Introduction
Neural stem cells (NSCs) are present in the central
nervous system (CNS) of adults and developing mammals (1,2).
They are self-renewing, multipotent and undifferentiated precursor
cells that have the ability to differentiate into glial and
neuronal lineages. NSCs may be useful in cell-based replacement
therapies for neurological disorders, including Alzheimer's
disease, traumatic brain injury, spinal cord injuries and retinal
disease (3–5). However, poor proliferation and
insufficient differentiation of the transplanted NSCs into neurons
limit the practical use of NSC-based therapies (6,7),
making further study necessary to realize the potential of NSCs in
clinical treatment (8–10). Therefore, it is important to
explore the molecular and cellular mechanisms that regulate NSC
proliferation and differentiation.
The Wnt/β-catenin pathway serves an important role
in regulating NSC fate. Activation of the Wnt/β-catenin pathway
promotes NSC proliferation and differentiation (9,10).
Hence the requirement for investigating the regulation of the
Wnt/β-catenin pathway.
MicroRNAs (miRNAs) are small noncoding RNAs ~22 nt
in length, which have been demonstrated to be involved in the gene
regulation process (11–13). miRNAs are involved in various
biological functions, including cell differentiation, proliferation
and apoptosis (14–17). miRNAs are involved in stem cell
fate and self-renewal, and regulate the expression of stem cell
genes (15,18,19).
In the present study, overexpression of miR-21 was
demonstrated to enhance the proliferation of rat NSCs and their
differentiation into neurons, and to reduce their differentiation
into astrocytes via the Wnt/β-catenin signaling pathway.
Materials and methods
Cell culture and transfection
All procedures requiring the use of laboratory
animals were approved and monitored by the Animal Care Committee of
Zhumadian Central Hospital (Zhumadian, China). Isolation, culture
and identification of NSCs was performed as previously described
(20). Pregnant Sprague-Dawley
rats (day E14 fetal; 200–250 g; n=6) were obtained from the
Shanghai Branch of National Rodent Laboratory Animal Resources
(Shanghai, China) and were housed in a controlled 22±1°C
environment under 14/10 h light/dark cycles with free access to
food and water. Following anesthesia with 50 mg/kg of ketamine and
10 mg/kg of xylazine administered intraperitoneally, NSCs were
isolated from the hippocampus of 40 embryonic day 14 fetal rats.
The tissues were transferred to cold phosphate buffered saline
(PBS), minced, and dissociated. The isolated NSCs were cultured in
Dulbecco's modified Eagle's medium-F12 medium (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 1% N2, 2%
B-27 supplement, 2 mmol/l glutamine, 20 ng/ml epidermal growth
factor (EGF) and 20 ng/ml basic fibroblast growth factor (bFGF;
Peprotech, Inc. Rocky Hill, NJ, USA). Primary neurospheres were
digested with 0.25% trypsin. EGF and bFGF were removed from the
growth medium, and the medium was supplemented with 1% fetal bovine
serum (FBS; Irvine Scientific, Santa Ana, CA, USA). Cells were
placed on poly-lysine-coated coverslips in 24-well plates in prior
to fixation. miR-21 mimic and scramble were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). The transfection of
miR-21 mimics and scramble miRNA (20 ng/ml) was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. NSCs were incubated with
1 µM of the Wnt/β-catenin pathway inhibitor FH535 (EMD Millipore,
Billerica, MA, USA) for 24 h, on day 7 post-transfection. miR-21
mimic and scramble sequences were: miR-21 mimic:
5′-UAGCUUAUCAGACUGAUGUUGA-3′; miR-21 scramble:
5′-UUCUCCGAACGUGUCACGUTT-3′.
Immunocytochemistry
Cells were fixed with paraformaldehyde (4%) for ~30
min at room temperature and permeabilized with 0.2% Triton X-100.
Following blocking with 10% goat serum, cells were incubated with
primary anti-β-tubulin III (AB15708A4; 1:200; Merck KGaA,
Darmstadt, Germany), anti-glial fibrillary acidic protein (GFAP;
04–1062; 1:1,000; Merck KGaA) and anti-nestin antibodies (ab134017;
1:100; Abcam, Cambridge, UK), at 4°C overnight. Subsequently, cells
were incubated with tetramethylrhodamine isothiocyanate-conjugated
goat polyclonal anti-rabbit immunoglobulin (Ig)G (ab145472; 1:300;
Abcam), fluorescein isothiocyanate (FITC)-conjugated goat
anti-rabbit IgG (bs-0519R-FITC; 1:100; BIOSS, Beijing, China), or
FITC-conjugated goat anti-mouse IgG (bs-2511R-FITC; 1:100; BIOSS)
for 3 h at room temperature. Nuclei were stained with DAPI.
