Introduction
With a prevalence of 0.2–1.4%, primary Sjogren's
syndrome (pSS) is one of the most common chronic, slowly
progressing systemic autoimmune diseases. Its main symptoms are
kerato-conjunctivitis sicca and xerostomia. The extremely various
clinical picture is characterized by multiple extraglandular
manifestations (EGMs) and associated diseases. The women accounting
for 90% of all cases in pSS. The onset age in 30–60 years. However,
both distributions by age and by sex show geographical and ethnic
differences (1–3).
The precise etiology remains unclear. Multiple
factors, including viral infection, hormonal balance and genetic
background were involved in the pathogenesis of pSS (4). The influence of abnormal apoptosis in
this disease has attracted considerable attention (5–8).
Cell apoptosis was a cell suicide under the control of gene in the
development of individual activities. It was a programmed cell
death process actively by the gene encoding with multicellular
organism to regulate the body growth and maintain the stability of
the internal environment. Cell apoptosis was involved in a variety
of physiological situations, including immunity, embryogenesis and
carcinogenesis. It was generally considered that cell apoptosis was
implemented through two different pathway (exogenous and endogenous
pathways). Exogenous pathway of apoptosis was regulated by the
family of the Bcl-2 protein, promoting material such as the
cytochrome c (Cytc), which induced apoptosis acts on the
mitochondria. The endogenous pathway of apoptosis was occurred by
Fas/FasL interactions (9). It is
generally believed that the regulatory mechanisms for pSS are
mainly through endogenous death receptor pathway. Kong et al
(10), Treviño-Talavera et
al (7) have already reported
the expression of Fas/FasL in the salivary glands of patients with
pSS, suggested that the endogenous death receptor pathway was
involved in pSS. However, it is not clear that whether the
exogenous plays an important role in the patients with pSS.
Cytc was a key factor of exogenous pathway. Once
released into the cytosol, Cytc binds to apoptotic protease
activating facter-1 (Apaf-1) and procaspase-9, apoptosome was
formed, apoptosis was initiated (11). However, the changes in Cytc
expression in pSS patients remain unclear. In the present study,
the expression of Cytc in the labial salivary glands of pSS
patients and healthy controls were examined to investigate the
possible role of Cytc in the pathogenesis and development of
pSS.
Materials and methods
Patients and group
The group comprised 35 patients (33 women and 2 men)
with a mean age of 44.42±2.62 years. All patients were recruited
from the Department of Emergency and Oral Medicine, School of
Stomatology, China Medical University between September 2014 and
September 2016 and diagnosed with pSS fulfilled the
American-European Consensus Group Criteria for this diagnosis
(12) and individuals with other
rheumatic diseases, infections, or malignant tumors were excluded
from the study. The control group included 35 cases (32 women and 3
men) with a mean age of 40.11±1.32 years. Healthy controls
specimens mainly come from the lip trauma (the 30 cases come from
lip trauma, the 5 cases come from the abnormal labial gland
surrounding the cyst during the operation of mucous cyst). There
were no significant differences in the ages or sex ratios between
the two groups. Both the research protocol and the consent forms
were approved by the Research Ethics Committee of School of
Stomatology, China Medical University.
Experimental specimen collection
Each patient was cutted down 0.6×0.6 cm labial gland
tissue of the lower lip under the local anesthesia. One part of
fresh specimens was immersed in 4% formaldehyde fixed, conventional
paraffin embedding. The other part was put into the liquid nitrogen
immediately, preservation in −70°C condition.
Experimental methods
Reverse transcription-polymerase chain reaction
(RT-PCR)
Total tissue RNA was extracted from tissue using the
TRIzol kits (Sangon Biotech Corporation, Shanghai, China),
according to the manufacturer's instructions. The quality and yield
of the RNA samples were determined by ultraviolet spectrophotometer
and the concentration was adjusted to 1 µg/µl for reverse
transcription. The Cytc and GADPH was run in the same reaction. RT
reaction system: RNA 2 µl, 10X RT buffer 2 µl, MgCL2 4
µl, dNTP 2 µl, RNase free H2O 7.5 µl, RNase inhibitor
0.5 µl, AMV 1 µl, random 9 primer 1 µl, reaction condition: 30°C
for 10 min, 42°C for 30 min, 99°C for 5 min, 5°C for 5 min. PCR
reaction system: cDNA 2 µl, forward primer 1 µl, reverse primer 1
µl, dNTP 0.5 µl, 5X RT buffer 5 µl, EsayTaq 0.125 µl. Reaction
condition: 95°C 1 min, 95°C 15 sec, 60°C 15 sec, 72°C 45 sec for 40
cycles. RT-PCR products was separated on 2% agarose gels. After
stained with ethidium bromide, gel images were photographed with
Chemi Imager™ 4400. The gray value of positive electrophoresis
strip were tested by professional digital analysis software. The
expression of mRNA was normalized against the expression of the
GAPDH gene.
