Introduction
Orthodontic tooth movement is achieved via the
impact of mechanical force on periodontal tissue, which is a
remodeling process accompanying bone regeneration in tension areas
and bone resorption in pressure areas (1,2). In
bone remodeling, the remodeling of alveolar bone is most important.
Periodontal tissue is a periosteum located between alveolar bone
and tooth root. It possesses the ability to form bone and serve a
key role in bone resorption induced by osteoclasts in the pressure
side and bone regeneration due to alveolar bone remodeling and
osteoblast generation in the stretching side (3,4).
Periodontal ligament cells (PDLCs) are primary cells in periodontal
tissue. They direct effector cells for mechanical force and
fibroblast-like cells with differentiation potential. Under
orthodontic force, PDLCs have an osteoblast phenotype and secrete
osteoblast-associated proteins; therefore, PDLCs translate
orthodontic force into biochemical signals for the reconstruction
of periodontal tissue and tooth movement (3–5).
Differentiation of PDLCs into osteoblast-like cells, which
participate in bone formation and bone resorption, is a critical
process in orthodontic tooth movement. Therefore, the translation
of mechanical force into biochemical signals is a biological
theoretical basis of modern orthodontic treatment.
Orthodontic force may cause adaptive changes in
microenvironments, including the cell matrix, cell membrane,
cytoskeleton, nucleoprotein and genome. Consequently, signals are
transduced to the cell nucleus, regulating the endonuclear genes
and stimulating a series of biological reactions. Various signaling
pathways, including the mitogen-activated protein kinase (MAPK)
pathway are involved in this biological process (6–8). The
MAPK pathway is composed of a series of Ser/Thr kinases, including
extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal
kinase (JNK), p38 and ERK5 subfamilies, which after cascade
phosphorylation, regulate the activity of certain transcription
factors. The MAPK signaling pathway is closely associated with bone
resorption of osteoclasts and the bone formation of osteoblasts.
In vitro cell studies demonstrated that intermittent
mechanical force and 10% cyclical tension stress activated the
ERK1/2 and p38 MAPK signaling pathway in human PDLCs (hPDLCs)
(9,10). However, to the best of our
knowledge, there are no reports demonstrating the time-dependent
expression and mechanism of ERK1/2 and p38 MAPK in orthodontic
tooth movement in animals. Therefore, in the present study, a rat
model of orthodontic tooth movement and ex vivo hPDLCs
exposed to centrifugal force were used to investigate the
involvement of ERK1/2 and p38 MAPK signaling pathways in
orthodontic tooth movement.
Materials and methods
Materials and animals
Collagenase I, neutral protease II, Dulbecco's
modified Eagle's medium (DMEM), penicillin-streptomycin, fetal
bovine serum (FBS), TRIzol and MTT reagent were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Hank's buffer,
radioimmunoprecipitation (RIPA) buffer and protease inhibitors were
purchased from Beijing Solarbio Science & Technology Co., Ltd.
(Beijing, China). Pentobarbital (Purity >99%; lot no. P3761) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Diethyl pyrocarbonate (DEPC) was purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China). The bicinchoninic acid (BCA) assay kit,
ERK1/2 inhibitor (PD098059), p38 MAPK inhibitor (SB203580),
enhanced chemiluminescent (ECL) plus detection reagent, HRP-labeled
goat anti-mouse IgG (A0216), HRP-labeled goat anti-rabbit IgG
(A0208), and mouse anti-GAPDH monoclonal antibody (AF0006) were
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Rabbit anti-ERK1/2 (ab54230), phosphorylated (p)-ERK1/2 (ab201015),
p38 MAPK (ab197348) and p-p38 (ab47363) polyclonal antibodies were
purchased from Abcam (Cambridge, UK). Orthodontic stainless steel
wire was from Innovative Material and Devices, Inc. (Shanghai,
China).
A total of 60 patients (35 male and 25 female) were
recruited in October 2015. Following obtainment of ethical approval
from the Medical Ethics Committee, Affiliated Stomatological
Hospital of Nanchang University (Jiangxi, China) and written
informed consent, human periodontal tissues were collected from
healthy premolar teeth, which were extracted from 11–15 year old
patients undergoing orthodontic treatment.
The animal studies were approved by the
Institutional Animal Care and Use Committee at the Affiliated
Stomatological Hospital of Nanchang University. A total of 60 male
Sprague Dawley rats (age, 4 weeks; weight, 200±20 g) were purchased
from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China)
with certificate number SCXK (Shanghai) 2012–0002. These rats were
housed at a temperature of 23±2°C and relative humidity of ~50%,
with natural light-dark cycle and free access to water and food.
All animal experiments were conducted in compliance with the Guide
for the Care and Use of Laboratory Animals of the Affiliated
Stomatological Hospital of Nanchang University.
Rats with orthodontic tooth
movement
A total of 60 Sprague Dawley rats were randomly
assigned into six groups (control, and orthodontic tooth movement
for 1, 3, 5, 7 and 14 days; n=10/group). The right first maxillary
molars of the rats were utilized as experimental teeth. Rats were
anesthetized with 0.1 ml pentobarbital (3%, w/v), and then
retention grooves at lengths of 0.5–1 mm were made at the near
medial surface of the maxillary incisor labial side and right first
molar in rats. Spiral springs were ligated with orthodontic
stainless steel wire between the maxillary incisor and first molar.
Tension force of ~40 Newtons produced by spiral springs pulled the
mesial movement of the first molar. Rats were euthanized at 1, 3,
5, 7 and 14 days. The first molars and surrounding alveolar bone
tissue were collected and stored at −80°C until further
analysis.
Ex vivo hPDLCs incubation and
characterization
Fresh healthy teeth collected from patients
following orthodontic treatment were washed with Hank's buffer
containing penicillin-streptomycin to remove bloodstains. One third
of the periodontal tissue in tooth roots was scraped using number
12 blades, cut into pieces, and incubated with collagenase I (3%,
w/v) and neutral protease II (4%, w/v) for digestion. After 1 h,
the mixture was centrifuged for 10 min at 1,000 × g at room
temperature, and the precipitate was resuspended in DMEM containing
15% (v/v) FBS to obtain a single cell suspension. The suspension
was incubated at 37°C and 5% CO2. The confluence of
hPDLCs reached 90% at day 7 and the cell morphology was observed
under a microscope (model TS100, Nikon Corporation, Tokyo, Japan).
hPDLCs were long spindle-shaped with a round or oval cell nucleus
with a clear nucleolus. Growth was maintained after cell
passages.