Coverslips were mounted and slides were analyzed by fluorescence
microscopy (Zeiss GmbH, Jena, Germany).
Cell proliferation
NSC proliferation was assessed using Cell Counting
kit 8 (CCK8) assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer's protocol. Proliferation
rates were analyzed 0, 1, 3, 5 and 7 days following transfection.
The optical density was measured at a wavelength of 450 nm. In
addition, a 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay was
used to assess cell proliferation. The single cell suspensions of
NSCs from the neurospheres (7 days following transfection) were
incubated with the proliferation marker EdU (0.2 mM/l) for 30 min
at 37°C. Following incubation, the dissociated cells were seeded
onto 100 µg/ml poly-L-lysine-coated coverslips and were stained for
the NSC marker nestin using the Click-iT EdU Imaging kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Cells were incubated with a rabbit anti-nestin polyclonal primary
antibody (ab134017; 1:200; Abcam) for 36 h at 4°C, and a
Cy3-conjugated goat anti-rabbit IgG secondary antibody
(bs-2511R-Cy3; 1:100; BIOSS) for 3 h at room temperature.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA samples were purified and had optical density 260/280
ratios between 1.8 and 2.0, as confirmed using a Nanodrop
Spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). The extracted RNA was reverse transcribed into cDNA using a
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.). For each sample 2 µg RNA was used to synthesize
the cDNA. The RT-qPCR reaction was conducted using iQ SYBR-Green
Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
regulated by the spectrofluorometric iQ5 Thermal iCycler (Bio-Rad
Laboratories, Inc.). qPCR detected the expression of miRNA-21 and
mRNA using the SYBR-Green PCR kit (Qiagen, Inc., Valencia, CA, USA)
on a 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Amplification was performed under the following
conditions: Initial 1 cycle at 95°C for 8 min, followed by 42
cycles at 95°C for 10 sec, at 60°C for 40 sec and at 72°C for 1
sec, and a final extension step at 72°C for 10 min. The relative
mRNA expression of each gene was calculated using the
2−ΔΔCq method and normalized to β-actin (21). The primers used were as follows:
miR-21 forward, 5-CCAGTGAAGATGTGTTCAGCT-3′ and reverse,
5-GCACAGCCAGTAGAAGTAGAT-3′; β-catenin forward,
5′-GACCACAAGCAGAGTGCTGA-3′ and reverse, 5′-ACTCGGGTCTGTCAGGTGAG-3′;
cyclin D1 forward, 5′-TGGAGCCCCTGAAGAAGAG-3′ and reverse,
5′-AAGTGCGTTGTGCGGTAGC-3′; p21 forward, 5′-CTGCTCTCCCTTCCTCAGAC-3′
and reverse, 5′-TGAGGTAGGACCAGGAAACC-3′; β-tubulin III forward,
5′-AGCAAGGTGCGTGAGGAGTA-3′ and reverse, 5′-AAGCCGGGCATGAAGAAGT-3′;
GFAP forward, 5′-CAACGTTAAGCTAGCCCTGGACAT-3′ and reverse,
5′-CTCACCATCCCGCATCTCCACAGT-3′; and β-actin forward,
5′-CCCGCGAGTACAACCTTCT-3′ and reverse,
5′-CGTCATCCATGGCGAACT-3′.
Western blot analysis
Cells were collected and washed twice with PBS.
Cells were lysed using lysis buffer containing 10 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, 1% sodium
deoxycholate and protease inhibitor cocktail tablet (Roche
Diagnostics, Basel, Switzerland) for 30 min at 37°C. The protein
content in each sample was determined using a Bio-Rad Protein assay
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts
of extracted protein samples (50 µg) were separated by 12% SDS-PAGE
and transferred onto a polyvinylidene fluoride membrane, which was
blocked with 5% milk overnight at 4°C. The membrane was incubated
with the following primary antibodies: β-catenin (Ab4176; 1:2,000),
cyclin D1 (ab134175; 1:100), p21 (ab109199; 1:500), β-tubulin III
(AB15708A4; 1:1,000), GFAP (04-1062; 1:2,000) and β-actin (Santa
Cruz Biotechnology, Inc. Ab3700; 1:500) overnight at 37°C.
Subsequently, the membrane was probed with horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat no. sc-2004; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and goat anti-mouse IgG
(cat no. sc-2005; Santa Cruz Biotechnology, Inc.) secondary
antibodies at 1:1,000 and room temperature for 1 h. Protein bands
were visualized using the Beyotime ECL Plus detection kit (Beyotime
Institute of Biotechnology, Haimen, China). The band intensities
were subsequently quantified using Quantity One software (version
4.62; Bio-Rad Laboratories, Inc.) and normalized to β-actin as an
internal standard.