The sequences of the primer pairs are: Cytc,
5′-GGGCGAGAGCTATGTAATGCAAG-3′ and 5′-TACAGCCAAAGCAGCAGCTCA-3′ (132
bp); GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ and
5′-TCCACCACCCTGTTGCTGTA-3′ (439 bp).
RT-qPCR
Total tissue RNA was extracted from tissue using the
TRIzol kits (Sangon Biotech Corporation), according to the
manufacturer's instructions. The quality and yield of the RNA
samples were determined by ultraviolet spectrophotometer and the
concentration was adjusted to 1 µg/µl for reverse transcription.
The RNA was reverse-transcribed to form cDNA using a ReverTra Ace
qPCR RT kit (Toyobo, Osaka, Japan). RT reaction system: RNA 1 µg,
5X PrimeScript RT Master Mix 4 µl, RNase Free dH2O was
added to reach 20 µl, reaction condition: 37°C for 15 min, 85°C for
5 min. qPCR was performed using the Light Cycler TaqMan Master kit
(Toyobo), according to the manufacturer's instructions, qPCR
reaction system: SYBR Premix Ex Taq II 10 µl, cDNA1 µl, forward
primer 0.5 µl, reverse primer 0.5 µl, Sterile water 8 µl. The
cycling conditions were as follows: Initial denaturation at 95°C
for 1 min, 30 cyles of denaturation at 94°C for 30 sec, annealing
at 58°C for 30 sec and extension at 72°C at 10 sec, followed by
72°C for 2 min and 16°C for 5 min. The relative mRNA expression
levels of Cytc were determined using the comparative Ct method,
using arithmetic formulae from the MxPro software tool. The
relative expression of Cytc was calculated using the ΔΔCt method.
The expression of mRNA was normalized against the expression of the
GAPDH gene.
Immunohistochemical staining for Cytc
The fixed biopsy specimen slides were fixed in
Carnoy's fixative and embedded in paraffin wax. Paraffinized
tissues were sectioned to 4 µm thickness, deparaffinized in xylene
and rehydrated through a series of concentrations of ethanol.
Antigen retrieval was carried out using an electric pressure cooker
(110°C, 20 min) in 10 mM citrate buffer (pH 6.0). The sections were
immersed in blocking solution (Dako Corp., Carpinteria, CA, USA)
for 10 min at room temperature followed by washing with phosphate
buffered saline (pH 7.4) plus 0.1% Tween-20 for blocking the
endogenous peroxidase activity. The sections were incubated with
rabbit anti-human Cytc (BD Pharmingen, Santiago, CA, USA) overnight
at 4°C which were diluted 1:50. The slides were washed for 5 min,
sections were then incubated with peroxidase-conjugated goat
anti-rabbit secondary antibody for 1 h at room temperature; both of
which were diluted 1:200. The reactions were developed using a DAB
substrate kit, with hematoxylin as counterstain. Each slide was
evaluated by one of the authors under a microscope (Nikon, Tokyo,
Japan).
At higher magnification (×400), five visual field
were selected randomly, the expression positive signal was analyzed
by Image-proplus software. Compared the level of Cytc protein in
pSS group and healthy control group of labial salivary gland
according to the integral optical density (IOD) as a parameter for
semi-quantitative detection.
Determination of the content of Cytc
The tissue homogenate was preparation and the
mitochondria was extracted: Reference Zydowo et al methods
(13). Labial gland tissue were
weight after rinsed with cold saline, 10% tissue homogenate were
made with precooling medium homogenate (0.1 mol/l Tris-HCl, 1 mol/l
KCl, 0.25 mol/l sucrose, pH 7.4). At low temperature with 15 min,
centrifugation of 600 g was conducted. Take the supernatant,
centrifugation of 1,800 × g was conducted again. The cold slurry
medium was added with the precipitation, the mitochondria
suspension was made and saved −20°C.
