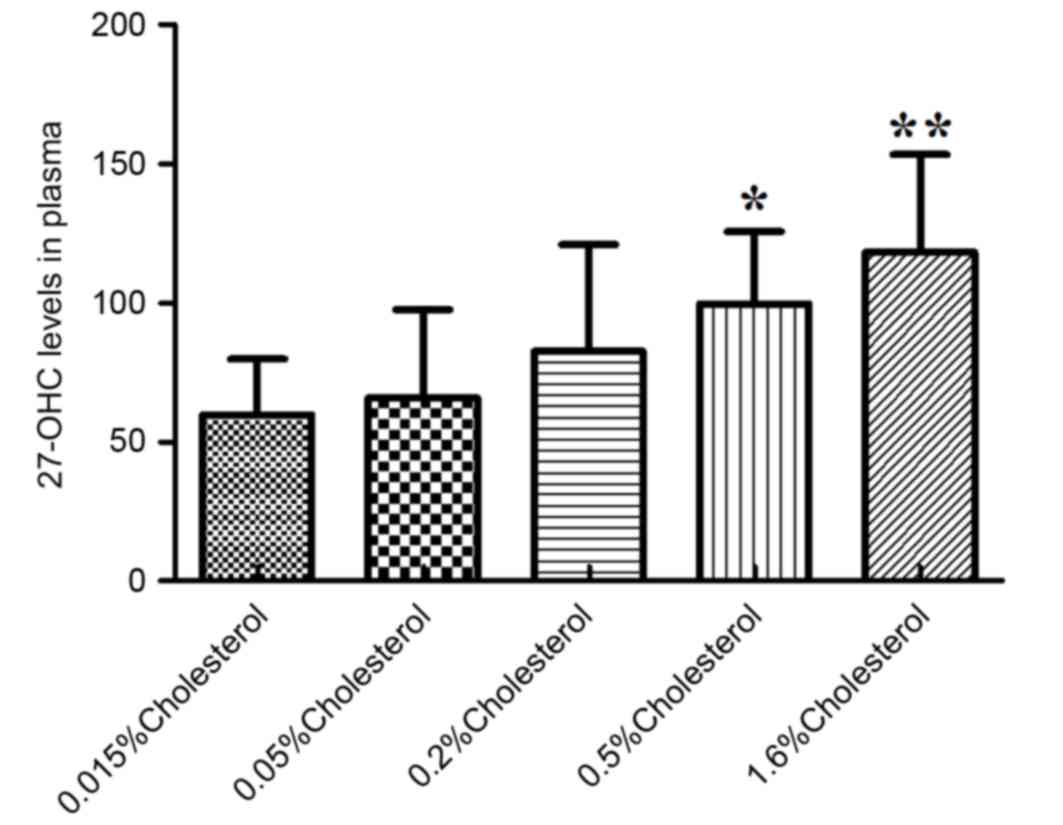
Following 8 weeks of diet intervention, analysis of oxysterol
levels in the plasma of rats revealed significant differences in
the levels of 7α-OHC (P<0.01; Table III), 7β-OHC (P<0.01; Table III) and 27-OHC (P<0.01;
Table III), but no significant
changes in 24S-OHC levels (P=0.56; Table III). At week 8 post-intervention,
the plasma levels of 27-OHC in the diet groups containing 0.5 and
1.6% cholesterol were significantly elevated compared with the
control diet group (Fig. 2).