Statistical analysis
All data are presented as the mean ± standard
deviation of three independent experiments. Statistical analyses
were performed using SPSS software version 20.0 (IBM SPSS, Armonk,
NY, USA). An unpaired Student's t-test or one-way followed by
Fisher's least square difference (LSD) post hoc test was used for a
statistical comparison of the mean values between two or four
groups, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of rat NSCs
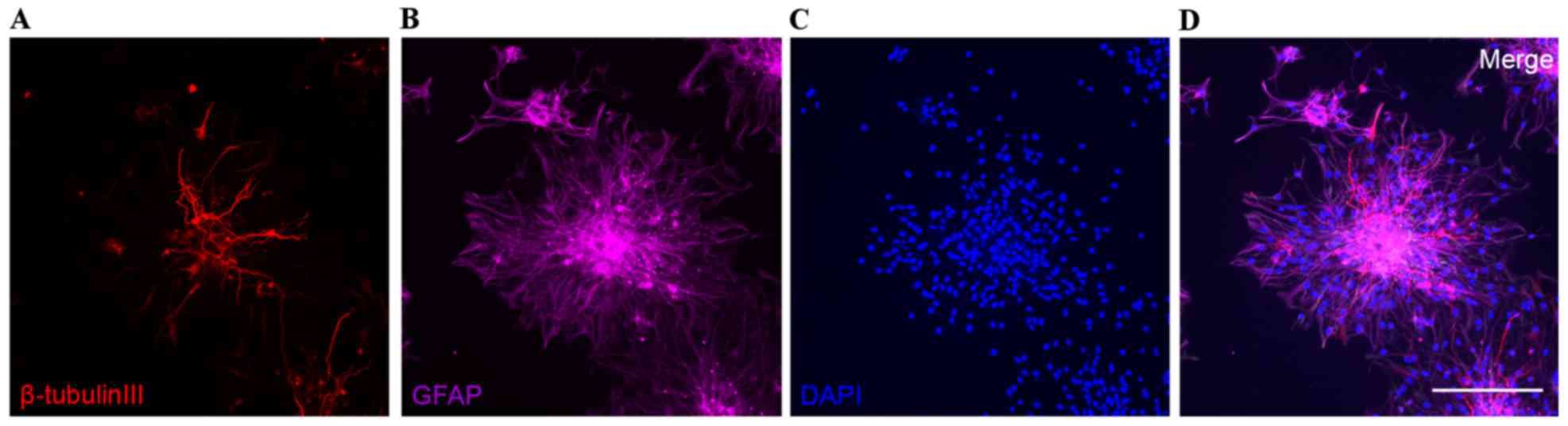
The rat NSCs formed neurospheres and differentiated
into neurons and astrocytes 3 days following removal of bFGF
(Fig. 1). In addition, the rat
NSCs expressed the NSC marker nestin (Fig. 2A), which identified the isolated
cells as rat NSCs.
miR-21 promotes rat NSC
proliferation
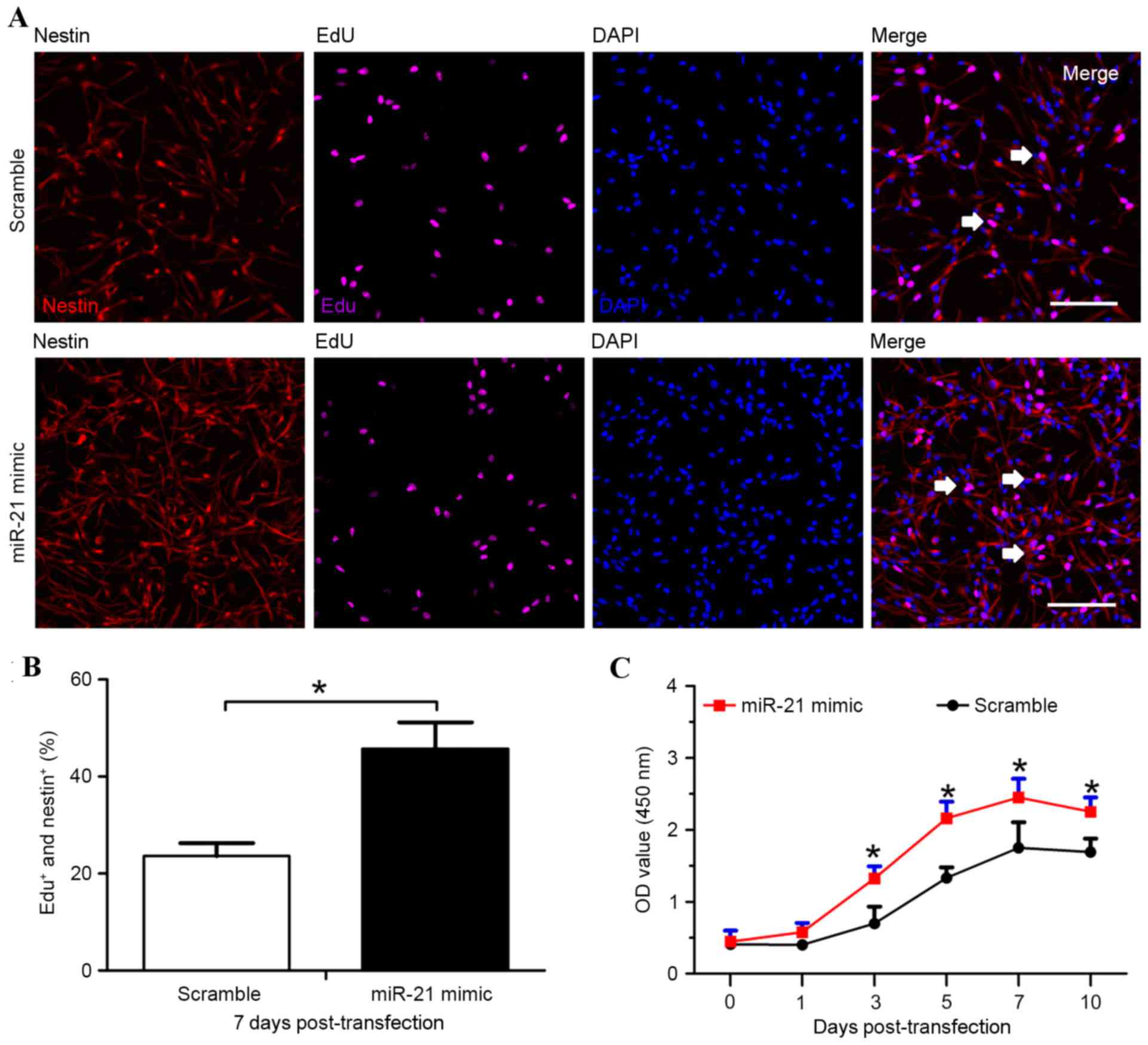
The EdU incorporation assay evaluated the effects of
miR-21 on rat NSC proliferation. Cells were treated with EdU (0.2
mM/l) for 30 min on day 7 post-transfection, and the incorporated
EdU was analyzed. The proportion of EdU-positive cells was enhanced
~2-fold in the miR-21 mimic group (45.72±5.39%) compared with the
scramble group (23.61±2.65%; P<0.05; Fig. 2B).
The CCK8 assay was also performed to determine the
