Introduction
Constant bone mass is maintained by bone remodeling
during adulthood and depends on the regulation of
osteoblast-osteoclast coupling (1,2).
Since resorption of old mineralized bone by osteoclasts is followed
by new bone formation by osteoblasts, the osteoblast-osteoclast
coupling can tightly regulate initiation, transition, and
termination phases of bone resorption (3). Loss of the coupling and resulting
disruption of bone homeostasis can result in several metabolic bone
diseases, including osteopetrosis, osteoporosis and
tumor-associated bone diseases (3). Therefore, therapies targeting these
diseases mainly fall into two categories: Anabolic drugs, which
stimulate bone formation, and antiresorptive drugs, which slow down
bone resorption (4).
Estrogen, a steroid hormone, is well-known to affect
the circulatory, reproductive and cardiovascular systems, and bone
homeostasis through inhibition of bone resorption and enhancement
of bone formation (5,6). Estrogen deficiency can cause early
and late forms of osteoporosis in postmenopausal women (7). There are three types of estrogen:
17β-estradiol (E2), estron and estriol. E2, secreted by the ovarium
of adult women with a normal menstrual cycle, has the highest
estrogenic potency (8). It has
been demonstrated that E2 supplementation effectively stimulates
the proliferative capacity of mesenchymal stem cells (MSCs)
(9). Further investigation
suggests that E2 induces bone formation by stimulating bone
morphogenetic protein-2 gene transcription in MSCs (9). E2 inhibits senescence of MSC via the
upregulation of telomerase, and improvement of osteogenic and
adipogenic differentiation of MSCs (10,11).
The impact of eph-ephrin bidirectional signaling on
bone homeostasis provides an explanation for cellular and molecular
mechanisms responsible for osteoblast-osteoclast coupling (1). Osteo-blasts and osteoclasts can
proliferate and differentiate from MSCs and macrophagocytes, and
ephrins and ephs coupling regulates these cell-cell interaction
processes (2). Ephrins are divided
into two classes, ephrinAs (ephrinA1-A5) and ephrinBs
(ephrinB1-B3). Ephs fall into the following two categories, ephAs
(ephA1-A10) and ephBs (ephB1-B6) (12). A previous study reported that
ephrinA2-ephA2 interaction facilitates the initiation phase of bone
remodeling through enhancing osteoclast differentiation and
suppressing osteoblast differentiation (1).
The present study aimed to investigate whether E2
exerts its bone protective effects through the ephA2/ephrinA2
signaling pathway in bone deterioration induced by ovariectomy
(OVX) in rats. Firstly, OVX was performed on rats and the effects
of estrogen on OVX-induced body weight gain, bone turnover markers
and key signaling molecules involved in the regulation of bone
metabolism were evaluated. Effects of estrogen on BMD, bone
histomorphology and trabecular bone microarchitecture were also
detected. Finally, the mechanism underlying the effect of E2 on
postmenopausal osteoporosis was explored. The present study
demonstrated that E2 attenuates OVX-induced bone deterioration
partially through the suppression of the ephA2/ephrinA2 signaling
pathway, a result which aids the prevention and treatment of
postmenopausal osteoporosis.
Materials and methods
Animals
A total of 45 Sprague-Dawley rats (12-week-old
females; weighing 220–240 g) were obtained from the Animal Center
of the Institute of Science and Technology (Shanghai, China) and
were housed in a controlled environment laboratory for 2 weeks
prior to the start of the experiment. Rats were kept at 22–24°C
with a 12-h light/dark cycle and free access to food and water. All
procedures involving animal welfare were reviewed and approved by
the Ethical Committee of Punan Hospital of Pudong New District
(Shanghai, China).
Experimental protocols
Rats were divided into three groups: i) Sham
operated (SHAM, n=15); ii) ovariectomized without treatment (OVX,
n=15); and iii) ovariectomized rats treated with E2 (OVX+E2, n=15).
All compounds were supplied by Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany) unless stated otherwise. Rats were
subcutaneously injected 5 times per week with 100 µl medium (10%
dimethyl sulfoxide, 90% sesame oil) in the SHAM and OVX groups, and
with E2 (20 µg/kg/day) in the OVX+E2 group. All three groups
received treatment at the same time each day. All rats were weighed
following 2, 4, 6 and 8 weeks of treatment.
Biochemical analysis
At 8 weeks of treatment, rats were deeply
anesthetized with urethane (5 ml/kg) intraperitoneally and
euthanized by exsanguination. Blood was collected following
overnight fasting and serum was separated by centrifugation at 500
× g for 15 min at 21°C and stored at −70°C. The level of serum
calcium (Ca) was measured on the Ciba Corning 550 EXPRESS using
Ciba Corning reagents (Ciba Corning Diagnostic Ltd., Sudbury, UK)
for the in vitro determination. The urine Ca and creatinine
(Cr) concentrations were analyzed by the same method as the serum
samples at 2, 4, 6 and 8 weeks following treatment. Serum
bone-specific alkaline phosphatase (b-ALP) and bone resorption
tartrate-resistant acid phosphatase-5b (TRAP-5b) were determined by
ELISA (cat no. SB-TR103; Immunodiagnostic Systems, Boldon, UK)
according to the manufacturer's protocol.
Bone mineral density (BMD)
analysis
BMD of left tibiae was measured using dual energy
X-ray absorptiometry (DEXA) and Lunar-DPX-IQbone densitometry (GE
Healthcare, Chicago, IL, USA).