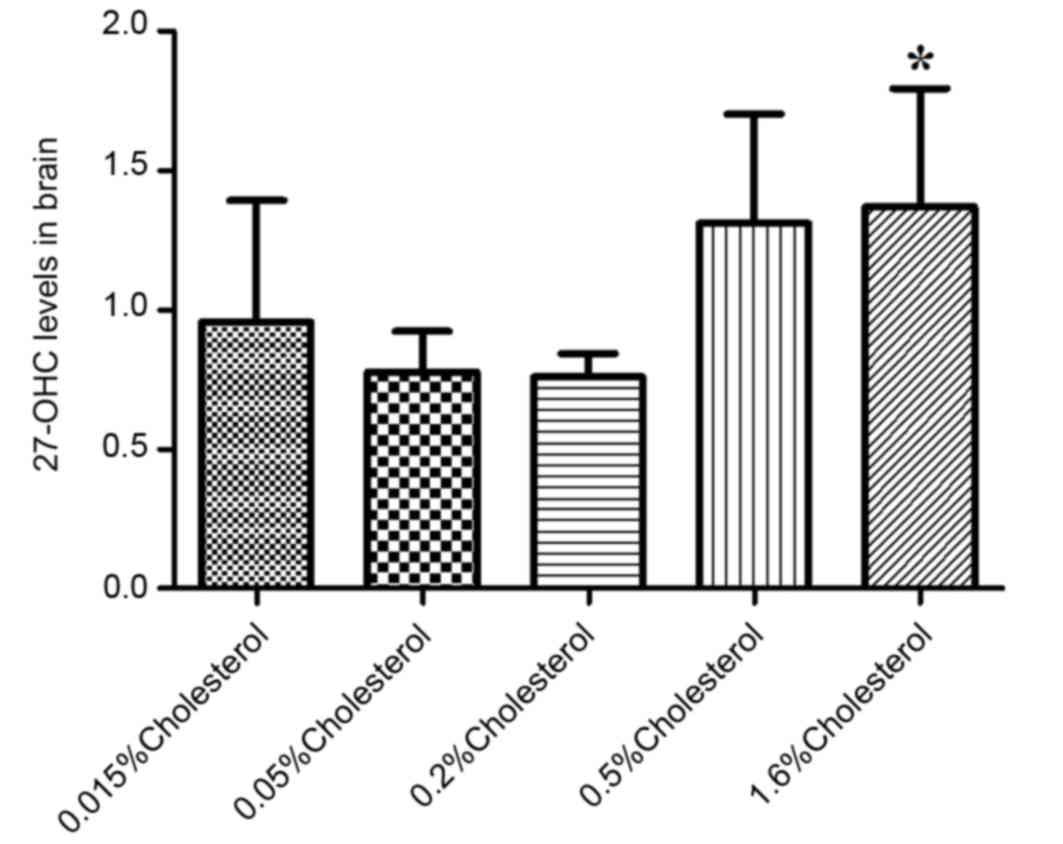
Similarly, the brain levels of 27-OHC in the 1.6%
cholesterol-containing diet group were significantly elevated
compared with the control diet group (Fig. 3).
 | Table III.Plasma and brain levels of oxysterols
in rats pre- and post-intervention. |
Table III.
Plasma and brain levels of oxysterols
in rats pre- and post-intervention.
|
| Dietary
cholesterol |
|---|
|
|
|
|---|
| Variable | 0.015% (n=7) | 0.05% (n=7) | 0.20% (n=7) | 0.50% (n=7) | 1.60% (n=7) | P-value |
|---|
| Pre-intervention |
|
|
|
|
|
|
|
Plasma |
|
|
|
|
|
|
| 27-OHC
(ng/ml) |
80.6±42.6 |
73.7±38.3 |
73.2±44.8 |
66.0±30.5 |
59.8±30.7 | 0.87 |
| 24S-OHC
(ng/ml) |
48.4±25.5 |
44.2±23.0 |
43.9±26.9 |
39.6±18.3 |
35.9±18.4 | 0.86 |
| 7β-OHC
(ng/ml) |
63.6±20.0 |
59.1±21.8 |
43.4±15.6 |
60.0±31.3 |
43.6±16.9 | 0.27 |
| 7α-OHC
(ng/ml |
53.9±12.3 |
44.4±6.7 |
52.5±19.0 |
52.7±18.8 |
47.4±12.2 | 0.70 |
|
Post-intervention |
|
|
|
|
|
|
|
Plasma |
|
|
|
|
|
|
| 27-OHC
(ng/ml) |
59.8±20.0 |
65.9±31.7 |
82.7±38.2 |
99.7±26.0 |
118.2±35.1 | <0.01 |
| 24S-OHC
(ng/ml) |
55.5±18.4 |
51.4±10.7 |
51.9±8.6 |
59.1±14.4 |
60.6±7.6 | 0.56 |
| 7β-OHC
(ng/ml) |
78.7±21.2 |
85.6±54.9 |
75.0±19.7 |
233.4±144.8 |
195.6±96.5 | <0.01 |
| 7α-OHC
(ng/ml) |
108.9±35.4 |