involvement of miR-21 in the proliferation of transfected rat NSCs.
The NSCs in the miR-21 mimic group exhibited faster growth and
proliferation rates compared with the scramble group 3, 5, 7 and 10
days post-transfection (P<0.05; Fig. 2C), indicating that overexpression
of miR-21 enhanced rat NSC proliferation.
Overexpression of miR-21 promotes rat
NSC proliferation through activation of the Wnt/β-catenin signaling
pathway
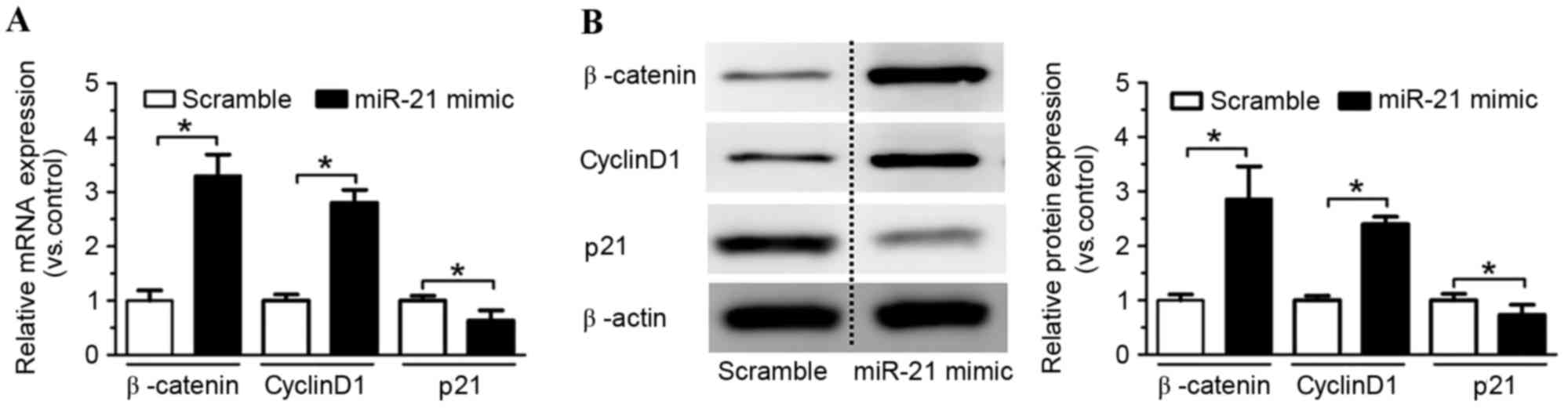
To determine whether the Wnt/β-catenin pathway was
involved in the miR-21 overexpression-induced increase of rat NSC
proliferation, the mRNA and protein expression levels of several
associated genes, including β-catenin, cyclin D1 and p21 were
detected by RT-qPCR and western blotting. Overexpression of miR-21
significantly increased β-catenin and cyclin D1 mRNA expression
levels compared with the scramble control (P<0.05; Fig. 3A), and reduced p21 mRNA expression
levels compared with the scramble control (P<0.05; Fig. 3A). β-catenin and cyclin D1 protein
expression levels were also increased in the miR-21 mimic group
compared with the scramble control group (P<0.05; Fig. 3B), whereas p21 protein expression
levels were decreased (P<0.05; Fig.