Determination of Cytc: 1 ml Cytc standard (80 mg/ml)
dilution was taken, add sodium sulfate when vibration, the density
of sodium sulfatewas measured at 520 nm. The standard curve was
draw with the concentration of the standard for horizontal
ordinate, optical density for longitudinal coordinate. The
concentration of sample were calculated by the standard curve.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software. The data are presented as the median ± interquartile. The
Mann-Whitney rank-sum test were used to compare Cytc mRNA and Cytc
protein between the pSS and the healthy controls. In addition,
correlations between the levels of IOD of Cytc protein with
clinical and laboratory variables were assessed using Spearman's
rank correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
RT-PCR and qRT-PCR
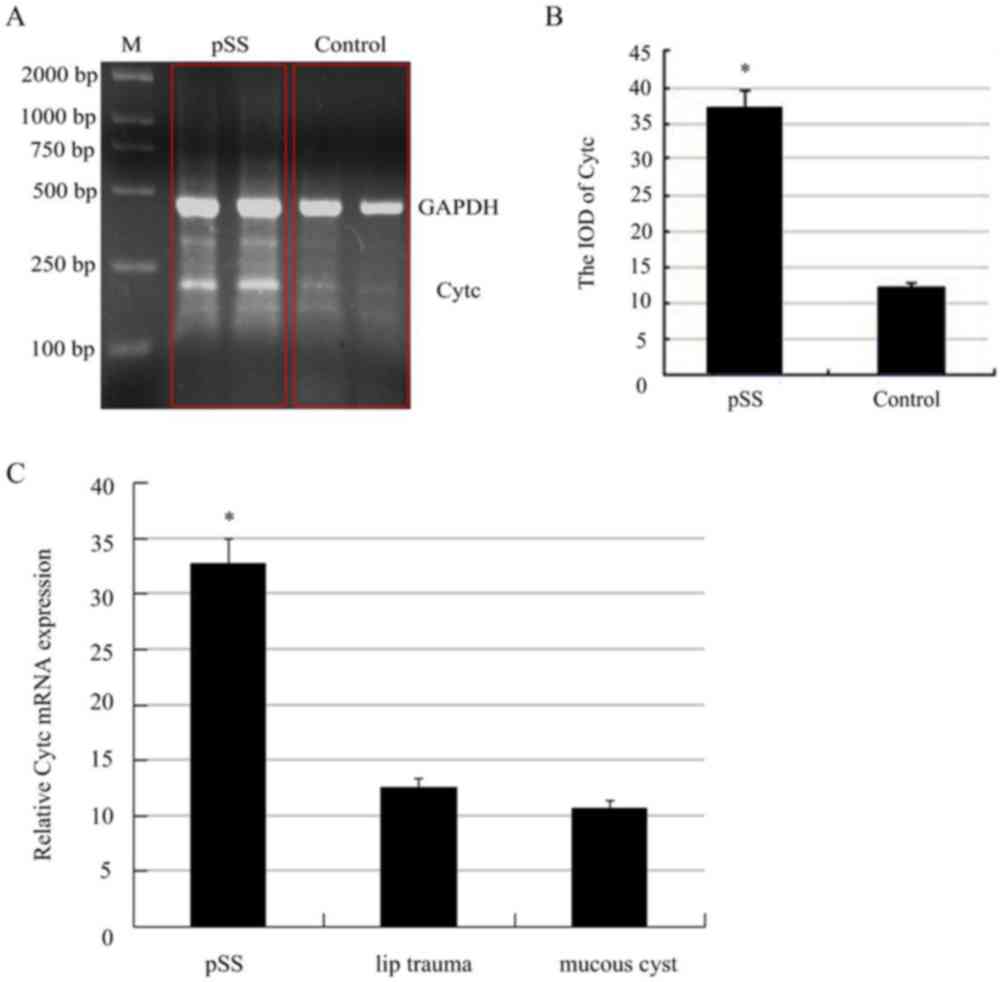
To determine the mRNA expression level of Cytc, the
specimen from the pSS patients and the healthy controls were
separated. The total mRNA was isolated and the mRNA expression
level of Cytc was investigated with RT-PCR. Using SPSS 17.0
software, the data are presented as the fold-change in the gene
expression normalized against GAPDH. As shown in Fig. 1A and B, there was a 3.45-fold
increase in the relative mRNA expression of Cytc in the pSS
patients prior to the control.
We also used qRT-PCR to explore the mRNA expression
level of Cytc. In this experiment, we divided the control group
into two groups according to the source of the sample. As shown in
Fig. 1C, there was a 2.66-fold
increase in the relative mRNA expression of Cytc in the pSS
patients prior to the control from the lip trauma, there was a
2.96-fold increase in the relative mRNA expression of Cytc in the
pSS patients prior to the control from the operation of mucous
cyst, but there was no significant difference between the two
control groups (Fig. 1C).
