Introduction
Type 2 diabetes mellitus (DM) is a disease that is
associated with high morbidity and mortality due to various
diabetic complications (1).
Diabetic cardiomyopathy, a severe diabetic complication, refers to
ventricular dysfunction independent of alterations in blood
pressure and coronary artery disease (2,3). In
the past decades, numerous studies have investigated the mechanisms
involved in diabetic cardiomyopathy (4–7). At
present, of the various etiologies that have been reported to be
associated the pathogenesis of diabetic cardiomyopathy, the
apoptosis of cardiomyocytes is considered to be one of the primary
causes of diabetic cardiomyopathy. Therefore, attenuation of
cardiomyocyte apoptosis has been investigated as a potential
therapeutic strategy for diabetic cardiomyopathy (8,9).
The endoplasmic reticulum (ER) has important roles
in the regulation of Ca2+ homoeostasis, and protein
synthesis and folding (10). ER
stress, which is characterized by a perturbation in the
Ca2+ or the redox balance of the ER, is the primary
cause of the accumulation of misfolded proteins (11,12).
At the initial stage of ER stress, a protective procedure termed
unfolded protein response (UPR) is initiated in the ER (13), which is mediated by three
transmembrane sensors, including protein kinase RNA-like
endoplasmic reticulum kinase (PERK), activated transcription factor
6 (ATF-6) and inositol-requiring enzyme 1 (IRE1). Under non-stress
conditions, the proteins are bound to the ER chaperone protein
glucose-regulated protein 78 (GRP78). Upon accumulation of unfolded
proteins in the ER, GRP78 becomes dissociated from these
transducers to assist with the folding of the accumulated proteins
(14). When ER stress is
prolonged, cell death is triggered by activating
CCAAT/enhancer-binding protein homologous protein (CHOP),
phosphorylated (p)-eukaryotic initiation factor 2α (eIF2α),
caspase-12, caspase-9 and caspase-3 (15–19).
Of these factors, CHOP, which exhibits low expression or is barely
detected under non-stress conditions, promotes apoptosis by
altering the Bcl-2/Bcl-2-associated X (Bax) ratio and generating
reactive oxygen species (20,21).
In addition, as previously described, cell apoptosis induced by ER
stress is reported to be independent of death receptor and
mitochondrial pathways (22). In
animals with hyperglycemia, which is associated with the
pathogenesis of diabetes, the level of ER stress was upregulated
and associated with myocardial cell apoptosis (3–5).
Therefore, the suppression of ER stress may serve as an effective
strategy to alleviate myocardial cell apoptosis to protect the
myocardial function.
Melatonin (MLT; N-acetyl-5-methoxytryptamine), an
endogenous substance that is primarily secreted by the pineal body,
has various properties, including antiapoptosis, anti-inflammatory
and antitumor effects, in addition to roles in anti-oxidative
stress (23–25). Previous studies have investigated
the effects of MLT in the treatment of cardiovascular diseases
(26–30). For example, Dominguez-Rodriguez
et al (29) indicated that
reduced MLT levels were associated with the development of heart
failure in patients with hypertensive cardiomyopathy. Furthermore,
convincing evidence also indicates that MLT is associated with
protective effects against ER stress (30,31).
Studies have previously investigated the role of MLT
in the protection of cardiomyopathy (26–29).
However, the mechanisms underlying these protective effects on
diabetic cardiomyopathy are not currently well defined. The present
study was designed to investigate whether MLT may relieve
myocardial apoptosis in long-term diabetic cardiomyopathy by
targeting ER stress.
Materials and methods
Experimental animals
All experimental protocols on animals were performed
in compliance with and approved by the Ethics Committee of Anhui
Medical University (Hefei, China). A total of 36 male
Sprague-Dawley rats (6 weeks old; weight, 160–200 g) were purchased
from the Experimental Animal Center of Anhui Medical University and
raised in a specific pathogen free environment. After one week of
adaptation, rats were randomized to control (n=12), DM (n=12) and
DM + MLT (n=12) groups. All rats were allowed free access to food
and water and were kept in conditions of 22–24°C with a relative
humidity of 40–70% on a 12 h light-dark cycle. All rats were fed a
high-fat diet containing 2% cholesterol, 10% lard and 88% normal
diet, apart from rats in the control group that were fed a normal
diet. Intraperitoneal glucose tolerance test (IPGTT) and
intraperitoneal insulin tolerance test (IPITT) were performed at
week 8 (see below for specific steps). At week 9, control rats were
injected with 1 mol/l sodium citrate saline buffer (Beijing
Chemical Reagent Co., Ltd, Beijing, China), while the rats in the
DM and DM + MLT groups were subject to intraperitoneal
administration of streptozocin (STZ; 25 mg/kg dissolved in 20 mM
sodium citrate saline buffer; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) once (26). The blood
glucose levels were tested 1 week after the streptozocin injection.
Animals with glucose levels ≥11.1 mmol/l were considered to be
diabetic. Rats in the DM + MLT group subsequently received 10
mg/kg/day MLT (Institute of Clinical Pharmacology of Anhui Medical
University, Hefei, China) by gavage for 24 weeks. The control and
DM rat groups were treated with intragastric saline administration.
At the end of week 33, animals were starved overnight and
anesthetized using a 10% chloral hydrate solution (300 mg/kg;
intraperitoneal injection). Cardiac function was evaluated under
anesthesia. Blood samples were collected from the abdominal aorta.
After the hearts from each rat were weighed and sliced, the heart
weight/body weight (HW/BW) was calculated and the left ventricle
(LV) was rapidly dissected and cut into two pieces. One piece was
immediately frozen in liquid nitrogen and stored at −80°C for
protein analysis and the other was fixed with 10% neutral formalin
for 6–8 days at room temperature for histological examination.
IPGTT
After a 12 h fast, all rats were intraperitoneally
administrated with glucose (1 g/kg bodyweight dissolved in a sodium
chloride injection; Sinopharm Chemical Reagent Co., Ltd, Shanghai,
China). Blood glucose concentrations were detected using a blood
glucose meter through a vein in the tail at 0, 15, 30, 60 and 120
min.
IPITT
After 12 h of abrosia, insulin (1I U/kg bodyweight
dissolved in a sodium chloride injection; Novolin 30R, Novo
Nordisk, Beijing, China) was intraperitoneally injected into rats.
Then blood glucose concentrations were detected through tail venous
blood 0, 15, 30, 60 and 120 min.
Cardiac function testing
Cardiac function was measured under constant flow
and constant pressure (32).
Briefly, a specific indwelling needle was intubated to the left
ventricle through via the right common carotid artery, which was
connected to a force-displacement transducer to record tension and
heart rate. Left ventricular end-diastolic pressure (LVEDP), left
ventricular systolic pressure (LVSP), maximal rate of increase of
left ventricular pressure (+dp/dtmax) and maximal rate of decrease
of left ventricular pressure (-dp/dtmax) as the rate of contraction
and relaxation were calculated using a BL-420 biological function
experimental system (Chengdu Techman Software Co., Ltd., Chengdu,
China).
