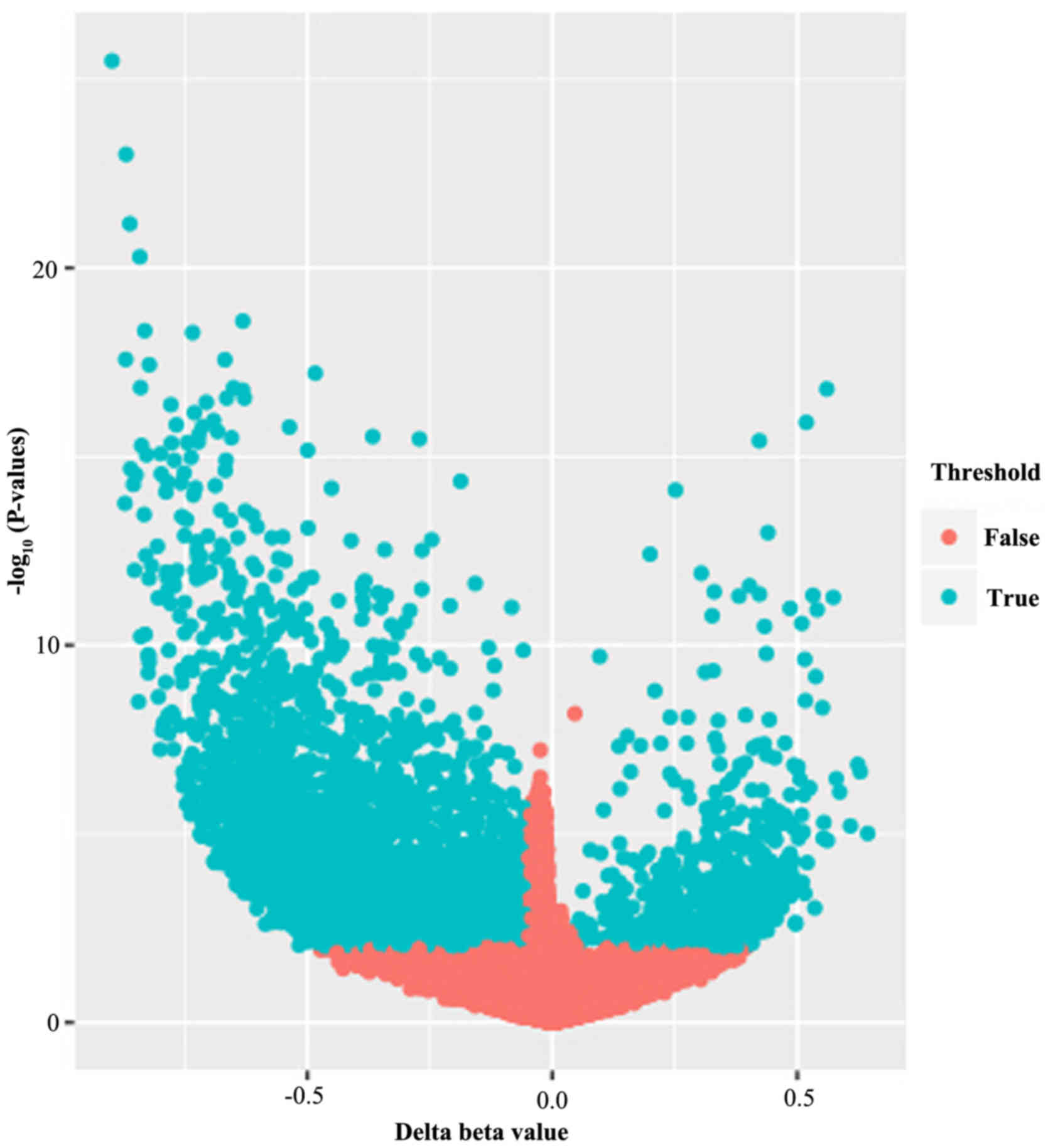
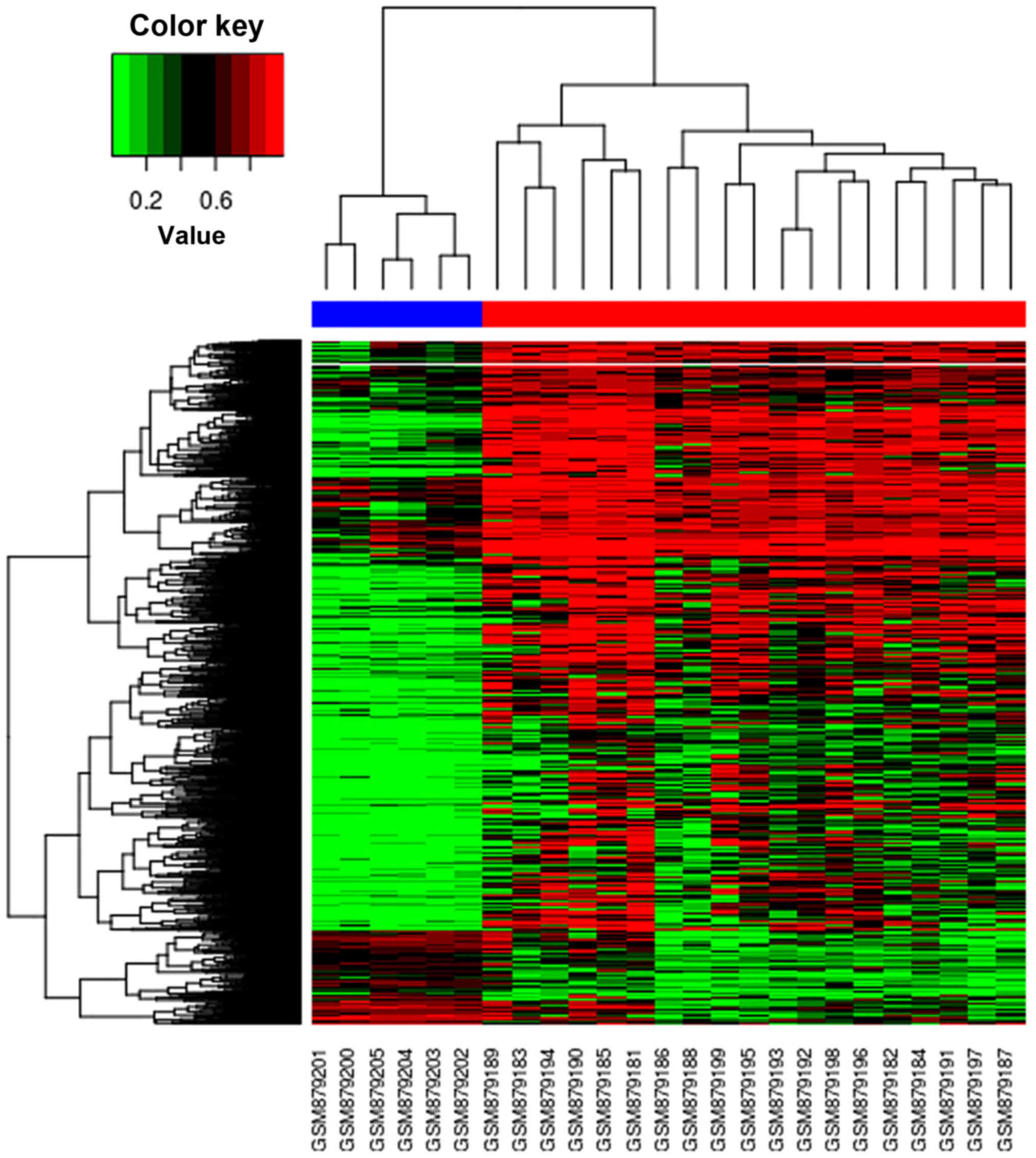
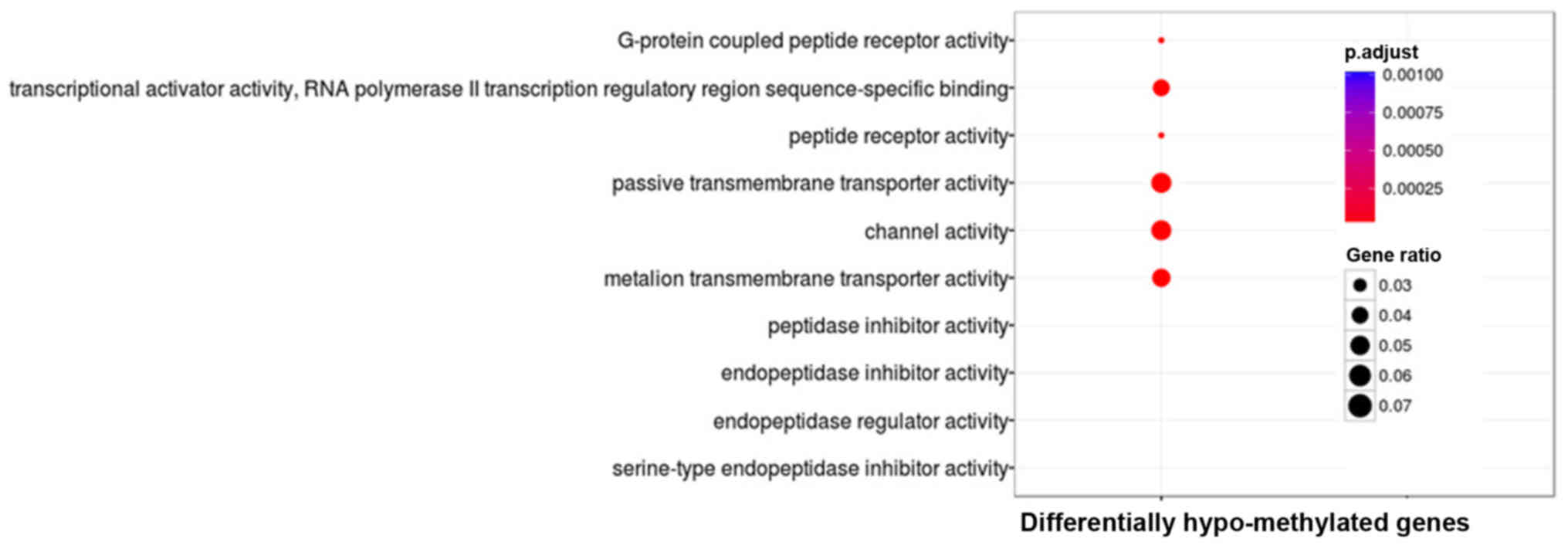
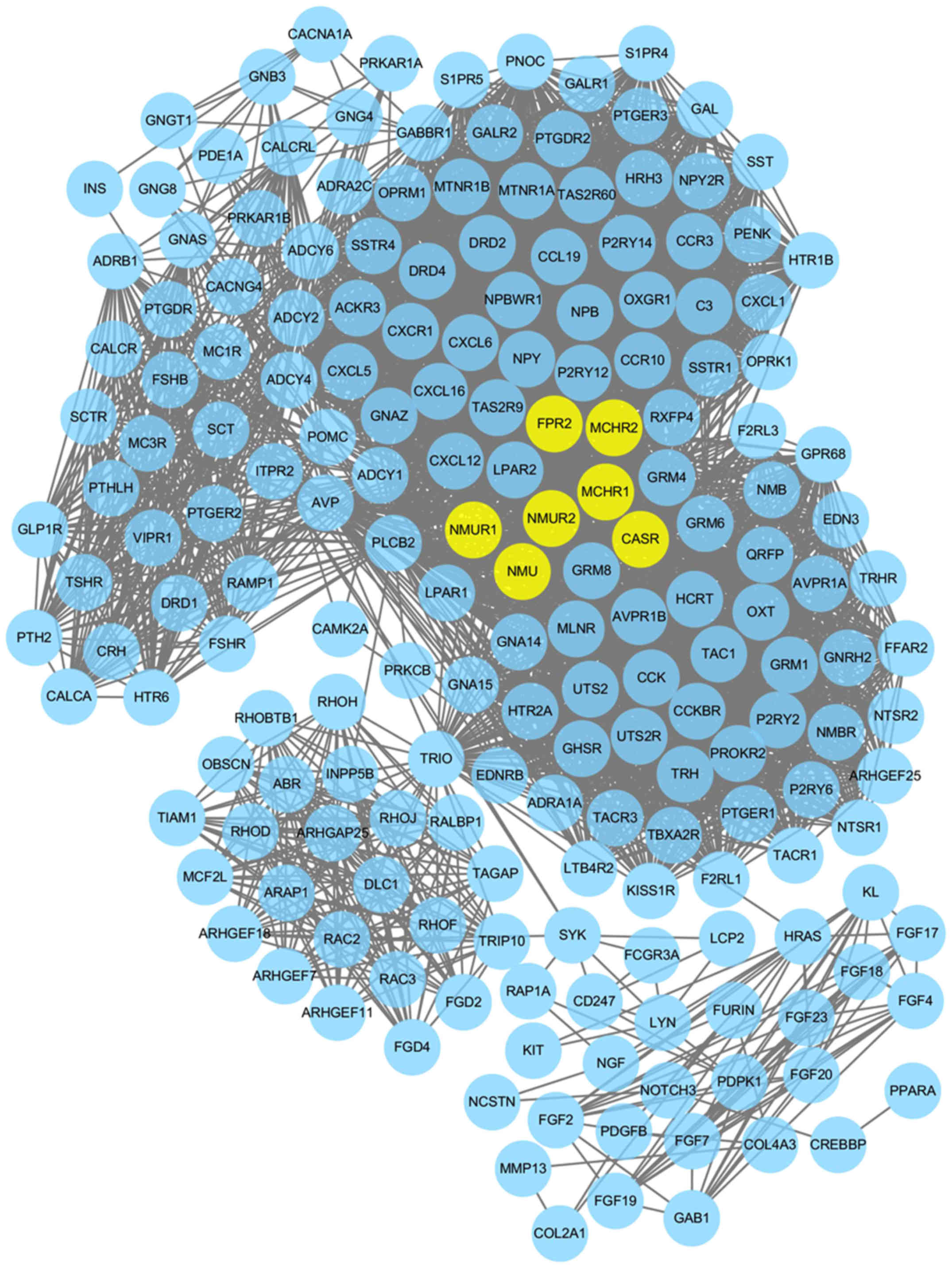
|
1
|
Kobayashi E, Hornicek FJ and Duan Z:
MicroRNA Involvement in Osteosarcoma. Sarcoma. 2012:3597392012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Posthumadeboer J, Witlox MA, Kaspers GJ
and van Royen BJ: Molecular alterations as target for therapy in
metastatic osteosarcoma: A review of literature. Clin Exp
Metastasis. 28:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Y, Cui Y, Wang W, Gu J, Guo S, Ma K and
Luo X: Hypomethylation of the hsa-miR-191 locus causes high
expression of hsa-mir-191 and promotes the
epithelial-to-mesenchymal transition in hepatocellular carcinoma.
