Introduction
Gastric cancer (GC) is the fifth most common cancer
and the third most common cause of cancer-related mortality
worldwide (1). As GC development
is a multistep carcinogenic process that involves numerous genetic
and epigenetic alterations, a better understanding of oncogenes and
tumor suppressor genes may provide additional clues for
understanding the underlying molecular mechanism for GC development
and for molecular targeted drug screening (2).
Long noncoding RNAs (lncRNAs) are a class of non
protein-coding transcripts that are >200 nucleotides in length
(3). A number of studies have
demonstrated the vital role of lncRNA expression dysregulation in
numerous diseases, particularly in cancer, owing to its extensive
biological functions in a wide variety of physiological and
pathological processes (4,5). lncRNA-activated by transforming
growth factor (TGF)-β (lncRNA-ATB), was identified during a screen
of lncRNAs that are regulated by TGF-β in hepatocellular carcinoma
(HCC); the study indicated that the lncRNA-ATB was upregulated in
HCC (6). In addition, lncRNA-ATB
was demonstrated to promote epithelial-to-mesenchymal transition
(EMT) and induce the invasion-metastasis cascade through the
TGF-β/microRNA (miR)-200s/zinc-finger E-box-binding homeobox (ZEB)
axis, which contributes to HCC progression (6). A series of subsequent studies
demonstrated that upregulated lncRNA-ATB expression was correlated
with poor prognosis in several cancer types, including GC (7), renal cell carcinoma (8), colorectal cancer (9) and breast cancer (10). However, the role of lncRNA-ATB in
EMT process and invasion-metastasis cascade in GC remains
unknown.
The present study investigated the expression of
lncRNA-ATB in GC tissues and cell lines, as well as its
relationship with clinicopathological features of patients with GC,
with a focus on the specific modulatory roles of lncRNA-ATB in the
proliferation, invasion and migration of GC cells.
Materials and methods
Specimens and relative clinical
data
A total of 40 pairs of GC tissues and adjacent
non-tumor tissues, along with the patients' clinical data were
obtained from the Tissue Bank in West China Hospital, Sichuan
University (Chengdu, China). All tissues were stored in liquid
nitrogen until used for RNA extraction. This study was approved by
the Research Ethics Committee of West China Hospital, and written
informed consent was received from all patients prior to enrollment
in the present study.
Cell culture
Five human GC cell lines (MGC-803, MKN-45, BGC-823,
MKN-28 and SGC-7901) and a normal gastric mucosal cell line (GES-1)
were acquired from our laboratory depository, originally obtained
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). All cell lines were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and incubated in a humid atmosphere of 5%
CO2 at 37°C. The medium was replaced every 2 days.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The NucleoZOL Reagent (Machery-Nagel GmbH, Düren,
Germany) was used to extract total RNA from the cell lines
(107 cells/ml) and patient tissue specimens (100 mg
tissue/ml). The concentration and purity of RNA were detected with
a NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA). Reverse
transcription of total RNA into cDNA was performed by PrimeScript
RT Reagent kit with gDNA Eraser (Takara Bio, Inc., Otsu, Japan),
following the manufacturer's instructions. qPCR was performed using
2X ChamQ SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing,
China) with a total reaction volume of 10 µl. qPCR was performed on
a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Thermocycling conditions
were as follows: One cycle of 95°C for 30 sec, followed by 40
cycles of 95°C for 10 sec and 60°C for 30 sec. The primer sequences
used were as follows: lncRNA-ATB forward,
5′-TCTGGCTGAGGCTGGTTGAC-3′ and reverse,
5′-ATCTCTGGGTGCTGGTGAAGG-3′; internal housekeeping gene GAPDH
forward, 5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-TGTCATCATATTTGGCAGGTT-3′. Relative expression levels of
lncRNA-ATB were calculated by the 2−ΔΔCq method,
normalized to GAPDH (11). The
ratio value of each sample was subsequently determined as the ratio
of expression between GC and normal tissue from the same patient.
Each experiment was performed in triplicate.
RNA interference
The lncRNA-ATB-targeted small interfering (si)RNA
and a non-specific negative control (si-NC) were both purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequence
of the si-lncRNA-ATB was: 5′-CCAUGAGGAGUACUGCCAATT-3′. MGC-803 and
MKN-45 cells were seeded in a 6 well plate (0.5×106
cells/well) and then transfected with 100 nM si-lncRNA-ATB or si-NC
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Following 48 h
incubation at 37°C, the transfected cells were harvested for
further analysis.