Treatment of hPDLCs
hPDLCs from passages 2–3 with a good growth status
were seeded at a density of 1×104 cells/well in 6-well
plates. The plates were placed in a centrifugal bracket at 37°C and
subjected to a centrifugal force of 80 × g for 1, 2, 6, 8 and 12 h.
hPDLCs without treatment served as a control. Subsequently, the
protein expression levels of ERK1/2 and p38 MAPK were measured by
western blotting. In addition, the mRNA expression levels of
ERK1/2, p38 MAPK and osteogenesis-associated genes, including
alkaline phosphatase (ALP), osteopontin (OPN), collagen I (Col I),
osteocalcin (OCN) and bone sialoprotein (BSP) were measured by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR).
hPDLCs were seeded at a density of 1×104
cells/well in 6-well plates and treated with 10 µM ERK1/2 inhibitor
(PD098059) and 10 µM p38 MAPK inhibitor (SB203580) at 37°C for 24
h. Cells without inhibitor treatment served as the control. Cells
with and without treatment were subjected to centrifugal force at
80 × g and 4°C for 6 h. Subsequently, mRNA expression levels of
osteogenesis-associated genes, including ALP, OPN, Col I, OCN and
BSP were determined by RT-qPCR.
RT-qPCR
In addition to hPDLCs, the mRNA expression levels of
ERK1/2 and p38 in homogenized periodontal tissue from rats were
determined by RT-qPCR. Total RNA of the periodontal tissue and
hPDLCs were extracted using TRIzol reagent and optical density (OD)
was measured at 260 nm (OD260) or 280 nm (OD280) using a
spectrophotometer (Evolution 201/220; Thermo Fisher Scientific,
Inc.). The OD260/OD280 ratio ranged between 1.7 and 2.0. RNA was
reverse transcribed to cDNA using BeyoRT first strand cDNA
synthesis kit (RNase H minus) (Beyotime Institute of Biotechnology)
and a PCR thermal cycler (PTC-200; MJ Research, Inc., Quebec,
Canada). Fluorescent quantitation using UltraSYBR mixture (CW0957M,
CWBiotech, Beijing, China) was measured on a
LightCycler® 96 instrument (Roche Diagnostics, Basel,
Switzerland). Primer sequences (Table
I) were designed by Sangon Biotech Co., Ltd. A total of 2 µl of
1 µg RNA, 12.5 µl UltraSYBR mixture, 2 µl dNTP mixture and 2 µl
MgCl2 were mixed and DEPC water (0.1%, v/v) was added to
obtain a final volume of 25 µl. Cycling conditions were as follows:
An initial predenaturation step at 95°C for 5 min, followed by 40
cycles of denaturation at 95°C for 30 sec, annealing at 58°C for 30
sec and extension at 72°C for 30 sec. Quantification cycle (Cq)
values were recorded and the relative expression level of target
genes were calculated using the 2−ΔΔCq method (11). The average value of three
experiments served as the Cq value of each sample. The Cq value of
the internal control gene subtracted from that of the target gene
to generate ΔCq, and the average ΔCq of each sample from that of
the control equaled ΔΔCq. The relative expression level of the
target gene was calculated using the 2−ΔΔCq method, and
therefore the relative expression level of the control was
20=1.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequence
(5′-3′) | Base pairs |
|---|
| ERK1/2 | F:
TCAAGCCTTCCAACCTC | 200 |
|
| R:
GCAGCCCACAGACCAAA |
|
| p38 MAPK | F:
AGGGCGATGTGACGTTT | 108 |
|
| R:
CTGGCAGGGTGAAGTTGG |
|
| ALP | F:
CTCGTTGACCACCTGGAAGAGCTTCAAACCG | 168 |
|
| R:
GGTCCGTCACGTTGTTCCTGTTCAGC |
|
| OPN | F:
CCAAGTAAGTCCAACGAAAG | 348 |
|
| R:
GGTGATGTCCTCGTCTGTA |
|
| OCN | F:
CATGAGAGCCCTCACA | 315 |
|
| R:
AGAGCGACACCCTAGAC |
|
| BSP | F:
AAAACGAAGAAAGCGAAGC | 331 |
|
| R:
TATTCATTGACGCCCGTGTA |
|
| Col I | F:
AGGGCTCCAACGAGATCGAGATCCG | 223 |
|
| R:
TACAGGAAGCAGACAGGGCCAACGTCG |
|
| GAPDH | F:
AGCCACATCGCTCAGACA | 314 |
|
| R:
TGGACTCCACGACGTACT |
|
Western blotting
Periodontal tissue of rats or hPDLCs were incubated
with 10 µg/ml RIPA buffer and protease inhibitors at 4°C. The
mixture was shaken for 10 min every 30 sec. After 40 min, samples
were centrifuged at 4°C and 7,000 × g for 10 min. The supernatant
was carefully collected to obtain total protein and the
concentration was determined with a BCA assay kit. Proteins (2
µg/µl; 10 µl) were loaded and subjected to 10% SDS-PAGE, prior to
transfer onto a PVDF membrane. The membrane was blocked with 5%
(w/v) non-fat milk at room temperature for 30 min The membrane was
incubated with primary antibodies (anti-GAPDH, anti-ERK1/2,
anti-p-ERK1/2, anti-p38 MAPK and anti-p-p38; all 1:1,000) at 4°C
overnight and subsequently with secondary antibodies (HRP-labeled
goat anti-mouse IgG and HRP-labeled goat anti-rabbit IgG; both
1:1,000) at room temperature for 2 h. ECL plus detection reagent
was added to the membrane and proteins were imaged on a ChemiDoc™
XRS gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Quantity One software (v4.62; Bio-Rad Laboratories, Inc.) was
used for densitometric analysis.
