Introduction
Dilated cardiomyopathy (DCM) is characterized by
dilatation of the ventricles, patchy interstitial fibrosis and
degenerated cardiomyocytes. Along with genetic abnormalities,
myocarditis has been considered to be a major factor that leads to
the development of DCM (1). There
is evidence for the role of the immune system in the pathogenesis
of myocarditis and subsequent development of DCM (2,3).
Experimental autoimmune myocarditis (EAM), which mimics human
fulminant myocarditis in the acute phase and human DCM in the
chronic phase, is induced by immunization of rats with cardiac
myosin (4).
DCM is a progressive disorder, and despite available
state-of-the art treatment such as diuretic or cardiac
resynchronization therapy (CRT), it is characterized by high
morbidity and mortality rates (5).
Mesenchymal stem cell (MSC) therapy may be a potential novel
approach for treatment of cardiovascular injury and for promotion
of tissue regeneration (6).
However, various stem cell trials for cardiovascular indications
have been disappointing, possibly due to of the use of autologous
stem cells (7). Cardiovascular
disease patients typically belong to the older age groups, where
numerous risk factors may compromise stem cell function (8,9).
Allogeneic MSCs may be easily scaled and quality-controlled, and
are immunologically relatively well-tolerated, allowing their use
for stem cell trials that has exceed the feasibility of autologous
strategies. Therefore, previous studies using allogeneic stem cells
have been established, including the Percutaneous Stem Cell
Injection Delivery Effects on Neomyogenesis in patients with DCM
trial revealed that the rate of major adverse cardiac events was
significantly lower in patients treated with allogenic vs.
autologous stem cells (10–12).
An additional source of MSCs are human umbilical cord-derived
mesenchymal stem cells (HuMSCs). They are generally discarded as
medical waste after delivery, thus, their use is of little ethical
concern. There are two arteries and a vein in the umbilical cord
which are surrounded by a hyaluronic acid-rich extracellular matrix
(ECM) also termed Wharton's jelly (WJ). MSCs from umbilical cord WJ
are easily isolated and cultured in vitro. Additionally,
they can be differentiated in vitro into several tissue
types (13). There are several
distinct advantages for HuMSCs over other MSCs: i) They have low
immunogenicity, attributable to low expression of human leukocyte
antigen major histocompatibility complex I (MHC I); and ii) they
lack MHC II molecules and co-stimulatory antigens, such as CD80 and
CD86. Therefore, HuMSCs are regarded as immunologically safe for
use in allogeneic clinical therapies (14,15).
Previous studies demonstrated that HuMSCs possess many potential
advantages for cell-based treatment of diseases, such as systemic
lupus erythematosus (16),
rheumatoid arthritis (17),
diabetes (18) and myocardial
ischemia (19,20). However, the potential beneficial
effects of HuMSCs on DCM and the underlying signaling events remain
speculative and remain to be fully elucidated.
A variety of signal transduction pathways are
involved in myocardial fibrogenesis leading to DCM. For example,
activation of the ERK/transforming growth factor-β1 (TGF-β1)
pathway was associated with upregulated collagen deposition
contributing to myocardial fibrosis (21–23).
In the present study, a DCM rat model was
established in order to investigate the therapeutic efficiency of
HuMSCs in DCM rats and to analyze the potential signaling
mechanisms.
Materials and methods
Animals
Lewis rats (male, 8-weeks old; weight, 180–200 g,
n=24) were obtained from Vital River Laboratories (Beijing, China)
and maintained in an air-conditioned animal facility at Shantou
University Medical College (Shantou, China) under 25°C and 70%
humidity conditions with a 12-h light/dark cycle. Throughout the
experiments for the current study, all animals were treated in
accordance with the institutional guidelines for animal
experiments. The Animal Care and Use Committee of the Shantou
University Medical College approved all experimental
procedures.