Cell proliferation assay
Cell proliferation was examined by Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Shanghai,
China), following the manufacturer's instructions. Briefly, MGC-803
and MKN-45 cells were seeded in a 96-well plate (1×104
cells/well) 24 h prior to transfection with 100 nM si-lncRNA-ATB or
si-NC using Lipofectamine 2000. Following 48 h of transfection at
37°C, serum free DMEM (100 µl) containing 10% CCK-8 reagent was
added to each well and cultured for 1 h at 37°C. The absorbance at
a wavelength of 450 nm was assessed with a microplate reader
(Bio-Rad Laboratories, Inc.). Experiments were repeated at least
three times.
Ethynyl-2-deoxyuridine (EdU)
incorporation assay
Cell proliferation was also examined by EdU
incorporation using the Cell-Light Apollo Stain kit (Guangzhou
RiboBio Co., Ltd., Guangzhou, China), according to the
manufacturer's instructions. MGC-803 and MKN-45 were seeded in a
96-well plate (1×104 cells/well) 24 h prior to
transfection with 100 nM si-lncRNA-ATB or si-NC using Lipofectamine
2000. Following 48 h incubation at 37°C, EdU (100 µl; 50 µM) was
added into each well and incubated for 2 h at 37°C. The cells were
fixed in of 4% paraformaldehyde in PBS (100 µl) for 20 min at room
temperature. Subsequently, the cells were incubated in glycine (50
µl; 2 mg/ml) for 5 min, followed by washes with PBS. Cells were
permeabilized with 1% Triton X-100 and incubated with 1X Apollo
Solution for 45 min at the room temperature in dark. Hoechst
solution (20 µl) was added into each well and incubated for an
additional 10 min at room temperature in dark, followed by washes
with PBS. Plates were observed and images captured under a Nikon
Eclipse TE2000-U fluorescence microscope (Nikon Corporation, Tokyo,
Japan); magnification, ×20. The excitation and emission parameters
of Apollo are 550 and 565 nm, respectively. The excitation and
emission parameters of Hoechst are 350 and 461 nm, respectively.
Experiments were repeated at least three times.
Transwell invasion and migration
assays
MGC-803 and MKN-45 cells were seeded in a 6-well
plate (0.5×106 cells/well) and transfected with 100 nM
si-lncRNA-ATB or si-NC using Lipofectamine 2000 according to the
manufacturer's instructions. Following 48 h incubation at 37°C, the
transfected cells were resuspended in serum-free DMEM and added in
the upper Transwell chamber with a Matrigel coated membrane. For
the migration assay, cells were seeded into the upper chamber
without Matrigel. The bottom chamber was filled with 400 µl DMEM
containing 20% FBS. Cells were incubated for 48 h at 37°C.
Non-invading cells were wiped off of the upper surface with a wet
cotton swab, and the cells that passed to the lower surface were
fixed in 4% paraformaldehyde at room temperature for 20 min and
then stained with 0.5% crystal violet solution at room temperature
overnight. Images of the invaded cells were captured under an
Eclipse T1-SM inverted microscope (Nikon Corporation) at ×10
magnification. Experiments were repeated at least three times.
Wound-healing assay
The migratory ability of GC cells was examined by
wound-healing assay. At 48 h post-siRNA transfection, MGC-803 and
MKN-45 were seeded in a 6-well plate (0.5×106
cells/well). The cells were allowed to reach 90% confluence, after
which a scratch was made through the center of each well using the
200 µl sterile pipette tip. The scratch was observed and imaged at
0, 24 and 48 h following the scratch using an Eclipse T1-SM
inverted microscope (Nikon Corporation) with ×10 magnification.
Experiments were repeated at least three times.
Western blot analysis
Cellular proteins of transfected MGC-803 and MKN-45
cells were lysed on ice with radioimmunoprecipitation assay lysis
buffer (BioTeke Corporation, Beijing, China) according to the
manufacturer's instructions. The concentration of protein was
detected using a NanoDrop 2000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA).
Equal amounts of protein extracts (20 µg/lane) were separated by 8%
SDS-PAGE and transferred electrophoretically to a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA) using the
Bio-Rad Semi-dry Transfer System (Bio-Rad Laboratories, Inc.).
