Introduction
The optic nerve is an extension of the central
nervous system (CNS). CNS trauma causes damage to neurons including
inflammation, neuronal apoptosis and functional recovery inhibition
(1,2). The impairment of the optic nerve
results in degeneration of the axon and death of retinal ganglion
cells (RGCs), as they convey visual signals from the retina along
their axons through the optic nerve to the brain, and even
irreversible loss of vision (3,4).
Optic nerve crush (ONC) is a well-established model of axonal
injury to the optic nerve, due to its accessibility, stability and
reproducibility (5,6). Following ONC, the injured RGCs suffer
from either early necrosis or delayed apoptotic death (7), and cellular apoptosis mainly occurred
within the first 2 weeks (8,9).
Carbon monoxide (CO) is a colorless and odorless gas
that has been known to be a toxic molecule for centuries, due to
its potent affinity to hemoglobin which results in hypoxia
(10). However a number of studies
have demonstrated that low or near-physiological doses of CO may
have anti-inflammatory (11),
anti-proliferative (12) and
anti-apoptotic (13) properties,
and neuroprotective effects (14).
In optic neuropathy, Biermann et al (15) demonstrated that preconditioning
with CO may protect RGCs from retinal ischemic/reperfusion injury.
A previous study indicated that inhaled CO exerts neuroprotective
effect against ONC (16). The
present study investigated the effects of preconditioning with CO
inhalation on ONC-induced RGC loss and the possible mechanisms.
Materials and methods
Animals and groups
A total of 108 adult male Sprague-Dawley rats (6
weeks old, 150–200 g) obtained from the experimental animal center
of Shanghai Jiao Tong University (certification: SCXK Shanghai
2012–0002) were used in the experiments. The animals were raised in
20 cages in a temperature (23°C) and humidity (50%) controlled room
with a 12-h light/dark cycle. Standard animal chow and water were
freely available. The experiments were performed according to the
Guide for the Care and Use of Laboratory Animals recommended by the
National Institutes of Health (Bethesda, MD, USA), and were
approved by the Ethics Committee for Animal Experimentation of
Shanghai Jiao Tong University (Shanghai, China). Animals were
randomly divided into three groups of 36: i) CO + crush group, in
which ONC was performed on the right eyes immediately following
exposure to 250 ppm CO for 1 h; ii) crush group, in which only ONC
was performed on the right eye of each rat; and iii) sham group, in
which sham surgery was performed on the right eye. When the rats
had been anesthetized with 10% chloral hydrate solution (400
mg/kg), the eyeballs were rapidly removed in 2 min. CO2
euthanasia (5 l/min) was subsequently administered to the rats.
Other procedures, including ONC, flash visual evoked potential
(FVEP) analysis and FluoroGold intravitreous injection, were
performed under deep anesthesia with 10% ketamine (95 mg/kg) and 2%
xylazine (7 mg/kg), injected intraperitoneally.
ONC injury model
ONC was performed as previously described (5,8). To
approach the optic nerve, an incision to the conjunctiva was made
on the right eye with the guidance of a binocular-operating
microscope. The optic nerve was exposed following separation of the
retractor bulbi muscle. The crush was performed at 2 mm posterior
the eyeball for 9 sec with a Sugita Titanium Aneurysm Clip II
Applier (Mizuho Medical Co., Ltd, Tokyo, Japan). Care was taken to
avoid affecting the retinal blood supply. An antibiotic ophthalmic
ointment was applied to the models after surgery. The sham group
received optic nerve exposure without the crush.
CO preconditioning
To examine the neuroprotective effect of inhaled CO,
animals were randomized to receive treatment either with air, or
air supplemented with 250 ppm CO (Shanghai Baosteel Gases Co.,
Ltd., Shanghai, China) for 1 h in an air-sealed chamber prior to
ONC. The temperature in the chamber was maintained within the range
of 22–25°C. During this procedure, the animals were awake and
freely moving in the chamber. Anesthesia was performed (the
procedure lasted ~10 min) immediately following gas inhalation,
following the ONC approach.
Hematoxylin and eosin (H&E)
staining
At 1 and 2 weeks post-surgery, six rats in each
group were euthanized with excessive anesthesia and CO2
inhalation. The eyeballs were dissected and fixed in 1%
glutaraldehyde and 4% paraformaldehyde (PFA) in 0.1 M PBS overnight
at 4°C. Then the entire eyeballs were paraffin-embedded. H&E
staining was performed using standard techniques for
paraffin-embedded tissues at 25°C for 10 min. Sections of 4-µm were
cut, and three sections with the sagittal plane through the optic
nerve head area were selected for each eye. The H&E-stained
retinas were imaged in 10 high power fields (HPF; magnification,
×400) on a Nikon microscope (Nikon Eclipse E100; Nikon Corporation,
Tokyo, Japan). Image Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used to perform
quantitative analysis of cell numbers in the retina ganglion cell
layer (GCL); six fields (two inner, two mid-periphery and two outer
retinal eccentricity) per eye were averaged. There were six rats in
every group at each time point.
RGC quantification
To evaluate the quantification of the RGCs at 2
weeks post-ONC, FluoroGold (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used 2 days prior to euthanasia, as previously
described (16–18). The FluoroGold was dissolved in
saline and injected into the intravitreal area using a Hamilton
syringe, with a final concentration of 3% in 1 µl for each eyeball.
At 2 weeks post-ONC, globes were removed and soaked in 4% PF for 2
h at 4°C. Following removal of the cornea and lens, the retinas
were extracted and flattened onto microscope slides by making four
radial cuts around the optic disc (19). We performed cell counting based on
observation under a fluorescence microscope (Olympus BX-51; Olympus
Corporation, Tokyo, Japan) at ×20 magnification. A grid frame
(0.5×0.5 mm) was used to choose the sample areas. The number of
RCGs was counted and the density calculated. RGCs were sampled at
the inner (1/6 retinal eccentricity), mid-periphery (1/2 retinal
eccentricity) and outer (5/6 retinal eccentricity) of each quadrant
of the retinal flat mount (20).
RGC densities were quantified by calculating the mean of the
samples. There were three animals in each group.