Neoplasia. 13:841–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geiman TM and Robertson KD: Chromatin
remodeling, histone modifications and DNA methylation-how does it
all fit together? J Cell Biochem. 87:117–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flores K, Wolschin F, Corneveaux JJ, Allen
AN, Huentelman MJ and Amdam GV: Genome-wide association between DNA
methylation and alternative splicing in an invertebrate. BMC
Genomics. 13:4802012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Sun J, Zhang H, Guo S, Gu J, Wang
W, Tang N, Zhou X and Yu J: High-frequency aberrantly methylated
targets in pancreatic adenocarcinoma identified via global DNA
methylation analysis using methylCap-seq. Clin Epigenetics.
6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang F, Todd NW, Li R, Zhang H, Fang H
and Stass SA: A panel of sputum-based genomic marker for early
detection of lung cancer. Cancer Prev Res (Phila). 3:1571–1578.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo S, Wang YL, Li Y, Jin L, Xiong M, Ji
QH and Wang J: Significant SNPs have limited prediction ability for
thyroid cancer. Cancer Med. 3:731–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J and Yao X: Use of DNA methylation
for cancer detection: Promises and challenges. Int J Biochem Cell
Biol. 41:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou P, Ji M, Yang B, Chen Z, Qiu J, Shi X
and Lu Z: Quantitative analysis of promoter hypermethylation in
multiple genes in osteosarcoma. Cancer. 106:1602–1609. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim S, Yang MH, Park JH, Nojima T,
Hashimoto H, Unni KK and Park YK: Inactivation of the RASSF1A in
osteosarcoma. Onco Rep. 10:897–901. 2003.
|
|
12
|
Patiño-García A, Piñeiro ES, Díez MZ,
Iturriagagoitia LG, Klüssmann FA and Ariznabarreta LS: Genetic and
epigenetic alterations of the cell cycle regulators and tumor
suppressor genes in pediatric osteosarcomas. J Pediatr Hematol
Oncol. 25:362–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sonaglio V, de Carvalho AC, Toledo SR,
Salinas-Souza C, Carvalho AL, Petrilli AS, de Camargo B and Vettore
AL: Aberrant DNA methylation of ESR1 and p14ARF genes could be
useful as prognostic indicators in osteosarcoma. OncoTargets Ther.