Serum biochemical analysis
Blood samples were collected from the abdominal
aorta and left for ~1 h at room temperature until it is completely
coagulated. The samples were then centrifuged at 1,000 × g for 10
min at room temperature, and serum was obtained and kept at −80°C.
Serum fasting blood glucose (FBG), total cholesterol (TC),
low-density lipoprotein (LDL) cholesterol, triglyceride (TG) and
insulin levels were determined using commercially available
spectrophotometric assay kits (Beijing BHKT Clinical Reagent Co.,
Ltd., Beijing, China), according to the manufacturer's protocol.
The insulin sensitivity index was calculated using the following
formula: 1 / (FBG × fasting insulin).
Histology
Formalin-fixed, paraffin-embedded myocardial tissue
samples were cut into 6-µm-thick serial sections and stained by
hematoxylin for 6 min and eosin for 2 min (H&E) or Masson's
trichrome staining at room temperature. Masson's trichome staining
was performed to detect myocardial fibrosis by sequential addition
of Bouin's, Weigert's and Biebrich solutions for 5 min each
according manufacturer's protocol (Masson trichrome stain kit; cat.
no. BSBA-4079A; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China). Morphological analysis was performed using
light microscopy and the mean optical density values for collagen
levels were measured using a JD-801 pathological image analysis
system (version 2013; Jiangsu JEDA Science-Technology Development
Co., Ltd., Nanjing, China).
Apoptosis assays
Myocardial apoptosis was detected in 6-µm-thick
serial sections of myocardial tissue samples using a One Step TUNEL
Apoptosis assay kit (Beyotime Institute of Biotechnology, Haimen,
China) following their fixation with 10% formalin and embedding in
paraffin. TUNEL staining was performed according to the
manufacturer's protocol. TUNEL-positive cells, which exhibited
green nuclear staining, were observed using a Leica fluorescence
microscope (DM4000). All cells with DAPI and/or TUNEL staining were
counted within five randomly selected fields in a blinded manner.
The index of apoptosis was expressed as the ratio of positively
stained apoptotic myocytes to the total number of myocytes
counted.
Immunohistochemistry analysis
Formalin-fixed, paraffin-embedded 6-µm-thick
cardiomyocyte sections were used for immunohistochemical staining.
The slides were deparaffinized by gradient concentration of ethanol
(anhydrous ethanol, then 95, 80 and 70% ethanol), rehydrated,
placed in citric acid buffer (0.01 mol/l; pH=6.0; Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) to boil for 10 min for
antigen retrieval, then cooled to room temperature. The slides were
blocked with 3% H2O2 solution for 20 min at
room temperature and washed with phosphate buffer solution (0.01
mol/l, pH=7.4; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.). Primary antibodies against caspase-3 (1:1,000; cat# 9662;
Cell Signaling Technology, Inc., Danvers, MA, USA) were incubated
with the sections overnight at 4°C. Subsequently, the slides were
washed in Tris-buffered saline (TBS containing 10 mM/l Tris HCl and
0.85% NaCl; pH=7.2) and incubated with anti-rat IgG biotinylated
antibody and horseradish peroxidase-conjugated streptavidin
(Universal IHC two-step test kit; PV-6000; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) according to the manufacturer's
protocol, for 10–20 min at room temperature. Finally, the samples
were incubated with 3,3′-diaminobenzidine (1:20) for 6 min as the
substrate and counterstained with hematoxylin for 3 min at room
temperature. Caspase-3-positive cells were observed by a light
microscope and the mean optical density values were measured using
JD-801 pathological imaging analysis system (version 2013).
Western blotting
The protein expression of the ER stress proteins,
GRP78, CHOP, ATF-6α, PERK, IRE1α and caspase-12, and the apoptotic
markers Bax, Bcl-2, caspase-3 and caspase-9, were assessed by
western blotting analysis. Cardiac muscle tissue was sheared and
ground with protein lysis solution (5X protein lysis solution:
21.75 g 150 mmol/l NaCl, 5 ml 1% Triton-X100, 0.5 g 0.1% SDS and 50
mmol/l Tris-HCl, the volume was made up to 500 ml with ultrapure
water) into homogenate on the ice. The 5X protein lysis solution,
physiological saline, phenylmethylsulfonyl fluoride (cat. no.
ST505; Beyotime Institute of Biotechnology) and leupeptin (cat. no.
L2884; Sigma-Aldrich; Merck KGaA) were used in the ratio
200:800:1:1. The homogenate was centrifuged at 14,000 × g for 15
min at 4°C. Then the supernatant fluid was collected, and protein
concentration was determined by BCA. Proteins (50 µg) were
separated by 8–15% SDS-PAGE and electrophoretically transferred
onto polyvinylidene fluoride membranes. Following blocking with 5%
non-fat dry milk for 2 h at room temperature, the membranes were
washed with TBS-Tween-20 [150 mM NaCl, 50 mM Tris (pH=7.5) and 0.1%
Tween-20] and incubated with primary antibodies against caspase-9
(1:1,000; cat# sc-133109), caspase-3 (1:1,000; cat# sc-373730),
Bcl-2 (1:500; cat# sc-23960), Bax (1:500; cat# sc-20067), GRP78
(1:1,000; cat# sc-376768), CHOP (1:1,000; cat# sc-71136), PERK
(1:50; cat# sc-9477), ATF-6α (1:500; cat# sc-22799), IRE1α
(1:1,000; cat# sc-10510), caspase-12 (1:500; cat# sc-21747) and
β-actin (1:1,000; cat# sc-130300) overnight at 4°C. All primary
antibodies were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The bound antibody was visualized with
horseradish peroxidase-conjugated secondary antibodies (goat
anti-mouse IgG; cat. no. AP124P; 1:1,000, goat anti-rabbit IgG,
cat. no. AP132P, 1:2,000 and rabbit anti-goat IgG; cat. no. AP106P;
1:4,000; EMD Millipore, Billerica, MA, USA) for 2 h at room
temperature. The bands were detected using an enhanced
chemiluminescence kit (BeyoECL Plus; Beyotime Institute of
Biotechnology) and rapidly exposed to an autoradiography film
(Amersham Hyperfilm ECL; GE Healthcare Life Sciences, Little
Chalfont, UK) to detect light emission by a nonradioactive method.