Preparation of HuMSCs
HuMSCs were prepared as previously described
(24). Briefly, human umbilical
cords from pregnant women (12 volunteers; age, 25–35 years;
recruited from February 2012 to November 2013) who underwent
full-term Caesarian sections were collected from the Second
Affiliated Hospital of Shantou University Medical College
immediately, washed, and cut into 2-3-cm thick sections. Written
informed consent was obtained from all participants. After
separating the arteries and veins, WJ was sliced into smaller
fragments and transferred to 75 cm2 flasks containing
Dulbecco's modified Eagle's medium/F12 media (Sigma-Aldrich; EMD
Millipore, Billerica, MA, USA) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
100 µg/ml penicillin/streptomycin (Shanghai Bioscience, Shanghai,
China), 1 g/ml amphotericin B (Gilead Sciences, Inc., San Dimas,
CA, USA), 5 ng/ml epidermal growth factor (Invitrogen; Thermo
Fisher Scientific, Inc.), and 5 ng/ml basic fibroblast growth
factor (Sigma-Aldrich; EMD Millipore). HuMSC were cultured for 5–7
days at 37°C in 5% CO2 to allow migration of cells from
the explants. After three passages the cells were used for
subsequent experiments. Ethical approval was obtained from the
Institutional Review Board of Shantou University Medical
College.
Generation of DCM rat model
Lewis rats were injected in the footpads with
antigen-adjuvant emulsion in accordance with a procedure described
previously (4). Briefly, purified
porcine cardiac myosin (Sigma-Aldrich; EMD Millipore) was dissolved
in 10 mM PBS and emulsified with an equal volume of complete
Freund's adjuvant with 10 mg/ml Mycobacterium tuberculosis
(Sigma-Aldrich; EMD Millipore). On day 0, rats received a single
immunization with a total of 0.2 ml emulsion per rat at two
subcutaneous sites (both footpads). At 28 days after immunization,
surviving DCM rats (n=16) were divided into two treatment groups:
i) 0.2 ml PBS only (vehicle control group, n=8), or ii) 0.2 ml
HuMSCs (1×106 cells/animal; experimental group, n=8).
HuMSCs or vehicle (PBS) was administered intravenously via the tail
vein. Age matched Lewis rats without immunization were used as
negative controls (negative control group, n=8). The
echocardiography and myocardial pathological section were used to
confirm the success of the DCM rat model (25).
Echocardiographic studies
Two-dimensional echocardiography was performed 56
days after myosin injections under isoflurane anesthesia (1.5–2.0%
volume in air), and using a 13-MHz transducer linked to an
ultrasound system (Acuson Antares, Siemens, Healthineers, Erlangen,
Germany). M-mode images were used to obtain measurements of the
left ventricular end systolic dimension (LVEDs), left ventricular
end diastolic dimension (LVEDd), interventricular septal thickness
(IVS), left ventricular posterior wall thickness (LVPW) and
fractional shortening (FS %). The average of three beats was used
for each parameter. FS (%) was calculated as follows [(LVEDd –
LVEDs)/LVEDd] ×100. All echocardiography analysis was performed
offline and investigators were blinded to the treatment groups.
Histopathological studies
Following echocardiographic analysis, rats were
sacrificed using cervical dislocation 56 days after myosin
injection. The hearts were excised and weighed to calculate the
heart/body weight (HW/BW) ratio. The hearts were subsequently fixed
in 4% paraformaldehyde, embedded in paraffin, and sectioned at 4-µm
thickness. These sections were stained with either hematoxylin and
eosin (H&E) for infiltration of inflammatory cells, or Masson's
trichrome stain for collagen fibers. Slides were viewed under a
high-power light microscope. The area of myocardial fibrosis (blue
color) in left ventricular (LV) tissue sections following Masson's
staining was quantified using a color image analyzer (Mac Scope;
Mitani Co., Fukui, Japan) and measured as the collagen volume
fraction (CVF) = (area of the collagen/area of field of vision)
×100. Ten randomly selected sections (magnification, ×100) from
each rat were analyzed and the results averaged.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Collagen I, III, TGF-β1 and tumor necrosis factor-α
(TNF-α) mRNA expression levels in myocardial tissue were detected
using RT-qPCR. The total RNA was isolated from 50 mg heart tissue
using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The primers were designed and
synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China).
The primer sequences are presented in Table I. Primer concentration was 200 pM.