Statistical analysis
Experiments were repeated at least three times and
data are presented as the mean ± standard deviation. Statistical
analysis was performed using one way analysis of variance followed
by a Tukey post-hoc test via SPSS version 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
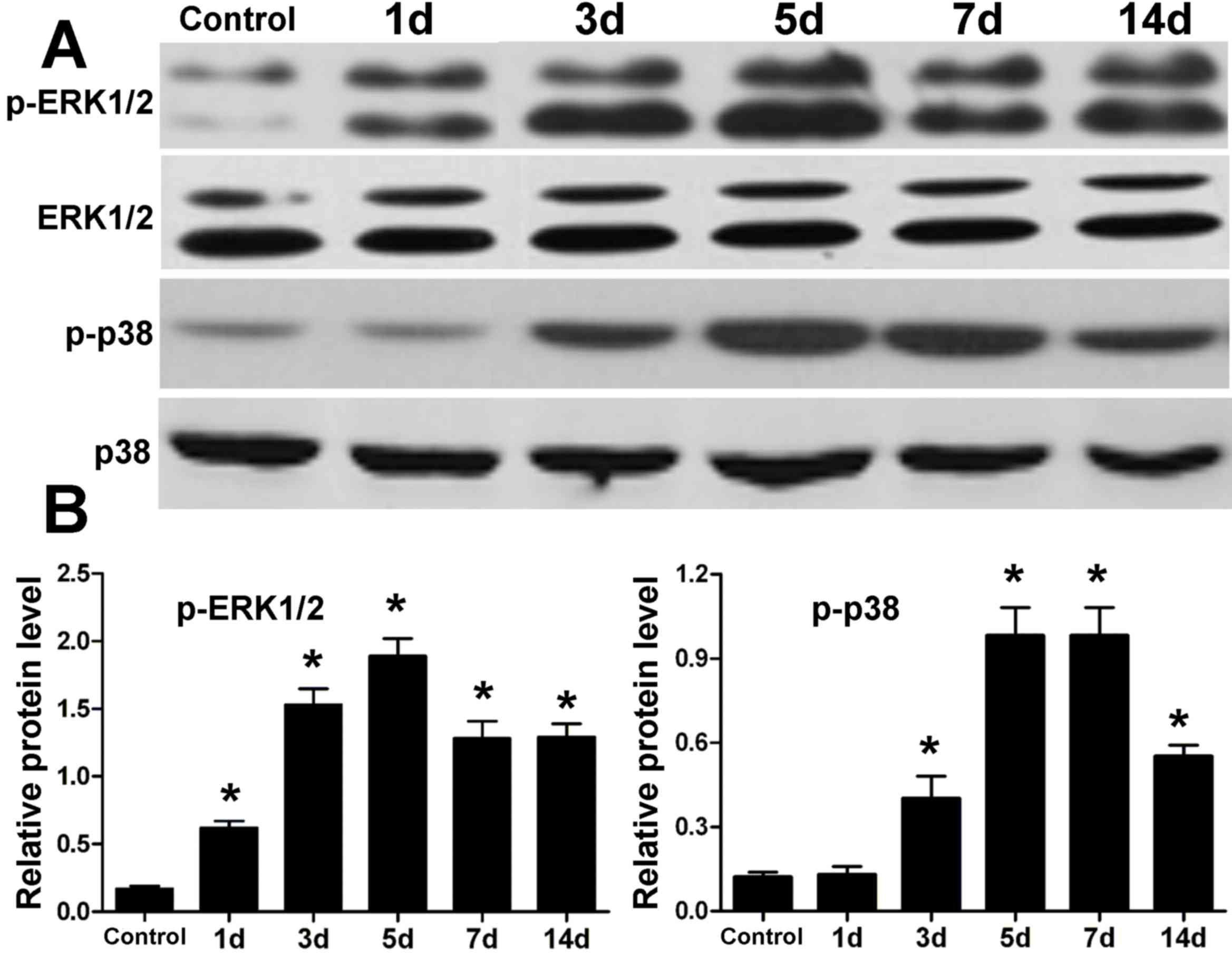
Protein expression levels of ERK1/2
and p38 MAPK in periodontal tissue of rats after tension force
The protein expression levels of ERK1/2 and p38 MAPK
in periodontal tissue were determined by western blotting (Fig. 1). Compared with the control, the
expression levels of ERK1/2 and p38 were similar following
orthodontic tooth movement; however, the expression levels of
p-ERK1/2 and p-p38 were elevated after tension force. In addition,
the increase in expression of p-ERK1/2 and p-p38 reached a peak
after 5 days of tooth movement. At 7 and 14 days, expression levels
of p-ERK1/2 and p-p38 were reduced to some extent, although they
were greater than the control.
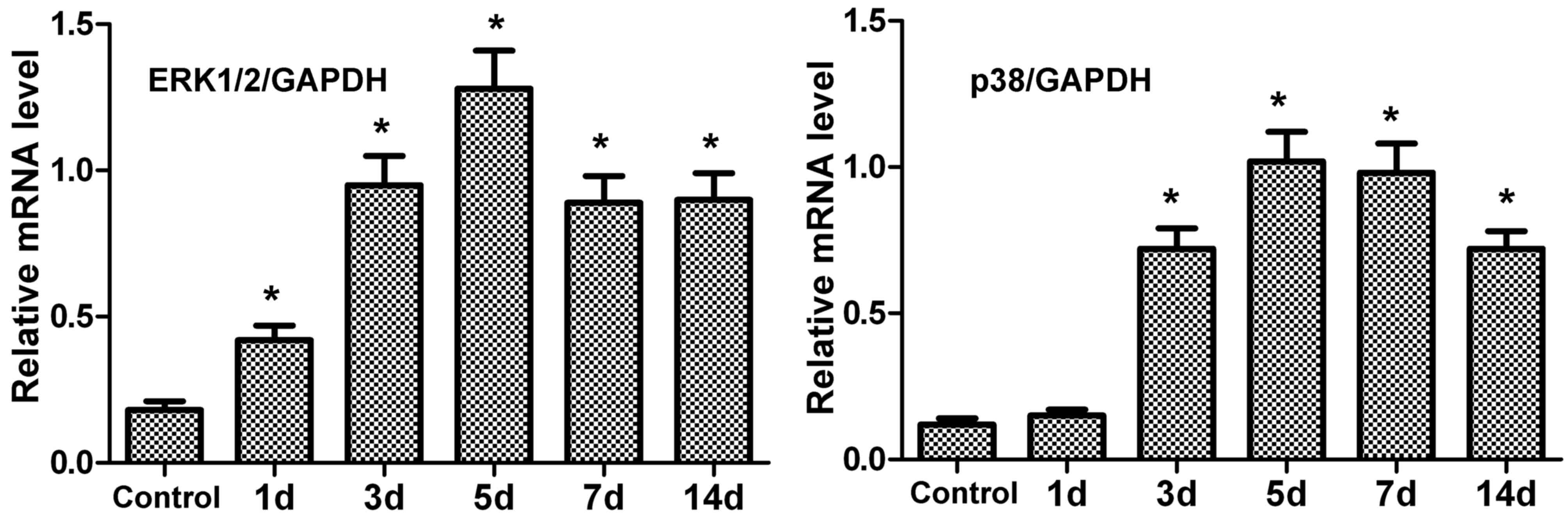
mRNA expression levels of ERK1/2 and
p38 in periodontal tissue of rats after tension force
The mRNA expression levels of ERK1/2 and p38 in
periodontal tissue were measured by RT-qPCR. Following tension
force, the mRNA expression levels of ERK1/2 and p38 in periodontal
tissue were upregulated compared with the control (Fig. 2). In addition, peak expression was
observed at 5 days.