3B). These findings suggested that the Wnt/β-catenin pathway
may be involved in mediating the effects of miR-21 overexpression
on rat NSC proliferation.
Suppression of the Wnt/β-catenin
signaling pathway inhibits the increased rat NSC proliferation
induced by overexpression of miR-21
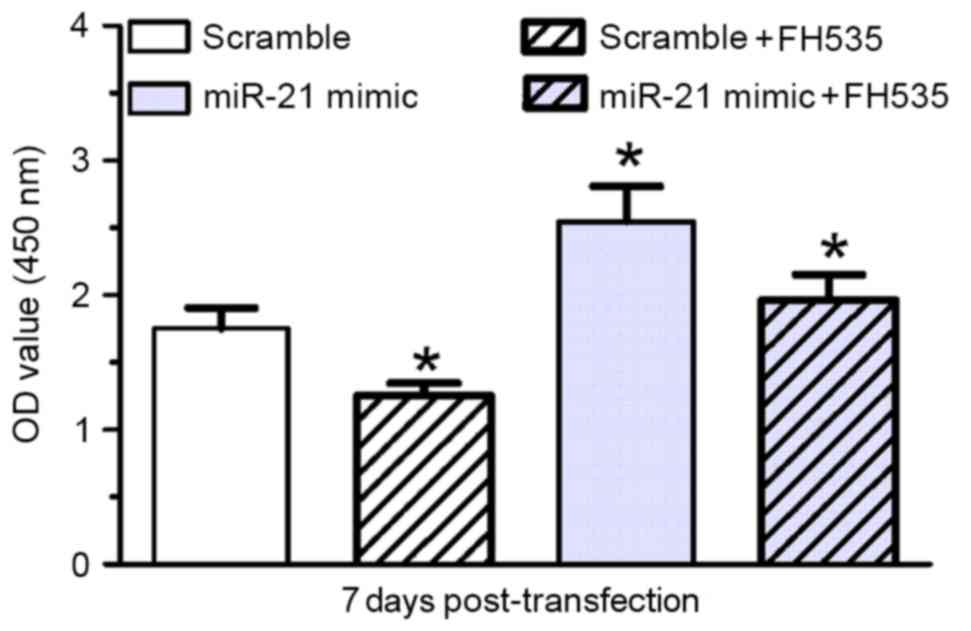
To confirm involvement of the Wnt/β-catenin pathway
in NSC proliferation, a pharmacological inhibitor, the β-catenin/T
cell factor (TCF) inhibitor (FH535), was used. Treatment with FH535
inhibited miR-21 mimic-induced rat NSC proliferation compared with
the scramble control (P<0.05; Fig.
4) and cells transfected with the miR-21 mimic only (P<0.05;
Fig. 4).