FVEP analysis
Visual electrophysiology was measured using an FVEP
analyzing instrument (BMLab 4.0; Second Military Medical
University, Shanghai, China) at 1 and 2 weeks post-ONC. Following
anesthesia, each animal was secured to a stereotaxic frame.
Subsequent to cutting the skin and drilling a hole above the visual
cortex on the skull, an active electrode was placed on the
pachymeninx and a passive electrode was placed on the skin in the
interorbital region. The settings were background illumination off,
25 sweeps were added up for a test in a single flash (10 db; 1.0
Hz). Only the latency of the first positive (P) wavelet, and the
amplitude between the first negative (N) and P wavelet of FVEP,
were measured and compared among the three groups. There were six
rats in every group at each time point.
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick end labeling (TUNEL) staining
TUNEL staining was performed on the cryosections
using the In Situ Cell Death Detection kit (Roche
Diagnostics, Indianapolis, IN, USA). A total of 2 weeks
post-surgery, eyeballs were rapidly enucleated following sacrifice
and postfixed in 4% PFA overnight at 4°C. Following dehydration
with sucrose, the eyes were frozen and cut into 12-µm sagittal
sections. Sections with the plane through the optic papilla were
permeabilized with 0.1% Triton X-100 for 2 min. The TUNEL reaction
was incubated in TdT enzyme and visualized by chromogenic staining
with diaminobenzidine (10 mg/ml) at room temperature for 15 min
(Sigma-Aldrich; Merck KGaA). TUNEL-positive (dark brown staining of
the diaminobenzidine) cells in the retinal GCL were analyzed in 10
HPF (magnification, ×400) and six fields were averaged for each
eye. There were three animals in each group.
Caspase-9 and caspase-3 activity
assay
To quantify the caspase-9 and caspase-3 activity
among the groups, the retinal caspase-9 and caspase-3 enzymatic
activity was measured using a Caspase-9 Activity Assay kit
(Beyotime Institute of Biotechnology, Haimen, China) and a
Caspase-3 Activity Assay kit (Beyotime Institute of Biotechnology)
at 2 weeks post-surgery. Following measurement of the protein
content using a protein assay kit (Beyotime Institute of
Biotechnology), each sample was mixed with Ac-DEVE-pNA and reaction
buffer to incubate at 37°C for 1 h. The retinal caspase-9 and
caspase-3 activity was calculated as the results of the calibrated
absorbance at 405 nm using a standard curve. There were three
animals in each group for each measurement.
Statistical analysis
For all analysis, the analyzers were blinded to
study group. All quantitative data are expressed as the mean ±
standard deviation. The results were analyzed by one-way analysis
of variance and Tukey's post hoc multiple comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Inhaled CO preconditioning increases
RGC density after ONC
The decreased neuronal count in the retinal GCL
following ONC was assessed by H&E staining (Fig. 1). The cell number in the GCL of the
sham group was 37.2±3.8 cells/HPF at 1 week and 34.5±3.9 cells/HPF
at 2 weeks post-surgery (Fig. 1B and
E). A sequential decline of GCL neurons was observed as early
as 1 week post-crush in the models, with aggravated decline by 2
weeks following ONC (Fig. 1C and
F). More H&E-stained cells in the GCL were observed in the
CO + crush group (Fig. 1D and G)
compared with the crush-only group (29.0±1.5 vs. 22.0±1.2 cells/HPF
at 1 week, P<0.001; and 22.6±3.0 vs. 16.6±0.6 cells/HPF at 2
weeks, P<0.01, respectively; Fig.
1H). It was apparent that preconditioning with inhaled CO
exerted a marked rescue effect on the neurons of rat retinas
following ONC insult.
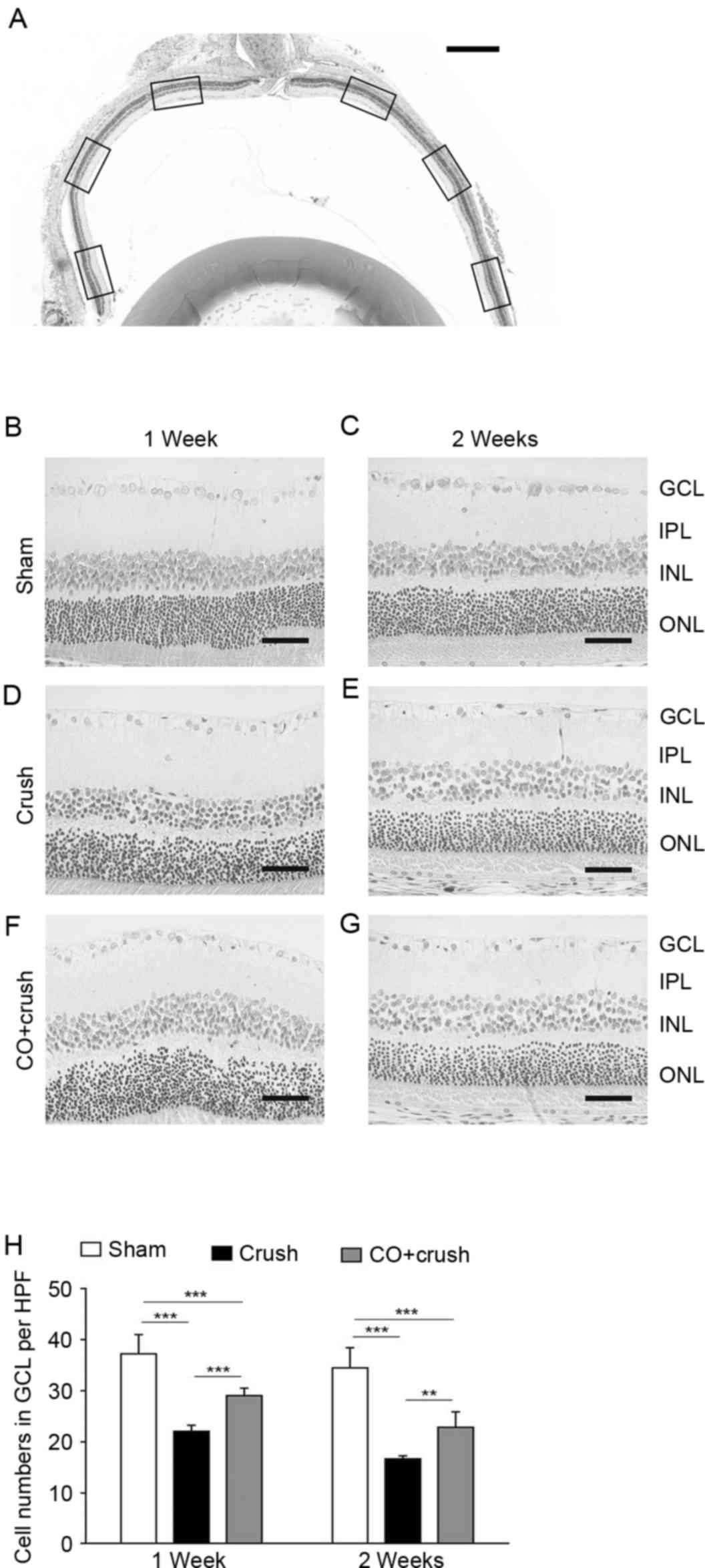 | Figure 1.H&E staining of retinas. (A)
Representative image of H&E of the retina with the optic nerve.