120.5±91.6 |
102.8±32.9 |
366.9±241.3 |
303.8±160.8 | <0.01 |
|
Brain |
|
|
|
|
|
|
| 27-OHC
(ng/ml) |
0.96±0.44 |
0.78±0.15 |
0.76±0.08 |
1.31±0.39 |
1.37±0.42 | <0.01 |
| 24S-OHC
(ng/ml) |
14.28±1.54 |
15.28±3.37 |
15.23±1.87 |
16.11±1.90 |
14.43±3.20 | 0.66 |
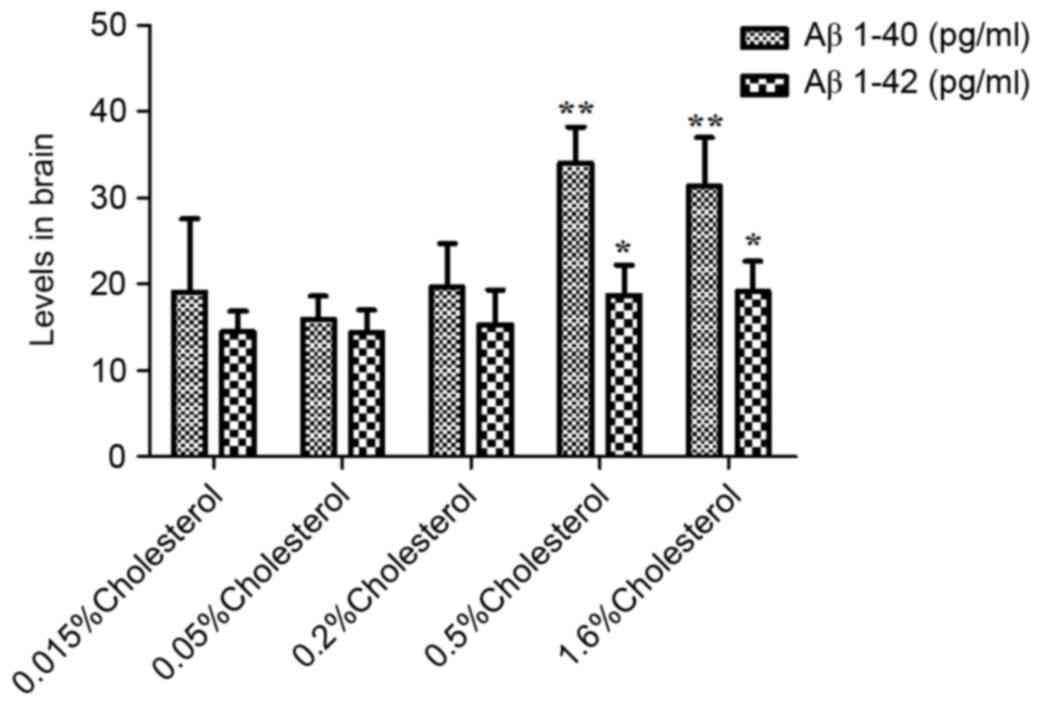
Aβ levels in plasma and brain
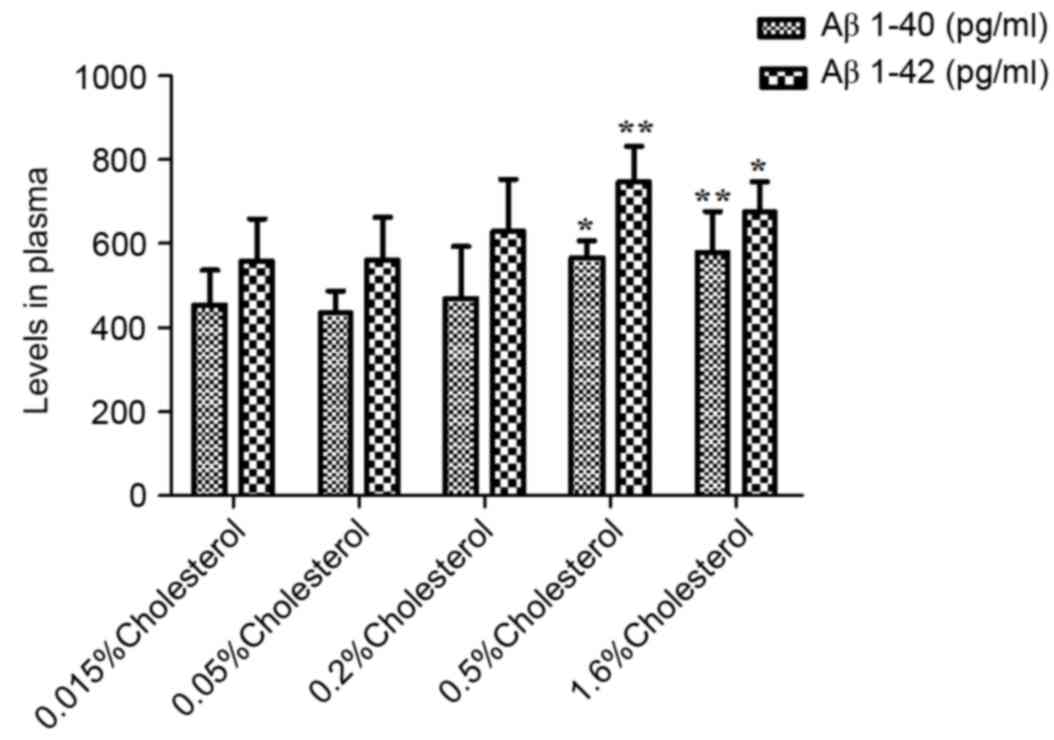
As illustrated in Table IV, the plasma levels of peptides
Aβ1-40 and Aβ1-40 in the five groups were not significantly
different prior to dietary intervention. However, at week 8
post-intervention, both plasma (Table
IV and Fig. 4) and brain
(Table IV and Fig. 5) levels were significantly elevated
in the 0.5 and 1.6% cholesterol-containing diet groups compared
with the control group.
 | Table IV.Plasma and brain levels of Ab1-40 and
Ab1-42 in rats pre- and post-intervention. |
Table IV.
Plasma and brain levels of Ab1-40 and
Ab1-42 in rats pre- and post-intervention.
|
| Dietary
cholesterol |
|---|
|
|
|
|---|
| Variable | 0.015% (n=7) | 0.05% (n=7) | 0.20% (n=7) | 0.50% (n=7) | 1.60% (n=7) | P-value |
|---|
|
Pre-intervention |
|
|
|
|
|
|
|
Plasma |
|
|
|
|
|
|
| Aβ1-40
(pg/ml) |
449.9±67.0 |
502.1±51.7 |
480.1±41.7 |
467.9±38.0 |
461.5±44.8 | 0.36 |
| Aβ1-42
(pg/ml) |
431.9±49.2 |
448.5±52.5 |