Membranes were blocked with 5% milk in PBS for 2 h at room
temperature and probed with specific primary antibodies (1:1,000)
against β-actin (cat. no. ab8227), vimentin (cat. no. ab45939),
N-cadherin (cat. no. ab18203) and c-Myc (cat. no. ab11917) (all
from Abcam, Cambridge UK), at 4°C overnight. Following 3 washes
with PBS (5 min each), IRDye 650-conjugated goat anti-mouse (cat.
no. 926–68070)/rabbit (cat. no. 926-32210) immunoglobulin G
secondary antibodies (1:200; LI-COR Biosciences, Lincoln, NE, USA)
were added and incubated for 1 h in the dark at room temperature.
Membranes were washed with PBS and bands were detected in a dark
room using a Fluorescence Odyssey Imaging System (LI-COR
Biosciences).
Statistical analysis
Analysis of the correlation between patient
clinicopathological characteristics and lncRNA-ATB expression was
performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA).
The remaining data analysis was performed by GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Comparison between
two groups was performed using a Student's t-test. Chi-square test
was used for analyzing the correlation between lncRNA-ATB
expression and clinicopathological features of the GC samples. Data
was presented as the mean ± standard deviation. All of the P-values
were two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
lncRNA-ATB is overexpressed in GC
tissues and cell lines
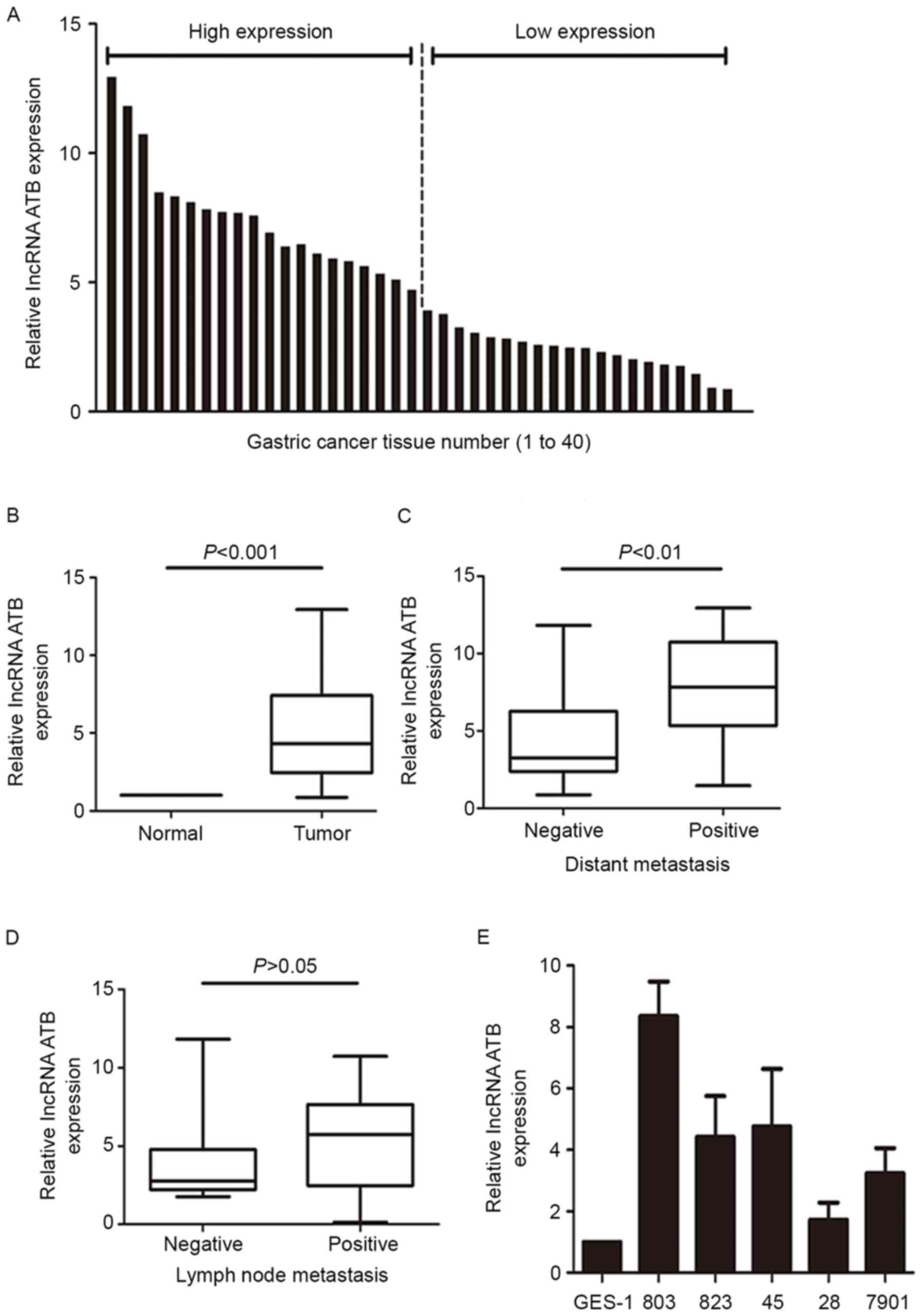
The expression levels of lncRNA-ATB in 40 pairs of