6:713–723. 2013.
|
|
14
|
Lu J, Song G, Tang Q, Zou C, Han F, Zhao
Z, Yong B, Yin J, Xu H, Xie X, et al: IRX1 hypomethylation promotes
osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J
Clin Invest. 125:1839–1856. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kresse SH, Rydbeck H, Skårn M, Namløs HM,
Barragan-Polania AH, Cleton-Jansen AM, Serra M, Liestøl K,
Hogendoorn PC, Hovig E, et al: Integrative analysis reveals
relationships of genetic and epigenetic alterations in
osteosarcoma. PLoS One. 7:e482622012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du P, Kibbe WA and Lin SM: Lumi: A
pipeline for processing Illumina microarray. Bioinformatics.
24:1547–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du P, Zhang X, Huang CC, Jafari N, Kibbe
WA, Hou L and Lin SM: Comparison of Β-value and M-value methods for
quantifying methylation levels by microarray analysis. BMC
Bioinformatics. 11:5872010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teschendorff AE, Marabita F, Lechner M,
Bartlett T, Tegner J, Gomez-Cabrero D and Beck S: A β-mixture
quantile normalization method for correcting probe design bias in
Illumina Infinium 450 k DNA methylation data. Bioinformatics.
29:189–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu P, Farrell WE, Haworth KE, Emes RD,
Kitchen MO, Glossop JR, Hanna FW and Fryer AA: Maternal genome-wide
DNA methylation profiling in gestational diabetes shows distinctive
disease-associated changes relative to matched healthy pregnancies.
Epigenetics. Mar 16–2016.(Epub ahead of print). View Article : Google Scholar :
|
|
20
|
Olson CF: Parallel Algorithms for
Hierarchical Clustering. Pattern An Mach Int IEEE Trans.
12:1088–1092. 1990. View
Article : Google Scholar
|
|
21
|
Sturn A, Quackenbush J and Trajanoski Z:
Genesis: Cluster analysis of microarray data. Bioinformatics.
18:207–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benjamini Y, Drai D, Elmer G, Kafkafi N
and Golani I: Controlling the false discovery rate in behavior
genetics research. Behav Brain Res. 125:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanehisa M and Goto S: KEGG: Kyoto
Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith SS: Female sex steroid hormones:
From receptors to networks to performance-actions on the
sensorimotor system. Prog Neurobiol. 44:55–86. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chisari M, Eisenman LN, Krishnan K,
Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF
and Mennerick S: The influence of neuroactive steroid lipophilicity
on GABAA receptor modulation: Evidence for a low-affinity
interaction. J Neurophysiol. 102:1254–1264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith SS: Withdrawal properties of a
neuroactive steroid: Implications for GABA A receptor gene
regulation in the brain and anxiety behavior. Steroids. 67:519–528.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe M, Maemura K, Oki K, Shiraishi N,
Shibayama Y and Katsu K: Gamma-aminobutyric acid (GABA) and cell
proliferation: Focus on cancer cells. Histol Histopathol.
21:1135–1141. 2006.PubMed/NCBI
|
|
32
|
Szczaurska K, Mazurkiewicz M and Opolski
A: The role of GABA-ergic system in carcinogenesis. Postepy Hig Med
Dosw. 57:485–500. 2003.PubMed/NCBI
|
|
33
|
Moon MS, Cho EW, Byun HS, Jung IL and Kim
IG: GAD 67KD antisense in colon cancer cells inhibits cell growth
and sensitizes to butyrate and pH reduction and H2O2 and
gamma-radiation. Arch Biochem Biophys. 430:229–236. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kleinrok Z, Matuszek M, Jesipowicz J,
Matuszek B, Opolski A and Radzikowski C: GABA content and GAD
activity in colon tumors taken from patients with colon cancer or
from xenografted human colon cancer cells growing as s.c. Tumors in
athymic nu/nu mice. J Physiol Pharmacol. 49:303–310.