Histopathological and
histomorphometric analyses
Following 60 days of treatment, left tibiae were
fixed by immersion in buffered formalin for 72 h at room
temperature, then decalcified in 10% ethylenediaminetetraacetic
acid for 4 weeks, dehydrated in a desiccator with graded ethanol
(25, 50, 70, 90 and 100%), defatted in xylene and embedded in
paraffin. Longitudinally oriented, 5-mm-thick sections were cut and
stained with H&E at room temperature for 10 min for
histopathological analysis, and with Safranin-O/Fast green dye for
10 min at room temperature for histomorphometrical analysis.
Measurements were taken using a light microscope (magnification,
×4; Leica Microsystems GmbH, Wetzlar, Germany) and an image
analyzer (Image Pro-Express; Media Cybernetics Inc., Rockville, MD,
USA). Static parameters including bone volume per tissue volume
(BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and
trabecular separation (Tb.Sp) were calculated and expressed as
previously described (13,14).
Osteoclast differentiation in
vitro
Monocytes were isolated from the tibia and femur
bone marrow of 8–10 weeks old rats, as previously described
(15). Bone marrow cells were
seeded in 96-well plates (3×104 cells/well) and washed
with 50 nmol/lmacrophage colony-stimulating factor 1 (M-CSF). After
4 days, the cells were considered bone-marrow-derived macrophages
(BMMs). To induce osteoclastogenesis, BMMs were seeded in 48-well
plates at a density of 15,000 cells/well and cultured with 100
nmol/l mouse receptor activator of NF-κB ligand (RANKL) and 50
nmol/l M-CSF for 4–6 days in the presence or absence of the bone
marrow sample. The medium was replaced every 2 days.
Small-interfering (si)RNA-based
knockdown of ehpA2 and ephrinA2
siRNA targeting ehpa2 (5′-CAAUCACCGACCACCGGAC-3′) or
ephrinA2 (5′-CGTACGCGGAATACTTCGA-3′) (all from Qiagen, Inc.,
Valencia, CA, USA) were used for transfection of cells using
Lipofectamine RNAi MAX Transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). A total of 500,000
cells were transfected with siRNA and harvested 48 h following
transfection for the assessment of knockdown efficiency, or other
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) measurement of gene
expression
At 8 weeks of treatment, rats were deeply
anesthetized with urethane (5 ml/kg) intraperitoneally and
euthanized by exsanguination. Total RNA was extracted from bone
marrow cells obtained from right tibiae using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Reactions were performed in an ABI7300
Real-Time quantitative instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: Initial denaturation at 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 31 sec. The expression levels
of the internal control GAPDH, was used as a housekeeping gene, and
the comparative 2−∆∆Cq method (16) was used to quantify gene expression
levels.
Western blot assay
Protein was collected from proximal tibias that were
lysed in radioimmunoprecipitation buffer (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) containing protease inhibitors at 4°C for 30
min, and the protein concentrations were quantified using a Bio-Rad
protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Proteins (30 µg) were separated on 8% SDS-PAGE gels and transferred
to polyvinylidene difluoride membranes (Amersham; GE Healthcare,
Chicago, IL, USA). The membranes were blocked in 5% non-fat milk
(Merck KGaA) overnight at 4°C. Transferred membranes were then
stained with the following primary antibodies: Anti- ehpa2 (cat no.
507183; 1:1,000; Zhejiang Kangchen Biotech Co., Ltd.), anti-
ephrinA2 (cat no. 123877; 1:2,000; R&D Systems, Inc.,
Minneapolis, MN, USA), and anti-β-actin (cat no. ab8227; 1:200;
Abcam) overnight at 4°C. Subsequently, protein bands were detected
by incubation with a horseradish peroxidase-conjugated secondary
antibody (cat no. A50-106P; 1:1,000; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) at room temperature
for 1 h. Signals were detected using an enhanced chemiluminescence
kit (cat. no. orb90504; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) and exposed to Kodak X-OMAT film (Kodak, Rochester,
NY, USA). Each experiment was performed at least three times and
the results were analyzed using Alpha View Analysis Tools
(AlphaView SA software, version 3.2.2; Protein Simple, Santa Clara,
CA, USA).
Statistical analysis
Results of the present study were expressed as the
mean ± standard deviation. Data analysis was performed using SPSS
(version 12.0; SPSS, Inc., Chicago, IL, USA). Differences between
multiple independent groups were determined using one-way analysis
of variance followed by a Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of E2 on OVX-induced body
weight gain
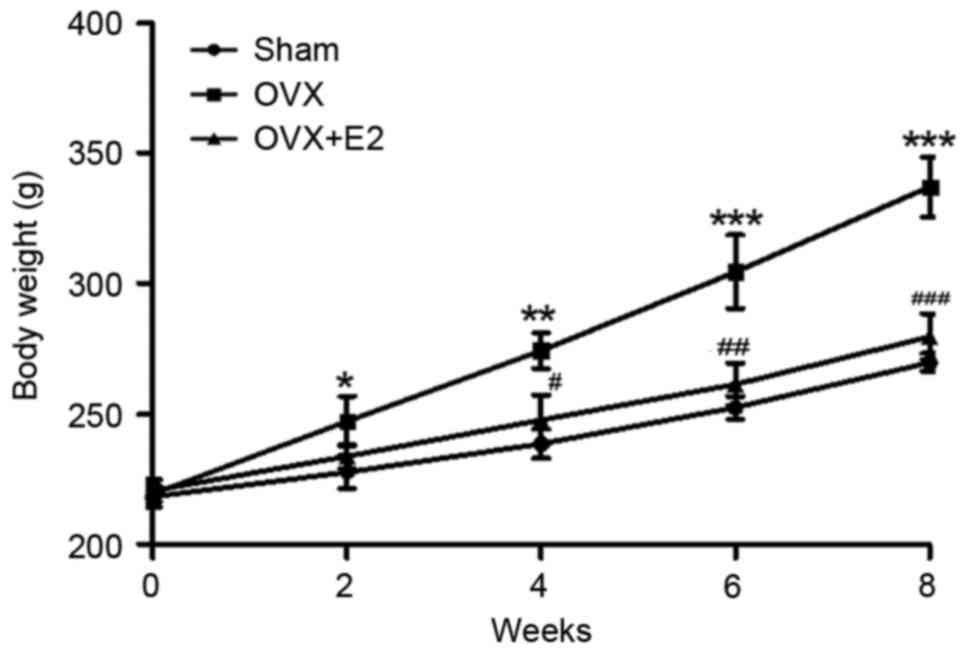
In the present study, the initial body weights of
the three groups were similar, 220 g. In the OVX group, body weight
gain was consistently elevated compared with the SHAM group. Even
though the daily food consumption in each group was nearly the
same, the body weight of the OVX group was significantly increased
compared with the SHAM group. Following E2 treatment, OVX-induced
body weight gain was significantly suppressed in a time-dependent
manner (Fig. 1). This outcome
suggests that E2 had a suppressive effect on OVX-induced body
weight gain.