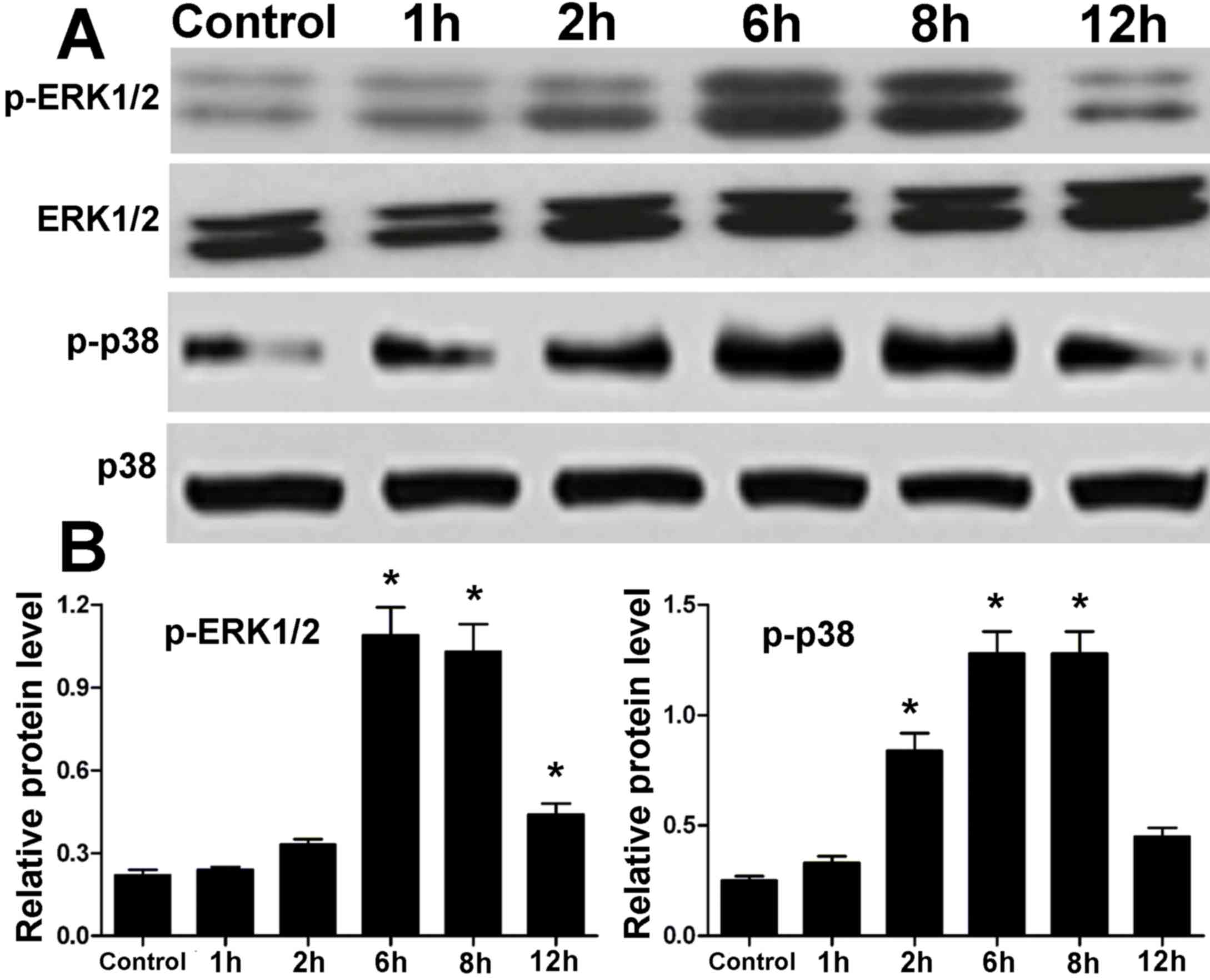
Protein expression levels of ERK1/2
and p38 MAPK in hPDLCs treated with centrifugal force
Protein expression levels of ERK1/2 and p38 MAPK in
hPDLCs treated with centrifugal force for various time points were
measured by western blotting. There was no difference in the
protein levels of ERK1/2 and p38 MAPK in hPDLCs between the control
and treatment groups (Fig. 3).
However, the expression levels of p-ERK1/2 and p-p38 were elevated
in the treatment groups compared with in the control group
(Fig. 3). After 1 to 6 h
centrifugal force, there was a gradual increase in the
phosphorylation levels of ERK1/2 and p38; however, at 12 h, the
phosphorylation of ERK1/2 and p38 MAPK proteins was reduced,
although still not to the levels of the control (Fig. 3). These results in hPDLCs were
similar to those observed in periodontal tissue.