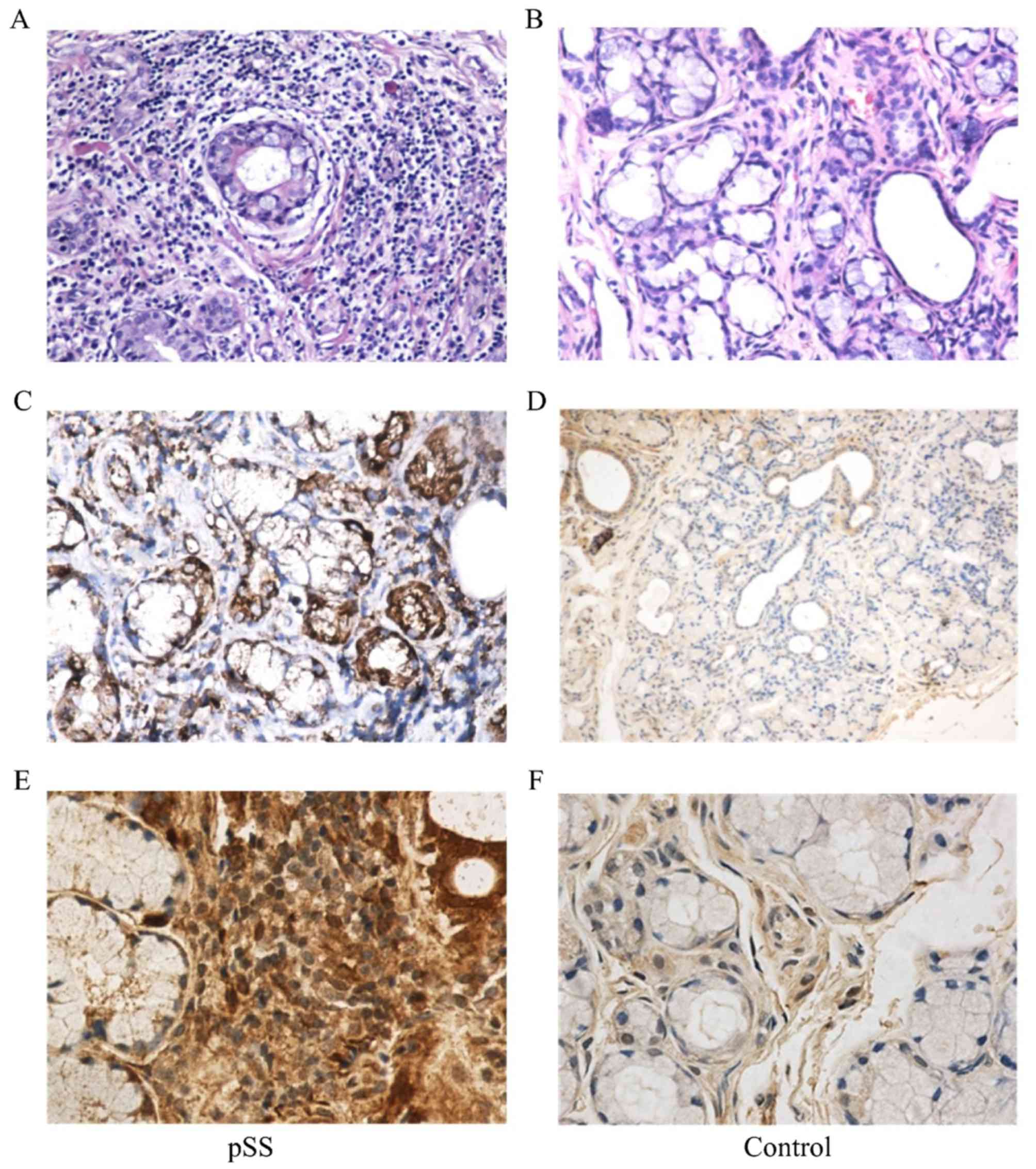
Immunohistochemistry staining
To evaluate the local effect of Cytc in pSS, we
applied immunohistochemical staining to determine the expression of
Cytc in the labial salivary glands from pSS. Freshly explanted
lower lip biopsy specimens were sectioned and stained with
anti-Cytc. All 35 pSS samples exhibited distinct expression of
Cytc. Brown particles were mainly distributed in the cytoplasm of
acinar epithelial cells, ductal epithelial cells and the
infiltration of focal lymphocytic. In contrast, the labial salivary
glands from the control exhibited lower expression of Cytc. It was
mainly expressed in mesenchymal cells and glandular epithelial
cells with a weak expression of Cytc. Overall, the expression of
Cytc in pSS was stronger and more widely distributed than the
control (Figs. 2 and 3A).