Effects of E2 on bone turnover
markers
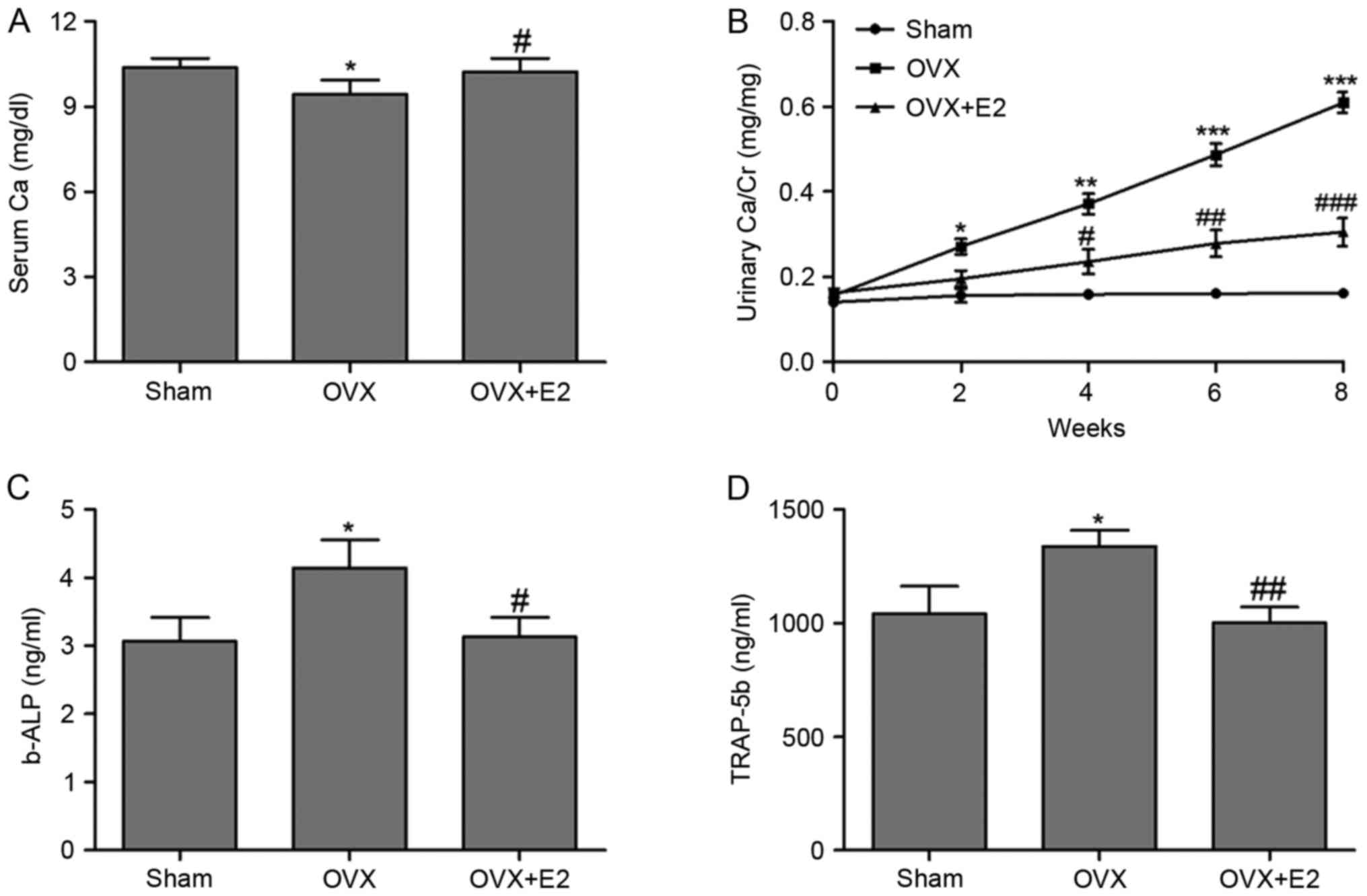
To further investigated the role of E2 in
OVX-induced bone deterioration, markers for bone turnover,
including serum Ca, urinary Ca/Cr, b-ALP and TRAP-5b were measured.