RNA (500 ng) in a 10 µl reaction mixture was reverse transcribed
using the PrimeScript RT reagent kit with gDNA Eraser (Perfect
Real-Time; Takara Biotechnology Co., Ltd., Dalian, China). The
reactions were incubated first at 37°C for 15 min, followed by
inactivation at 85°C for 5 sec and finally held at 4°C. The qPCR
reaction was performed using SYBR Premix Ex Taq™ II (Tli RNaseH
Plus; Takara Biotechnology Co., Ltd.) and detection was performed
with the CFX96™ PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The reaction cycles were: Denaturation at 95°C
for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec
and annealing and extension at 60°C for 30 sec. The relative level
of gene expression was normalized to the expression of the
housekeeping gene GAPDH using the 2−ΔΔCq method
(26).
 | Table I.List of quantitative polymerase chain
reaction primers. |
Table I.
List of quantitative polymerase chain
reaction primers.
| Primer | Forward
(3′-5′) | Reverse
(3′-5′) |
|---|
| Col I |
CGTGGAAACCTGATGTATGCT |
CCTATGACTTCTGCGTCTGG |
| Col III |
GATCCTAACCAAGGCTGCAA |
ATCTGTCCACCAGTGCTTCC |
| TGF-β1 |
ATTCCTGGCGTTACCTTGG |
AGCCCTGTATTCCGTCTCCT |
| TNF-α |
GCTCCCTCTCATCAGTTCCA |
GCTTGGTGGTTTGCTACGAC |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC |
Western blot analysis
Heart tissues were treated in
radioimmunoprecipitation assay lysis buffer [100 mM NaCl, 20 mM
Tris (pH 8.0), 1 mM EDTA (pH 8.0), 0.5% Triton X-100, 0.5% Nonidet
P-40] to extract total protein, which were quantified using
bicinchoninic acid method (Beyotime Institute of Biotechnology,
Haimen, China). Total protein (30 µg) was separated by 10% SDS-PAGE
gel and transferred electrophoretically to polyvinylidene
difluoride membranes (EMD Millipore) for western blot analysis.
Membranes were blocked with 5% non-fat dry milk in Tris-buffered
saline [20 mM Tris (pH 6.8), 137 mM NaCl] with 0.1% Tween-20,
washed, and incubated at 4°C for 16 h with the following primary
antibodies: GAPDH (catalog no. D4C6R, 1:10,000), extracellular
signal-regulated kinase (ERK)-1/2 (catalog no. 9258, 1:1,000),
phosphorylated (p)-ERK-1/2 (catalog no. 4668, 1:2,000), p38 mitogen
activated protein kinase (MAPK) (catalog no. 8690, 1:1,000) and
p-p38 MAPK (catalog no. 4511, 1:1,000) were all purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Collagen III
(catalog no. ab7778, 1:5,000) and connective tissue growth factor
(CTGF, catalog no. ab6992, 1:1,000) were purchased from Abcam
(Cambridge, UK). Membranes were washed and incubated with a 1:2,000
dilution of horseradish peroxidase-labeled goat anti-rabbit IgG
secondary antibody (catalog no. 4050–05; Southern Biotechnology
Associates, Inc., Birmingham, AL, USA) for 1 h at room temperature.
Immunoreactive protein bands were visualized using the ECL Plus
chemiluminescence kit (EMD Millipore). Bands were analyzed using
Image Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
and protein expression quantities were determined according to the
following calculation: Integrated optical density (IOD)=density
(mean) × area.
Statistical analysis
Data are expressed as mean ± standard deviation.
Analyses of the differences between groups were performed using
one-way analysis of variance followed by Tukey's multiple
comparison test. P<0.05 was considered statistically
significant.