415.9±58.7 |
412.4±46.3 |
412.6±59.5 | 0.66 |
|
Post-intervention |
|
|
|
|
|
|
|
Plasma |
|
|
|
|
|
|
| Aβ1-40
(pg/ml) |
455.0±80.5 |
434.6±52.2 |
469.1±123.6 |
565.9±40.9 |
579.8±96.9 | <0.01 |
| Aβ1-42
(pg/ml) |
558.9±99.2 |
560.4±101.7 |
629.0±123.5 |
746.9±84.2 |
674.9±72.5 | <0.01 |
|
Brain |
|
|
|
|
|
|
| Aβ1-40
(pg/ml) |
19.06±8.53 |
15.90±2.69 |
19.65±5.06 |
34.01±4.18 |
31.36±5.58 | <0.01 |
| Aβ1-42
(pg/ml) |
14.49±2.37 |
14.38±2.58 |
15.29±4.06 |
18.69±3.55 |
19.13±3.49 | 0.02 |
Discussion
The main finding of the present study is that the
levels of cholesterol and 27-OHC in the peripheral blood were
increased by dietary cholesterol intervention in a dose-dependent
manner. In addition, 7α-OHC and 7β-OHC levels in the blood and
27-OHC in the brain were elevated following high-cholesterol diets
compared with control diet, while 24S-OHC levels were not affected
by dietary cholesterol in SD rats. Furthermore, blood Aβ1-40 and
Aβ1-42 levels, commonly involved in Alzheimer's disease (AD)
pathogenesis, were also upregulated as a result of accumulated
cholesterol. Finally, high-cholesterol diet resulted in elevated
blood 27-OHC levels as well as elevated Aβ1-40 and Aβ1-42 levels,
suggesting that 27-OHC may be linked to Aβ accumulation in the
brain.