1998.PubMed/NCBI
|
|
35
|
Kunzelmann K: Ion Channels and Cancer. J
Membr Biol. 205:159–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pardo LA and Stuhmer W: Eag1: An emerging
oncological target. Cancer Res. 68:1611–1613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Voloshyna I, Besana A, Castillo M, Matos
T, Weinstein IB, Mansukhani M, Robinson RB and Cordon-Cardo C:
TREK-1 is a novel molecular target in prostate cancer. Cancer Res.
68:1197–1203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weaver AK, Bomben VC and Sontheimer H:
Expression and function of calcium-activated potassium channels in
human glioma cells. Glia. 54:223–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pluznick JL and Sansom SC: BK channels in
the kidney: Role in K(+) secretion and localization of molecular
components. Am J Physiol Renal Physiol. 291:F517–F529. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weaver AK, Liu X and Sontheimer H: Role
for calcium-activated potassium channels (BK) in growth control of
human malignant glioma cells. J Neurosci Res. 78:224–234. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei WC, Akerman CJ, Newey SE, Pan J,
Clinch NW, Jacob Y, Shen MR, Wilkins RJ and Ellory JC: The
potassium-chloride cotransporter 2 promotes cervical cancer cell
migration and invasion by an ion transport-independent mechanism. J
Physiol. 589:5349–5359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rezzonico R, Schmid-Alliana A, Romey G,
Bourget-Ponzio I, Breuil V, Breittmayer V, Tartare-Deckert S, Rossi
B and Schmid-Antomarchi H: Prostaglandin E2 induces interaction
between hSlo potassium channel and Syk tyrosine kinase in
osteosarcoma cells. J Bone Miner Res. 17:869–878. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu J, Zhong D, Wu X, Sha M, Kang L and
Ding Z: Voltage-gated potassium channel Kv1.3 is highly expressed
in human osteosarcoma and promotes osteosarcoma growth. Int J Mol
Sci. 14:19245–19256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moley KH and Mueckler MM: Glucose
transport and apoptosis. Apoptosis. 5:99–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lorenz HM, Herrmann M, Winkler T, Gaipl U
and Kalden JR: Role of apoptosis in autoimmunity. Apoptosis.
5:443–449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schulze-Bergkamen H and Krammer PH:
Apoptosis in cancer-implications for therapy. Semin Oncol.
31:90–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wyllie AH: Apoptosis and the regulation of
cell numbers in normal and neoplastic tissues: An overview. Cancer
Metastasis Rev. 11:95–103. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deeley RG, Westlake C and Cole SP:
Transmembrane transport of endo-and xenobiotics by mammalian
ATP-binding cassette multidrug resistance proteins. Physiol Rev.
86:849–899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fujii R, Hosoya M, Fukusumi S, Kawamata Y,
Habata Y, Hinuma S, Onda H, Nishimura O and Fujino M:
Identification of neuromedin U as the cognate ligand of the orphan
G protein-coupled receptor FM-3. J Biol Chem. 275:21068–21074.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Moriyama M, Sato T, Inoue H, Fukuyama S,
Teranishi H, Kangawa K, Kano T, Yoshimura A and Kojima M: The
neuropeptide neuromedin U promotes inflammation by direct
activation of mast cells. J Exp Med. 202:217–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Harten SK, Esteban MA, Shukla D, Ashcroft
M and Maxwell PH: Inactivation of the von Hippel-Lindau tumour
suppressor gene induces Neuromedin U expression in renal cancer
cells. Mol Cancer. 10:892011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kohno Y: Oral cancer in vivo gene
expression profiling assisted by laser capture microdissection and
microarray analysis. Oncogene. 20:6196–6204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shetzline SE, Rallapalli R, Dowd KJ, Zou
S, Nakata Y, Swider CR, Kalota A, Choi JK and Gewirtz AM:
Neuromedin U: A Myb-regulated autocrine growth factor for human
myeloid leukemias. Blood. 104:1833–1840. 2004. View Article : Google Scholar : PubMed/NCBI
|