Images were capture of the inner, mid-periphery and outer areas of
the sections (scale bar, 500 µm). Representative images are
presented of retinas in the (B) sham group at 1 week, (C) sham
group at 2 weeks, (D) crush group at 1 week, (E) crush group at 2
weeks, (F) CO + crush group at 1 week and (G) CO + crush group at 2
weeks (scale bars, 50 µm). (H) The number of cells/HPF in the GCL
of the CO + crush group was significantly increased compared with
that of the crush-only group at the two time-points. **P<0.01,
***P<0.001. n=6. HPF, high power field; GCL, ganglion cell
layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL,
outer nuclear layer; H&E, hematoxylin and eosin. |
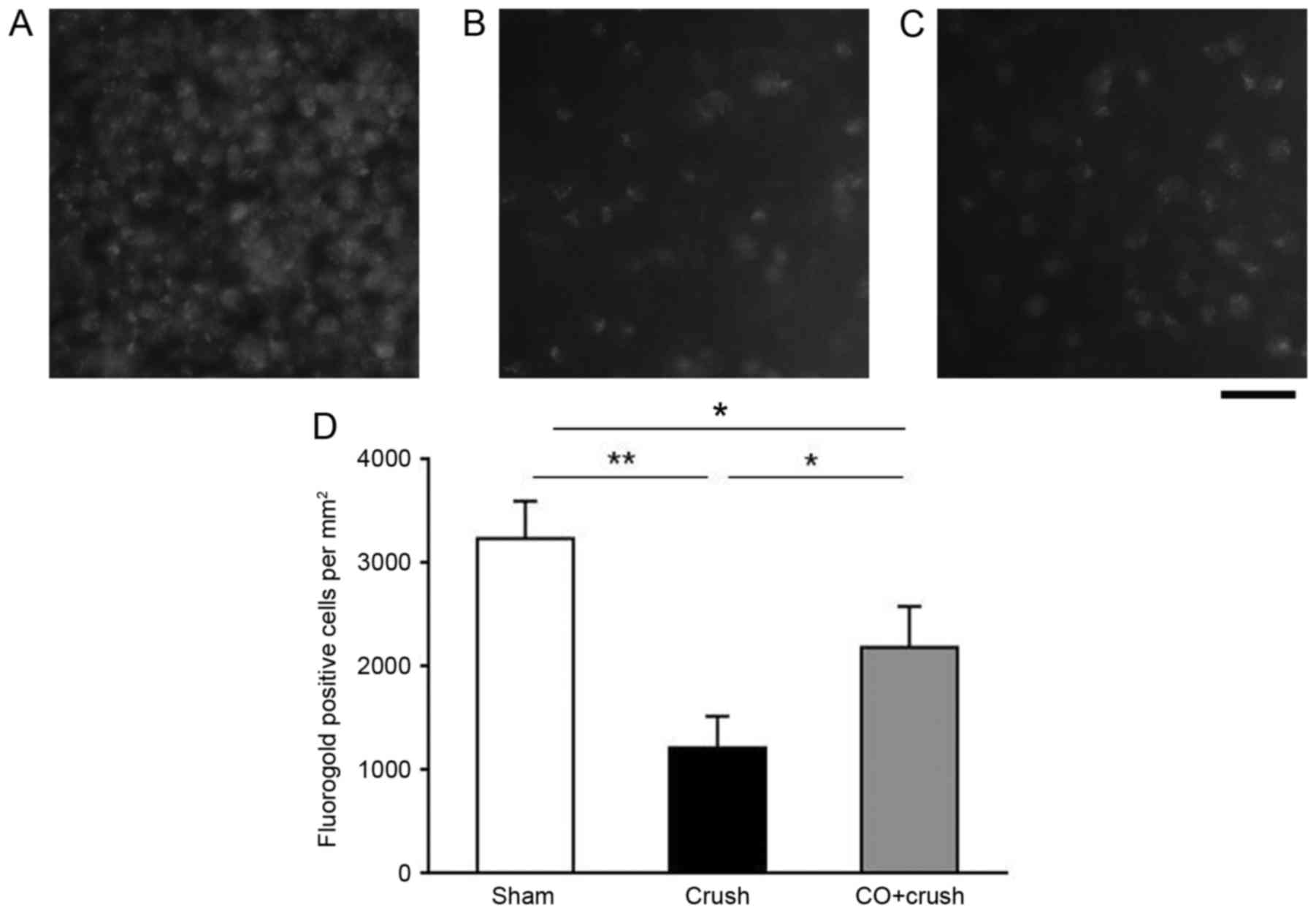
Additionally, the RGC survival rate was assessed by
FluoroGold labeling to count the surviving RGC number at 2 weeks
post-ONC (Fig. 2). The average RGC
density in the retinas of the sham-operated group was 3,236.2±360.0
cells/mm2 (Fig. 2A). At
2 weeks post-crush, the RGC densities decreased to 1,216.6±306.1
and 2,186.1±394.0 cells/mm2 in the crush-only group and
the CO + crush group, respectively (Fig. 2B and C; P<0.05). Therefore, the
RGC survival rates of the crush-only group were 48.2 and 37.6% as
determined by H&E staining and FluoroGold labeling at 2 weeks,
respectively. Increasing survival rates were observed in the CO +
crush group of 66.2 and 67.6%, respectively. These results
demonstrated that the RGC survival rate was increased in the CO +
crush group compared with the corresponding crush-only group.
