Introduction
As a result of current lifestyle and dietary habits,
the morbidity of atherosclerotic coronary heart disease (CHD) is
constantly increasing, placing CHD among the most serious threats
to human health worldwide (1). In
2008, in the Global Health Statistical Report of the World Health
Organization (WHO), cardiovascular and cerebrovascular diseases
were reported to be the second leading cause of human mortality,
following malignant tumors (2). In
May 2014, the WHO updated the evaluation report regarding global
health, indicating that ischemic heart disease, apoplexy, chronic
obstructive pulmonary disease and lower respiratory infection are
the four major causes of human mortality (3). Globally, ~7 million people succumb to
ischemic heart disease and ~50% of patients succumb to myocardial
ischemia/reperfusion (I/R) injury. The morbidity of myocardial I/R
injury increases every year, thus posing a serious threat to global
health and making myocardial infarction one of the most important
public health concerns currently (4).
Apoptosis is a type of programmed cell death; it is
a highly-regulated process involving specific mechanisms, that is
activated in mammalian cells under certain conditions. During
apoptosis, endogenous DNA-degrading enzymes are activated resulting
in cell death (5). Somatic cell
death is a result of necrosis or apoptosis (6); these two types of cell death differ
in their functions, molecular mechanisms and biochemistry.
Under ischemic conditions, the enzymatic activity of
myocardial cells is severely compromised; as a result, during
reperfusion, the restoration of oxygen supply further damages
myocardial cells (7). During
reperfusion, the generation of reactive oxygen species (ROS) is
enhanced (7). ROS can destroy the
structure of cell membranes through lipid peroxidation (8), thus causing an increase in membrane
permeability, resulting in cellular edema. The structure of the
mitochondrial membrane is also compromised, thus damaging
mitochondrial function and impairing energy production, further
aggravating cellular injury. ROS can oxidize proteins and impair
their functions (9). When membrane
protein functions are impaired, the ion exchange processes are
inhibited; this is one of the primary causes of intracellular
Ca2+ overload. ROS promote the chemotaxis of white blood
cells; they also activate various enzymes and promote the release
of prostaglandins and leukotrienes, thus further aggravating tissue
damage (9). In addition, ROS can
destroy nucleic acids and chromosomes; the resulting DNA damage
also activates the pathways leading to cell apoptosis or necrosis,
thus further promoting myocardial I/R injury (10).
Inflammatory processes are among the most important
mechanisms implicated in the pathophysiology of myocardial I/R
injury. During inflammatory reactions, the infiltration of
polymorphonuclear neutrophils (PMNs) is critical (11). In I/R injury, microvascular
endothelial cells and the blood vessel endothelium are damaged,
attracting the adhesion of PMNs in the endothelial tissue (12). The inflammatory reaction is
initiated by PMNs attachment to the blood vessel endothelium
(12). This results in the
increased generation of oxygen free radicals and the activation of
various proteases, thus altering vascular permeability (13,14).
Once activated, PMNs change shape, making it difficult to penetrate
through the tissues; thus, they are concentrated in the blood
vessel endothelium, resulting in tissue damage and endothelial
dysfunction (12,13). Activated PMBs also secrete
proinflammatory cytokines, including interleukin (IL)-1, IL-6 and
tumor necrosis factor (TNF)-α. Cytokines further activate the
inflammatory response through nuclear factor-κB signaling,
potentiating the release of inflammatory mediators, thus forming a
vicious cycle, and promoting the development and progression of I/R
injury (12).
Anisodamine is a cholinergic and α1
adrenergic receptor antagonist, which has been used in China for
the treatment of coronary heart disease, for the prevention and
treatment of angina, pulmonary hypertension and chronic heart
failure, in combination with cardiac glycosides or diuretic agents
(15,16). The present study aimed to
investigate the putative cardioprotective effects of anisodamine
against myocardial I/R injury, and to explore the underlying
molecular mechanisms, using an in vivo rat model of I/R
injury.
Materials and methods
Animal experiments and ethical
approval
Adult male Sprague-Dawley rats (n=24; weight,
220–250 g; 8–10 weeks) were provided by the Experimental Animal
Center of the Third Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China). All experimental protocols used in
the present study were approved by Committee on Animal Research and
Ethics of Guangzhou Medical University (Guangzhou, China). The rats
were maintained in a temperature-controlled facility, at 22±2°C,
with a relative humidity of 55±10%, under a 12-h light/dark cycle,
and food and water ad libitum.
Myocardial I/R
All rats were randomly distributed into three groups
(8 rats/group): Control group (sham-operated group); MI/R group
(myocardial ischemia/reperfusion injury group); and ANI group
(anisodamine treatment group). Rats were anesthetized with sodium
pentobarbital (30 mg/kg, intraperitoneally), and the following
surgical procedure was performed: The trachea was cannulated with a
PE-90 catheter and rats were ventilated with O2 and
CO2, maintaining a tidal volume of 0.8–1.2 ml. Following
a left thoracotomy, the left anterior descending was exposed and
occluded by ligation with a 5–0 silk suture. Myocardial ischemia
was maintained for 1 h, then the ligation was removed, and rats
were reperfused for 2 h and treated with anisodamine. In control
group, rat was anesthetized with sodium pentobarbital (30 mg/kg,
intraperitoneally) and the surgical procedure was performed without
ligation. In anisodamine treatment group, the rats were gavaged
with 25 mg/kg of anisodamine (Shanghai Xinyijinzhu Pharmaceutical
Co., Ltd., Shanghai, China) for 7 days. In sham group, rat was only
anesthetized with sodium pentobarbital (30 mg/kg,
intraperitoneally).
Myocardial infarct size
Hearts were harvested following treatment with
anisodamine for 7 days and washed 3 times with normal saline. Heart
tissue was fixed with 5% paraformaldehyde for 24 h and sliced into
1-mm transverse sections and stained with 1%
2,3,5-triphenyltetrazolium chloride at room temperature for 1 h.
Subsequently, infarct sizes were observed using inverted microscope
(Leica Microsystems GmbH, Wetzlar, Germany) and analyzed using
ImageJ software version 1.43 (National Institutes of Health,
Bethesda, MD, USA). Necrotic area was the white area and area at
risk was determined as white area/total area ×100%.
Assessment of cardiac function
Following anesthesia, the rats were secured in a
supine position, an indwelling arterial needle was inserted into
the right carotid artery, and the BL-420 Biological data
acquisition and analysis system (Chengdu Thaimeng Software Co.,
Ltd., Chengdu, China) was used to measure the left ventricular (LV)
systolic pressure (LVSP) and the LV end-diastolic pressure (LVEDP).
The LV pressure maximum rising and falling rates
(+dp/dtmax, -dp/dtmax) were analyzed using
Isoheart Software, version 1.5 (Hugo Sachs Electronic,
March-Hugstetten, Germany).