A previous study has also investigated the effect of
dietary cholesterol on plasma cholesterol levels, and reported a
dose-dependent increase of plasma cholesterol concentrations
following 6 months of high cholesterol-containing diet (15). However, the effect of cholesterol
intake on production and distribution of oxysterols remains unknown
and the present study for the first time investigated these
effects. 27-OHC is catalyzed by the cytochrome P450 family 27
subfamily A member 1 (CYP27A1) enzyme, which is expressed in the
liver (16). Unlike 27-OHC, 7β-OHC
is generated by non-enzymatic oxidation, whereas 7α-OHC is
generated by both non-enzymatic and enzymatic oxidation, which is
catalyzed by cytochrome P450 family 7 subfamily A member 1
(CYP7A1). It is hypothesized that increased cholesterol levels
contribute to 27-OHC, 7α-OHC and 7β-OHC production (17). 24S-OHC is converted from
cholesterol via the cytochrome P450 family 46 subfamily A member 1
(CYP46A1) enzyme, which is primarily expressed in neurons (18). Cholesterol in the central nervous
system (CNS) is not directly influenced by cholesterol in the
peripheral blood. Consequently, 24S-OHC levels in brain and blood
are less likely affected by dietary cholesterol, as demonstrated in
the present results.
There are accumulating studies regarding the
association of cholesterol and Aβ production and aggregation with
controversial results. Some studies from several animal species
suggest that Aβ accumulation in the brain is promoted by
high-cholesterol diet (19). By
contrast, another study reported that Aβ1-42 levels are negatively
correlated with plasma cholesterol in APP/PS1 transgenic mice
receiving a cholesterol diet (20). The age of the animals examined is
considered as the main cause of these controversies, because
cholesterol may serve different roles during the development and
maturation of the brain. Therefore, in the present study,
10-month-old SD rats instead of young rats were selected for
experiments, in order to avoid confounding effects of cholesterol
on brain development. The present results demonstrated that Aβ1-40
and Aβ1-42 levels, in both plasma and brain, were significantly
affected by a cholesterol-rich diet in rats.
Cholesterol cannot freely cross into the brain,
owing to the impermeability of the blood-brain barrier (BBB).
Subsequently, brain cholesterol metabolism is separated from the
peripheral metabolism (21). The
question as to how peripheral cholesterol influences Aβ generation
and accumulation in the brain remains to be answered. A previous
study from our group focused on the impact of cholesterol
metabolism on the rats' brains and revealed that 27-OHC was
involved in learning and memory disorders (13). Of note, the amyloidogenic pathway
of amyloid precursor protein processing is upregulated in the human
neuroblastoma cell line SH-SY5Y following treatment with 27-OHC
(12). Based on the ability of
27-OHC to cross the BBB, the present results indicated that
accumulating 27-OHC in the peripheral blood, induced by
cholesterol-enriched diets, may contribute to increased 27-OHC and
Aβ generation in the CNS, and may subsequently be involved in the
pathogenesis of AD or cognitive decline.
In conclusion, the present study suggested that
cholesterol-enriched diets induced increased Aβ production in the
plasma and the brain and that this effect may be mediated via
oxysterols, especially 27-OHC. The present study indicated that it
may be possible to prevent the generation of Aβ by targeting
27-OHC. Deciphering the molecular mechanism involving oxysterols
may lead to novel therapeutic strategies for neurodegenerative
diseases in the future.
Acknowledgements
The present study was supported by the State Key
Program of the National Natural Science Foundation of China (grant
no. 81330065).
References
|
1
|
van Vliet P: Cholesterol and late-life
cognitive decline. J Alzheimers Dis. 30 Suppl 2:S147–S162.
2012.PubMed/NCBI
|
|
2
|
Wood WG, Li L, Müller WE and Eckert GP:
Cholesterol as a causative factor in Alzheimer's disease: A
debatable hypothesis. J Neurochem. 129:559–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anstey KJ, Lipnicki DM and Low LF:
Cholesterol as a risk factor for dementia and cognitive decline: A
systematic review of prospective studies with meta-analysis. Am J
Geriatr Psychiatry. 16:343–354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purnell C, Gao S, Callahan CM and Hendrie
HC: Cardiovascular risk factors and incident Alzheimer disease: A
systematic review of the literature. Alzheimer Dis Assoc Disord.
23:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu X and Zheng J: Cholesterol promotes the
interaction of Alzheimer β-amyloid monomer with lipid bilayer. J
Mol Biol. 421:561–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drolle E, Gaikwad RM and Leonenko Z:
Nanoscale electrostatic domains in cholesterol-laden lipid
membranes create a target for amyloid binding. Biophys J.
103:L27–L29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Scala C, Chahinian H, Yahi N, Garmy N
and Fantini J: Interaction of Alzheimer's β-amyloid peptides with
cholesterol: Mechanistic insights into amyloid pore formation.
Biochemistry. 53:4489–4502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McGuinness B, O'Hare J, Craig D, Bullock
R, Malouf R and Passmore P: Statins for the treatment of dementia.
Cochrane Database Syst Rev: CD007514. 2010. View Article : Google Scholar
|
|
9
|
Daugvilaite V, Arfelt KN, Benned-Jensen T,
Sailer AW and Rosenkilde MM: Oxysterol-EBI2 signaling in immune
regulation and viral infection. Eur J Immunol. 44:1904–1912. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olkkonen VM, Beaslas O and Nissilä E:
Oxysterols and their cellular effectors. Biomolecules. 2:76–103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Björkhem I, Cedazo-Minguez A, Leoni V and
Meaney S: Oxysterols and neurodegenerative diseases. Mol Aspects
Med. 30:171–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prasanthi JR, Huls A, Thomasson S,
Thompson A, Schommer E and Ghribi O: Differential effects of
24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid
precursor protein levels and processing in human neuroblastoma
SH-SY5Y cells. Mol Neurodegener. 4:12009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang DD, Yu HL, Ma WW, Liu QR, Han J,
Wang H and Xiao R: 27-Hydroxycholesterol contributes to disruptive
effects on learning and memory by modulating cholesterol metabolism
in the rat brain. Neuroscience. 300:163–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burkard I, Rentsch KM and von Eckardstein
A: Determination of 24S- and 27-hydroxycholesterol in plasma by
high-performance liquid chromatography-mass spectrometry. J Lipid
Res. 45:776–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karu K, Hornshaw M, Woffendin G, Bodin K,
Hamberg M, Alvelius G, Sjövall J, Turton J, Wang Y and Griffiths
WJ: Liquid chromatography-mass spectrometry utilizing multi-stage
fragmentation for the identification of oxysterols. J Lipid Res.
48:976–987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zurkinden L, Solcà C, Vögeli IA, Vogt B,
Ackermann D, Erickson SK, Frey FJ, Sviridov D and Escher G: Effect
of Cyp27A1 gene dosage on atherosclerosis development in
ApoE-knockout mice. FASEB J. 28:1198–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito Y and Noguchi N: 7-Hydroxycholestrol
as a possible biomarker of cellular lipid peroxidation: Difference
between cellular and plasma lipid peroxidation. Biochem Biophys Res
Commun. 446:741–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shafaati M, Olin M, Båvner A, Pettersson
H, Rozell B, Meaney S, Parini P and Björkhem I: Enhanced production
of 24S-hydroxycholesterol is not sufficient to drive liver X
receptor target genes in vivo. J Intern Med. 270:377–387. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YL, Wang LM, Chen Y, Gao JY, Marshall
C, Cai ZY, Hu G and Xiao M: Changes in astrocyte functional markers
and β-amyloid metabolism-related proteins in the early stages of
hypercholesterolemia. Neuroscience. 316:178–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wirths O, Thelen K, Breyhan H, Luzón-Toro
B, Hoffmann KH, Falkai P, Lütjohann D and Bayer TA: Decreased
plasma cholesterol levels during aging in transgenic mouse models
of Alzheimer's disease. Exp Gerontol. 41:220–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marwarha G and Ghribi O: Does the
oxysterol 27-hydroxycholesterol underlie Alzheimer's
disease-Parkinson's disease overlap? Exp Gerontol. 68:13–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|