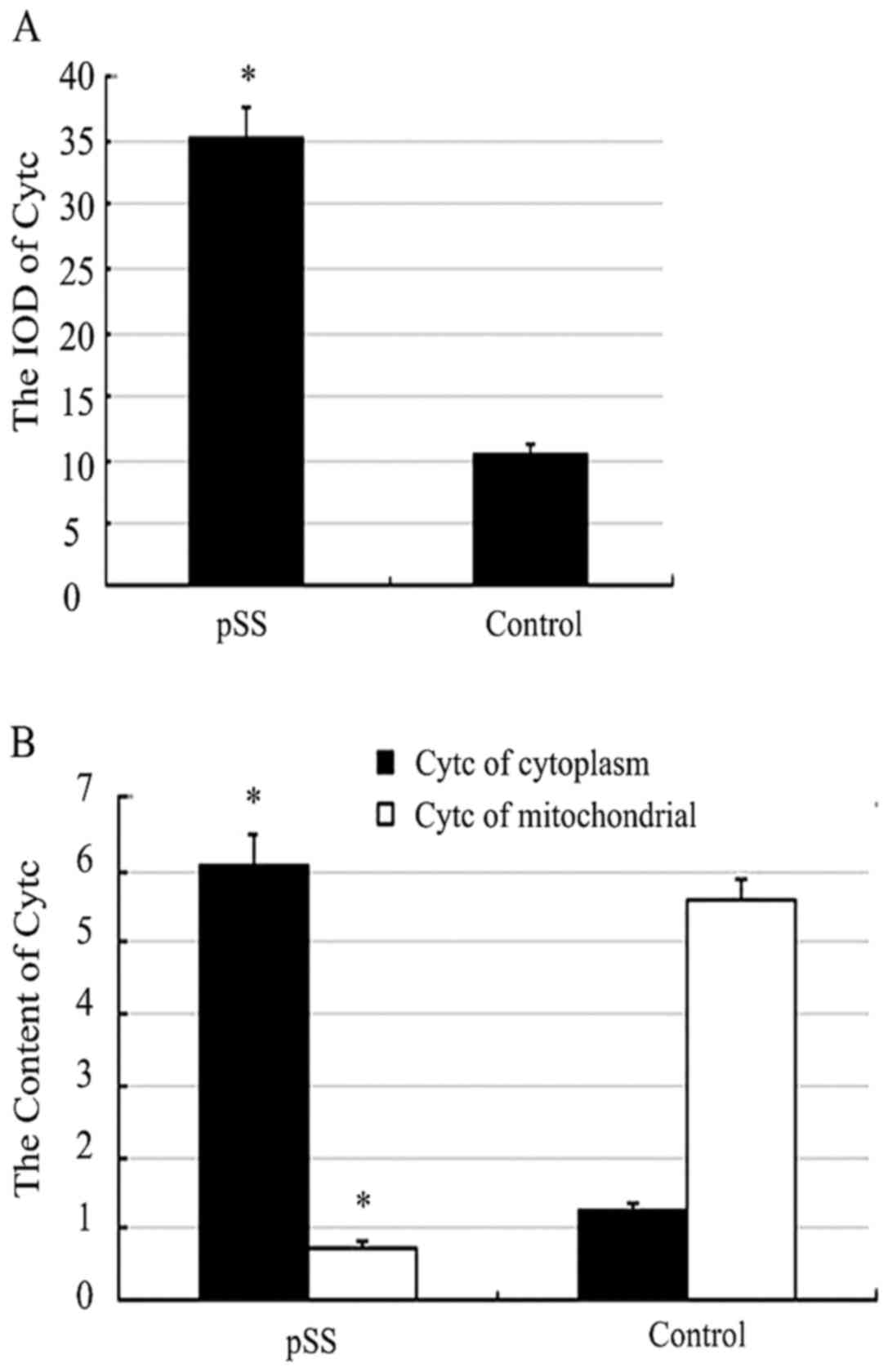
The content of Cytc
There was great differences between the two groups
(P<0.05). The cytoplasm content of Cytc in pSS was 6.07±1.84,
while the control group was 1.25±1.45. The mitochondrial content of
Cytc in pSS was 0.76±1.32, while the control group was 5.57±0.59
(Fig. 3B).