Serum Ca concentration in the OVX+E2 group was significantly
increased compared with the OVX group (Fig. 2A). Detection of urinary Ca/Cr for 8
weeks revealed increased levels the in OVX group compared with the
SHAM group, while an immediate decrease was observed following E2
treatment (Fig. 2B). b-ALP and
TRAP-5b concentrations in serum were also detected, revealing that
E2 treatment effectively attenuated the enhancement of b-ALP and
TRAP-5b concentrations induced by OVX in rats (Fig. 2C and D).
Effects of E2 on expression levels of
bone metabolism-associated genes
ALP, TRAP, runt-related transcription factor 2
(Runx2), Sp7 transcription factor (osterix), collagen alpha-1(I)
chain (col1a1), osteoprotegerin (OPG) and RANKL are key signaling
molecules involved in the regulation of bone metabolism. ALP is a
marker for osteoblast activity and TRAP is a bone resorption
factor. Runx2 is a master transcription factor of bone formation
and osteoblast differentiation, and osterix, acting downstream of
Runx2, regulates the transcription of bone-specific Col1a1 gene
(17,18). RANKL regulates osteoclast
differentiation and function, and OPG is a soluble decoy receptor
for RANKL, which competes with RANKL for binding to RANK and
therefore suppresses osteoclast formation and activation (19,20).
E2 treatment effectively and significantly
attenuated the upregulation of ALP and TRAP mRNA induced by OVX
(Fig. 3A and B). Runx2, osterix
and Col1a1 were markedly downregulated in the OVX group compared
with the SHAM and OVX+E2 groups (Fig.
3C). Additionally, E2 significantly suppressed the OVX-induced
RANKL upregulation but had no evident effect on the OPG level
(Fig. 3D). Therefore, the
OPG/RANKL ratio was decreased in the OVX group compared with the
SHAM group, indicating that OVX activated generation and
differentiation of osteoclasts. The aforementioned results
suggested that E2 inhibited bone resorption function of osteoclasts
by inhibiting the production of TRAP and RANKL, and induced bone
formation function of osteoblasts by inducing Runx2, osterix and
Col1a1 expression.
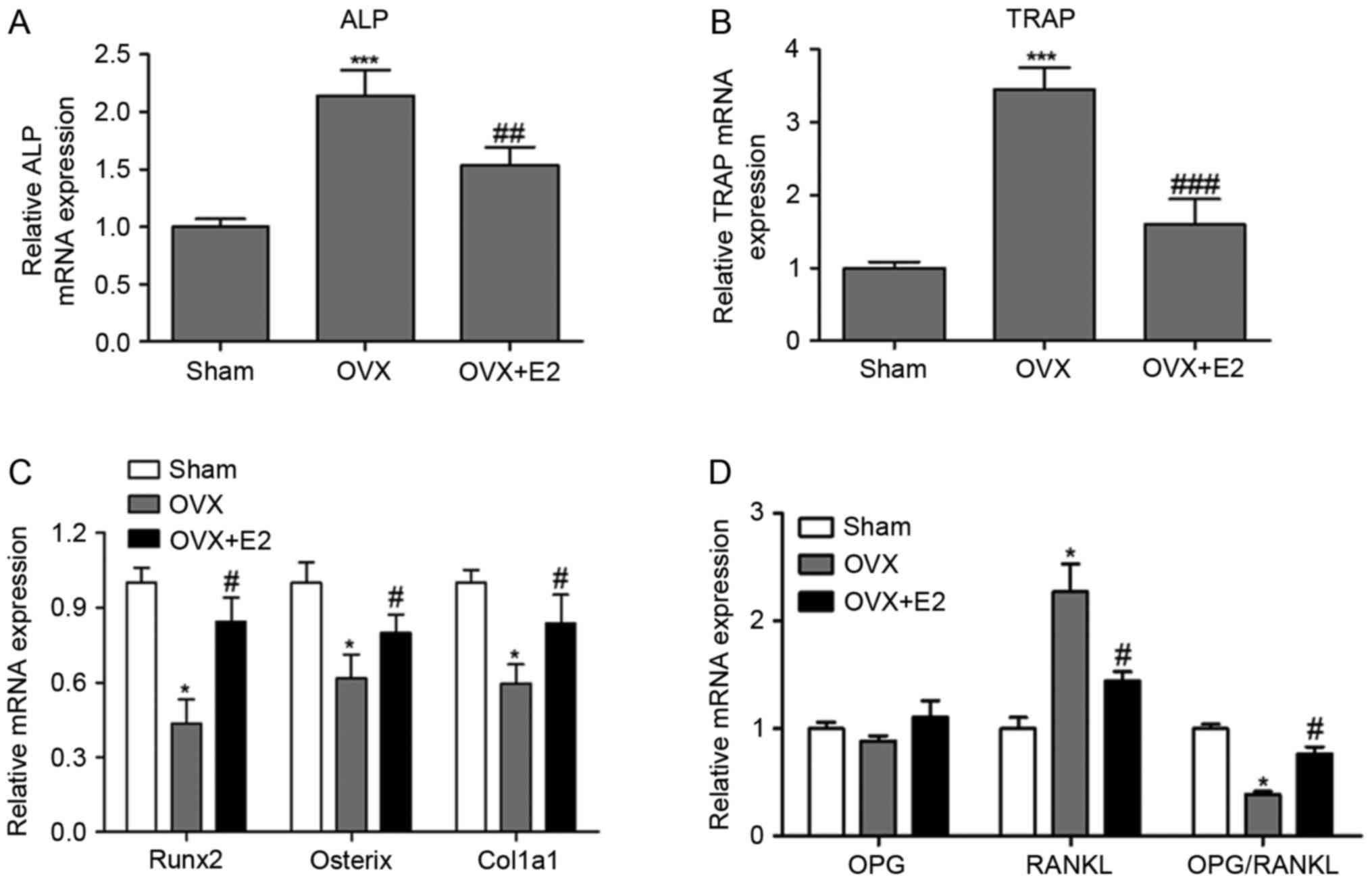 | Figure 3.Effects of E2 on expression levels of
bone metabolism-associated genes. (A) Relative ALP; (B) TRAP; (C)
Runx2, osterix and col1a1; (D) OPG and RANKL mRNA expression. Data
are presented as the mean ± standard deviation. *P<0.05,
***P<0.001 vs. the SHAM group. #P<0.05,
##P<0.01, ###P<0.001 vs. the OVX group.