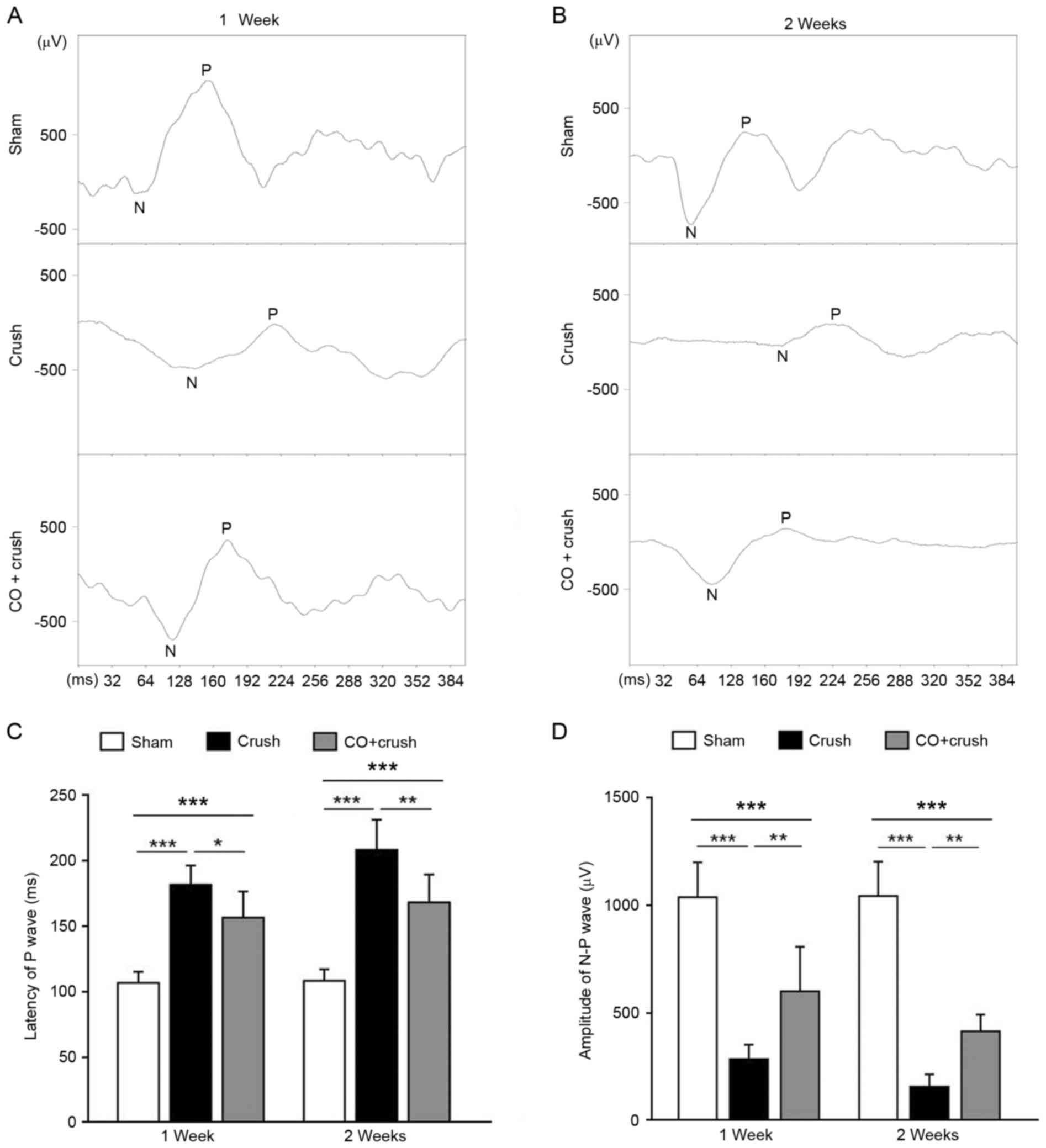
CO preconditioning ameliorated
abnormal FVEP induced by ONC
In order to evaluate visual function among the
groups, FVEP was tested at 1 and 2 weeks post-surgery (Fig. 3). The latency of the first P wave
was 106.5±8.6 msec (sham group), 181.2±14.9 msec (crush-only group)
and 156.3±19.9 msec (CO + crush group) (crush-only group vs. sham
group, P<0.001; CO+crush group vs. crush-only group, P<0.05;
CO + crush group vs. sham group, P<0.001; Fig. 3A and C) 1 week post-surgery.
Simultaneously, the amplitude of the N-P wave was 1,035.2±162.9 µV
(sham group), 283.9±67.3 µV (crush-only group) and 599.0±206.5 µV
(CO + crush group) (crush-only group vs. sham group, P<0.001; CO
+ crush group vs. crush-only group, P<0.01; CO + crush group vs.
sham group, P<0.001; Fig. 3A and
D). A total of 2 weeks post-surgery, the visual function had
deteriorated, as demonstrated by the presentation of prolonged
latency (207.8±23.2 msec) and shortened amplitude (157.1±55.9 µV)
in the crush-only group compared with the sham group (108.1±8.8
msec of latency, P<0.001; and 1,041.6±159.1 µV of amplitude,
P<0.001; Fig. 3B-D). CO
preconditioning led to a shorter latency (1,67.7±1.4 msec;
P<0.01 CO + crush group vs. crush-only group; P<0.001 CO +
crush group vs. sham group; Fig. 3B
and C) and a larger amplitude (414.1±77.0 µV; P<0.01 CO +
crush group vs. crush-only group; P<0.001 CO + crush group vs.
sham group; Fig. 3B-D) compared
with the corresponding crush-only group at 2 weeks post-surgery.
These data demonstrated that CO preconditioning preserved visual
function to a certain degree following ONC injury.