gastric tissues were measured by RT-qPCR. A total of 40 ratios
value were obtained from 40 pairs of tissues, and the average value
as 4.37. The 40 pairs were divided into a high-expression group
(n=20; ratio >4.37) and a low-expression group (n=20; ratio
<4.37) according to the average ratio value (average ratio=4.37;
Fig. 1A). lncRNA-ATB expression
was significantly higher in GC tumor tissues compared with
expression in the adjacent non-tumor tissues (P<0.001; Fig. 1B). In addition, lncRNA-ATB
expression was significantly higher in GC tissues from patients
with distant metastasis (P<0.01; Fig. 1C), whereas lncRNA-ATB expression
was not significantly correlated with lymph node metastasis
(P>0.05; Fig. 1D). The
expression of lncRNA-ATB was also examined in different GC cell
lines and the GES-1 normal gastric cell line. The results
demonstrated that lncRNA-ATB expression was higher in the GC cell
lines compared with expression in GES-1 (Fig. 1E).
Correlation between lncRNA-ATB
expression and clinicopathological characteristics in patients with
GC
High lncRNA-ATB expression levels were positively
correlated with invasion depth (P=0.003), distant metastasis
(P=0.008) and tumor-node-metastasis (TNM) stage (P=0.001), compared
with the low-expression group (Table
I). However, no significant correlation was identified between
the lncRNA-ATB expression and lymph node metastasis, distant
metastasis, patient age and sex (P>0.05). Therefore, it was
concluded that the overexpression of lncRNA-ATB may be associated
with GC progression.