Sham, placebo operated rats; OVX, ovariectomy; E2, 17β-estradiol;
TRAP, tartrate-resistant acid phosphatase-5b; ALP, alkaline
phosphatase; Runx2, runt-related transcription factor 2; osterix,
Sp7 transcription factor; col1a1, collagen alpha-1(I) chain; OPG,
osteoprotegerin; RANKL, receptor activator of nuclear factor κB
ligand. |
Effects of E2 on BMD and bone
histomorphology of the experimental rats
As presented in Fig.
4A, the BMD of tibia differed significantly between the three
groups at 4 and 8 weeks following treatment. Tibiae BMD decreased
notably in the OVX compared with the SHAM group. E2 treatment
significantly increased the OVX+E2 group tibia BMD compared with
the OVX group.
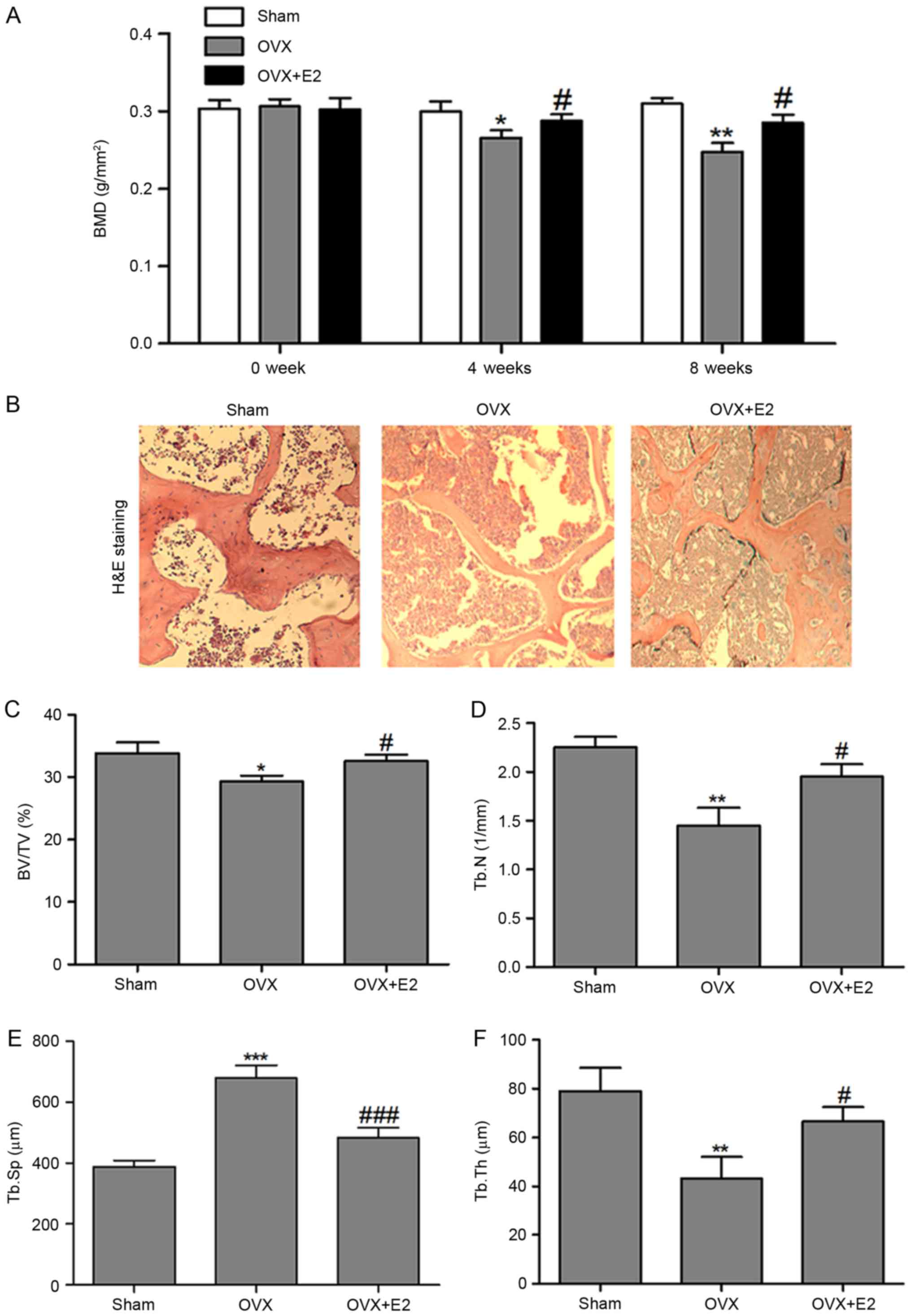 | Figure 4.Effects of E2 on bone morphology. (A)
Effect of E2 on BMD in left tibiae of OVX rats evaluated by DXA (B)
Effect of E2 on bone histomorphology analyzed by H&E staining
(magnification, ×100). (C) Effects of E2 on the BV/TV; (D) Tb.N;
(E) Tb.Sp; and (F) Tb.Th in the left tibiae of rats. Data are
presented as the mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001 vs. the SHAM group.