The values were quantified using Quantity One software (version,
4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin
antibody was used as the internal reference.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Each experiment was
repeated ≥3 times. Data are presented as the mean ± standard
deviation. The differences among the groups were evaluated using
one-way analysis of variance followed by the Student-Newman-Keuls
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
IPGTT and IPITT results
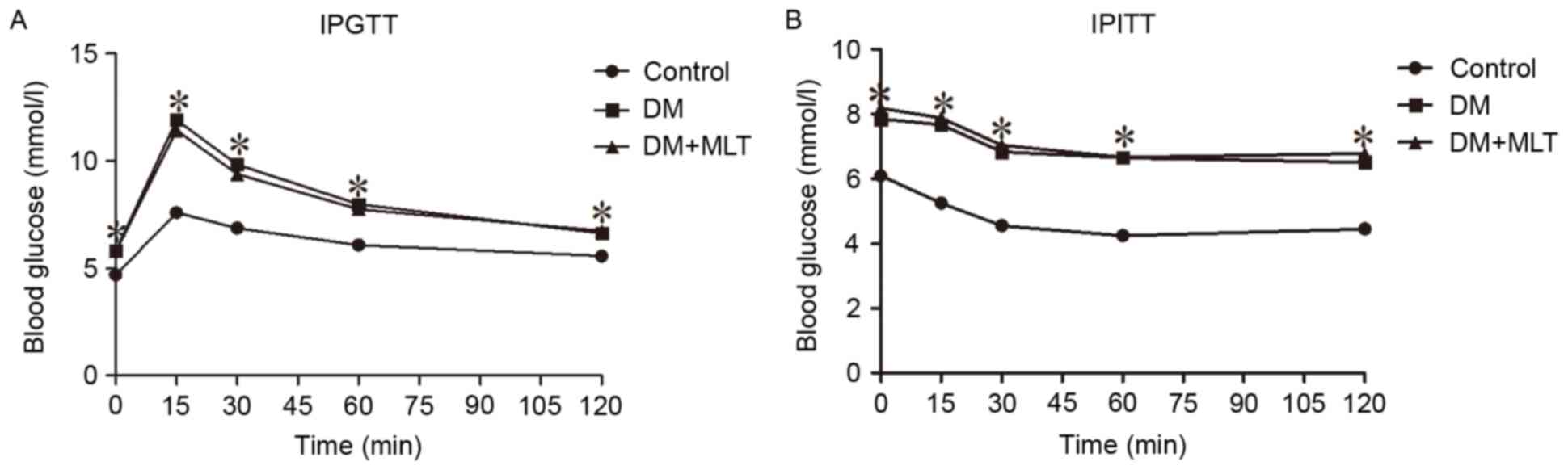
After week 8, IPGTT and IPITT results demonstrated
that the blood glucose levels in DM rats were significantly higher
compared with those in control rats at baseline and at 15, 30, 60
and 120 min time points (P<0.05; Fig. 1).
Myocardial dysfunction in diabetic
models
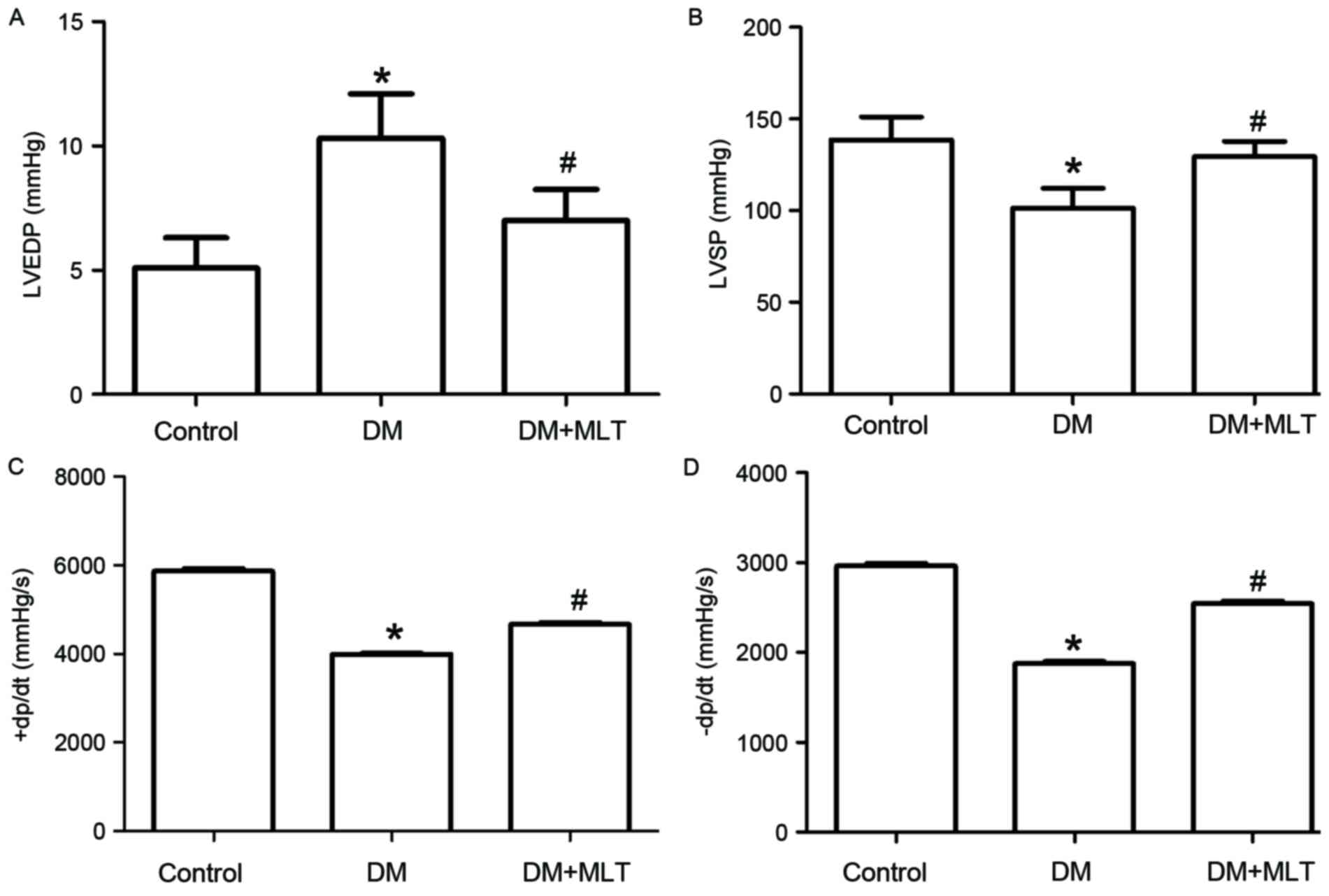
When investigating cardiac function at the end of
week 33, the results demonstrated that LVSP, +dp/dtmax and
-dp/dtmax were significantly decreased, while the LVEDP was
significantly increased, in DM rats compared with control rats
(P<0.05; Fig. 2). These results
indicate that long-term hyperglycemia led to myocardial
dysfunction. However, MLT improved cardiac function significantly
compared with DM rats without MLT treatment (P<0.05; Fig. 2).
Basic parameters in diabetic
models
After 24 weeks of MLT treatment, FBG, TC, LDL and TG
levels, and HW/BW and insulin resistance, were significantly higher
in DM rats compared with control rats (P<0.05; Table I). However, MLT therapy reduced
FBG, lipid levels and the HW/BW (Table
I). Additionally, MLT marginally reduced insulin resistance,
although this change was not significant (Table I).
 | Table I.Basic parameters of rats in different
experimental groups at the end of 33 weeks. |
Table I.