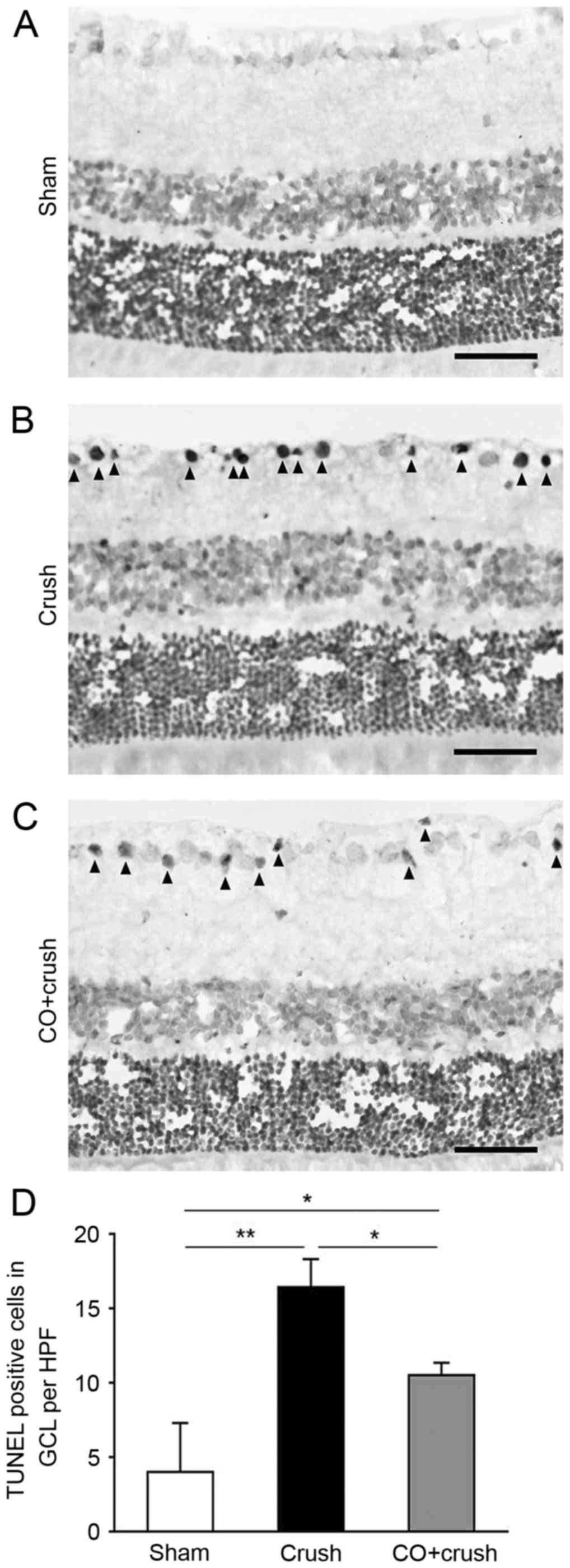
Anti-apoptotic effect of CO
preconditioning
TUNEL staining was performed to determine the extent
of apoptosis in the retina. The nuclei of cells were clearly
stained and few TUNEL-positive cells were observed in sham-operated
rats (Fig. 4A). As illustrated in
Fig. 4B, TUNEL-positive cells were
markedly increased in the GCL of the crush-only group. CO
preconditioning reduced the number of TUNEL-positive cells
(Fig. 4C). The TUNEL assay
demonstrated that the numbers of TUNEL-positive cells/HPF in the
GCL were 4.1±3.3 cells in the sham group, 16.4±1.9 cells in the
crush-only group and 10.6±0.8 cells in the CO + crush group (sham
group vs. crush-only group, P<0.01; crush-only group vs. CO +
crush group, P<0.05; Fig. 4D) 2
weeks following surgery, demonstrating that CO exerted a
significant anti-apoptotic effect on RGCs following ONC.
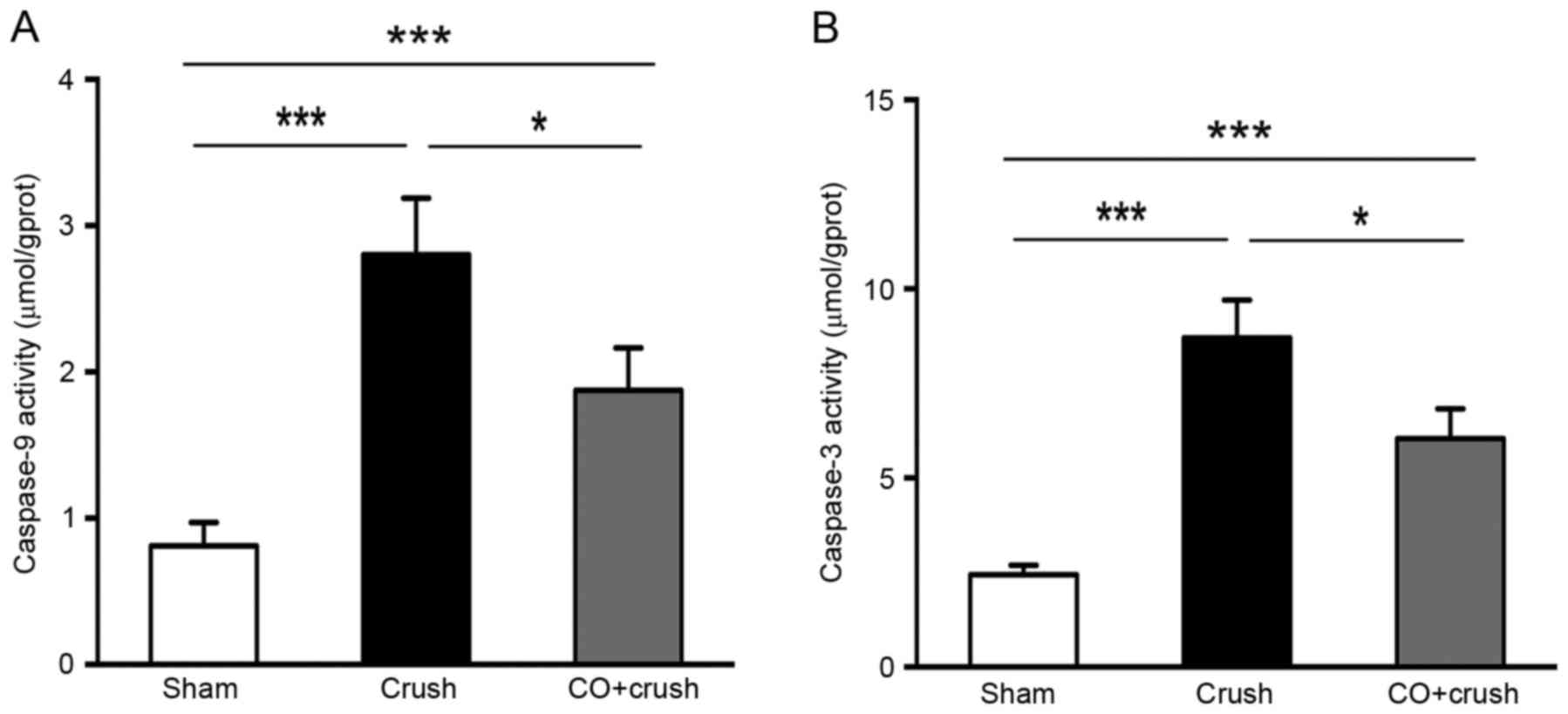
As presented in Fig.