#P<0.05, ###P<0.001 vs. the OVX group.
DEXA, Dual-energy X-ray absorptiometry; Sham, placebo operated
rats; OVX, ovariectomy; E2, 17β-estradiol; BV/TV, bone volume per
tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular
number; Tb.Sp, trabecular separation; BMD, bone mineral density;
H&E, hematoxylin and eosin. |
H&E staining was performed to assess the effects
of E2 on bone histomorphology. As presented in Fig. 4B, following OVX, the trabecular
bone became thinner, sparse and interrupted, which showed reduced
area in visual field/unit, and demonstrated enlargement of the
medullary canal compared with the SHAM tissue. E2 reversed the
effects of OVX stimulation on the bone structure, and the
photomicrograph of the trabecular bone in E2-treated rats appeared
similar to the SHAM group trabecular bone.
Histomorphometry was performed to evaluate bone
quality and architecture. As presented in Fig. 4C-F, OVX rats had a reduced BV/TV,
Tb.N and Tb.Th compared with the SHAM group. E2
treatmentreversedthose reductions and prevented the increase of the
Tb.S P-value, which differed significantly between the OVX and E2
groups. Low BV/TV, Tb.N and Tb.Th, and high Tb.Sp in the OVX group
indicated bone loss, mainly due to trabecular perforation and
thinning, and loss of trabecular connectivity. All these effects
were attenuated by treatment with E2.
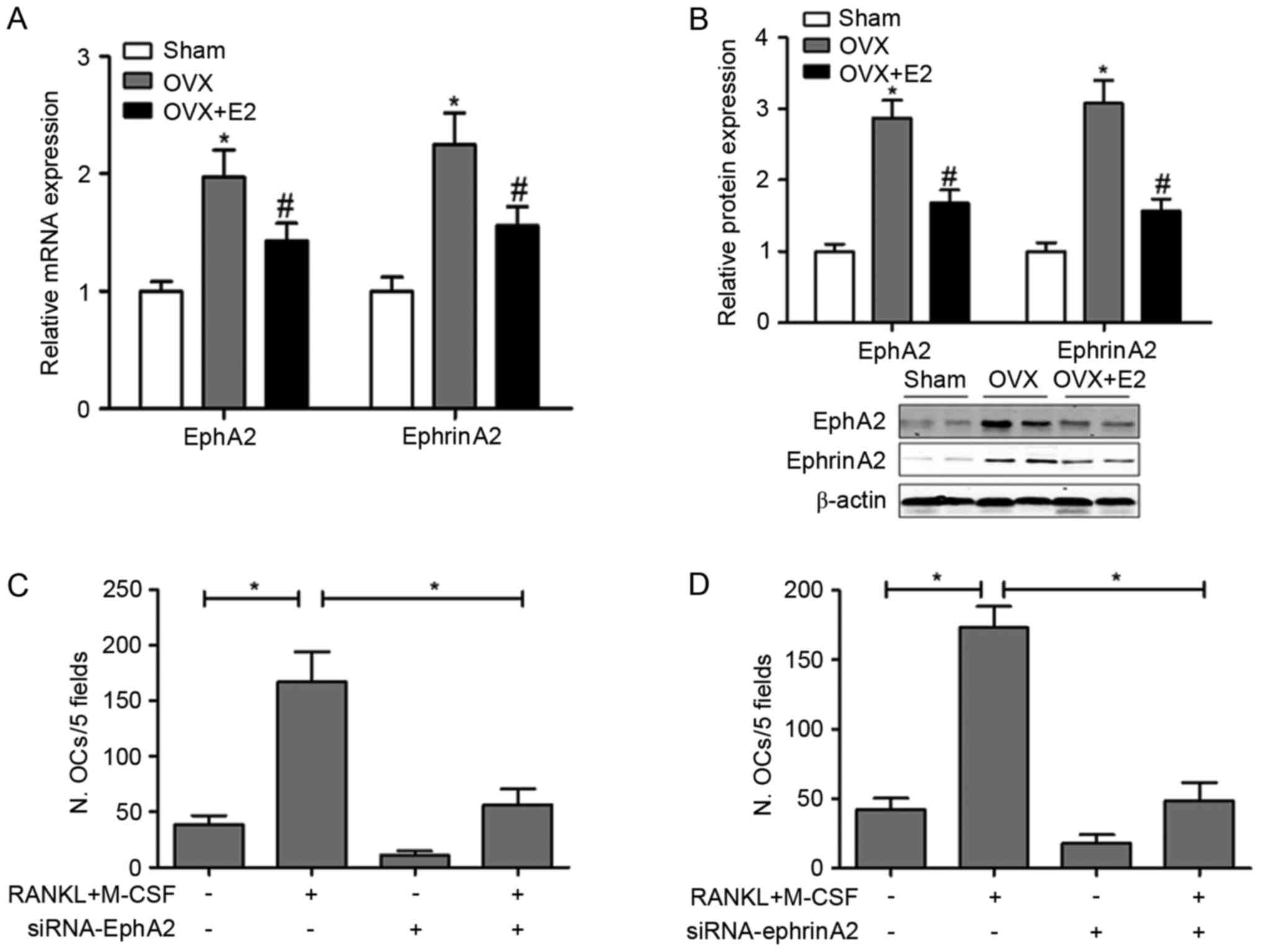
E2 suppresses ephA2 and ephrinA2
expression in the experimental rats
It is commonly accepted that bone homeostasis is
regulated by various genes and signaling pathways in response to
osteotropic agents. Among these pathways, ephA2-ephrinA2 signaling
was reported to stimulate osteoclast and inhibit osteoblast
differentiation (12). Therefore,
the present study investigated whether the effect of E2 on bone
formation/resorption regulation is associated with the expression
of ephA2 and ephrin A2. RT-qPCR and western blot assays
demonstrated that E2 significantly decreased the OVX-stimulated
expression of ephA2 and ephrinA2 protein (Fig. 5A and B).