 | Table I.Correlation between lncRNA-ATB
expression level and clinicopathological features in patients with
gastric cancer. |
Table I.
Correlation between lncRNA-ATB
expression level and clinicopathological features in patients with
gastric cancer.
|
|
| LncRNA-ATB
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristics | n | Low | High | P-value |
|---|
| Age (years) |
|
|
| 0.751 |
|
>60 | 18 | 10 | 8 |
|
|
<60 | 22 | 10 | 12 |
|
| Sex |
|
|
| 0.514 |
| Male | 25 | 14 | 11 |
|
|
Female | 15 | 6 | 9 |
|
| Invasion depth |
|
|
| 0.003 |
| T1 | 4 | 3 | 1 |
|
| T2 | 11 | 8 | 3 |
|
| T3 | 12 | 8 | 4 |
|
| T4 | 13 | 1 | 12 |
|
| Lymph node
metastasis |
|
|
| 0.070 |
| 0 | 16 | 12 | 4 |
|
| 1 | 13 | 4 | 9 |
|
| 2 | 7 | 3 | 4 |
|
| 3 | 4 | 1 | 3 |
|
| Metastasis |
|
|
| 0.008 |
| Yes | 7 | 0 | 7 |
|
| No | 33 | 20 | 13 |
|
| TNM stage |
|
|
| 0.001 |
| I | 13 | 11 | 2 |
|
| II | 12 | 7 | 5 |
|
| III | 8 | 2 | 6 |
|
| IV | 7 | 0 | 7 |
|
lncRNA-ATB-knockdown suppresses
cellular proliferation
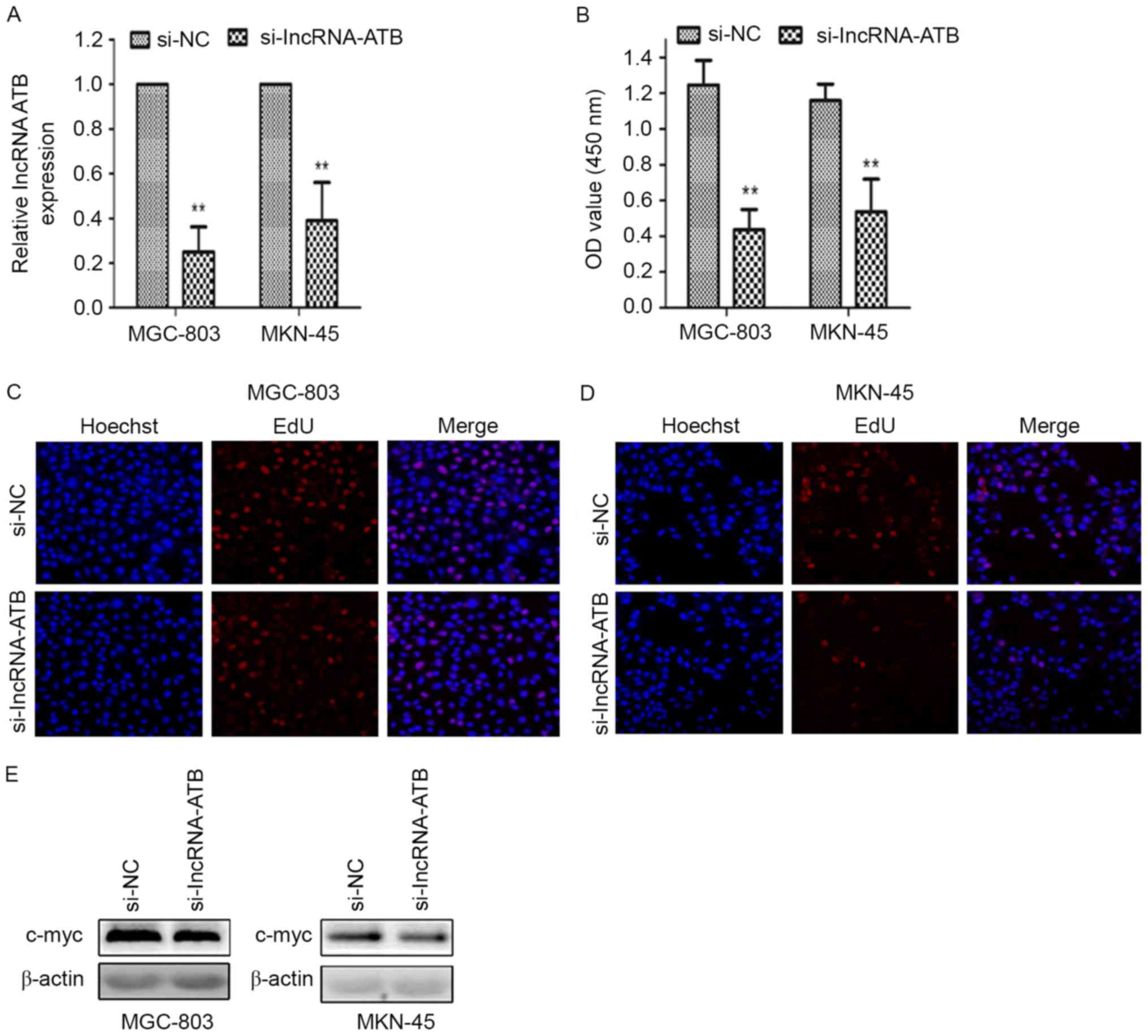
RT-qPCR results indicated that the level of
lncRNA-ATB expression was significantly reduced in GC cells
transfected with si-lncRNA-ATB compared with cells transfected with
si-NC (P<0.01; Fig. 2A), which
indicated that si-lncRNA-ATB could be used in further in
vitro experiments. CCK-8 assay results demonstrated that
cellular proliferation was significantly suppressed in MGC-803 and
MKN-45 cells transfected with si-lncRNA-ATB compared with si-NC
transfected cells (P<0.01; Fig.