The correlation between the expression
of Cytc protein and clinical and laboratory variables
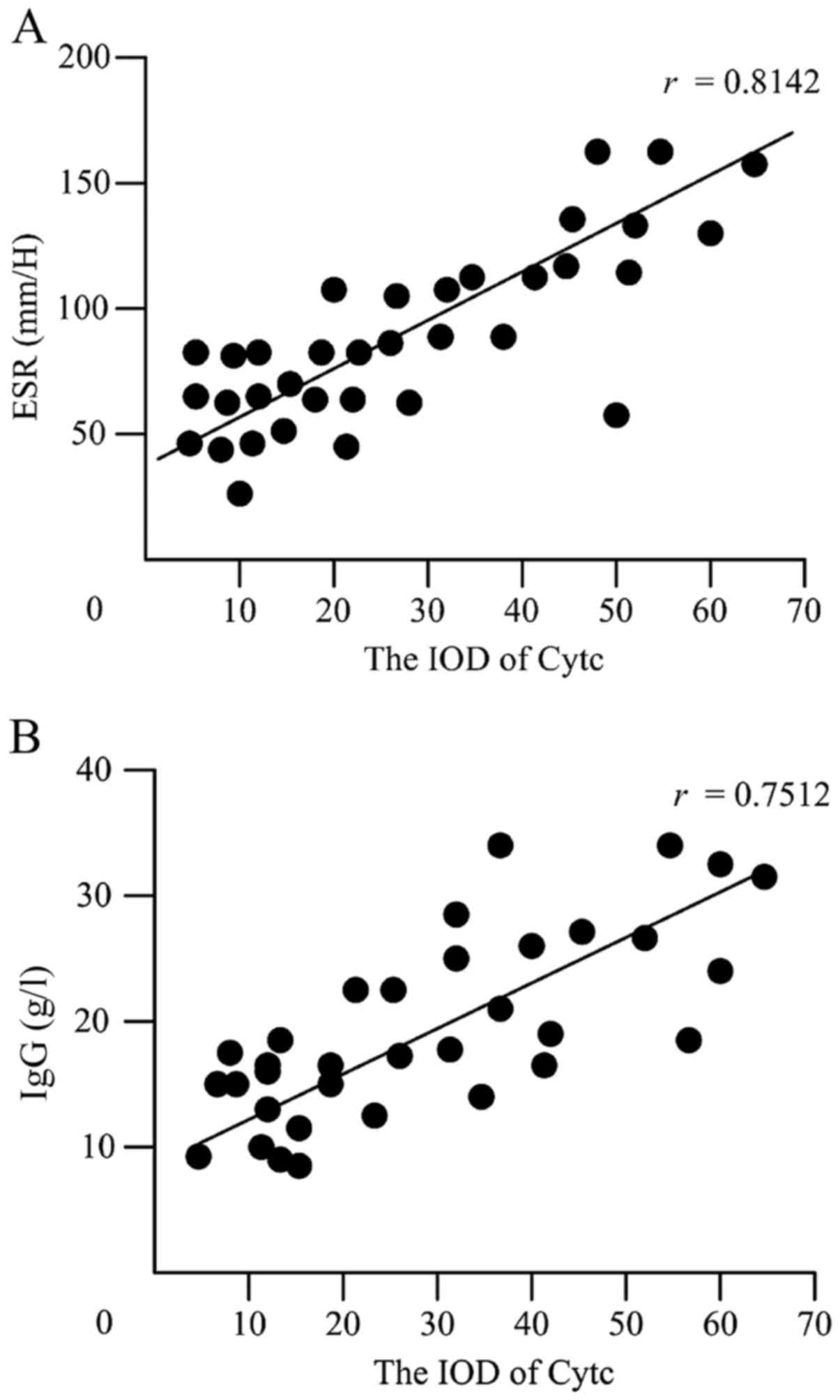
To further determine the relationship between the
IOD of Cytc protein levels and laboratory test results including
the titers of SSA, SSB, ANA, ESR, CRP, RF and IgG levels. It was
found that the IOD of Cytc protein levels was positively correlated
with ESR (r=0.8142, P<0.05) (Fig.
4A) and IgG (r=0.7512, P<0.05) (Fig. 4B). However, no significant
correlations were found between the IOD of Cytc protein levels and
the other laboratory values (data not shown). Interestingly, when
patients were grouped according to test results normal or abnormal,
elevated the IOD of Cytc protein levels exhibited were found in the
groups with high titers of ANA (7.88±0.22 vs. 1.22±0.12, P=0.0032)
or high concentration of CRP (16.11±0.12 vs. 3.22±0.45, P=0.0054),
RF (7.21±2.34 vs. 1.67±0.12, P=0.0035), as shown in Table I.
 | Table I.Comparison of the IOD levels of Cytc
protein between pSS patients with normal or abnormal laboratory
values. |
Table I.