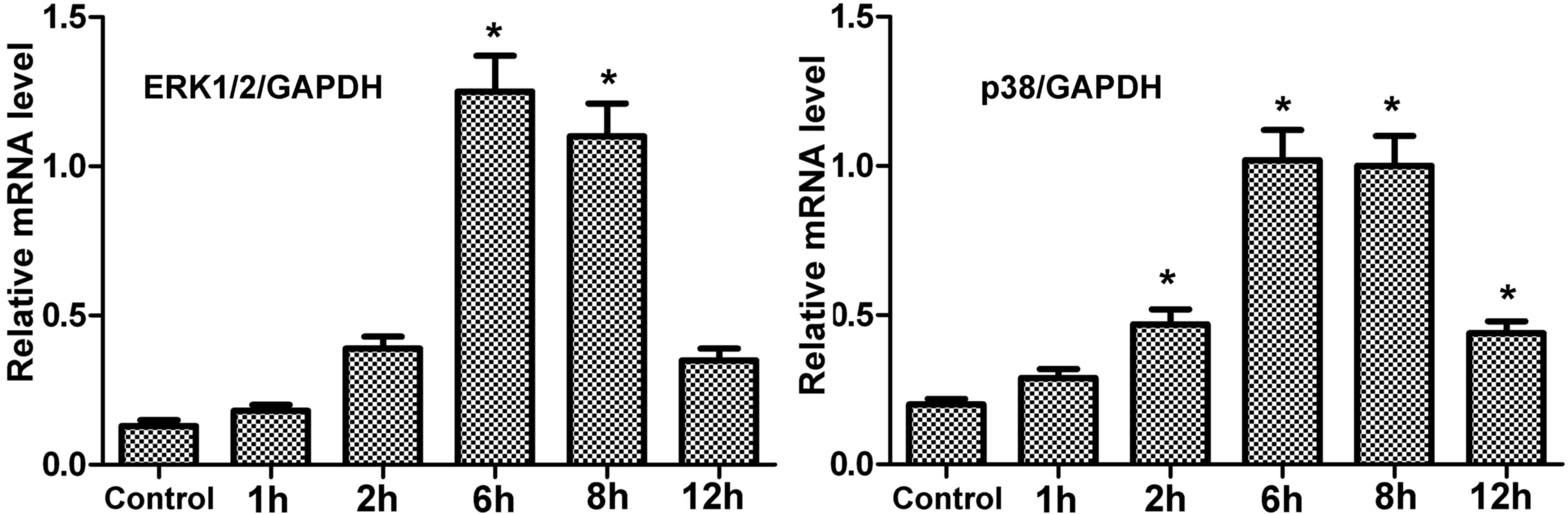
mRNA expression levels of encoding
ERK1/2 and p38 in hPDLCs treated with centrifugal force
The mRNA expression levels of ERK1/2 and p38 in
treated hPDLCs were greater than the control (Fig. 4). Under centrifugal force, mRNA
expression of these genes gradually increased with time, reaching a
peak at 6 h. However, at 12 h, the expression was reduced, although
not to the level of the control. Therefore, the results in hPDLCs
concurred with those of periodontal tissue.
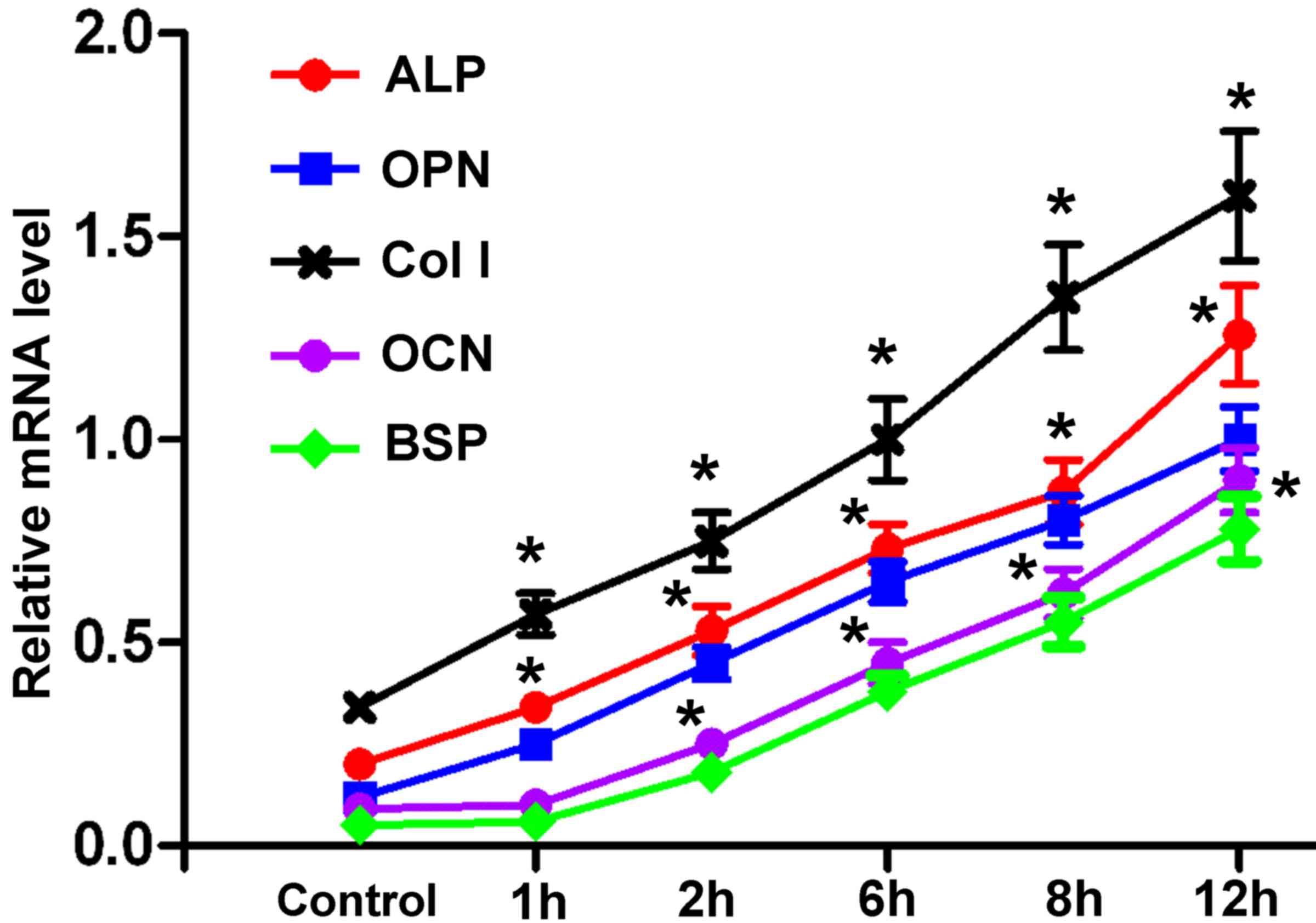
Expression of osteogenesis-associated
genes in hPDLCs exposed to centrifugal force
Following force treatment, the mRNA expression
levels of ALP, OPN, Col I, OCN and BSP were markedly upregulated in
hPDLCs compared with in the control group, in a time-dependent
manner (Fig. 5). The increase in
ALP, OPN, Col I was evident at 1 h after force treatment, whereas a
marked increase in OCN and BSP was observed at 6 h post
treatment.