2B). The EdU assay also demonstrated reduced proliferative
ability in MGC-803 cells (Fig. 2C)
and in MKN-45 cells (Fig. 2D)
transfected with si-lncRNA-ATB. Western blotting revealed that the
expression of c-Myc, an indicator of cell proliferation, was
notably reduced in the si-lncRNA-ATB group compared with the si-NC
group (Fig. 2E).
lncRNA-ATB-knockdown reduces the
invasion and migration ability of GC cells
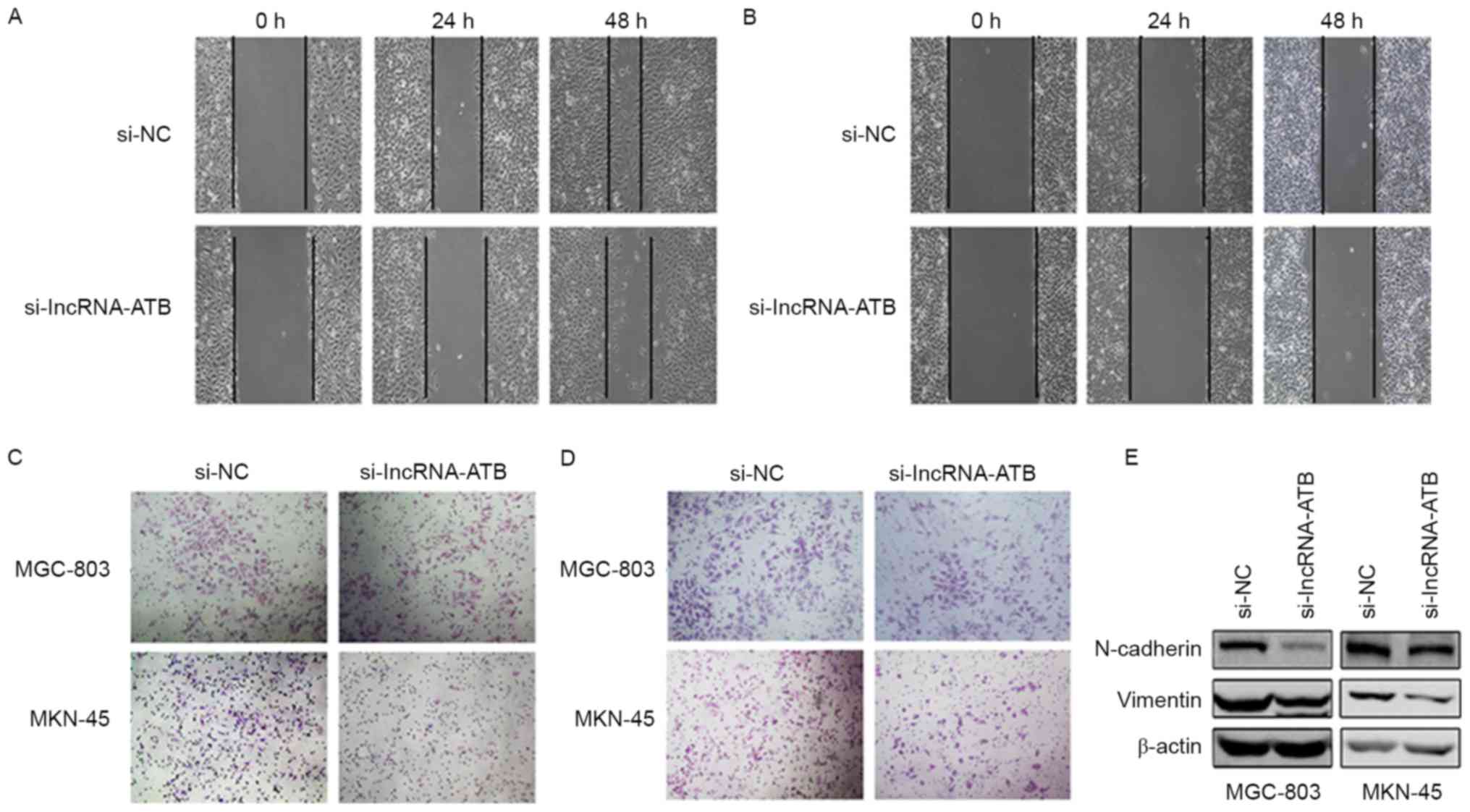
In the wound healing assays, the migratory ability
of both the MGC-803 cells (Fig.