Comparison of the IOD levels of Cytc
protein between pSS patients with normal or abnormal laboratory
values.
|
| IOD levels of Cytc
protein |
|
|---|
|
|
|
|
|---|
| Parameter | Normal mean ± SD
(n) | Abnormal mean ± SD
(n) | P-value |
|---|
| A-SSA | 3.22±1.02 (5) | 5.17±4.03 (30) | 0.1142 |
| A-SSB | 5.22±2.21 (18) | 3.53±0.12 (17) | 0.6433 |
| ANA | 1.22±0.12 (5) | 7.88±0.22 (30) | 0.0032b |
| CRP | 3.22±0.45 (25) | 16.11±0.12 (10) | 0.0054b |
| RF | 1.67±0.12 (15) | 7.21±2.34 (20) | 0.0035b |
| IgM | 6.23±3.20 (18) | 10.08±2.14 (17) | 0.0335a |
| IgA | 2.16±2.11 (30) | 7.24±1.24 (5) | 0.0132a |
Discussion
pSS is a type of autoimmune disease concerning
exocrine glands of whole body, which is characterized by the
infiltration of lymphocytes and plasma cell, mainly affecting
lacrimal gland as well as salivary gland. Hyposecretion of gland
and some symptoms can be caused, for instance, xerostomia,
xerophthalmia. On the early stage of disease, the local exocrine
gland was injured, with the aggravation of local inflammation,
uncontrollable immune system will induce systemic disease and
develop into malignancy eventually. At present, the etiology and
pathogenic mechanisms are not very clear. In recent years, from a
large number of literature, we can find that pSS may related to the
endogenous pathway of apoptosis (7,10).
Sisto M (14) found that
ectodysplasin-A2 (EDA-A2) and its receptor X-linked ectodermal
dysplasia receptor (XEDAR) are overexpressed in pSS salivary gland
epithelial cells in comparison with healthy individuals and that
the EDA-A2/XEDAR system in these cells is involved in the induction
of apoptosis via caspases activation. Horai (15) observed that polyinosinic: Cytidylic
acid induced apoptosis of primary salivary gland epithelial cells
in vitro compared with a relatively low prevalence of
apoptosis found in the ducts and alveoli of labial salivary glands
in vivo. Katsiougiannis (16) discovered that endoplasmic reticulum
stress is activated in minor salivary gland epithelial cells from
pSS patients and controls. Endoplasmic reticulum stress-induced
apoptosis in human salivary gland cells leads to cell surface and
apoptotic blebs relocalization of Ro/SSA and La/SSB
autoantigens.
Cytc is a mitochondrial protein responsible for
transferring electrons between electron transport chain complexes
III and IV. Under normal circumstances, Cytc was intergrated into
the inner membrane of mitochondrial. When stimulated by the
apoptosis, Bax forms oligomers and intergrated into the outer
membrane of mitochondrial, inducing and changing permeability of
mitochondrial membrane, promoting the release of Cytc. It also
contribute to the formation of apoptosome (which consists of
Apaf-1, Cytc and caspase-9), make effector caspases alive to induce
apoptosis (17,18). The release of Cytc from the
mitochondria has been considered as a commitment step in intrinsic
apoptosis (19,20).
The present study demonstrated that the expression
of Cytc mRNA turns enhanced in the labial gland of pSS-patients and
it has statstical signaficance when compared with the group of
health patients (P<0.05). But it notable that the Cytc in
mitochondria is lower than control-group (P<0.05). Cytc was
released from mitochondria to cytoplasm, not only reduce the
respiratory function of mitochondria but also induce the key
procedure of apoptosis, activating caspases reaction and apoptosis.
In addition, our investigations have revealed a close correlation
of Cytc levels with the disease activity and severity in pSS
patients. Our data have clearly shown that the levels of Cytc
protein positively correlate with ESR and IgG. We provide new
evidence indicating involvement of Cytc overactivation in the
disease pathophysiology.
In conclusion, the present study demonstrated that
Cytc is upregulated in pSS patients, indicating a possible role of
Cytc in the pathogenesis and development of pSS.
Acknowledgements
This study was supported by grants from the Youth
Scientific Research Fund Project of the School of Stomatology,
China Medical University (no. K101593-16-05).
References
|
1
|
Olsson P, Turesson C, Mandl T, Jacobsson L
and Theander E: Cigarette smoking and the risk of primary Sjögren's
syndrome: A nested case control study. Arthritis Res Ther.