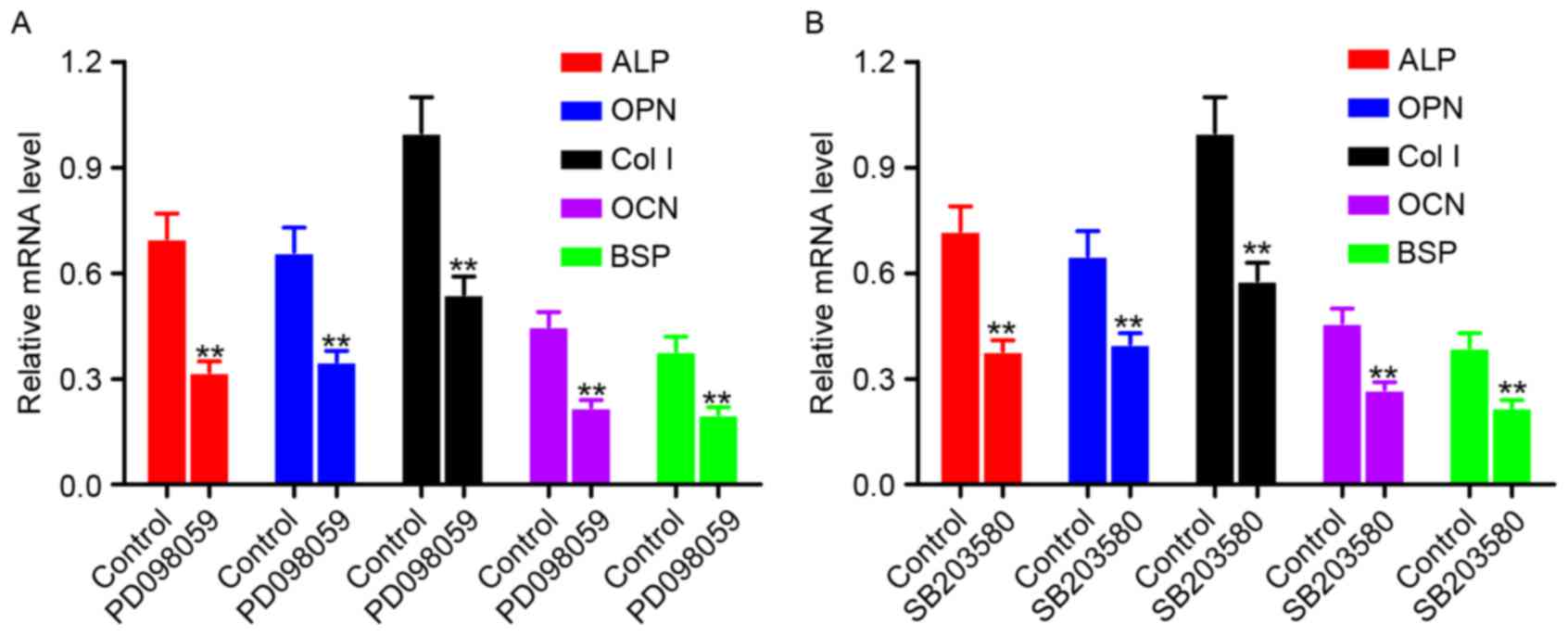
Expression of osteogenesis-associated
genes in hPDLCs following treatment with an ERK1/2 inhibitor and
p38 MAPK inhibitor
hPDLCs were treated with an ERK1/2 inhibitor
(PD098059) (Fig. 6A) or a p38 MAPK
inhibitor (SB203580) (Fig. 6B),
and the mRNA expression levels of osteogenesis-associated genes
were measured by RT-qPCR. Cells without inhibitor treatment served
as the control. Compared with the control group, the mRNA
expression levels of ALP, OPN, Col I, OCN and BSP were
significantly downregulated in the PD098059 or SB203580 treatment
groups (P<0.01).
Discussion
MAPK is a Ser/Thr protein kinase that is widely
expressed in various cells. Through a cascade reaction, the MAPK
pathway transduces signals to the cell nucleus to regulate
transcription, and therefore influences cell proliferation,
apoptosis and differentiation (12). In the MAPK family, ERK1/2, p38 and
JNK subfamilies have been extensively investigated. ERK1/2 and p38
are involved in osteogenesis-associated gene expression and bone
formation in vivo (13,14).
However, the full underlying mechanism of ERK1/2 and p38 pathways
in osteogenic differentiation remains contradictory. Li et
al (14) revealed that when
the ERK1/2 and p38 signaling pathways were inhibited in dental
follicle cells, osteogenic differentiation in early, middle and
advanced stages was promoted. However, Xiao et al (15) reported that inhibition of ERK1/2
resulted in restrained osteoblast activity and bone formation.
Therefore, ERK1/2 may exert positive or negative effects on
osteogenic differentiation, which may result from diverse external
stimuli, cell types and action time.
Kang et al (16) demonstrated that numerous pathways,
including MAPK, were activated in hPDLCs under mechanical pressure.
Tsutsumi et al (9) revealed
that intermittent mechanical force induced activation of the ERK1/2
and p38 MAPK pathways in hPDLCs. Cyclical tension stress of 10%
promoted the differentiation of hPDLCs via activation of the ERK1/2
signaling pathway and the expression of osteogenic
differentiation-associated genes (10). In addition, after continuous stress
with 0.25 and 0.5 Newtons in rats, the expression levels of
p-ERK1/2 were upregulated (17).
It was suggested that in in vitro cell experiments,
mechanical force had a positive effect on the ERK1/2 and p38 MAPK
signaling pathways. Therefore, to further clarify the role of
ERK1/2 and p38 MAPK pathways in orthodontic bone remodeling, the
present study used a rat model of orthodontic tooth movement via a
spring tension method and a hPDLCs model with centrifugal force.