Results
Effects of HuMSCs on myocardial
fibrosis and cardiac function in DCM rats
HuMSCs treatment significantly reduced myocardial
fibrosis (Fig. 1A and B). HW/BW
was significantly greater in vehicle control rats compared with
negative control rats. HuMSC treatment significantly reduced HW/BW
when compared with those in vehicle control rats (Fig. 1C, Table II, P<0.01 vs. normal, P<0.05
vs. control). Echocardiographic analyses revealed a significant
impairment in systolic and diastolic function (Fig. 1A). DCM rats demonstrated LV
remodeling with increased LVEDd, LVEDs and reduced FS in
vehicle-treated DCM rats compared with untreated rats (Fig. 1C, Table II, P<0.01 vs. normal),
indicating impaired myocardial function. HuMSC treatment
significantly reversed these changes (Fig. 1C, Table II, P<0.01 vs. control).
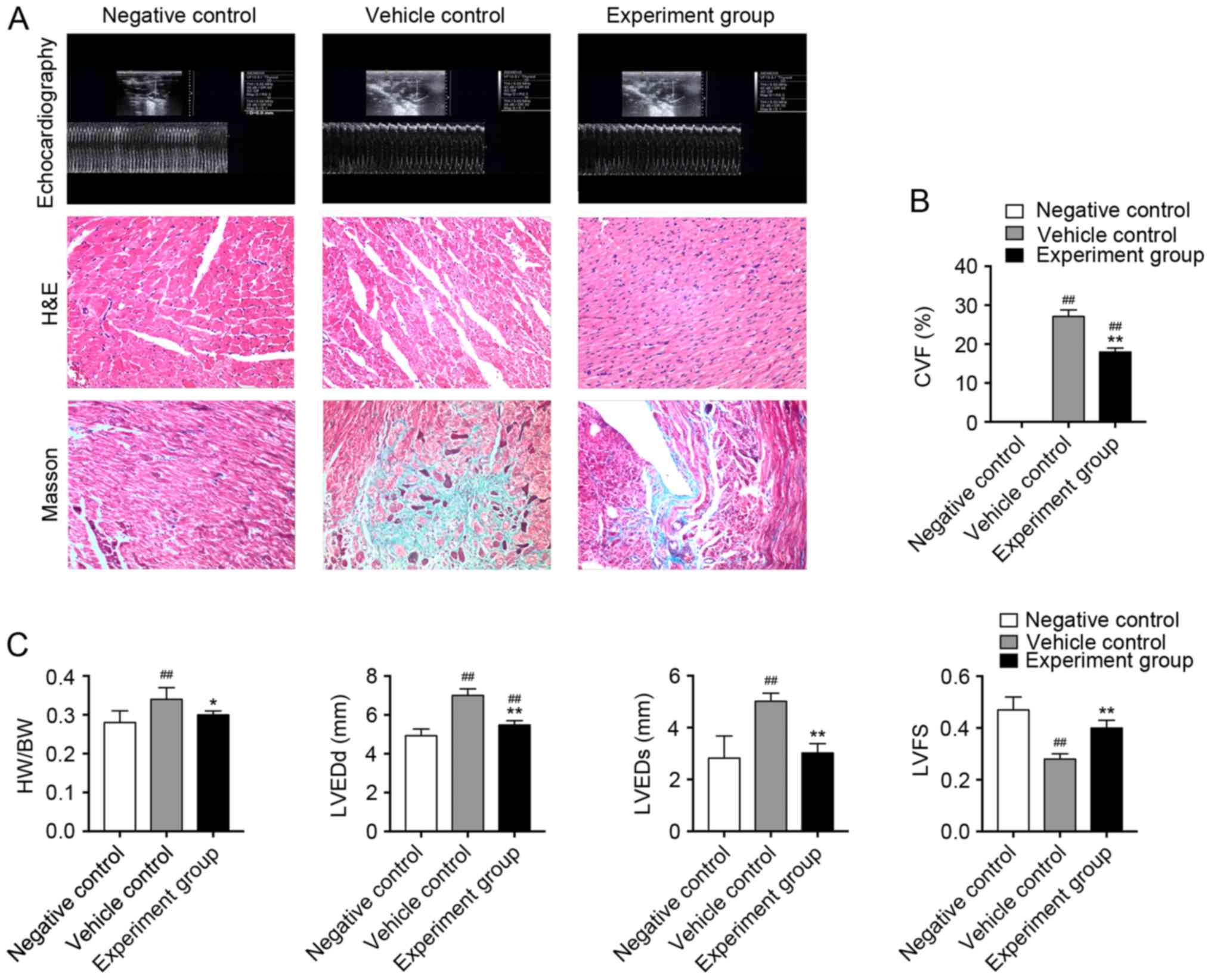 | Figure 1.(A) H&E and Masson's staining in
left ventricular tissue slices in age-matched untreated rats,
immunized rats treated with vehicle and immunized rats treated with
HuMSCs. Magnification, ×100. (B) CVF for Masson's staining in left
ventricular tissue slices from the different treatment groups.