5, Caspase-9 and Caspase-3 Activity Assay kits were used to
measure the caspase-9 and caspase-3 activity in the retina. The
caspase-9 activity was 0.8±0.2, 2.8±0.4, 1.8±0.3 µmol/grams of
protein in the sham, crush-only and CO + crush groups, respectively
(P<0.05, CO + crush vs. crush-only group). The caspase-3
activity was 2.4±0.2, 8.7±1.0, 6.0±0.8 µmol/gprot in the sham,
crush-only and CO + crush group, respectively (P<0.05, CO +
crush vs. crush-only group). The results demonstrated that the
reduction of caspase-9 and caspase-3 activity may be involved in
the mechanism of CO-induced neuroprotection.
Discussion
The principal findings of the present study
regarding inhaled CO preconditioning were as follows: i)
Preconditioning significantly rescued the loss of RGCs due to ONC
injury, as evidenced by the improvement in RGC density in
H&E-stained and FluoroGold-labeled flat-mounted retinas; ii)
preconditioning ameliorated visual function, as demonstrated by the
shortened latency of P waves and increased amplitude of N-P waves
of FVEPs at 1 and 2 weeks post-ONC; and iii) preconditioning
significantly inhibited the apoptotic process in the retina, as
demonstrated by the reduction in TUNEL staining and caspase-3/9
expression. The results of the present study demonstrated that CO
inhalation had a neuroprotective effect on RGCs in a rat ONC
model.
RGCs are well-characterized CNS neurons, with the
soma located in the inner part of the retina and the axon processed
along the optic nerve that reach the superior colliculus in the
brain (21,22). The crush operation directly damages
the optic nerve, in addition to causing rapid injury to the RGCs
(23). ONC is suitable for
studying the neurodegeneration of traumatic optic neuropathy and
glaucomatous damage. The development of strategies for
neuroprotection in response to neuropathy has involved gaseous
therapies, including CO.
Similar to nitric oxide (NO), CO is regarded as a
neurotransmitter and a signal molecule which mediates vasodilation,
although its capacity to activate soluble guanylyl cyclase is
markedly lower compared with NO (24). Inhaled CO, in addition to CO
donors, including HO, or CO releasing molecules (CORMs), has been
investigated for its potential neuroprotective effect in a number
of studies. Ex vivo, CO derived from heme protects against
amyloid β peptide toxicity following inhibition of 5′-AMP-activated
protein kinase catalytic subunit α-1 activation, and this
protective effect exists concurrently in CORM-2 in a
concentration-dependent manner (25). The study of Wang et al
(14) demonstrated a benefit of
decreasing infarct size using 250 ppm CO inhalation immediately
following permanent middle cerebral artery occlusion. In the
present study, the morphological results demonstrated that inhaled
CO preconditioning attenuated RGC loss due to ONC injury. In
addition, FVEP measurements demonstrated that visual function was
better-preserved in the CO + crush group compared with the
crush-only group, indicating a protective effect on the ocular
structures. These results indicated that CO preconditioning may
prevent RGC damage due to ON injury. CO or its donors may be
applied in sophisticated retinal or ON surgery to protect against
ON damage. The present study was different from previous work that
focused on precautions to be taken prior to neuronal injury to
avoid increased damage.
The mechanisms behind low concentration CO-induced
protective effects are various. One possibility is promoting the
anti-apoptotic pathway to prevent cell and tissue injury (26–28).
The present study used TUNEL staining, a method involving an in
situ test with TdT-specific binding to the 3′-OH ends of
fragment DNA (29), to investigate
the apoptotic alterations following ONC. The results of the present
study suggested that the neuroprotection of CO preconditioning was
mediated by a reduction in apoptosis of the retina in the rat model
of ONC. The apoptotic pathway remains complex and poorly
understood; it is considered that the caspase cascade executes this
process, in which casepase-9 is an important amplifier of the
mitochondrial signaling downstream and caspase-3 acts as the
trigger of the apoptosis pathway (30). Inhibition of excessive caspase
activity has been demonstrated to be neuroprotective for RGCs
(31). In the present study,
compared with the crush-only group, the attenuated caspase-9 and
caspase-3 expression in the CO + crush group demonstrated that
inhaled CO preconditioning suppressed caspase-9 and caspase-3
enzymatic activity, thereby protecting retina by mitigating
apoptosis.
There are various pathways underlying CO-mediated
protection against apoptosis. Brouard et al (28) reported that 10,000 ppm CO
preconditioning protected endothelial cells from tumor necrosis
factor-α-induced apoptosis by the activation of nuclear
factor-κB-dependent genes, including baculoviral IAP
repeat-containing protein 3 and Bcl-2 family member A1.