EphA2 or ephrinA2 knockdown
significantly suppresses osteoclastogenesis
The involvement of the ephA2-ephrinA2 signaling
pathway in the E2-induced effects on bone metabolism, especially
bone resorption function, was evaluated. Osteoclast differentiation
was induced by RANKL and M-CSF in vitro and the number of
osteoclasts in 5 fields was detected when ephA2 or ephrinA2 were
knocked down. As presented in Fig. 5C
and D, osteoclast differentiation was successfully induced by
RANKL and M-CSF. However, ephA2 or ephrinA2 knockdown significantly
suppressed osteoclastogenesis, indicating their stimulating role on
osteoclast differentiation under normal conditions.
Discussion
The results of the present study indicate that E2
can attenuate OVX-induced bone deterioration partially through the
suppression of the eph A2/ephrin A2 signaling pathway. E2
demonstrated suppressive effects on OVX-induced body weight gain
and increased bone turnover. E2 inhibited the bone resorption
function of osteoclasts and induced the bone formation function of
osteoblasts by stimulating the expression of bone
metabolism-related genes. E2 treatment significantly increased the
tibia BMD and prevented bone loss compared with the OVX group. The
underlying mechanism was mediated, at least partially, by the
suppression of the ephA2/ephrinA2 signaling pathway.
As demonstrated in previous studies, OVX rats
exhibit significantly higher body mass compared with sham-operated
rats, mainly due to the estrogen deficiency that stimulates fat
deposition (21,22). Previous studies have demonstrated
that increased body mass provides an additional stimulus for bone
neo-formation, acting as a partial protection against the
osteopenia of long bones (23).
Excess body weight gain was completely prevented E2 treatment in
the present study, which is consistent with previous studies
(24,25).
Biochemical markers of bone turnover are important
research tools to measure the effects of various agents on bone
remodeling (26). Results of the
present study demonstrated a reduction in the bone turnover rate
following the treatment with E2, evidenced by alterations in the
serum Ca, urinary Ca/Cr, b-ALP and TRAP-5b concentrations, which
are consistent with a previous study (22). The protective role of E2 on bone
homeostasis was exerted through inhibition of bone resorption via
suppression of the production of TRAP and RANKL and induction of
bone formation function by promoting Runx2, osterix and Col1a1
expression, which are consistent with previous studies (7,27).
Decreased bone mass is one of the major factors jeopardizing bone
integrity, leading to reduced bone strength and an increased
susceptibility to fractures (28).
Therefore, E2 treatment prevents the deterioration of trabecular
microarchitecture and enhances the bone strength.
The present study demonstrated that mRNA and protein
expression of ephA2 and ephrinA2 was signifi-cantly decreased in
the OVX+E2 group, and ephA2 or ephrinA2 knockdown significantly
suppressed osteoclastogenesis. Similar to the effect of the
ephA2-ephrinA2 signaling, the interaction between homeobox protein
P pHB1/B3 and ephrin B1 suppressed osteoblast differentiation
(29). By contrast, ephB4-ephrinB2
signaling inhibited osteoclast differentiation and promoted
osteoblast differentiation, inducing a shift from bone resorption
to bone formation (30).
In osteoblastogenic cultures, ephA2 was found to
inhibit differentiation and mineralization of osteoblasts (31). The present study indicated that
ephrinA2 and other osteoclast-efferent factors negatively regulated
bone formation. Furthermore, reverse signaling through ephrinA2
into osteoclasts enhances osteoclastogenesis, most likely via
phospholipase Cγ2 activation (12,31).
In addition to osteoclast-osteoblast interactions,
osteoblast-osteoblast or osteoclast-osteoclast interactions through
ephrinA2, ephA2 and A4 have also been reported (12,31).
In conclusion, the present study indicates that E2
can attenuate OVX-induced bone deteriorations partially through the
suppression of the ephA2/ephrinA2 signaling pathway. The
ephA2/ephrinA2 signaling pathway maybe a potential target for
osteoporosis treatment.
Acknowledgements
The present study was supported by the Shanghai
Pudong New Area of Science and Technology Development Innovation
Fund (grant no. PKJ2016-Y03) and the Shanghai Municipal Commission
of Health and Family Planning Research Grant (grant no.
201640177).
References
|
1
|
Jin S, Yan Z, Yang T, Liu S, Liang W and
Hui Y: Eph-ephrin bidirectional signalling: A promising approach
for osteoporosis treatment. J Med Hypotheses Ideas. 7:40–42. 2013.
View Article : Google Scholar
|
|
2
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar
|
|
3
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar
|
|
4
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar :
|
|
5
|
Sarrel PM, Lufkin EG, Oursler MJ and Keefe
D: Estrogen actions in arteries, bone and brain. Sci Med.
1:441994.
|
|
6
|
Nappi C, Bifulco G, Tommaselli GA, Gargano
V and Di Carlo C: Hormonal contraception and bone metabolism: A
systematic review. Contraception. 86:606–621. 2012. View Article : Google Scholar
|
|
7
|
Riggs BL: The mechanisms of estrogen
regulation of bone resorption. J Clin Invest. 106:1203–1204. 2000.