ELISA assays
The levels of creatine kinase (CK), lactate
dehydrogenase (LDH), proinflammatory factors and oxidative stress
markers, as well as the activity of caspase-3, were assessed in
serum samples isolated from rats. Following anesthesia, blood
samples were acquired and serum was isolated by centrifugation at
4°C for 10 min at 10,000 × g. The serum levels of CK (A032) and LDH
(A020-2), of the endogenous antioxidative enzyme superoxide
dismutase (SOD, A001-3), of the lipid peroxidation product
malondialdehyde (MDA, A003-1) and ROS (E004), of the inflammatory
factors TNF-α (H052) and IL-6 (H007), Nox (A116) and the activity
of caspase-3 (G015) were determined using commercially available
ELISA kits (Nanjing Jiancheng Bio-Engineering Institute Co., Ltd.,
Nanjing, China).
Western blot analysis
Heart tissue samples were washed with normal saline
and lysed in radioimmunoprecipitation assay lysis buffer (RIPA; EMD
Millipore, Billerica, MA, USA). The supernatants were harvested by
centrifugation at 4°C for 10 min at 10,000 × g. The protein
concentration was determined using a bicinchoninic acid protein
assay kit (EMD Millipore). Equal amounts (50 µg) of extracted
protein samples were separated by 6–12% SDS-PAGE, depending on
molecular mass, and transferred onto polyvinylidene difluoride
membranes (EMD Millipore). Membranes were blocked at room
temperature with 5% fat-free milk in TBS containing Tween-20 (TBST)
for 1 h and then incubated overnight at 4°C with the following
primary antibodies: Anti-apoptosis regulator Bcl-2-like protein 4
(Bax; sc493; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-B-cell lymphoma 2 (Bcl-2; sc783; 1:500; Santa Cruz
Biotechnology, Inc.), anti-inducible (i)nitric oxide synthase
(iNOS; sc49055; 1:500; Santa Cruz Biotechnology, Inc.),
anti-endothelial (e)NOS, anti-nicotinamide-adenine dinucleotide
phosphate (NADPH) oxidase 4 (Nox4; ab109225; 1:2,000; Abcam,
Cambridge, UK) and anti-GAPDH (1:500, sc-25778; Santa Cruz
Biotechnology, Inc.). The membranes were washed 3 times with TBST
and then incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (sc-2004, 1:1,000; Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h. Protein
bands were visualized using an enhanced chemiluminescence kit (ECL;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Blots were
semi-quantified using an image analyzer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and Image Lab version 3.0 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). The
statistical significance of the differences between groups was
assessed by one-way analysis of variance (ANOVA) followed by a post
hoc Student-Newman-Keuls test for multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of anisodamine on myocardial
infarct size
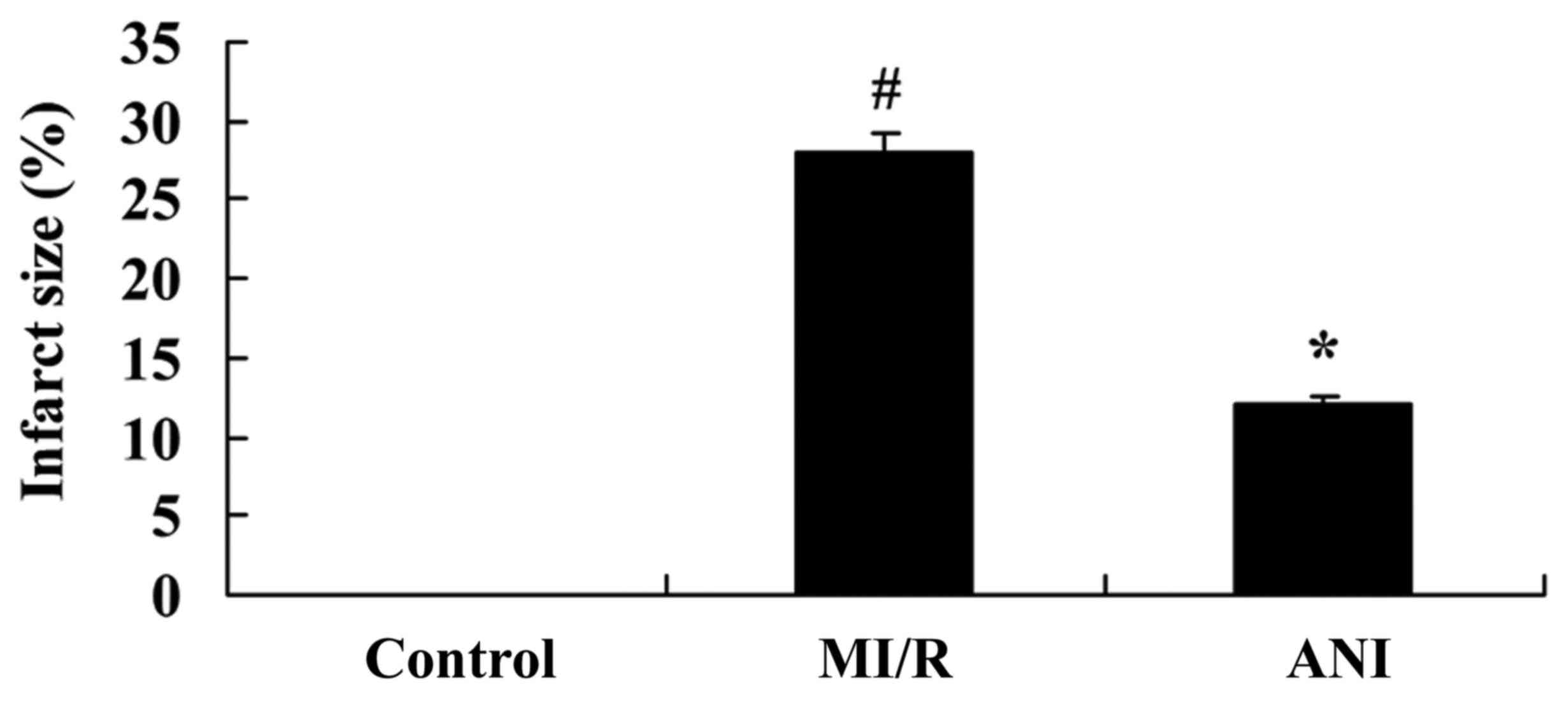
The effects of anisodamine on myocardial infarct
sizes were investigated using a rat model of myocardial I/R. As
presented in Fig. 1, myocardial
infarct sizes in rats in the I/R model group were significantly
increased compared with in rats of the control group. However,
treatment with anisodamine was revealed to significantly limit the
size of the infarcted area in rat hearts compared with the I/R
model group (Fig. 1).