3A) and the MKN-45 (Fig. 3B)
cells transfected with si-lncRNA-ATB was notably reduced compared
with the control si-NC transfected cells. To further examine the
effects on cell migration and invasion, Transwell assays were
performed. Knockdown of lncRNA-ATB resulted in fewer cells
migrating through the chambers in both MGC-803 and MKN-45 cells
(Fig. 3C). Similar results were
obtained with Matrigel invasion assays (Fig. 3D). As lncRNA-ATB is a TGF-β-induced
lncRNA, the effects of lncRNA-ATB on the EMT process were analyzed.
It was demonstrated that the silencing of lncRNA-ATB resulted in
the downregulated protein expression of the mesenchymal markers
N-cadherin and vimentin (Fig.
3E).
Discussion
The clinical outcome of patients with GC remains
dismal with the 5-year survival rate as low as 25%, or less,
despite the rapid progress in developing surgical techniques and
molecular-targeted therapies (12). This may be attributed to the
underlying highly complex molecular mechanisms, which involve
multiple tumor suppressors and oncogenes, as well as some
well-studied tumor-related signaling pathways (13). Therefore, it is essential to
identify reliable molecular biomarkers of GC to improve the early
diagnosis and overall survival rate. In addition, it may also
provide additional clues to the development of individualized
therapy.
Noncoding RNA was previously regarded as
transcriptional ‘noise’ in the conventional opinion of gene
regulation (14); however, an
increasing number of studies have demonstrated their regulatory
potential in a range of physiological and pathological processes
(15). lncRNA-ATB was originally
reported as a novel TGF-β-induced lncRNA that was highly
overexpressed in HCC. lncRNA-ATB competitively binds to members of
the miR-200 miRNA family, which results in the upregulated
expression of ZEB1 and ZEB2 mRNA and protein, thus inducing EMT
(6). Notably, knock down
lncRNA-ATB expression was revealed to sufficiently abolish
TGF-β-induced EMT in HCC cells, even with the involvement of many
other TGF-β-induced EMT drivers, such as Snail, Twist and Slug
(6,16). These results indicated an essential
role of lncRNA-ATB in EMT regulation. Upregulated lncRNA-ATB
expression levels were also demonstrated in GC (7) renal cell carcinoma (8), colorectal cancer (9) as well as breast cancer (10) tissues compared with the levels of
expression in the paired normal tissues. Survival analysis also
indicated the inverse relationship between lncRNA-ATB expression
and cancer prognosis (17).
However, lncRNA-ATB expression was reported to be decreased in
pancreatic cancer tissues compared with the adjacent normal
tissues, which suggested the role of lncRNA-ATB in tumor
progression may be pro-tumorigenic or tumor suppressive (18). Interestingly, the similar duality
of TGF-β signaling was also observed in the pancreatic ductal
adenocarcinoma (19).
In the present study, lncRNA-ATB expression levels
in GC tissues and GC cell lines were significantly higher compared
with adjacent normal tissues and normal gastric mucosal cells.
These results were consistent with a previous study (7). In addition, patients with distant
metastasis exhibited high expression of lncRNA-ATB. Further
correlational analysis with the clinicopathological features
indicated that higher lncRNA-ATB expression was correlated with
increased invasion depth, more distant metastasis and advanced TNM
stage. However, a previous study reported no significant
association between high lncRNA-ATB expression and depth of tumor
invasion, distant metastasis or clinical stage (7). This contradiction may be due to
differences in the high and low expression ratio groupings;
analysis with a larger sample size is necessary to further
investigate this.
The present study also performed in vitro
experiments to investigate the role of lncRNA-ATB in GC cellular
processes. As the MGC-803 and MKN-45 cell lines exhibited the
highest expression of lncRNA-ATB, they were selected for in
vitro experiments. The results indicated that the knockdown of
lncRNA-ATB expression significantly suppressed the proliferation,
migration and invasion abilities of GC cell lines. In addition,
reduced expression levels of mesenchymal markers N-cadherin and
vimentin were observed in cells with knocked-down lncRNA-ATB
expression. These data suggested that the regulatory role of
lncRNA-ATB in GC progression was fulfilled partially through the
induction of EMT. However, the underlying regulatory mechanism
requires further clarification.