Basic parameters of rats in different
experimental groups at the end of 33 weeks.
| Parameter | Control (n=6) | DM (n=6) | DM + MLT (n=8) |
|---|
| FBG, mmol/l |
4.75±0.38 |
19.27±1.03a |
16.59±1.45a,b |
| TC, mmol/l |
2.53±0.08 |
8.05±1.54a |
5.25±0.43a,b |
| LDL, mmol/l |
1.14±0.09 |
6.12±1.96a |
3.81±0.48a,b |
| TG, mmol/l |
1.15±0.06 |
5.63±0.77a |
4.08±0.48a,b |
| Insulin,
µIU/ml |
11.40±2.15 |
34.79±7.97a |
30.30±6.22a |
| Ln (ISI) |
−3.97±0.20 |
−6.48±0.23a |
−6.04±0.42a,b |
| HW/BW
(×10−2) |
0.23±0.02 |
0.35±0.04a |
0.29±0.04a,b |
Morphological characteristics
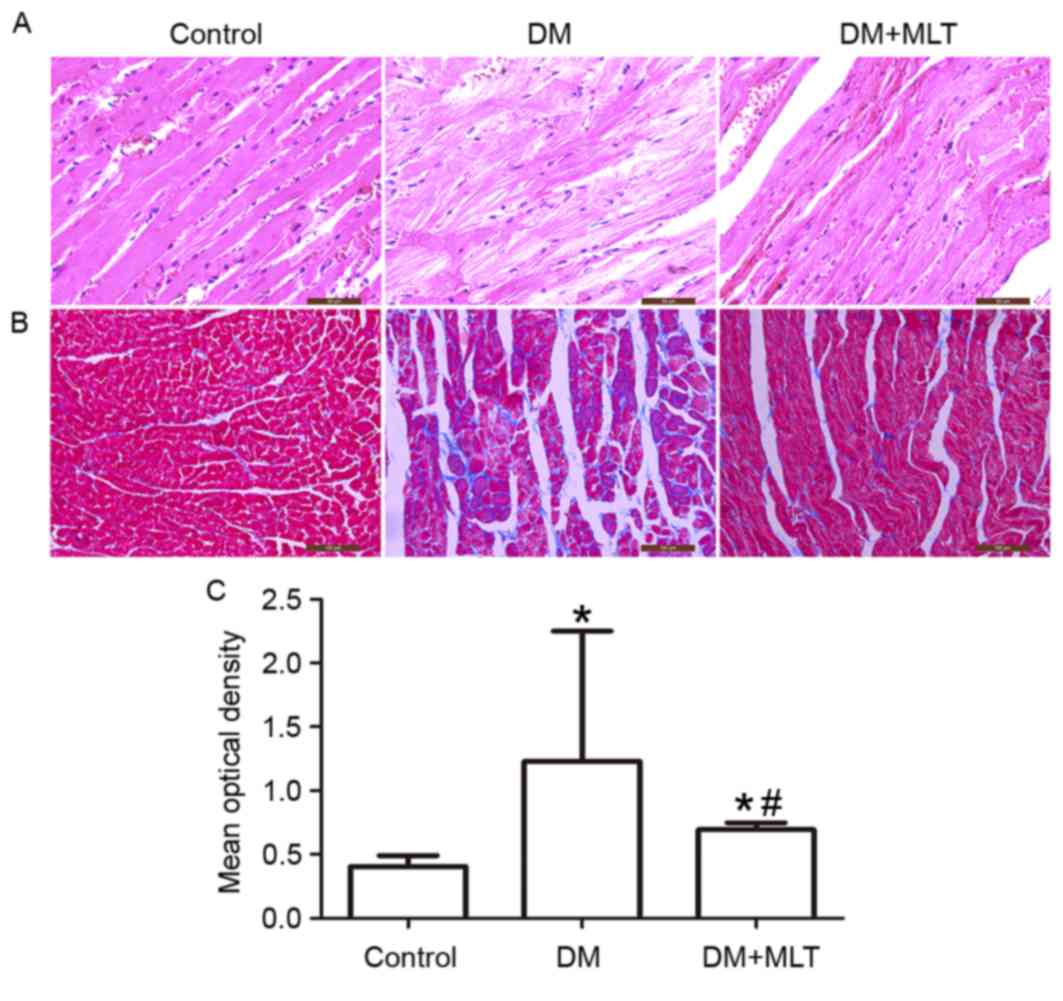
The morphological characteristics of the myocardial
lesions were examined by H&E (Fig.
3A) and Masson's trichome staining (Fig. 3B and C). Compared with the control
rats, myocardial fibers were arranged irregularly and the staining
was uneven in the rats of the DM group (Fig. 3A). MLT treatment improved the
structural impairments of the myocardium and the disarrangement of
myocardial fibers was corrected (Fig.
3A). Masson's staining demonstrated that collagen content was
significantly increased and thick collagen fibers formed complex
network structures in the heart of DM rats (P<0.05; Fig. 3B and C). However, the collagen
content was significantly reduced in DM rats treated with MLT
(P<0.05, Fig. 3B and C).