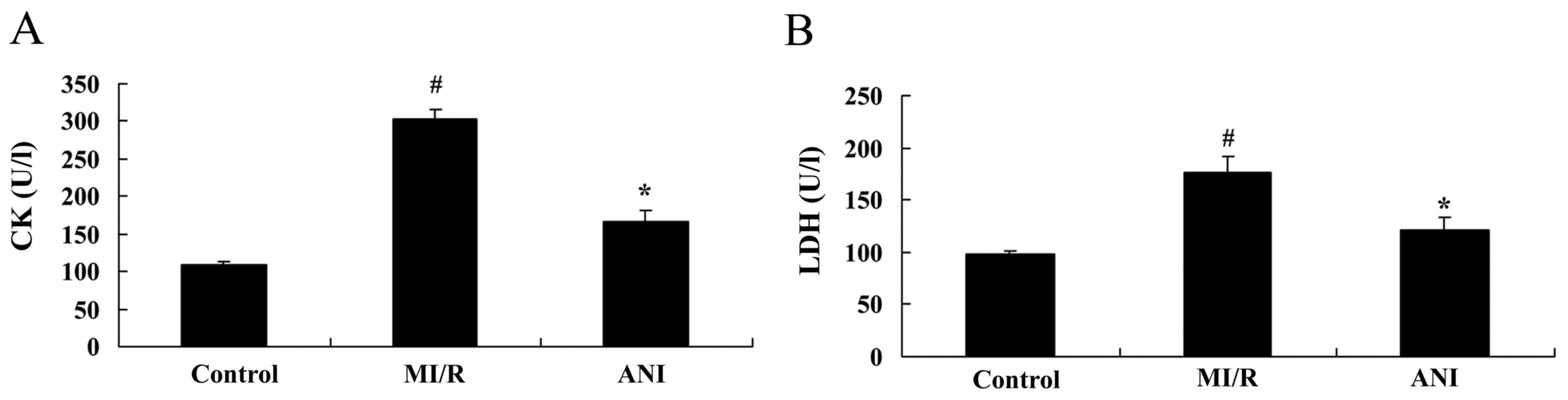
Effects of anisodamine on CK and
LDH
The effects of anisodamine on the levels of CK and
LDH were investigated in serum samples isolated from I/R model
rats. The present results demonstrated a significant increase in CK
and LDH levels in rats from the myocardial I/R model group compared
with in rats in the control group (Fig. 2). Notably, treatment with
anisodamine significantly suppressed the I/R-induced increase in CK
and LDH serum levels compared the I/R model group (Fig. 2).
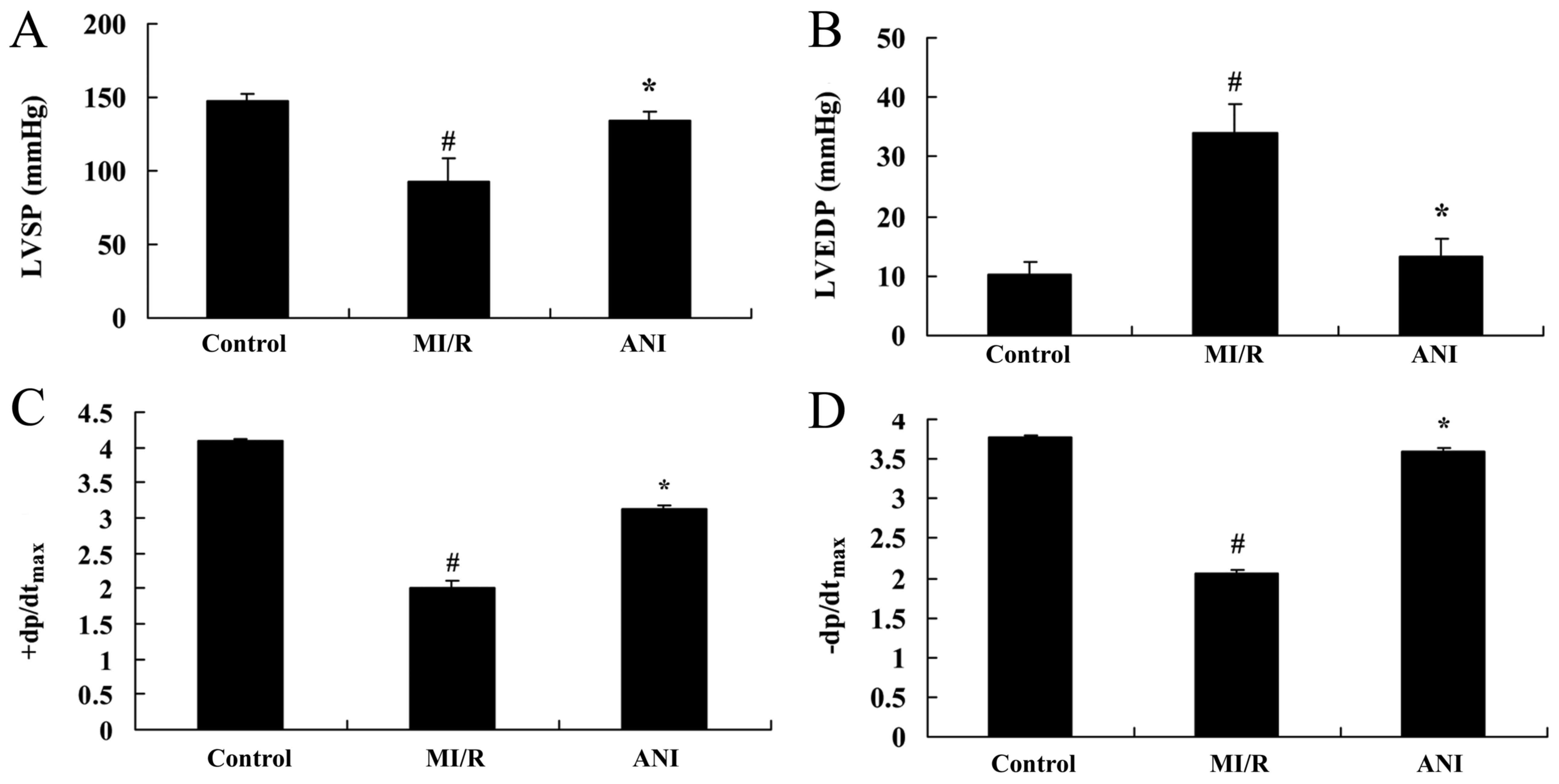
Effects of anisodamine on cardiac
function
The effects of anisodamine on cardiac function were
investigated in rats following myocardial I/R. As presented in
Fig. 3, a significant decrease in
LVSP, +dp/dtmax and -dp/dtmax was observed in
rats of the I/R model group, whereas LVEDP was significantly
increased compared with in the control group. Treatment with
anisodamine was revealed to attenuate the I/R-induced alterations
in all parameters of cardiac function: LVSP, +dp/dtmax
and -dp/dtmax were significantly increased, whereas the
increase in LVEDP was significantly suppressed following
anisodamine administration compared with rats in the I/R model
group (Fig. 3).