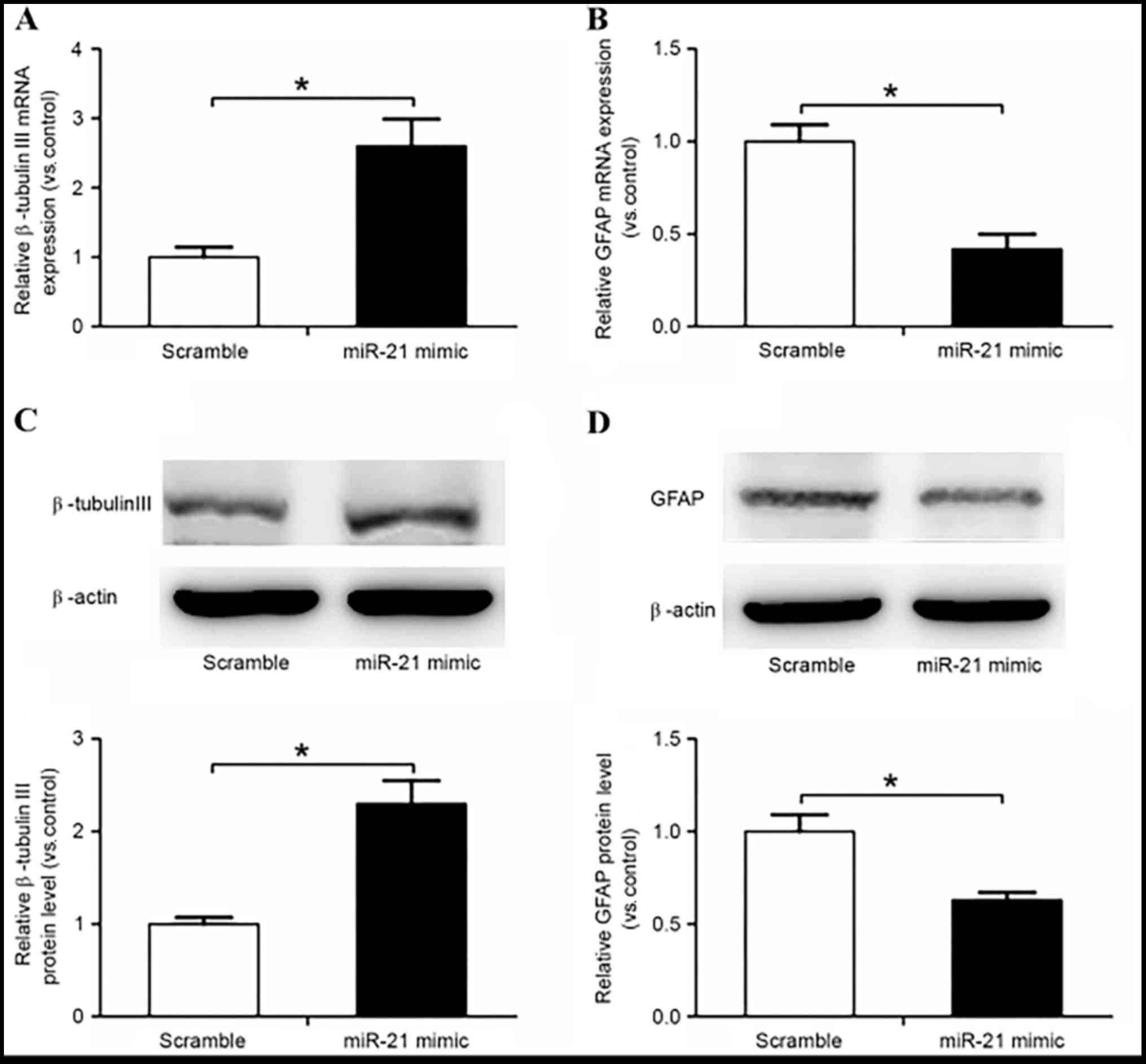
miR-21 promotes rat NSC
differentiation to neurons and inhibits differentiation to
astrocytes
miR-21 promoted the differentiation of rat NSCs to
neurons and inhibited the differentiation to astrocytes, as
confirmed by increased β-tubulin III expression and decreased GFAP
expression compared with in the scramble control group (P<0.05;
Fig. 5).
Discussion
In the present study, miR-21 overexpression was
demonstrated to promote rat NSC proliferation and neural
differentiation, and to reduce differentiation to astrocytes, via
the Wnt/β-catenin signaling pathway. Furthermore, inhibition of the
Wnt/β-catenin signaling pathway impaired the miR-21-induced
increase in NSC proliferation. Therefore, the present study
revealed that miR-21 is involved in NSC proliferation and
differentiation via the Wnt/β-catenin signaling pathway.