View Article : Google Scholar :
|
|
8
|
Eastell R: Role of oestrogen in the
regulation of bone turnover at the menarche. J Endocrinol.
185:223–234. 2005. View Article : Google Scholar
|
|
9
|
Zhou S, Turgeman G, Harris SE, Leitman DC,
Komm BS, Bodine PV and Gazit D: Estrogens activate bone
morphogenetic protein-2 gene transcription in mouse mesenchymal
stem cells. Mol Endocrinol. 17:56–66. 2003. View Article : Google Scholar
|
|
10
|
Hong L, Colpan A and Peptan IA:
Modulations of 17-beta estradiol on osteogenic and adipogenic
differentiations of human mesenchymal stem cells. Tissue Eng.
12:2747–2753. 2006. View Article : Google Scholar
|
|
11
|
Hong L, Colpan A, Peptan IA, Daw J, George
A and Evans CA: 17-Beta estradiol enhances osteogenic and
adipogenic differentiation of human adipose-derived stromal cells.
Tissue Eng. 13:1197–1203. 2007. View Article : Google Scholar
|
|
12
|
Irie N, Takada Y, Watanabe Y, Matsuzaki Y,
Naruse C, Asano M, Iwakura Y, Suda T and Matsuo K: Bidirectional
signaling through ephrinA2-EphA2 enhances osteoclastogenesis and
suppresses osteoblastogenesis. J Biol Chem. 284:14637–14644. 2009.
View Article : Google Scholar :
|
|
13
|
Difford J: A simplified method for the
preparation of methyl methacrylate embedding medium for
undecalcified bone. Med Lab Technol. 31:79–81. 1974.
|
|
14
|
Baron R, Vignery A, Neff L, Silverglate A
and Maria Santa A: Processing of undecalcified bone specimens for
bone histomorphometryBone Histomorphometry: Teachniques and
Interpretation. Recker R: CRC Press; Boca Raton, FL: 1983
|
|
15
|
Witwicka H, Hwang SY, Reyes-Gutierrez P,
Jia H, Odgren PE, Donahue LR, Birnbaum MJ and Odgren PR: Studies of
OC-STAMP in osteoclast fusion: A new knockout mouse model, rescue
of cell fusion and transmembrane topology. PLoS One.
10:e01282752015. View Article : Google Scholar :
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar
|
|
18
|
Ortuño MJ, Susperregui AR, Artigas N, Rosa
JL and Ventura F: Osterix induces Col1a1 gene expression through
binding to Sp1 sites in the bone enhancer and proximal promoter
regions. Bone. 52:548–556. 2013. View Article : Google Scholar
|
|
19
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: a novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar
|
|
20
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar
|
|
21
|
Shuid AN, Ping LL, Muhammad N, Mohamed N
and Soelaiman IN: The effects of Labisia pumila var. alata on bone
markers and bone calcium in a rat model of post-menopausal
osteoporosis. J Ethnopharmacol. 133:538–542. 2011. View Article : Google Scholar
|
|
22
|
Zhao X, Wu ZX, Zhang Y, Yan YB, He Q, Cao
PC and Lei W: Anti-osteoporosis activity of cibotium barometz
extract on ovariectomy-induced bone loss in rats. J Ethnopharmacol.
137:1083–1088. 2011. View Article : Google Scholar
|
|
23
|
Notomi T, Okimoto N, Okazaki Y, Nakamura T
and Suzuki M: Tower climbing exercise started 3 months after
ovariectomy recovers bone strength of the femur and lumbar
vertebrae in aged osteopenic rats. J Bone Miner Res. 18:140–149.
2003. View Article : Google Scholar
|
|
24
|
Davidge ST, Zhang Y and Stewart KG: A
comparison of ovariectomy models for estrogen studies. Am J Physiol
Regul Integr Comp Physiol. 280:R904–R907. 2001.
|
|
25
|
Redick JH, Nussbaum AI and Mook DG:
Estradiol induced suppression of feeding in the female rat:
Dependence on body weight. Physiol Behav. 10:543–547. 1973.
View Article : Google Scholar
|
|
26
|
Bahlous A, Kalai E, Salah Hadj M, Bouzid K
and Zerelli L: Biochemical markers of bone remodeling: Recent data
of their applications in managing postmenopausal osteoporosis.
Tunis Med. 84:751–757. 2006.(In French).
|
|
27
|
Zheng MH, Lau TT, Prince R, Criddle A,
Wysocki S, Beilharz M, Papadimitriou JM and Wood DJ: 17
beta-estradiol suppresses gene expression of tartrate-resistant
acid phosphatase and carbonic anhydrase II in ovariectomized rats.
Calcif Tissue Int. 56:166–169. 1995. View Article : Google Scholar
|
|
28
|
Park JA, Ha SK, Kang TH, Oh MS, Cho MH,
Lee SY, Park JH and Kim SY: Protective effect of apigenin on
ovariectomy-induced bone loss in rats. Life Sci. 82:1217–1223.
2008. View Article : Google Scholar
|
|
29
|
Shimizu E, Tamasi J and Partridge NC:
Alendronate affects osteoblast functions by crosstalk through
EphrinB1-EphB. J Dent Res. 91:268–274. 2012. View Article : Google Scholar :
|
|
30
|
Matsuo K: Eph and ephrin interactions in
bone. Adv Exp Med Biol. 658:95–103. 2010. View Article : Google Scholar
|
|
31
|
Matsuo K and Otaki N: Bone cell
interactions through Eph/ephrin: Bone modeling, remodeling and
associated diseases. Cell Adh Migr. 6:148–156. 2012. View Article : Google Scholar :
|