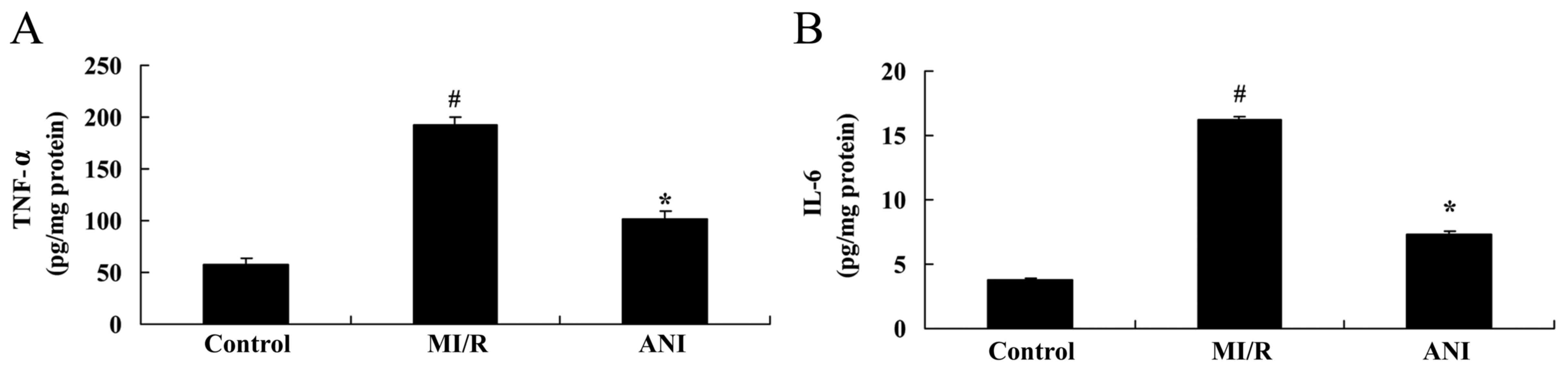
Effects of anisodamine on the
production of inflammatory factors
Compared with in rats of the control group, TNF-α
and IL-6 levels in serum samples isolated from I/R rats were
revealed to be significantly upregulated (Fig. 4). Notably, treatment with
anisodamine was demonstrated to significantly attenuate the
I/R-induced increase in the serum levels of TNF-α and IL-6 in rats
compared with the I/R model group (Fig. 4).
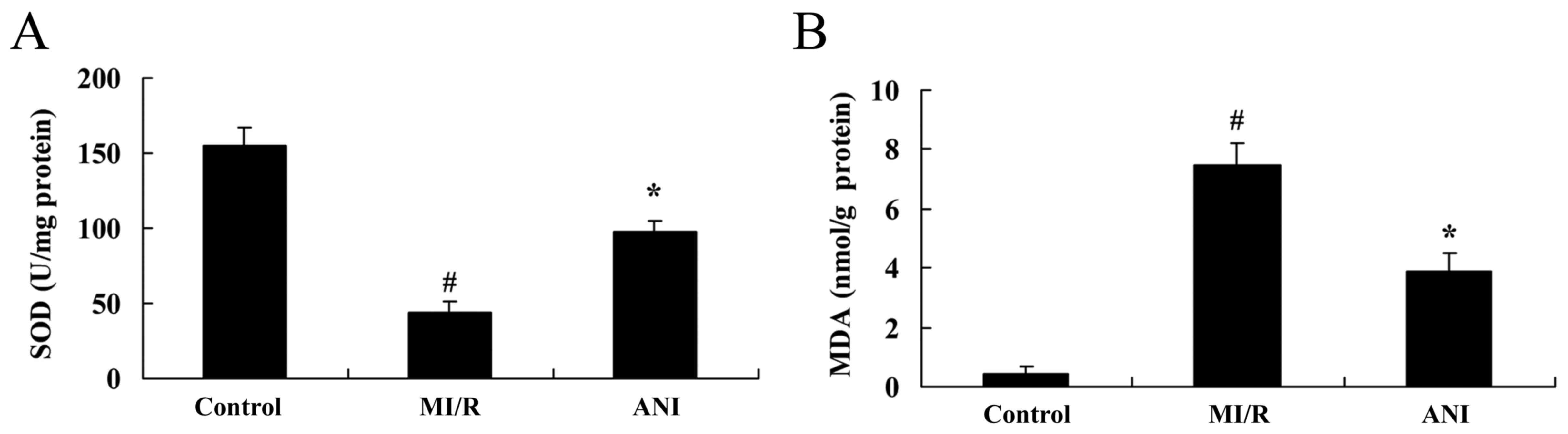
Effects of anisodamine on oxidative
stress
In order to investigate the putative antioxidative
properties of anisodamine during I/R injury, the levels of SOD and
MDA were measured in serum samples isolated from rats following I/R
using ELISA. As presented in Fig.
5, a significant downregulation in the levels of SOD, and an
increase in MDA levels was detected in rats from the I/R model
group compared with in the control group. However, treatment with
anisodamine was revealed to reverse the I/R-induced alterations, as
SOD serum levels were upregulated and MDA levels were decreased