##P<0.01 vs. negative control; *P<0.05,
**P<0.01 vs. vehicle control, n=8. (C) Heart cavity
measurements. ##P<0.01 vs. negative control;
*P<0.05, **P<0.01 vs. vehicle control, n=8. H&E,
hematoxylin and eosin; HuMSCs, human umbilical cord-derived
mesenchymal stem cells; CVF, collagen volume fraction; HW/BW, heart
weight/body weight; LVEDd, left ventricular dimension in end
diastole; LVEDs, left ventricular dimension in end systole. |
 | Table II.Echocardiographic parameters 56 days
after treatment of DCM rats with HuMSCs in. |
Table II.
Echocardiographic parameters 56 days
after treatment of DCM rats with HuMSCs in.
| Characteristic | Negative
control | Vehicle
control | Experiment |
|---|
| HW/BW |
0.28±0.03 |
0.34±0.03a |
0.30±0.01b |
| LVEDd (mm) |
4.93±0.35 |
7.00±0.34a |
5.48±0.22a,c |
| LVEDs (mm) |
2.82±0.86 |
5.02±0.31a |
3.02±0.36c |
| IVS (mm) |
2.16±0.20 |
2.12±0.19 |
2.10±0.20 |
| LVWP (mm) |
2.34±0.11 |
2.12±0.26 |
2.22±0.15 |
| LVFS |
0.47±0.05 |
0.28±0.02a |
0.40±0.03c |
Histopathological examination revealed that the
hearts of DCM rats showed severe fibrosis compared with negative