Following tension stress for 1, 3, 5, 7 and 14 days in vivo
or centrifugal force for 1, 2, 6, 8 and 12 h ex vivo, the
protein and mRNA expression levels of ERK1/2 and p38 in periodontal
tissue or hPDLCs were measured by western blotting and RT-qPCR,
respectively. The results demonstrated that the mRNA and protein
expression levels of p-ERK1/2 and p-p38, and the mRNA expression
levels of ERK1/2 and p38, in periodontal tissue and hPDLCs were
markedly elevated. Only the mRNA expression levels of ERK1/2 and
p38 were increased; total protein levels were not increased. This
might result from the increased phosphorylation of ERK1/2 and p38
proteins following activation of the signal pathways. The
expression levels of these genes were affected in a time-dependent
manner, and the greatest expression was observed at 5 days (in
rats) or 6 h (in hPDLCs). These findings indicated that orthodontic
tension stress in rats and hPDLCs exposed to centrifugal force
resulted in the phosphorylation of ERK1/2 and p38. Although the
transcription of mRNA was initiated at a time that was earlier than
the expression time of proteins, the expression of mRNA and protein
were continuous and quick processes. This may not always be in a
time-dependent manner. Therefore, in the present study, the
time-dependent alterations of MAPK protein and mRNA were
consistent.
Bone formation involves osteoblast proliferation,
extracellular matrix formation and matrix mineralization, and is
associated with the expression of numerous genes, including ALP,
OPN, Col I, OCN and BSP (18). ALP
is primarily involved in initiating matrix mineralization and has a
suppressive effect on calcification inhibition. Its expression is
an early indicator of osteogenic differentiation and is a
precondition for OCN expression and calcium nodule formation
(19). As an early-to-mid
osteogenic marker, OPN binds to cells or the matrix surface, and
promotes the interaction between cells and the extracellular matrix
and hydroxyapatite production, thereby facilitating the
organization of osteoblast-like-cells, calcium compound deposition
and bone formation (20). Col I is
a primary scaffold of matrix mineralization and promotes osteoblast
adhesion and differentiation (21). As a vitamin K-dependent low
molecular weight protein, OCN exhibits a strong affinity for
calcium and hydroxyapatite, and is considered an indicator of
middle or advanced stage osteogenesis (22). As an extracellular matrix protein,
BSP is a marker of osteoblast maturation and is expressed at the
advanced stages of bone formation (23). Therefore, the present study
measured the mRNA expression levels of osteogenesis-associated
genes, including ALP, OPN, Col I, OCN and BSP, in hPDLCs following
centrifugal force treatment for 1, 2, 6, 8 and 12 h. The expression
levels of these genes increased by 6 h. These results suggested
that mechanical force positively regulated the expression of
osteogenesis-associated genes in hPDLCs. The increase in mRNA
expression levels of ALP, OPN, Col I, OCN and BSP in hPDLCs induced
by mechanical force was associated with activation of the ERK1/2
and p38 signaling pathways. Expression levels of OCN, BSP, p-ERK1/2
and p-p38 were greatest at 12 h under stress, and this suggested
that the phosphorylation of ERK1/2 and p38 was required for
transcription of OCN and BSP. After 6 h, the expression levels of
p-ERK1/2 and p-p38 were reduced; however, the expression of
osteogenesis-associated genes continued to be upregulated. It was
suggested that these five genes may be influenced by other
signaling pathways, including bone morphogenetic protein/Smad,
Wnt/β-catenin, Notch and Hedgehog (24–26).
In addition, Tang et al (27) revealed that flow shear stress on
hPDLCs for 2 h resulted in enhancement of cell viability, ALP
activity, Col I mRNA levels, p-ERK1/2, p-p38 MAPK, generation of
osteoid-like nodules and rearrangement of filamentous actin.
However, following administration of MAPK inhibitors U0126 and
SB203580, the alterations induced by flow shear stress were
suppressed (24). It was suggested
that flow shear stress stimulated osteogenic differentiation of
hPDLCs via the ERK1/2 and p38 MAPK signaling pathways. Activation
of the ERK1/2 pathway and upregulation of osteogenesis-associated
genes may accelerate osteogenesis and calcification of hPDLCs
(8,28). Therefore, in the present study, the
effects of an ERK1/2 inhibitor and p38 MAPK inhibitor on
osteogenesis-associated genes in hPDLCs were investigated. RT-qPCR
demonstrated that the ERK1/2 inhibitor and p38 MAPK inhibitor
significantly suppressed the mRNA expression levels of ALP, OPN,
Col I, OCN and BSP, which suggested that suppression of the ERK1/2
and p38 MAPK pathways may inhibit expression of these
osteogenesis-associated genes and bone formation of hPDLCs.
In conclusion, the expression and regulation of
ERK1/2 and p38 MAPK signaling pathways were investigated in
periodontal tissue following orthodontic tooth movement in
vivo and in hPDLCs exposed to centrifugal force ex vivo.
The protein expression levels of p-ERK1/2 and p-p38, rather than
total ERK1/2 and p38, were elevated in orthodontic periodontal
tissue in vivo and hPDLCs ex vivo. The mRNA
expression levels of ERK1/2 and p38 in orthodontic periodontal
tissue in vivo and hPDLCs ex vivo were additionally
upregulated and positively regulated the expression levels of
osteogenesis-associated genes, which was further demonstrated using
specific inhibitors of ERK1/2 and p38 signaling. ERK1/2 and p38
MAPK pathways were positively and closely associated with
periodontal tissue remodeling of orthodontic tooth movement. These
findings aid further investigation of the underlying molecular
mechanisms in periodontal tissue following orthodontic tooth
movement, and the development of drugs that target the ERK1/2 and
p38 MAPK signaling pathways.
References
|
1
|
Liu X and Li Z: Research progress of
biochemical markers changes of bone turnover in orthodontic tooth
movement. J Oral Sci Res. 29:487–489. 2013.
|
|
2
|
Adusumilli S, Yalamanchi L and
Yalamanchili PS: Periodontally accelerated osteogenic orthodontics:
An interdisciplinary approach for faster orthodontic therapy. J
Pharm Bioallied Sci. 6 Suppl 1:S2–S5. 2014. View Article : Google Scholar :
|
|
3
|
Zhou H and Cai P: Research progress on
periodontal conditions in subjects following orthodontic therapy.
Int J Oral Sci. 38:109–111. 2011.
|
|
4
|
Peng P and Wang W: Biological behavior of
periodontal ligament in orthodontic tooth movement. Beijing J Stom.
20:354–357. 2012.
|
|
5
|
Bao X and Hu M: Research progress on
mechanisms and regulations of bone resorption in orthodontic tooth
movement. Int J Oral Sci. 39:187–189. 2012.
|
|
6
|
Kim IS, Jeong BC, Kim OS, Kim YJ, Lee SE,
Lee KN, Koh JT and Chung HJ: Lactone form
3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors
(statins) stimulate the osteoblastic differentiation of mouse
periodontal ligament cells via the ERK pathway. J Periodontal Res.