following anisodamine administration compared with in untreated I/R
model rats (Fig. 5).
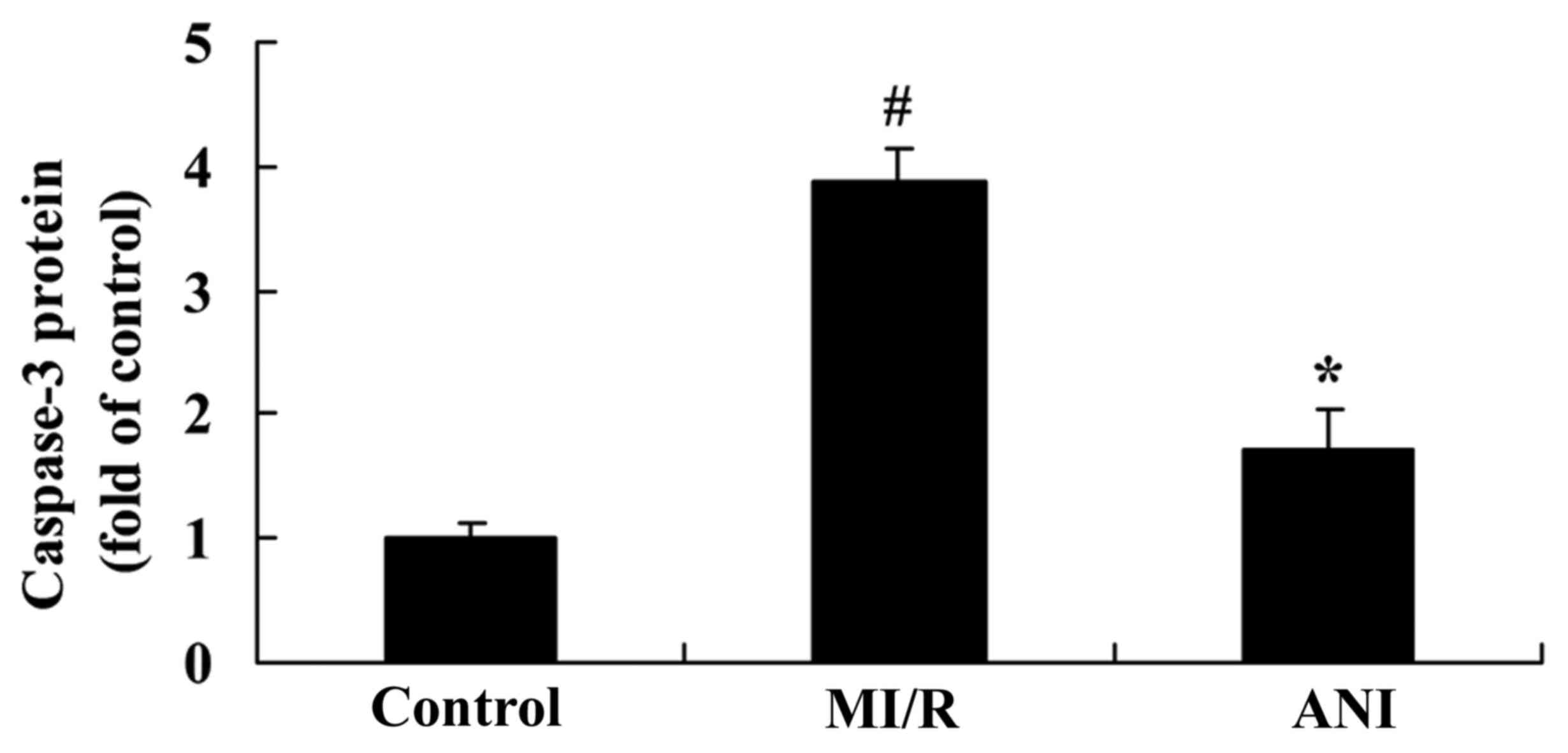
Effects of anisodamine on caspase-3
serum levels
To further investigate the effects of anisodamine on
the apoptosis of myocardial cells following I/R injury, the levels
of the proapoptotic protein caspase-3 were measured in serum
samples using an ELISA kit. As demonstrated in Fig. 6, caspase-3 serum levels were
significantly upregulated in rats following myocardial I/R compared
with the control group. However, anisodamine administration was
revealed to significantly suppress the increase in caspase-3 levels
compared with in untreated rats of the I/R group (Fig. 6).
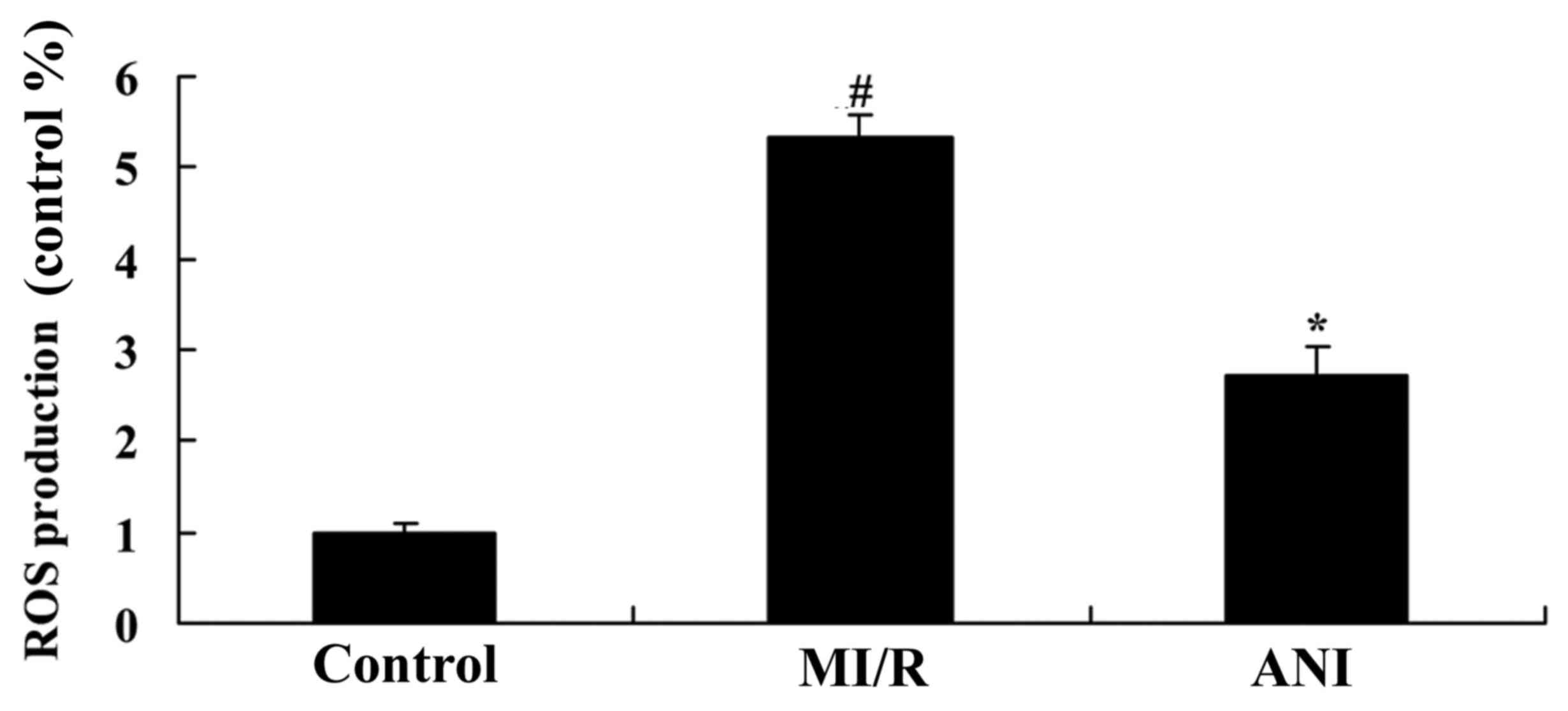
Effects of anisodamine on ROS
generation
As presented in Fig.
7, ROS levels in serum samples from I/R model rats were
significantly increased compared with in control rats. The
I/R-induced increase in ROS generation was revealed to be
significantly suppressed following anisodamine treatment compared
with in untreated rats from the I/R model group (Fig. 7).