In conclusion, the present study reported that the
higher expression of lncRNA-ATB were positively correlated with
invasion depth, distant metastasis and advanced TNM stage. In
addition, it was demonstrated that knocking down lncRNA-ATB
expression reduced the proliferation, migration and invasion
ability of GC cell lines. These results suggested the potential
role of lncRNA-ATB in clinical outcome prediction and
molecular-targeted therapy in GC.
Acknowledgements
We would like to thank the contributions of all
authors who participated in this study. This study was supported by
the National Natural Science Foundation of China (grant no.
81572731).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN. Int J Cancer. 136:E359–E386. 2015. View Article : Google Scholar
|
|
2
|
Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J
and Sung JJ: Dysregulation of cellular signaling in gastric cancer.
Cancer Lett. 295:144–153. 2010. View Article : Google Scholar
|
|
3
|
Rönnau CG, Verhaegh GW, Luna-Velez MV and
Schalken JA: Noncoding RNAs as novel biomarkers in prostate cancer.
Biomed Res Int. 2014:5917032014. View Article : Google Scholar :
|
|
4
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar :
|
|
5
|
Brunner AL, Beck AH, Edris B, Sweeney RT,
Zhu SX, Li R, Montgomery K, Varma S, Gilks T, Guo X, et al:
Transcriptional profiling of long non-coding RNAs and novel
transcribed regions across a diverse panel of archived human
cancers. Genome Biol. 13:R752012. View Article : Google Scholar :
|
|
6
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar
|
|
7
|
Saito T, Kurashige J, Nambara S, Komatsu
H, Hirata H, Ueda M, Sakimura S, Uchi R, Takano Y, Shinden Y, et
al: A long non-coding RNA activated by transforming growth factor-β
is an independent prognostic marker of gastric cancer. Ann Sug
Oncol. 3 Suppl 22:S915–S922. 2015. View Article : Google Scholar
|
|
8
|
Xiong J, Liu Y, Jiang L, Zeng Y and Tang
W: High expression of long non-coding RNA lncRNA-ATB is correlated
with metastases and promotes cell migration and invasion in renal
cell carcinoma. Jpn J Clin Oncol. 46:378–384. 2016. View Article : Google Scholar :
|
|
9
|
Iguchi T, Uchi R, Nambara S, Saito T,
Komatsu H, Hirata H, Ueda M, Sakimura S, Takano Y, Kurashige J, et
al: A long noncoding RNA, lncRNA-ATB, is involved in the
progression and prognosis of colorectal cancer. Anticancer Res.
35:1385–1388. 2015.
|
|
10
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015. View Article : Google Scholar :
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Saka M, Morita S, Fukagawa T and Katai H:
Present and future status of gastric cancer surgery. Jpn J Clin
Oncol. 41:307–313. 2011. View Article : Google Scholar
|
|
13
|
Kang W, Cheng AS, Yu J and To KF: Emerging
role of Hippo pathway in gastric and other gastrointestinal
cancers. World J Gastroenterol. 22:1279–1288. 2016. View Article : Google Scholar :
|
|
14
|
Fang XY, Pan HF, Leng RX and Ye DQ: Long
noncoding RNAs: Novel insights into gastric cancer. Cancer Lett.
356:357–366. 2015. View Article : Google Scholar
|
|
15
|
Mattick JS: RNA regulation: A new
genetics? Nat Rev Genet. 5:316–323. 2004. View Article : Google Scholar
|
|
16
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar :
|
|
17
|
Yue B, Qiu S, Zhao S, Liu C, Zhang D, Yu
F, Peng Z and Yan D: LncRNA ATB mediated E-cadherin repression
promotes the progression of colon cancer and predicts poor
prognosis. J Gastroenterol Hepatol. 31:595–603. 2016. View Article : Google Scholar
|
|
18
|
Qu S, Yang X, Song W, Sun W, Li X, Wang J,
Zhong Y, Shang R, Ruan B, Zhang Z, et al: Downregulation of
lncRNA-ATB correlates with clinical progression and unfavorable
prognosis in pancreatic cancer. Tumour Biol. 37:3933–3938. 2016.
View Article : Google Scholar
|
|
19
|
David CJ, Huang YH, Chen M, Su J, Zou Y,
Bardeesy N, Iacobuzio-Donahue CA and Massagué J: TGF-β tumor
suppression through a lethal EMT. Cell. 164:1015–1030. 2016.
View Article : Google Scholar :
|