46:204–213. 2011. View Article : Google Scholar
|
|
7
|
Chen X, Shi J, Ye Q, Cai X and Zhang T:
Effect of p38 MAPK signaling pathway on BMP-2-induced osteogenic
differentiation of human dental follicle cells. Chin J Cell Bio.
35:816–823. 2013.(In Chinese). View Article : Google Scholar
|
|
8
|
Liu C and Sun J: Hydrolyzed tilapia fish
collagen induces osteogenic differentiation of human periodontal
ligament cells. Biomed Mater. 10:0650202015. View Article : Google Scholar
|
|
9
|
Tsutsumi T, Kajiya H, Fukawa T, Sasaki M,
Nemoto T, Tsuzuki T, Takahashi Y, Fujii S, Maeda H and Okabe K: The
potential role of transient receptor potential type A1 as a
mechanoreceptor in human periodontal ligament cells. Eur J Oral
Sci. 121:538–544. 2013. View Article : Google Scholar
|
|
10
|
Li L, Han M, Li S, Wang L and Xu Y: Cyclic
tensile stress during physiological occlusal force enhances
osteogenic differentiation of human periodontal ligament cells via
ERK1/2-Elk1 MAPK pathway. DNA Cell Biol. 32:488–497. 2013.
View Article : Google Scholar :
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Gerdts J, Summers DW, Milbrandt J and
DiAntonio A: Axon self-destruction: New links among SARM1, MAPKs,
and NAD+ metabolism. Neuron. 89:449–460. 2016. View Article : Google Scholar :
|
|
13
|
Kim HK, Kim MG and Leem KH: Effects of egg
yolk-derived peptide on osteogenic gene expression and MAPK
activation. Molecules. 19:12909–12924. 2014. View Article : Google Scholar
|
|
14
|
Li C, Yang X, He Y, Ye G, Li X, Zhang X,
Zhou L and Deng F: Bone morphogenetic protein-9 induces osteogenic
differentiation of rat dental follicle stem cells in P38 and ERK1/2
MAPK dependent manner. Int J Med Sci. 9:862–871. 2012. View Article : Google Scholar :
|
|
15
|
Xiao G, Jiang D, Gopalakrishnan R and
Franceschi RT: Fibroblast growth factor 2 induction of the
osteocalcin gene requires MAPK activity and phosphorylation of the
osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem.
277:36181–36187. 2002. View Article : Google Scholar
|
|
16
|
Kang KL, Lee SW, Ahn YS, Kim SH and Kang
YG: Bioinformatic analysis of responsive genes in two-dimension and
three-dimension cultured human periodontal ligament cells subjected
to compressive stress. J Periodontal Res. 48:87–97. 2013.
View Article : Google Scholar
|
|
17
|
Pavlidis D, Bourauel C, Rahimi A, Götz W
and Jäger A: Proliferation and differentiation of periodontal
ligament cells following short-term tooth movement in the rat using
different regimens of loading. Eur J Orthod. 31:565–571. 2009.
View Article : Google Scholar
|
|
18
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar
|
|
19
|
Guo Z, Kang S, Chen D, Wu Q, Wang S, Xie
W, Zhu X, Baxter SW, Zhou X, Jurat-Fuentes JL and Zhang Y: MAPK
signaling pathway alters expression of midgut ALP and ABCC genes
and causes resistance to Bacillus thuringiensis Cry1Ac toxin in
diamondback moth. PLoS Genet. 11:e10051242015. View Article : Google Scholar :
|
|
20
|
Zhang R, Zhang Z, Pan X, Huang X, Huang Z
and Zhang G: ATX–LPA axis induces expression of OPN in hepatic
cancer cell SMMC7721. Anat Rec (Hoboken). 294:406–411. 2011.
View Article : Google Scholar
|
|
21
|
Wang C: The effect of Asiaticoside on the
expression of TGF-β on thIand Col III in rats after myocardial
infarction. J Comm Medi. 2015.(In Chinese).
|
|
22
|
Rocha ÉD, de Brito NJ, Dantas MM, Silva
Ade A, Almeida Md and Brandão-Neto J: Effect of zinc
supplementation on GH, IGF1, IGFBP3, OCN, and ALP in
non-zinc-deficient children. J Am Coll Nutr. 34:290–299. 2015.
View Article : Google Scholar
|
|
23
|
Bouet G, Bouleftour W, Juignet L,
Linossier MT, Thomas M, Vanden-Bossche A, Aubin JE, Vico L, Marchat
D and Malaval L: The impairment of osteogenesis in bone
sialoprotein (BSP) knockout calvaria cell cultures is cell density
dependent. PLoS One. 10:e01174022015. View Article : Google Scholar :
|
|
24
|
Xu L and Kong Q: Research progress of key
signaling pathways in osteoblast differentiation and bone formation
regulation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 28:1484–1489.
2014.(In Chinese).
|
|
25
|
Yang Z, Ren L, Deng F, Wang Z and Song J:
Low-intensity pulsed ultrasound induces osteogenic differentiation
of human periodontal ligament cells through activation of bone
morphogenetic protein-smad signaling. J Ultrasound Med. 33:865–873.
2014. View Article : Google Scholar
|
|
26
|
Matsuzawa M, Sheu TJ, Lee YJ, Chen M, Li
TF, Huang CT, Holz JD and Puzas JE: Putative signaling action of
amelogenin utilizes the Wnt/beta-catenin pathway. J Periodontal
Res. 44:289–296. 2009. View Article : Google Scholar
|
|
27
|
Tang M, Peng Z, Mai Z, Chen L, Mao Q, Chen
Z, Chen Q, Liu L, Wang Y and Ai H: Fluid shear stress stimulates
osteogenic differentiation of human periodontal ligament cells via
the extracellular signal-regulated kinase 1/2 and p38
mitogen-activated protein kinase signaling pathways. J Periodontol.
85:1806–1813. 2014. View Article : Google Scholar
|
|
28
|
Lee SK, Chung JH, Choi SC, Auh QS, Lee YM,
Lee SI and Kim EC: Sodium hydrogen sulfide inhibits nicotine and
lipopolysaccharide-induced osteoclastic differentiation and
reversed osteoblastic differentiation in human periodontal ligament
cells. J Cell Biochem. 114:1183–1193. 2013. View Article : Google Scholar
|