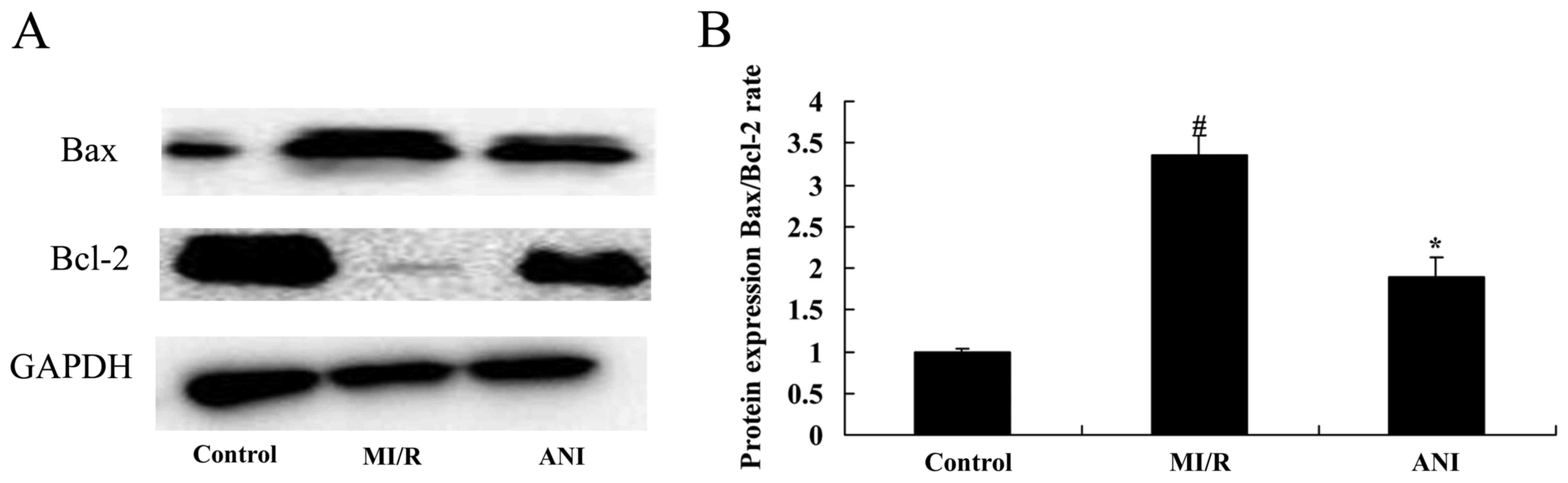
Effects of anisodamine on
apoptosis-associated protein expression
In order to investigate the effects of anisodamine
on the mechanisms of myocardial apoptosis following I/R injury
in vivo, the protein expression levels of the
apoptosis-associated proteins apoptosis regulator Bcl-2 and Bax
were assessed using western blot analysis. The Bax/Bcl-2 ratio in
rats from the I/R model group was significantly increased compared
with in control rats (Fig. 8).
Notably, treatment with anisodamine resulted in a significant
downregulation in the Bax/Bcl-2 expression ratio compared with in
the untreated I/R model rats (Fig.
8).
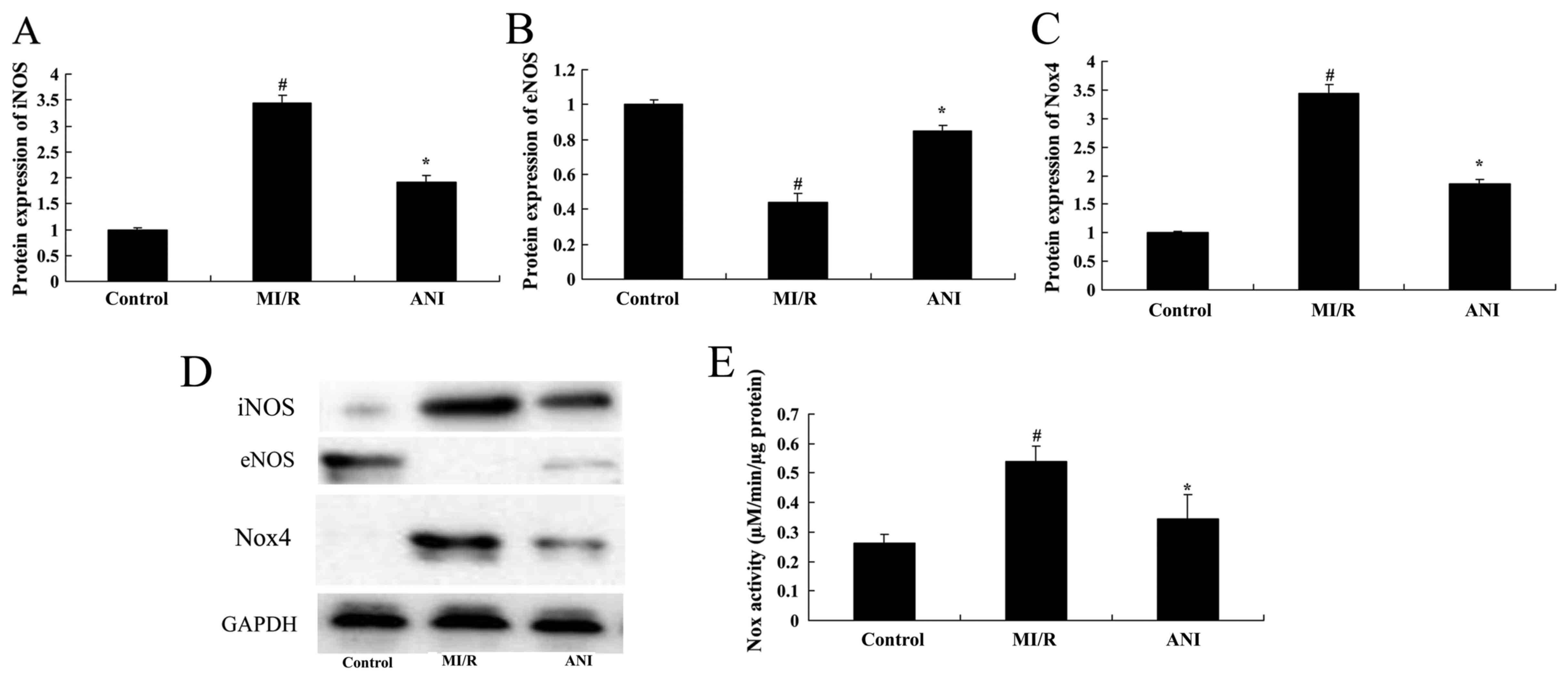
Effects of anisodamine on iNOS and
eNOS protein expression
The protein expression levels of iNOS and eNOS in
rats following myocardial I/R were assessed using western blot
analysis (Fig. 9). Compared with
the control group, a significant upregulation in iNOS and a
downregulation in eNOS protein expression was detected in
myocardial tissue samples from I/R rats (Fig. 9A, B and D). Treatment with
anisodamine was demonstrated to significantly inhibit the protein
expression of iNOS and induce the protein expression of eNOS
following I/R compared with in untreated rats from the I/R model
group (Fig. 9A, B and D).
Effects of anisodamine on Nox4 protein
expression and Nox activity
Following myocardial I/R in vivo, the protein
expression levels of Nox4 were significantly upregulated and the
activity of Nox was significantly potentiated compared with in rats
from the control group (Fig. 9C and
E). Notably, treatment with anisodamine appeared to suppress
the I/R-induced alterations. Western blot analysis revealed
anisodamine administration significantly downregulated Nox4 protein
expression, and Nox activity was also suppressed compared with in
untreated I/R rats (Fig. 9C and
E).