Cardiac apoptosis
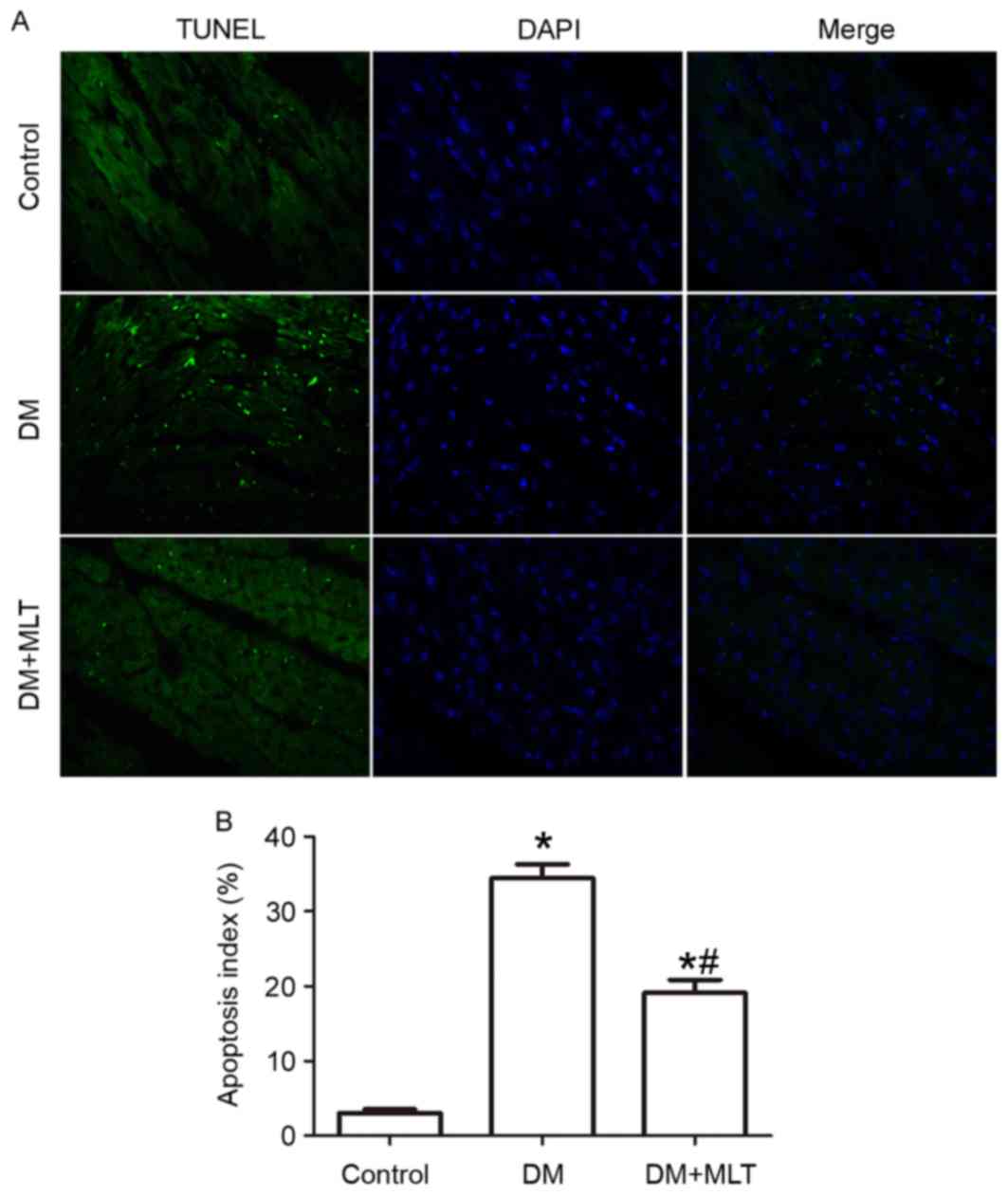
TUNEL assay results demonstrated that the percentage
of apoptotic myocardial cells was significantly increased in DM
rats compared with the control rats (P<0.05; Fig. 4). However, a significant decrease
in the percentage of apoptotic myocardial cells was observed in DM
rats treated with MLT (Fig. 4).
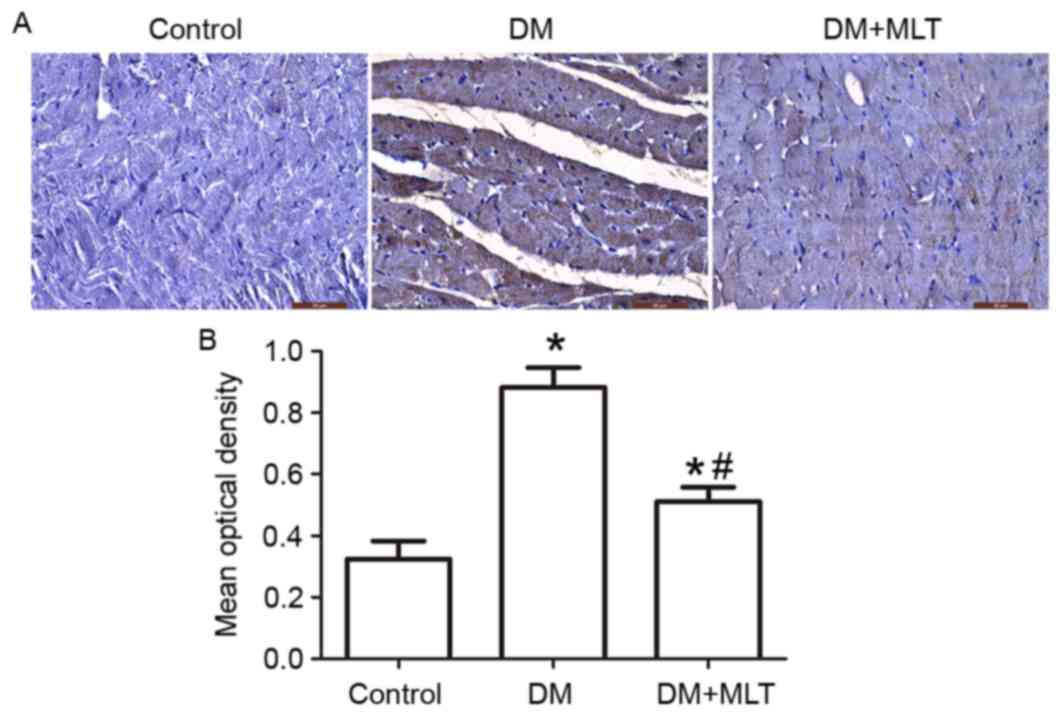
Caspase-3 is the primary terminal cleavage enzyme in the process of
cell apoptosis. In the present study, immunohistochemical analysis
demonstrated that the expression of caspase-3 was markedly
upregulated in the cardiac muscle tissues of DM rats compared with
the control rats (P<0.05; Fig.
5), while MLT treatment reduced DM-induced upregulation of
caspase-3 (P<0.05; Fig. 5).