19:502017. View Article : Google Scholar :
|
|
2
|
Xin Y, Cai H, Li Y and Cui Y: Thymic
hyperplasia associated with primary Sjogren's syndrome cured by
thymectomy. J Thorac Dis. 9:E130–E132. 2017. View Article : Google Scholar :
|
|
3
|
Moerman RV, Arends S, Meiners PM, Vissink
A, Spijkervet FK, Kroese FG, Brouwer E and Bootsma H: Detailed
analysis of the articular domain in patients with primary sjögren
syndrome. J Rheumatol. 44:292–296. 2017. View Article : Google Scholar
|
|
4
|
Zhang N, Yang N, Chen Q, Qiu F and Li X:
Upregulated expression level of the growth factor, progranulin, is
associated with the development of primary Sjögren's syndrome. Exp
Ther Med. 8:1643–1647. 2014. View Article : Google Scholar :
|
|
5
|
Manganelli P and Fietta P: Apoptosis and
Sjögren syndrome. Semin Arthritis Rheum. 33:49–65. 2003. View Article : Google Scholar
|
|
6
|
Nakamura H, Koji T, Tominaga M, Kawakami
A, Migita K, Kawabe Y, Nakamura T, Shirabe S and Eguchi K:
Apoptosis in labial salivary glands from Sjögren's syndrome (SS)
patients: Comparison with human T lymphotropic virus-I
(HTLV-I)-seronegative and -seropositive SS patients. Clin Exp
Immunol. 114:106–112. 1998. View Article : Google Scholar :
|
|
7
|
Treviño-Talavera BA, Palafox-Sánchez A,
Muñoz-Valle JF, Orozco-Barocio G, Navarro-Hernández RE, Vázquez-Del
Mercado M, de la Torre García I and Oregon-Romero E: FAS −670A>G
promoter polymorphism is associated with soluble Fas levels in
primary Sjögren's syndrome. Genet Mol Res. 13:4831–4838. 2014.
View Article : Google Scholar
|
|
8
|
Nakamura H: Cell death of salivary gland
epithelial cells and involvement of HTLV-I in Sjögren's syndrome.
Nihon Rinsho Meneki Gakkai Kaishi. 37:117–124. 2014.(In Japanese).
View Article : Google Scholar
|
|
9
|
Zeng W, Wang X, Xu P, Liu G, Eden HS and
Chen X: Molecular imaging of apoptosis: From micro to macro.
Theranostics. 5:559–582. 2015. View Article : Google Scholar :
|
|
10
|
Kong L, Ogawa N, Nakabayashi T, Liu GT,
D'Souza E, McGuff HS, Guerrero D, Talal N and Dang H: Fas and fas
ligand expression in the salivary glands of patients with primary
Sjögren's syndrome. Arthritis Rheum. 40:87–97. 1997. View Article : Google Scholar
|
|
11
|
Green DR: Apoptotic pathways: Paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar
|
|
12
|
Vitali C, Bombardieri S, Jonsson R,
Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox
RI, Kassan SS, et al: Classification criteria for Sjögren's
syndrome: A revised version of the European criteria proposed by
the American-European Consensus Group. Ann Rheum Dis. 61:554–558.
2002. View Article : Google Scholar :
|
|
13
|
Zydowo MM, Swierczyński J, Nagel G and
Wrzołkowa T: The respiration and calcium content of heart
mitochondria from rats with vitamin D induced carionecrosis.
Biochem J. 226:155–161. 1985. View Article : Google Scholar :
|
|
14
|
Sisto M, Lorusso L and Lisi S: X-linked
ectodermal dysplasia receptor (XEDAR) gene silencing prevents
caspase-3-mediated apoptosis in Sjögren's syndrome. Clin Exp Med.
17:111–119. 2017. View Article : Google Scholar
|
|
15
|
Horai Y, Nakamura H, Nakashima Y, Hayashi
T and Kawakami A: Analysis of the downstream mediators of toll-like
receptor 3-induced apoptosis in labial salivary glands in patients
with Sjögren's syndrome. Mod Rheumatol. 26:99–104. 2016. View Article : Google Scholar
|
|
16
|
Katsiougiannis S, Tenta R and Skopouli FN:
Endoplasmic reticulum stress causes autophagy and apoptosis leading
to cellular redistribution of the autoantigens Ro/Sjögren's
syndrome-related antigen A (SSA) and La/SSB in salivary gland
epithelial cells. Clin Exp Immunol. 181:244–252. 2015. View Article : Google Scholar :
|
|
17
|
Zhang Y, Liu H, Jin J, Zhu X, Lu L and
Jiang H: The role of endogenous reactive oxygen species in
oxymatrine-induced caspase-3-dependent apoptosis in human melanoma
A375 cells. Anticancer Drugs. 21:494–501. 2010. View Article : Google Scholar
|
|
18
|
Yokogawa Y, Yamauchi R, Saito A, Yamato Y
and Toma T: Kinetic modelling of cytochrome c adsorption on SBA-15.
Biomed Mater Eng. 28:37–46. 2017.
|
|
19
|
Song R, Harris LD and Pettaway CA:
Downmodulation of Bcl-2 sensitizes metastatic LNCaP-LN3 cells to
undergo apoptosis via the intrinsic pathway. Prostate. 70:571–583.
2010.
|
|
20
|
Lo YT, Huang HW, Huang YC, Chan JF and Hsu
YH: Elucidation of tRNA-cytochrome c interactions through
hydrogen/deuterium exchange mass spectrometry. Biochim Biophys
Acta. 1865:539–546. 2017. View Article : Google Scholar
|