Discussion
During myocardial I/R injury, the intracellular
concentration of Ca2+ is abnormally increased, resulting
in the induction of myocyte apoptosis (17). Intracellular Ca2+
overload promotes the generation of oxygen free radicals, which can
damage organelle membranes, enhance intracellular acidosis and
impair the mitochondrial electron transport chain. In addition,
proteases, lipases and nucleases are activated, and cause plasma
membrane damage, lysis of structural proteins and chromosomal
damage, resulting in impaired cellular metabolism and function,
which promotes I/R-induced injury (18). The results of the present study
demonstrated that treatment with anisodamine attenuated myocardial
infarct sizes, decreased serum CK and LDH levels, and improved
cardiac function parameters, including LVSP, LVEDP,
+dp/dtmax and -dp/dtmax, in rats following
the induction of I/R injury.
During myocardial I/R injury, the complement system
is activated, which increases the production of adherence factors
and the secretion of chemotactic factors (19). Inflammatory mediators, including
leukotrienes, are attracted through chemotaxis, to ischemic
tissues, and PMNs are recruited and activated in the site of
injury. During reperfusion, ROS generation is potentiated in
cardiac muscle tissue (9).
Activated hemamebas adhere, transform and deposit in blocked
myocardiac microvessels, causing neighboring cells to enter a
hypoxic state and impacting cellular metabolism (20). Activated white blood cells in the
lumen of blood vessels attach to the endothelium, where they
synthesize and release vasoactive substances and inflammatory
mediators, thus increasing vascular permeability, triggering
inflammatory responses, and ultimately contributing to endothelial
and cardiomyocyte damage (20).
The present study demonstrated that treatment with anisodamine
significantly suppressed the I/R-induced increase in TNF-α and IL-6
serum levels in rats following the induction of I/R injury. In
accordance with the present results, Xu et al (21) reported that anisodamine suppressed
T helper cell type 2-associated responses and eosinophil-mediated
inflammatory processes in a murine model of allergic asthma.
The mitochondrial electron transport chain and Nox
are primarily responsible for ROS generation under physiological
conditions. During myocardial I/R injury, ROS production from the
mitochondria and Nox is implicated in oxidative stress injury in
cardiomyocytes (22). Nox is an
enzyme also expressed in phagocytes; however, Nox in myocardial
cells differs in its catalytic characteristics and biological
functions: Cardiac muscle Nox is weakly active under physiological
conditions, whereas ROS production is a critical function of
phagocytosis; cardiac Nox and NADPH or nicotinamide adenine
dinucleotide (NADH) are electron donors, whereas only NADPH is an
electron donor in phagocytes; in phagocytes, Nox-generated ROS
mainly participate in the host mechanisms of defense (23,24),
whereas in cardiomyocytes ROS function as a second messenger during
the regulation of cellular proliferation and differentiation
(22). The results of the present
study revealed that anisodamine significantly reversed the
I/R-induced decrease in SOD levels and increase in MDA levels in
serum samples isolated from rats following I/R. In accordance with
the present results, Liu et al (25) reported that anisodamine attenuated
oxidative stress-induced mitochondrial injury in swine with cardiac
arrest.
Nox4 exhibits characteristics that distinguish it
from other members of the Nox family of enzymes: Nox2 and Nox1
share a high sequence homology, whereas the amino acid sequence of
Nox4 exhibits only a 39% homology with other Nox members,
suggesting a corresponding difference in Nox4 structure. In
addition, under physiological conditions, Nox4 activity is
independent of regulatory subunits and is constitutively active;
the activity of Nox4 is mainly controlled by its expression levels.
Due to differences in their activation mechanisms, Nox2 needs to be
induced in order to generate O2−, whereas Nox4 can
constitutively generate low levels of H2O2
(26). Furthermore, Nox4 in
cardiac muscle cells can catalyze O2− generation
effectively, using NADH as a hydrogen donor (23). Due to the autonomous activity of
Nox4, NADH is highly used in cardiac muscle cells (26). The results of the present study
demonstrated that anisodamine significantly suppressed the
I/R-induced upregulation in Nox4 protein expression and Nox
activity in I/R model rats in vivo.
ROS and ROS-mediated oxidative stress responses have
been implicated in cardiac hypertrophy induced by α-adrenergic
agonists, angiotensin II, endothelin (ET)-1 and TNF-α (23). Nox2 and Nox4 in cardiac muscle
cells have also been reported to participate in the development and
progression of cardiac hypertrophy and fibrosis (18). Nox2 has been demonstrated to
exacerbate hypertension triggered by cardiac hypertrophy,
interstitial fibrosis and aldosterone/salt overload, whereas the
expression of Nox4 can increase following stimulation by
angiotensin II, α-adrenergic agonists and hypertension (18). Nox4 is the main source of ROS in
the hypertrophic heart (23). The
results of the present study demonstrated that treatment with
anisodamine significantly attenuated the I/R-induced increase in
ROS generation in rat myocardial tissue in vivo.
Under physiological conditions, NO is released by
the vascular endothelium and serves an important protective role
for endothelial function (27). ET
contents in the circulation are low under physiological conditions,
and its effects on the endothelium are negligible (27). During I/R injury, the endothelial
synthesis and release of NO is reduced, weakening antagonistic
oxygen radicals, and its protective effects on the endothelium are
lost, thus further aggravating vascular endothelial injury and
forming a vicious cycle during the pathogenesis of I/R injury
(28). Previous studies have
reported that during myocardial I/R, the plasma NO concentrations
are reduced and the ET concentrations are increased (27,28).
In the present study, anisodamine was revealed to significantly
downregulate the protein expression of iNOS and upregulate the
expression of eNOS, thus counteracting the I/R-induced dysfunctions
in NO production in vivo.
In conclusion, the present study demonstrated that
anisodamine exerted cardioprotective effects against myocardial I/R
injury, through the inhibition of oxidative stress, inflammation
and apoptosis, via targeting the expression of NOS and Nox, and the
production of ROS. The present results suggested that anisodamine
may have potential as an alternative therapeutic strategy for the
treatment of patients with myocardial infarction.
References
|
1
|
Woodcock A, Bakerly ND, New JP, Gibson JM,
Wu W, Vestbo J and Leather D: The salford lung study protocol: A
pragmatic, randomised phase III real-world effectiveness trial in
asthma. BMC Pulm Med. 15:1602015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gafarov VV, Panov DO, Gromova EA, Gagulin
IV and Gafarova AV: Workplace stress and its impact on the 16-year
risk of myocardial infarction and stroke in an open female
population aged 25–64 years in Russia/Siberia (WHO
MONICA-psychosocial program). Ter Arkh. 87:71–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horspool MJ, Julious SA, Boote J, Bradburn
MJ, Cooper CL, Davis S, Elphick H, Norman P, Smithson WH and
VanStaa T: Preventing and lessening exacerbations of asthma in
school-age children associated with a new term (PLEASANT): Study
protocol for a cluster randomised control trial. Trials.