Measurement of relative protein
expression levels
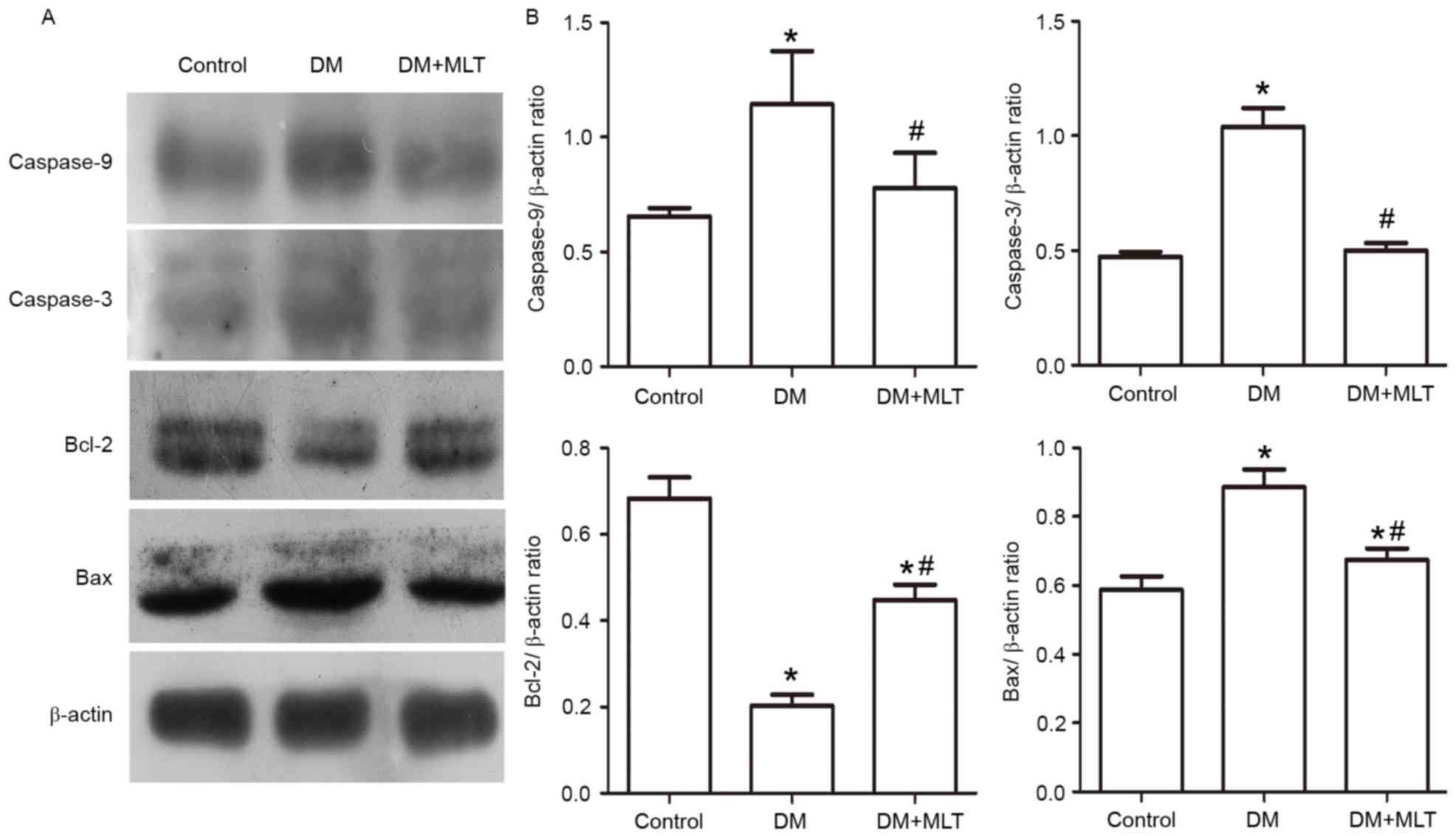
The expression of caspase-3, caspase-9 and the
proapoptotic protein Bax were significantly upregulated in the
myocardium of DM rats compared with control rats, while the
expression of the antiapoptotic protein Bcl-2 was significantly
downregulated (P<0.05; Fig. 6).
These alterations were reversed in DM rats treated with MLT
(P<0.05; Fig. 6). Furthermore,
in rats in the DM group, the protein expression of GRP78, PERK,
ATF-6α, caspase-12 and CHOP was significantly increased in the
myocardium compared with control rats (P<0.05; Fig. 7). However, in DM rats that received
MLT treatment, the expression of these proteins was significantly
downregulated (P<0.05; Fig. 7).
Notably, although the expression of IRE1α was significantly
increased in DM rats compared with control rats (P<0.05), the
expression of IRE1α was not significantly reduced in DM rats with
MLT treatment compared with the DM group (Fig. 7).
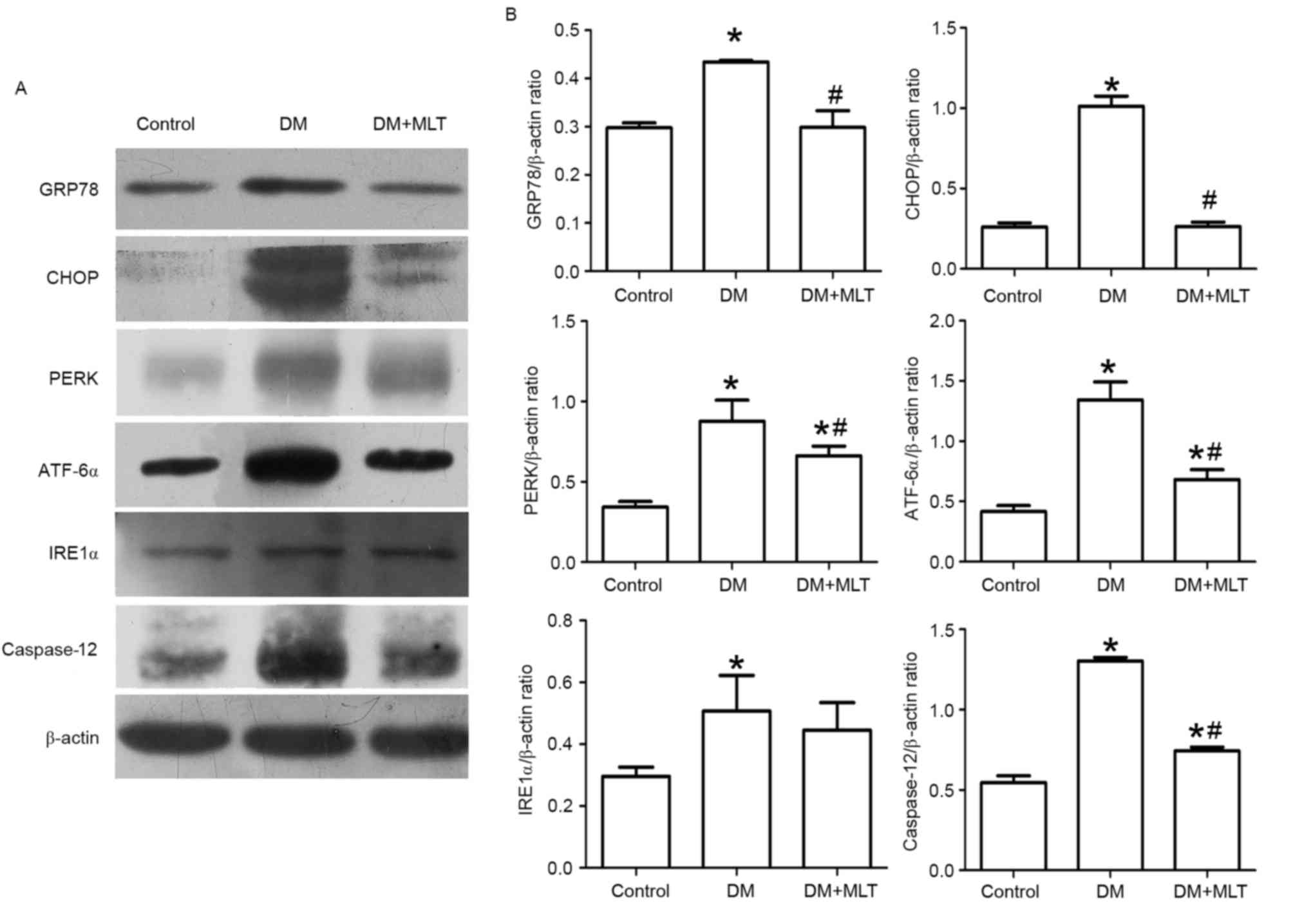 | Figure 7.(A) Western blot was performed to
measure GRP78, CHOP, PERK, ATF-6α, IRE1α and Caspase-12 in
myocardial tissue. 1: Control, 2: DM group, 3: DM + MLT group. (B)
Data are expressed as mean ± SD, n≥6 per group. *P<0.05 vs.
control, #P<0.05 DM + MLT group vs. DM group. DM,
diabetes mellitus; MLT, melatonin; SD, standard deviation. |
Discussion
The present study established a rat model of type 2
DM to investigate the therapeutic effects of MLT in diabetic
cardiomyopathy. The results of this study indicate that MLT may
exert a protective effect on cardiomyopathy by suppressing ER
stress.