Overexpressing heme oxygenase 1 (HO-1) had ameliorating effects on
amyloid β1-42 toxicity in Alzheimer's disease via the production of
CO (25). Mitochondria triggered
the anti-apoptotic function of mesenchymal stem cells, and
inhibiting HO activity prevented mitochondrial biogenesis and
attenuated the protective response in a co-culture of mesenchymal
stem cells and distressed somatic cells (32). These beneficial effects of HO-1 and
CO suggested that CO and HO-1 may interact with each other and the
underlying mechanism for its precondition neuroprotection will be
explored. In addition, Abe et al (33) applied high-pressure CO in kidney
transplantation and demonstrated its anti-apoptotic effects by
increasing phosphatidylinositol-3 kinase and p38-mitogen-activated
protein kinase. The study used mixed gas composed of 2,000 hPa CO
and 1,500 hPa O2 to precondition the isolated kidney
prior to transplantation, which led to improved renal function and
tubular epithelial cellular apoptosis compared with 2,000 hPa
N2 and 1,500 hPa O2. Therefore, methods of
applying CO, and parameters including pressure and dosage, are
considerations for future research.
CO has a primarily negative connotation as a
poisonous gas, which is acknowledged so widely that working against
this dogma difficult. However, CO gas and its molecular release
have exhibited protective effects in a number of animal models
(34–37). CO has also demonstrated benefits in
suppressing proliferation and inflammation in cell cultures
(11,12). Being a well-known toxic gas, safety
and efficacy are considerations when translating the use of CO to a
therapeutic setting. A single-blind crossover study of 12
volunteers indicated that the increase in COHb level following
1,200 or 1,500 ppm CO administration was comparable to smoking 20
cigarettes per day, and CO inhalation had no significant effect on
blood pressure or heart rate (38). Given that CO has a potent influence
on the delivery of oxygen, partial pressure of oxygen and oxygen
saturation were measured by Liu et al (39), and the results demonstrated that
250 ppm CO did not affect oxygenation, although COHb significantly
increased by 5.5, 2.8 and 1.5% following CO saline intraperitoneal
administration for 1, 3 and 6 h respectively. In addition, CO
application alone did not affect intestinal cellular apoptosis. In
a study of CO preconditioning with retinal ischemic/reperfusion
injury, there was no difference in FluoroGold retrograde labeling
RGC densities between the control group and the CO group (15). In the present study experiments,
all animals were free and active during the CO exposure; it
therefore appears that the dose of CO was safe for rats.
In conclusion, the results of the present study
suggested that preconditioning with a low concentration CO
inhalation provided neuroprotection following ONC, with an
increased RGC survival rate, and ameliorated visual function via a
caspase-dependent anti-apoptotic pathway.
Acknowledgements
The present study was supported by the General
Program of Shanghai Municipal Health and Family Planning Commission
(grant no. M201440522) and the National Natural Science Foundation
of China (grant no. 81300777).
References
|
1
|
Sharma TP, McDowell CM, Liu Y, Wagner AH,
Thole D, Faga BP, Wordinger RJ, Braun TA and Clark AF: Optic nerve
crush induces spatial and temporal gene expression patterns in
retina and optic nerve of BALB/cJ mice. Mol Neurodegener. 9:142014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz M: Optic nerve crush: Protection
and regeneration. Brain Res Bull. 62:467–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stutzki H, Leibig C, Andreadaki A, Fischer
D and Zeck G: Inflammatory stimulation preserves physiological
properties of retinal ganglion cells after optic nerve injury.
Front Cell Neurosci. 8:382014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmitt HM, Pelzel HR, Schlamp CL and
Nickells RW: Histone deacetylase 3 (HDAC3) plays an important role
in retinal ganglion cell death after acute optic nerve injury. Mol
Neurodegener. 9:392014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang R and Xu J, Xie J, Kang Z, Sun X,
Chen N, Liu L and Xu J: Hyperbaric oxygen preconditioning promotes
survival of retinal ganglion cells in a rat model of optic nerve
crush. J Neurotrauma. 27:763–770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levkovitch-Verbin H, Harris-Cerruti C,
Groner Y, Wheeler LA, Schwartz M and Yoles E: RGC death in mice
after optic nerve crush injury: Oxidative stress and
neuroprotection. Invest Ophthalmol Vis Sci. 41:4169–4174.
2000.PubMed/NCBI
|
|
7
|
Bien A, Seidenbecher CI, Böckers TM, Sabel
BA and Kreutz MR: Apoptotic versus necrotic characteristics of
retinal ganglion cell death after partial optic nerve injury. J
Neurotrauma. 16:153–163. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun JC, Xu T, Zuo Q, Wang RB, Qi AQ, Cao
WL, Sun AJ, Sun XJ and Xu J: Hydrogen-rich saline promotes survival
of retinal ganglion cells in a rat model of optic nerve crush. PLoS
One. 9:e992992014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mansour-Robaey S, Clarke DB, Wang YC, Bray
GM and Aguayo AJ: Effects of ocular injury and administration of
brain-derived neurotrophic factor on survival and regrowth of
axotomized retinal ganglion cells. Proc Natl Acad Sci USA.
91:1632–1636. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weaver LK: Carbon monoxide poisoning. Crit
Care Clin. 15:297–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Otterbein LE, Bach FH, Alam J, Soares M,
Lu Tao H, Wysk M, Davis RJ, Flavell RA and Choi AM: Carbon monoxide
has anti-inflammatory effects involving the mitogen-activated
protein kinase pathway. Nat Med. 6:422–428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song R, Mahidhara RS, Liu F, Ning W,
Otterbein LE and Choi AM: Carbon monoxide inhibits human airway
smooth muscle cell proliferation via mitogen-activated protein
kinase pathway. Am J Respir Cell Mol Biol. 27:603–610. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brouard S, Otterbein LE, Anrather J,
Tobiasch E, Bach FH, Choi AM and Soares MP: Carbon monoxide
generated by heme oxygenase 1 suppresses endothelial cell
apoptosis. J Exp Med. 192:1015–1026. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Cao W, Biswal S and Doré S: Carbon
monoxide-activated Nrf2 pathway leads to protection against
permanent focal cerebral ischemia. Stroke. 42:2605–2610. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biermann J, Lagrèze WA, Dimitriu C,
Stoykow C and Goebel U: Preconditioning with inhalative carbon
monoxide protects rat retinal ganglion cells from
ischemia/reperfusion injury. Invest Ophthalmol Vis Sci.