14:2972013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim DE, Lee Y, Kim M, Lee S, Jon S and Lee
SH: Bilirubin nanoparticles ameliorate allergic lung inflammation
in a mouse model of asthma. Biomaterials. 140:37–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YP, Zhang JH, Li CQ, Sun QX and Jiang
XH: Obesity enhances Th2 inflammatory response via natural killer T
cells in a murine model of allergic asthma. Int J Clin Exp Med.
8:15403–15412. 2015.PubMed/NCBI
|
|
6
|
Zambalde ÉP, Teixeira MM, Favarin DC, de
Oliveira JR, Magalhães ML, Cunha MM, Silva WC Junior, Okuma CH,
Rodrigues V Junior, Levy BD and Rogerio AP: The anti-inflammatory
and pro-resolution effects of aspirin-triggered RvD1 (AT-RvD1) on
peripheral blood mononuclear cells from patients with severe
asthma. Int Immunopharmacol. 35:142–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao L, Guo C, Chen J, Chen Z and Yan Z:
Free vascularized fibular grafting improves vascularity compared
with core decompression in femoral head osteonecrosis: A randomized
clinical trial. Clin Orthop Relat Res. 475:2230–2240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Celik O, Celik E, Turkcuoglu I, Yilmaz E,
Ulas M, Simsek Y, Karaer A, Celik N, Aydin NE, Ozerol I and Unlu C:
Surgical removal of endometrioma decreases the NF-kB1 (p50/105) and
NF-kB p65 (Rel A) expression in the eutopic endometrium during the
implantation window. Reprod Sci. 20:762–770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Yu JN and Tao XJ: Systemic lupus
erythematosus complicated with femoral head ischemic necrosis
treated by Chinese medicine therapy for activating blood and
dredging collaterals method. Chin J Integr Med. 17:105–110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei L, Zhang H, Li X, Yang C, Wang G,
Zhang L, Cui M and Han L: Efficacy and safety evaluation of
intravenous infusion of cervus and cucumis polypeptides for
treatment of avascular necrosis of the femoral head: A randomized
clinical trial. J Tradit Chin Med. 36:39–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan JB, Ruan JW, Liu W, Zhu LQ, Zhu XH, Yi
H, Cui SY, Zhao JN and Cui ZM: miR-135b expression downregulates
Ppm1e to activate AMPK signaling and protect osteoblastic cells
from dexamethasone. Oncotarget. 7:70613–70622. 2016.PubMed/NCBI
|
|
12
|
Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu
X, Feng Y and Dai Z: MiR-17-5p modulates osteoblastic
differentiation and cell proliferation by targeting SMAD7 in
non-traumatic osteonecrosis. Exp Mol Med. 46:e1072014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan HF, Christina VR, Guo CA, Chu YW, Liu
RH and Yan ZQ: Involvement of MicroRNA-210 demethylation in
steroid-associated osteonecrosis of the femoral head. Sci Rep.
6:200462016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Y, Du Z, Ren M, Yang Q, Wang Q, Chen
G, Zhao H, Li Z, Wang J and Zhang G: Association of gene variants
of transcription factors PPARγ, RUNX2, Osterix genes and COL2A1,
IGFBP3 genes with the development of osteonecrosis of the femoral
head in Chinese population. Bone. 101:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan P, Su W, Zhang Y, Li Z, Deng C and
Zhuo Y: Trimetazidine protects retinal ganglion cells from acute
glaucoma via the Nrf2/Ho-1 pathway. Clin Sci (Lond). 131:2363–2375.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XR, Shi GX, Yang JW, Yan CQ, Lin LT,
Du SQ, Zhu W, He T, Zeng XH, Xu Q and Liu CZ: Acupuncture
ameliorates cognitive impairment and hippocampus neuronal loss in
experimental vascular dementia through Nrf2-mediated antioxidant
response. Free Radic Biol Med. 89:1077–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milger K, Götschke J, Krause L, Nathan P,
Alessandrini F, Tufman A, Fischer R, Bartel S, Theis FJ, Behr J, et
al: Identification of a plasma miRNA biomarker signature for
allergic asthma: A translational approach. Allergy. May
17–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou ZC, Gu SZ, Wu J and Liang QW: VEGF,
eNOS, and ABCB1 genetic polymorphisms may increase the risk of
osteonecrosis of the femoral head. Genet Mol Res. 14:13688–13698.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jonsson BA, Kadar T, Havelin LI, Haugan K,
Espehaug B, Indrekvam K, Furnes O and Hallan G: Oxinium modular
femoral heads do not reduce polyethylene wear in cemented total hip
arthroplasty at five years: A randomised trial of 120 hips using
radiostereometric analysis. Bone Joint J. 97-B:1463–1469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun SJ, Nam DH and Ryu JK: Femoral artery
access using the US-determined inguinal ligament and femoral head
as reliable landmarks: Prospective study of usefulness and safety.
J Vasc Interv Radiol. 26:552–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu ZP, Wang H, Hou LN, et al: Modulatory
effect of anisodamine on airway hyper-reactivity and eosinophilic
inflammation in a murine model of allergic asthma. Int
Immunopharmacol. 11:260–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tingart M, Beckmann J, Opolka A, Matsuura
M, Schaumburger J, Grifka J and Grässel S: Analysis of bone matrix
composition and trabecular microarchitecture of the femoral
metaphysis in patients with osteonecrosis of the femoral head. J
Orthop Res. 27:1175–1181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong X, Li X, Zhang C, Zhu L, Liu C, Qin
Q, Liu C, Wang Q, Zhu J, Wu X, et al: Ethyl acetate fraction of
Huogu formula inhibits adipogenic differentiation of bone marrow
stromal cells via the BMP and Wnt signaling pathways. Int J Biol
Sci. 13:480–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin D, Zuo S, Li L, Wang L and Lian K:
Treatment of neglected femoral neck fractures using the modified
dynamic hip screw with autogenous bone and bone morphogenetic
protein-2 composite materials grafting. Indian J Orthop.
49:342–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YH, Zhang J, Dai Z, et al: [Protection
of anisodamine on the mitochondrial injury induced by oxidative
stress in swine with cardiac arrest]. Zhonghua Wei Zhong Bing Ji
Jiu Yi Xue. 25:290–293. 2013.PubMed/NCBI
|
|
26
|
Renkawitz T, Santori FS, Grifka J,
Valverde C, Morlock MM and Learmonth ID: A new short uncemented,
proximally fixed anatomic femoral implant with a prominent lateral
flare: Design rationals and study design of an international
clinical trial. BMC Musculoskelet Disord. 9:1472008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma XL, Liu ZP, Ma JX, Han C and Zang JC:
Dynamic expression of Runx2, Osterix and AJ18 in the femoral head
of steroid-induced osteonecrosis in rats. Orthop Surg. 2:278–284.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Wang Y, Li Y, Sun J and Zhao G: The
effect of combined regulation of the expression of peroxisome
proliferator-activated receptor-γ and calcitonin gene-related
peptide on alcohol-induced adipogenic differentiation of bone
marrow mesenchymal stem cells. Mol Cell Biochem. 392:39–48. 2014.
View Article : Google Scholar : PubMed/NCBI
|