Apoptosis is considered to be closely associated
with the pathogenesis of diabetic cardiomyopathy (33). It has also been reported that
cardiomyocytes undergo apoptosis in response to diabetic
hyperglycemia, inflammation and ER stress (34). In addition, evidence indicates that
the apoptosis of cardiomyocytes was increased in animals with DM
(8). Furthermore, cardiomyocytes
apoptosis is also the key initiating factor for cardiac dysfunction
by stimulating cardiomyocyte hypertrophy and fibroblast
proliferation. The most recognized marker proteins of apoptosis are
Bax, caspase-3 and the antiapoptotic protein Bcl-2. Of these,
caspase-3, a member of the caspase family that is involved in
programmed cell death, is a frequently activated death protease
that catalyzes the cleavage of key cellular proteins (35,36).
In the present study, TUNEL assays demonstrated increased apoptosis
in cardiac myocytes of the DM group compared with the control
group. In addition, the expression of caspase-3 and Bax were
significantly upregulated in the DM group compared with control
rats. With regards to the expression of Bcl-2, an antiapoptotic
member of the Bcl-2 family (37),
significant downregulation was observed in the DM rats compared
with the control group. Consistent with previous studies, the
results of the current study indicate that apoptosis is elevated
was increased in rats with diabetic myocardiopathy.
MLT has been reported to exert protective effects on
the heart against myocardial infarction (38). Notably, it is able to prevent
apoptosis in several biological processes, including the induction
of interleukin release. In a recent study, Amin et al
(28) reported that MLT
ameliorated apoptosis through extrinsic and intrinsic pathways. In
the present study, melatonin ameliorated the apoptosis of
cardiomyocytes through upregulation of Bcl-2, and downregulation of
Bax and Caspase-3, compared with the DM group.
Various studies have focused on the mechanisms of
regulating the apoptosis of cardiomyocytes in DM (39,40).
The identification of agents capable of interfering with apoptosis
is clinically important to target the specific pathways that are
activated during diabetic cardiomyopathy. ER stress may be a
potential cause of apoptosis in diabetic cardiomyopathy.
Previously, hyperglycemia and insulin resistance were reported to
enhance ER stress in diabetic cardiomyocytes. Other than
hyperglycemia, the diabetic myocardium may experience other adverse
factors, including oxidative stress, hyperhomocysteinemia, hypoxia
and lipid deposition, which may lead to ER stress (41). According to previous studies
(42,43), ER stress may induce apoptosis
through a complex mechanism in which UPR-mediated signals have
important roles in the initiation, commitment and execution of the
process. The present study primarily focused on the function of
PERK, CHOP and ATF-6α. Previous studies have reported that
signaling via PERK and ATF-6α contributed to the triggering of
proapoptotic signals (42–44). Regarding the mechanism, PERK is not
active under normal conditions, however, in the presence of ER
stress, dissociation of GRP78 from PERK initiates the dimerization
and autophosphorylation of the kinase, which activates PERK. Upon
dissociation of GRP78, ATF-6α is also activated, which moves to the
nucleus and induces genes with an ER stress response element.
Furthermore, downstream elements such as CHOP or c-Jun N-terminal
kinase may induce apoptosis in cells. The results of the current
study demonstrated that the expression of PERK, CHOP and ATF-6α in
DM rats was downregulated when treated with MLT. On this basis, we
hypothesized that MLT may attenuate the apoptosis of cardiomyocytes
by suppressing the ER stress.
Previously, MLT was demonstrated to protect against
the cardiac toxicity of doxorubicin in rats (45). Furthermore, MLT improved
cardiovascular function and attenuated damage in the heart of rats
with renovascular hypertension, although the exact mechanism
remains to be established (46).
In the present study, the results indicated that MLT reduced LVEDP,
and increased LVSP, +dp/dt and -dp/dt, compared with DM rats
without MLT treatment. These results demonstrate that MLT may
improve cardiac function DM rats.
In conclusion, ER stress has an important role in
the activation of apoptosis in the streptozocin-induced diabetic
rat model. The results of the present study may provide a novel
therapeutic strategy for treating diabetic myocardial injury. The
beneficial effects of MLT may, at least partially, occur via the
PERK/ATF-6α/CHOP pathway. Therefore, MLT may have potential for
clinical use as an adjuvant therapy to treat diabetic
cardiomyopathy. Further in vitro studies are required to
fully elaborate the effect of MLT on diabetic ER stress.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570419, 81470568
and 81270372), the Anhui Provincial Natural Science Foundation
(grant no. 1608085MH168) and the Research Project for Practice
Development of National TCM Clinical Research Bases (grant no.
JDZX2015133).
References
|
1
|
Gregg EW, Li Y, Wang J, Burrows NR, Ali
MK, Rolka D, Williams DE and Geiss L: Changes in diabetes-related
complications in the United States, 1990–2010. N Engl J Med.
370:1514–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duckworth WC: Hyperglycemia and
cardiovascular disease. Curr Atheroscler Rep. 3:383–391. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cicek FA, Toy A, Tuncay E, Can B and Turan
B: Beta-blocker timolol alleviates hyperglycemia-induced cardiac
damage via inhibition of endoplasmic reticulum stress. J Bioenerg
Biomembr. 46:377–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Ma X, Zhao M, Zhang B, Chi J, Liu
W, Chen W, Fu Y, Liu Y and Yin X: H2 and H3 relaxin inhibit high
glucose-induced apoptosis in neonatal rat ventricular myocytes.
Biochimie. 108:59–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun
Y, Zhang Y and Ge Z: Endoplasmic reticulum stress is involved in
myocardial apoptosis of streptozocin-induced diabetic rats. J
Endocrinol. 196:565–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sari FR, Watanabe K, Widyantoro B,
Thandavarayan RA, Harima M, Zhang S, Muslin AJ, Kodama M and Aizawa
Y: Partial inactivation of cardiac 14-3-3 protein in vivo elicits
endoplasmic reticulum stress (ERS) and activates ERS-initiated
apoptosis in ERS-induced mice. Cell Physiol Biochem. 26:167–178.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lakshmanan AP, Harima M, Suzuki K,
Soetikno V, Nagata M, Nakamura T, Takahashi T, Sone H, Kawachi H
and Watanabe K: The hyperglycemia stimulated myocardial endoplasmic
reticulum (ER) stress contributes to diabetic cardiomyopathy in the
transgenic non-obese type 2 diabetic rats: A differential role of
unfolded protein response (UPR) signaling proteins. Int J Biochem
Cell Biol. 45:438–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai L and Kang YJ: Cell death and diabetic
cardiomyopathy. Cardiovasc Toxicol. 3:219–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Q, Wang XM, Ye HW, Yu Y, Kang PF, Wang
HJ, Guan SD and Li ZH: Changes in the expression of cardiac
mitofusin-2 in different stages of diabetes in rats. Mol Med Rep.