The stimulatory effects of miR-21 on rat NSC
proliferation have been confirmed in the present study. Compared
with in the scramble control group, overexpression of miR-21
promoted NSC proliferation. In the present study cyclin D1 was
upregulated and p21 was downregulated in response to miR-21
overexpression, as determined by RT-qPCR; p21 is a universal
inhibitor of the cyclin/cyclin-dependent kinase (CDK) family
(22). The cyclin D1 gene is a
marker of cell cycle progression from G1 to S phase, and
acts by binding to and activating CDKs (23). Furthermore, these findings were
confirmed by western blot analysis. Cyclin D1 protein expression
levels were upregulated, whereas p21 protein expression levels were
downregulated in rat NSCs transfected with miR-21 mimic compared
with the scramble controls. In addition, increased rat NSC
proliferation was observed by EdU assay and CCK8 assay. These
results are concordant with those of previous reports regarding
other cell types. In lung carcinoma, the proliferative action of
miR-21 was revealed to be associated with upregulation of cyclin D1
and downregulation of p21 (24).
miR-21 is also considered to be a proliferative factor for human
tubular epithelial cells (25),
colorectal cancer cells (26) and
colon cancer stem cells (27). The
results from the present study, as well as from previous studies,
confirm that miR-21 is involved in the regulation of NSC
proliferation and differentiation.
The Wnt/β-catenin signaling pathway is involved in
cell proliferation and the differentiation of NSCs in the CNS
during embryonic and adult neurogenesis (28). In mammals, the Wnt/β-catenin
signaling pathway is involved in the regulation of cellular growth,
as well as numerous pathophysiological processes, including stroke
and Alzheimer's disease (29–31).
The present study revealed a potential association between miR-21
and the Wnt/β-catenin signaling pathway. Overexpression of miR-21
was able to activate the Wnt/β-catenin signaling pathway in rat
NSCs, as determined by measuring the expression levels of the
Wnt/β-catenin pathway-associated markers β-catenin, cyclin D1 and
p21. β-catenin in the Wnt signaling pathway is a coactivator of
TCF/lymphoid enhancer factor (LEF)-dependent transcription, which
is normally phosphorylated by glycogen synthase kinase 3β and
degraded quickly. In activation of the canonical Wnt pathway,
increased levels of β-catenin are translocated to the nucleus.
Nuclear β-catenin associates with the TCF/LEF transcription factor,
and this complex subsequently binds to the promoter of Wnt-pathway
genes, including cyclin D1, to activate transcription and promote
proliferation. Increased levels of β-catenin indicate the
activation of Wnt signaling (32).
The results of the present study demonstrated that overexpression
of miR-21 contributed to the increased β-catenin levels, and
therefore activated the Wnt/β-catenin pathway. Furthermore, a
pharmacological inhibitor of β-catenin/TCF (FH535) was used to
validate the involvement of the Wnt/β-catenin signaling pathway in
miR-21-induced rat NSC proliferation. Inhibition of the
Wnt/β-catenin pathway significantly attenuated the effects of
miR-21 on rat NSC proliferation, as determined using a CCK8 assay.
These findings confirmed that the Wnt/β-catenin signaling pathway
is required for miR-21-induced regulation of proliferation and
differentiation of rat NSCs.
In conclusion, these data revealed the involvement
of miR-21 in regulating proliferation and differentiation of rat
NSCs. The present study also demonstrated that miR-21 regulated rat
NSC proliferation and differentiation via the Wnt/β-catenin
signaling pathway. The present results suggested that miR-21 may
have potential as a clinically useful tool and may represent a
promising molecular target for the regulation of NSC
proliferation.
Acknowledgements
The present study was supported by the Natural
Science Fund of Henan Province (grant no. HN2013ZA042).
References
|
1
|
Aranha MM, Santos DM, Solá S, Steer CJ and
Rodrigues CM: miR-34a regulates mouse neural stem cell
differentiation. PLoS One. 6:e213962011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi Y, Sun G, Zhao C and Stewart R: Neural
stem cell self-renewal. Crit Rev Oncol Hematol. 65:43–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koliatsos VE, Xu L and Cummings BJ: Stem
cell therapies for traumatic brain injury. Regen Med. 10:917–920.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bi YY, Feng DF and Pan DC: Stem/progenitor
cells: A potential source of retina-specific cells for retinal
repair. Neurosci Res. 65:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piltti KM, Avakian SN, Funes GM, Hu A,
Uchida N, Anderson AJ and Cummings BJ: Transplantation dose alters
the dynamics of human neural stem cell engraftment, proliferation
and migration after spinal cord injury. Stem Cell Res. 15:341–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu H, Li M, Song T, Qian Y, Xiao X, Chen
X, Zhang P, Feng X, Parker T and Liu Y: Retrovirus delivered
neurotrophin-3 promotes survival, proliferation and neuronal
differentiation of human fetal neural stem cells in vitro. Brain
Res Bull. 77:158–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou XM, Sun JB, Yuan HP, Wu DL, Zhou XR,
Sun DW, Li HY, Shao ZB and Zhang ZR: A rat model for studying
neural stem cell transplantation. Acta Pharmacol Sin. 30:1496–1504.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo Y and Zhu D: Combinatorial control of
transgene expression by hypoxia-responsive promoter and microrna
regulation for neural stem cell-based cancer therapy. Biomed Res
Int. 2014:7513972014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez-Ramirez MA and Nicoli S: Role of
miRNAs and epigenetics in neural stem cell fate determination.