51:3784–3791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Wang R, Wu J, Xia F, Sun Q, Xu J
and Liu L: Low-dose carbon monoxide inhalation protects neuronal
cells from apoptosis after optic nerve crush. Biochem Biophys Res
Commun. 469:809–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, He H, Zhu Y, Wan X, Zhou LF, Wang
J, Wang WF, Liu L and Li B: Chemical and material communication
between the optic nerves in rats. Clin Exp Ophthalmol. 43:742–748.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu L, Sun Q, Wang R, Chen Z, Wu J, Xia F
and Fan XQ: Methane attenuates retinal ischemia/reperfusion injury
via anti-oxidative and anti-apoptotic pathways. Brain Res.
1646:327–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vigneswara V, Berry M, Logan A and Ahmed
Z: Pigment epithelium-derived factor is retinal ganglion cell
neuroprotective and axogenic after optic nerve crush injury. Invest
Ophthalmol Vis Sci. 54:2624–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mead B, Thompson A, Scheven BA, Logan A,
Berry M and Leadbeater W: Comparative evaluation of methods for
estimating retinal ganglion cell loss in retinal sections and
wholemounts. PLoS One. 9:e1106122014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carter DA, Bray GM and Aguayo AJ:
Regenerated retinal ganglion cell axons can form
well-differentiated synapses in the superior colliculus of adult
hamsters. J Neurosci. 9:4042–4050. 1989.PubMed/NCBI
|
|
22
|
Osterhout JA, El-Danaf RN, Nguyen PL and
Huberman AD: Birthdate and outgrowth timing predict cellular
mechanisms of axon target matching in the developing visual
pathway. Cell Rep. 8:1006–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mawrin C, Pap T, Pallas M, Dietzmann K,
Behrens-Baumann W and Vorwerk CK: Changes of retinal glutamate
transporter GLT-1 mRNA levels following optic nerve damage. Mol
Vis. 9:10–13. 2003.PubMed/NCBI
|
|
24
|
Barañano DE and Snyder SH: Neural roles
for heme oxygenase: Contrasts to nitric oxide synthase. Proc Natl
Acad Sci USA. 98:10996–11002. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hettiarachchi N, Dallas M, Al-Owais M,
Griffiths H, Hooper N, Scragg J, Boyle J and Peers C: Heme
oxygenase-1 protects against Alzheimer's amyloid-β(1–42)-induced
toxicity via carbon monoxide production. Cell Death Dis.
5:e15692014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Qin W, Qiu X, Cao J, Liu D and Sun
B: A novel role of exogenous carbon monoxide on protecting cardiac
function and improving survival against sepsis via mitochondrial
energetic metabolism pathway. Int J Biol Sci. 10:777–788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nikolic I, Saksida T, Mangano K, Vujicic
M, Stojanovic I, Nicoletti F and Stosic-Grujicic S: Pharmacological
application of carbon monoxide ameliorates islet-directed
autoimmunity in mice via anti-inflammatory and anti-apoptotic
effects. Diabetologia. 57:980–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brouard S, Berberat PO, Tobiasch E, Seldon
MP, Bach FH and Soares MP: Heme oxygenase-1-derived carbon monoxide
requires the activation of transcription factor NF-kappa B to
protect endothelial cells from tumor necrosis factor-alpha-mediated
apoptosis. J Biol Chem. 277:17950–17961. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirata H, Takahashi A, Kobayashi S,
Yonehara S, Sawai H, Okazaki T, Yamamoto K and Sasada M: Caspases
are activated in a branched protease cascade and control distinct
downstream processes in Fas-induced apoptosis. J Exp Med.
187:587–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seki M, Soussou W, Manabe S and Lipton SA:
Protection of retinal ganglion cells by caspase substrate-binding
peptide IQACRG from N-methyl-D-aspartate receptor-mediated
excitotoxicity. Invest Ophthalmol Vis Sci. 51:1198–1207. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahrouf-Yorgov M, Augeul L, Da Silva CC,
Jourdan M, Rigolet M, Manin S, Ferrera R, Ovize M, Henry A, Guguin
A, et al: Mesenchymal stem cells sense mitochondria released from
damaged cells as danger signals to activate their rescue
properties. Cell Death Differ. 24:1224–1238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abe T, Yazawa K, Fujino M, Imamura R,
Hatayama N, Kakuta Y, Tsutahara K, Okumi M, Ichimaru N, Kaimori JY,
et al: High-pressure carbon monoxide preserves rat kidney grafts
from apoptosis and inflammation. Lab Invest. 97:468–477. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fagone P, Mangano K, Mammana S, Cavalli E,
Di Marco R, Barcellona ML, Salvatorelli L, Magro G and Nicoletti F:
Carbon monoxide-releasing molecule-A1 (CORM-A1) improves clinical
signs of experimental autoimmune uveoretinitis (EAU) in rats. Clin
Immunol. 157:198–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caumartin Y, Stephen J, Deng JP, Lian D,
Lan Z, Liu W, Garcia B, Jevnikar AM, Wang H, Cepinskas G and Luke
PP: Carbon monoxide-releasing molecules protect against
ischemia-reperfusion injury during kidney transplantation. Kidney
Int. 79:1080–1089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferrándiz ML, Maicas N, Garcia-Arnandis I,
Terencio MC, Motterlini R, Devesa I, Joosten LA, van den Berg WB
and Alcaraz MJ: Treatment with a CO-releasing molecule (CORM-3)
reduces joint inflammation and erosion in murine collagen-induced
arthritis. Ann Rheum Dis. 67:1211–1217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sato K, Balla J, Otterbein L, Smith RN,
Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G,
et al: Carbon monoxide generated by heme oxygenase-1 suppresses the
rejection of mouse-to-rat cardiac transplants. J Immunol.
166:4185–4194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zevin S, Saunders S, Gourlay SG, Jacob P
and Benowitz NL: Cardiovascular effects of carbon monoxide and
cigarette smoking. J Am Coll Cardiol. 38:1633–1638. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu SH, Ma K, Xu XR and Xu B: A single
dose of carbon monoxide intraperitoneal administration protects rat
intestine from injury induced by lipopolysaccharide. Cell Stress
Chaperones. 15:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|