6:811–814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Groenendyk J, Agellon LB and Michalak M:
Coping with endoplasmic reticulum stress in the cardiovascular
system. Annu Rev Physiol. 75:49–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prins D and Michalak M: Endoplasmic
reticulum proteins in cardiac development and dysfunction. Can J
Physiol Pharmacol. 87:419–425. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H and Liu R: ER stress and hepatic
lipid metabolism. Front Genet. 9:1122014.
|
|
13
|
Senft D and Ronai ZA: UPR, autophagy, and
mitochondria crosstalk underlies the ER stress response. Trends
Biochem Sci. 40:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Lai E, Teodoro T and Volchuk A:
GRP78, but not protein-disulfide isomerase, partially reverses
hyperglycemia induced inhibition of insulin synthesis and secretion
in pancreatic {beta}-cells. J Biol Chem. 284:5289–5298. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kepp O, Semeraro M, Pedro Bravo-San JM,
Bloy N, Buqué A, Huang X, Zhou H, Senovilla L, Kroemer G and
Galluzzi L: eIF2α phosphorylation as a biomarker of immunogenic
cell death. Semin Cancer Biol. 33:86–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scull CM and Tabas I: Mechanisms of ER
stress-induced apoptosis in atherosclerosis. Arterioscler Thromb
Vasc Biol. 31:2792–2797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu L, Cai B, Zheng S, Liu X, Cai H and Li
H: Effect of emodin on endoplasmic reticulum stress in rats with
severe acute pancreatitis. Inflammation. 36:1020–1029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Araki E, Oyadomari S and Mori M:
Endoplasmic reticulum stress and diabetes mellitus. Intern Med.
42:7–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hill SM, Frasch T, Xiang S, Yuan L,
Duplessis T and Mao L: Molecular mechanisms of melatonin anticancer
effects. Integr Cancer Ther. 8:337–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mauriz JL, Collado PS, Veneroso C, Reiter
RJ and González-Gallego J: A review of the molecular aspects of
melatonins anti-inflammatory actions: Recent insights and new
perspectives. J Pineal Res. 54:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galano A, Tan DX and Reiter RJ: Melatonin
as a naturally against oxidative stress: A physiochemical
examination. J Pineal Res. 51:1–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai
M, Yang Y, Chen W, Liu J, Yi W, et al: Reduced silent information
regulator 1 signaling exacerbates myocardial ischemia-reperfusion
injury in type 2 diabetic rats and the protective effect of
melatonin. J Pineal Res. 59:376–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang
S, Wang N, Liang Z, Li Y, Chen W, et al: JAK2/STAT3 activation by
melatonin attenuates the mitochondrial oxidative damage induced by
myocardial ischemia/reperfusion injury. J Pineal Res. 55:275–286.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amin AH, El-Missiry MA and Othman AL:
Melatonin ameliorates metabolic risk factors, modulates apoptotic
proteins, and protects the rat heart against diabetes-induced
apoptosis. Eur J Pharmacol. 747:166–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dominguez-Rodriguez A, Abreu-Gonzalez P
and Reiter RJ: The potential usefulness of serum melatonin level to
predict heart failure in patients with hypertensive cardiomyopathy.
Int J Cardiol. 174:415–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hadj Ayed Tka K, Mahfoudh Boussaid A,
Zaouali MA, Kammoun R, Bejaoui M, Mazgar Ghoul S, Catafau Rosello J
and Ben Abdennebi H: Melatonin modulates endoplasmic reticulum
stress and Akt/GSK3-beta signaling pathway in a rat model of renal
warm ischemia reperfusion. Anal Cell Pathol (Amst).
2015:6351722015.PubMed/NCBI
|
|
31
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Layland J, Cave AC, Warren C, Grieve DJ,
Sparks E, Kentish JC, Solaro RJ and Shah AM: Protection against
endotoxemia-induced contractile dysfunction in mice with
cardiac-specific expression of slow skeletal troponin I. FASEB J.
19:1137–1139. 2005.PubMed/NCBI
|
|
33
|
Ouyang C, You J and Xie Z: The interplay
between autophagy and apoptosis in the diabetic heart. J Mol Cell
Cardio. 71:71–80. 2014. View Article : Google Scholar
|
|
34
|
Liu Q, Wang S and Cai L: Diabetic
cardiomyopathy and its mechanisms: Role of oxidative stress and
damage. J Diabetes Investig. 5:623–634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roos WP and Kaina B: DNA damage-induced
cell death by apoptosis. Trends Mol Med. 12:440–450. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JY: DNA damage and apoptosis. Cell
Death Differ. 8:1047–1048. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Acikel M, Buyukokuroglu ME, Aksoy H,
Erdogan F and Erol MK: Protective effects of melatonin against
myocardial injury induced by isoproterenol in rats. J Pineal Res.
35:75–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sheu JJ, Chang LT, Chiang CH, Sun CK,
Chang NK, Youssef AA, Wu CJ, Lee FY and Yip HK: Impact of diabetes
on cardiomyocyte apoptosis and connexin43 gap junction integrity:
Role of pharmacological modulation. Int Heart J. 48:233–245. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghosh S, Pulinilkunnil T, Yuen G,
Kewalramani G, An D, Qi D, Abrahani A and Rodrigues B:
Cardiomyocyte apoptosis induced by short-term diabetes requires
mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol.
289:H768–H776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun
Y, Zhang Y and Ge Z: Involvement of endoplasmic reticulum stress in
myocardial apoptosis of streptozocin-induced diabetic rats. J Clin
Biochem Nutr. 41:58–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sovolyova N, Healy S, Samali A and Logue
SE: Stressed to death-mechanisms of ER stress-induced cell death.
Biol Chem. 395:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Di Sano F, Ferraro E, Tufi R, Achsel T,
Piacentini M and Cecconi F: Endoplasmic reticulum stress induces
apoptosis by an apoptosome-dependent but Caspase 12-independent
mechanism. J Biol Chem. 281:2693–2700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu MF, Ho S, Qian ZM and Tang PL:
Melatonin protects against cardiac toxicity of doxorubicin in rat.
J Pineal Res. 31:301–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Erşahin M, Sehirli O, Toklu HZ,
Süleymanoglu S, Emekli-Alturfan E, Yarat A, Tatlidede E, Yeğen BC
and Sener G: Melatonin improves cardiovascular function and
ameliorates renal, cardiac and cerebral damage in rats with
renovascular hypertension. J Pineal Res. 47:97–106. 2009.
View Article : Google Scholar : PubMed/NCBI
|