Epigenetics. 9:90–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rolando C and Taylor V: Neural stem cell
of the hippocampus: Development, physiology regulation, and
dysfunction in disease. Curr Top Dev Biol. 107:183–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia T, Li J, Cheng H, Zhang C and Zhang Y:
Small-molecule regulators of MicroRNAs in biomedicine. Drug Dev
Res. 76:375–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen B, Li H, Zeng X, Yang P, Liu X, Zhao
X and Liang S: Roles of microRNA on cancer cell metabolism. J
Transl Med. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwakawa HO and Tomari Y: The functions of
MicroRNAs: mRNA decay and translational repression. Trends Cell
Biol. 25:651–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Zhang SY, Gao YM, Liu YF, Liu YB,
Zhao ZG and Yang K: MicroRNAs as oncogenes or tumour suppressors in
oesophageal cancer: Potential biomarkers and therapeutic targets.
Cell Prolif. 47:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang S, Deng Y, Gu P and Fan X: MicroRNAs
regulate bone development and regeneration. Int J Mol Sci.
16:8227–8253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, You T and Jing J: MiR-125b inhibits
cell biological progression of Ewing's sarcoma by suppressing the
PI3K/Akt signalling pathway. Cell Prolif. 47:152–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Yu M, Liu C, Zhu H, He X, Peng S and
Hua J: miR-34c works downstream of p53 leading to dairy goat male
germline stem-cell (mGSCs) apoptosis. Cell Prolif. 46:223–231.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gangaraju VK and Lin H: MicroRNAs: Key
regulators of stem cells. Nat Rev Mol Cell Biol. 10:116–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi E, Choi E and Hwang KC: MicroRNAs as
novel regulators of stem cell fate. World J Stem Cells. 5:172–187.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu
JH, Zhu Y and Yang GY: Transplantation of vascular endothelial
growth factor-transfected neural stem cells into the rat brain
provides neuroprotection after transient focal cerebral ischemia.
Neurosurgery. 57:325–333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methds. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sherr CJ: D-type cyclins. Trends Biochem
Sci. 20:187–190. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu D, Shi M and Fan XD: Mechanism of
miR-21 via Wnt/β-catenin signaling pathway in human A549 lung
cancer cells and Lewis lung carcinoma in mice. Asian Pac J Trop
Med. 8:479–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XJ, Hong Q, Wang Z, Yu YY, Zou X and
Xu LH: MicroRNA21 promotes interstitial fibrosis via targeting
DDAH1: A potential role in renal fibrosis. Mol Cell Biochem.
411:181–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi C, Yang Y, Xia Y, Okugawa Y, Yang J,
Liang Y, Chen H, Zhang P, Wang F, Han H, et al: Novel evidence for
an oncogenic role of microRNA-21 in colitis-associated colorectal
cancer. Gut. 65:1470–1481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Nangia-Makker P, Farhana LG,
Rajendra S, Levi E and Majumdar AP: miR-21 and miR-145 cooperation
in regulation of colon cancer stem cells. Mol Cancer. 14:982015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Munji RN, Choe Y, Li G, Siegenthaler JA
and Pleasure SJ: Wnt signaling regulates neuronal differentiation
of cortical intermediate progenitors. J Neurosci. 31:1676–1687.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Ferrari GV, Avila ME, Medina MA,
Perez-Palma E, Bustos BI and Alarcon MA: Wnt/β-catenin signaling in
Alzheimer's disease. CNS Neurol Disord Drug Targets. 13:745–754.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mazur M, Bujak A, Matloka M, Janowska S,
Gunerka P, Bojarski L, Stanczak A, Klejman A, Bednarek A,
Lamparska-Przybysz M and Wieczorek M: Cell-based assay for low-and
high-scale screening of the Wnt/β-catenin signaling modulators.
Anal Biochem. 475:56–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun FL, Wang W, Zuo W, Xue JL, Xu JD, Ai
HX, Zhang L, Wang XM and Ji XM: Promoting neurogenesis via
Wnt/β-catenin signaling pathway accounts for the neurorestorative
effects of morroniside against cerebral ischemia injury. Eur J
Pharmacol. 738:214–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jamieson C, Sharma M and Henderson BR: Wnt
signaling from membrane to nucleus: β-catenin caught in a loop. Int
J Biochem Cell Biol. 44:847–850. 2012. View Article : Google Scholar : PubMed/NCBI
|