control rats (Fig. 1A).
Inflammatory cellular infiltration was not observed in the hearts
of the three groups as identified by H&E staining (Fig. 1A). The area of myocardial fibrosis
as quantified by Masson's staining of collagen deposits was
approaching 30% in DCM rats (Fig.
1B).
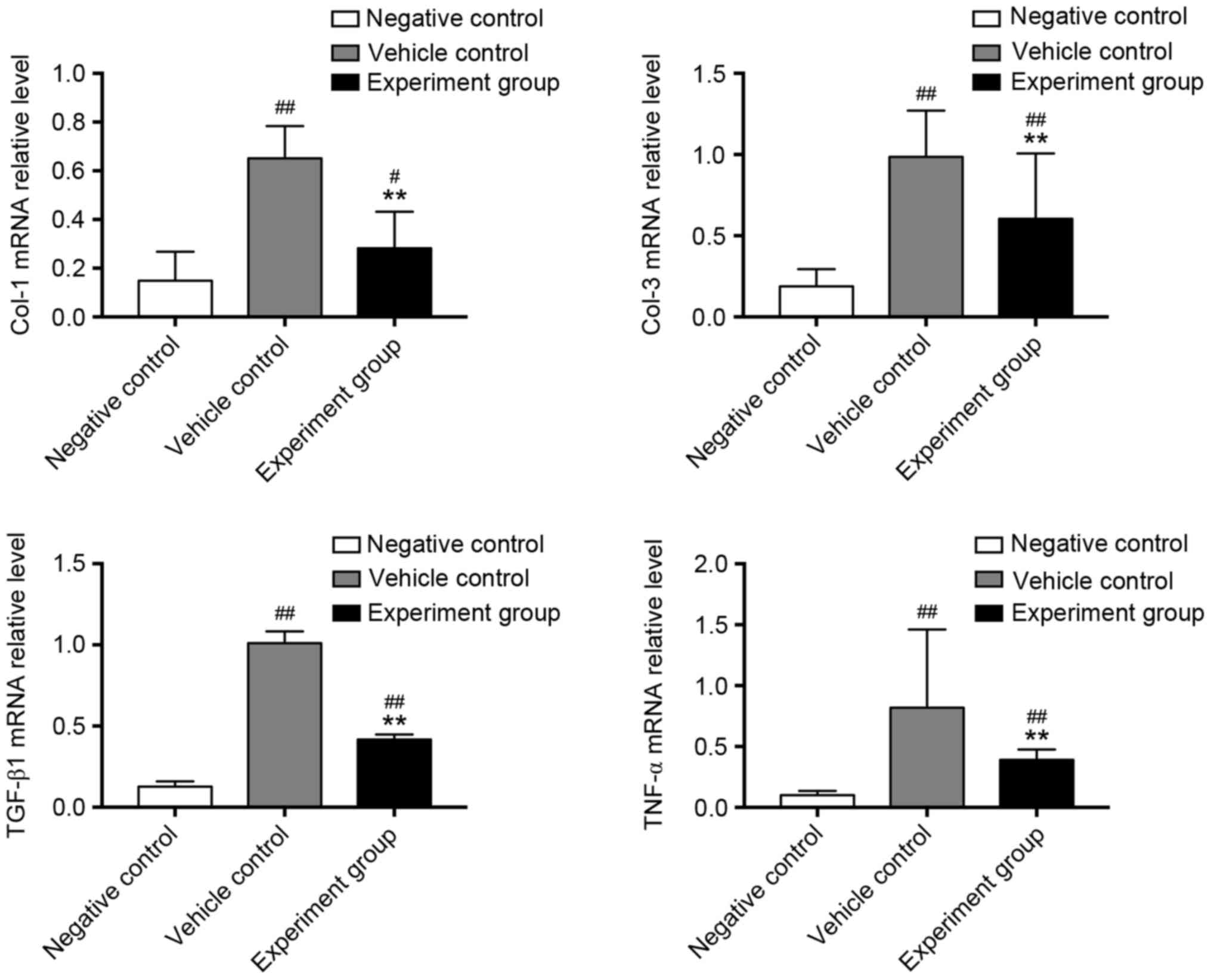
Effects of HuMSCs on molecular markers
of myocardial fibrosis in DCM rats
To confirm the positive effect of HuMSCs on
myocardial fibrosis, RT-qPCR analysis on molecular markers of
myocardial fibrosis was performed (Fig. 2). Collagen I, III and the
profibrotic factors TGF-β1 and TNF-α were significantly upregulated
in vehicle-treated DCM rats, compared with negative control rats
(Fig. 2, P<0.01). By contrast,
treatment with HuMSCs significantly reduced mRNA expression of
collagen-I, III, TGF-β1 and TNF-α when compared with
vehicle-treated DCM rats (Fig. 2,
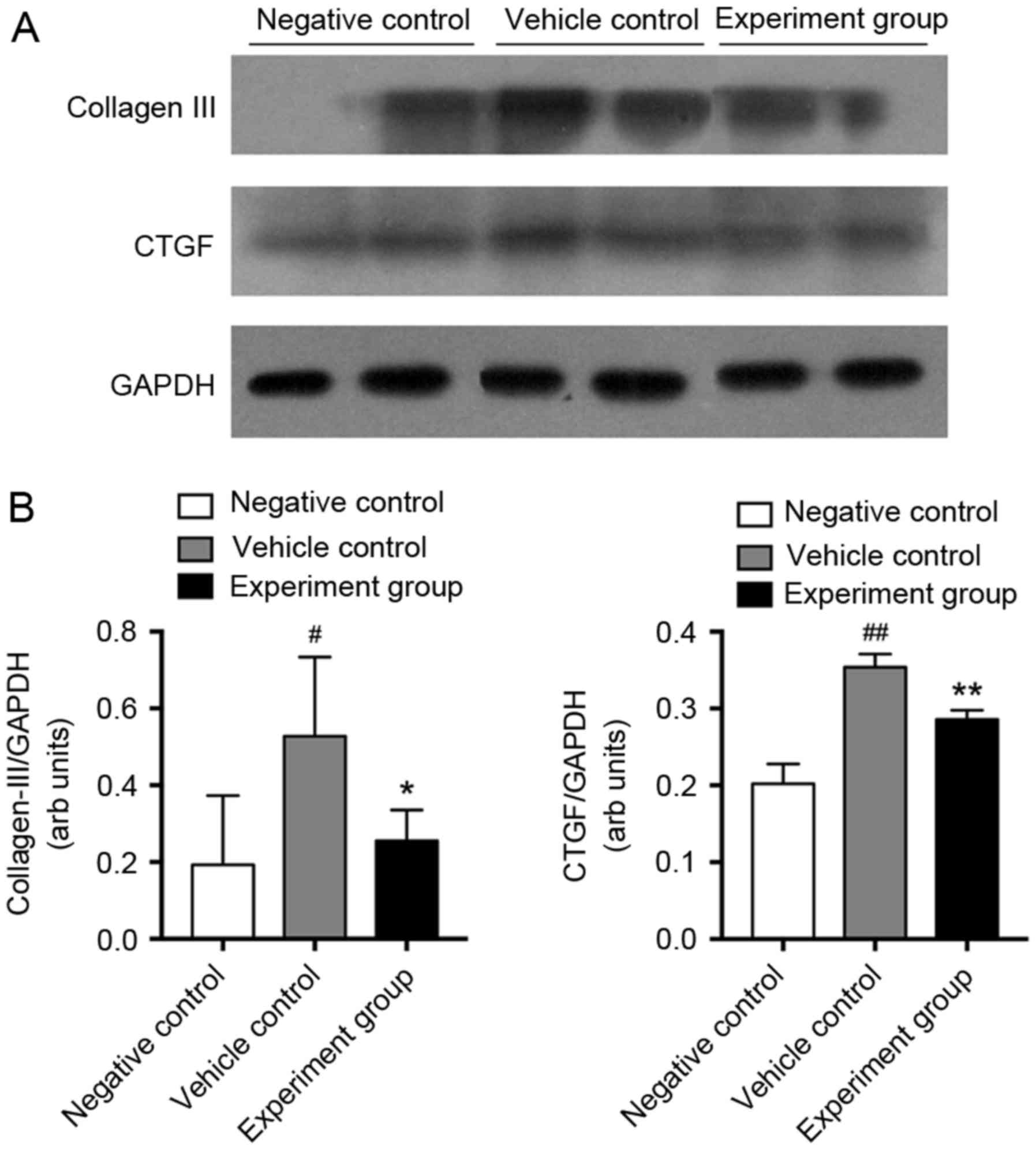
P<0.01). At the protein level, CTGF and collagen-III were
significantly upregulated in vehicle-treated DCM rats compared with
negative control rats (Fig. 3,
P<0.05, P<0.01). However, treatment with HuMSCs significantly
reduced the myocardial protein expression of collagen-III and CTGF
(Fig. 3, P<0.05,
P<0.01).
Effects of HuMSCs on activation of
MAPK signaling in DCM rats
TGF-β1 activates numerous non-canonical signaling
pathways, including MAPK pathways. Therefore, in order to
investigate the signaling mechanisms, which HuMSCs use to reduce
myocardial fibrosis in DCM rats, activation of p38-MAPK and ERK1/2
was quantified (Fig. 4).
Myocardial protein expression of p38-MAPK, ERK1/2, p-p38-MAPK and
p-ERK1/2 were quantified using western blot analysis. P-ERK1/2
levels significantly increased in vehicle control rats compared
with negative control rats, whereas p-p38-MAPK did not change
(Fig. 4B, P<0.01). HuMSCs
treatment alleviated these changes in DCM rats. Protein expression
of p-ERK1/2; however, not p-p38-MAPK was attenuated significantly
in the HuMSCs treated DCM rats compared with vehicle-treated DCM
rats (Fig. 4B, P<0.01),
indicating that HuMSCs may alter myocardial fibrosis via TNF-α,
TGF-β and ERK1/2 activation.
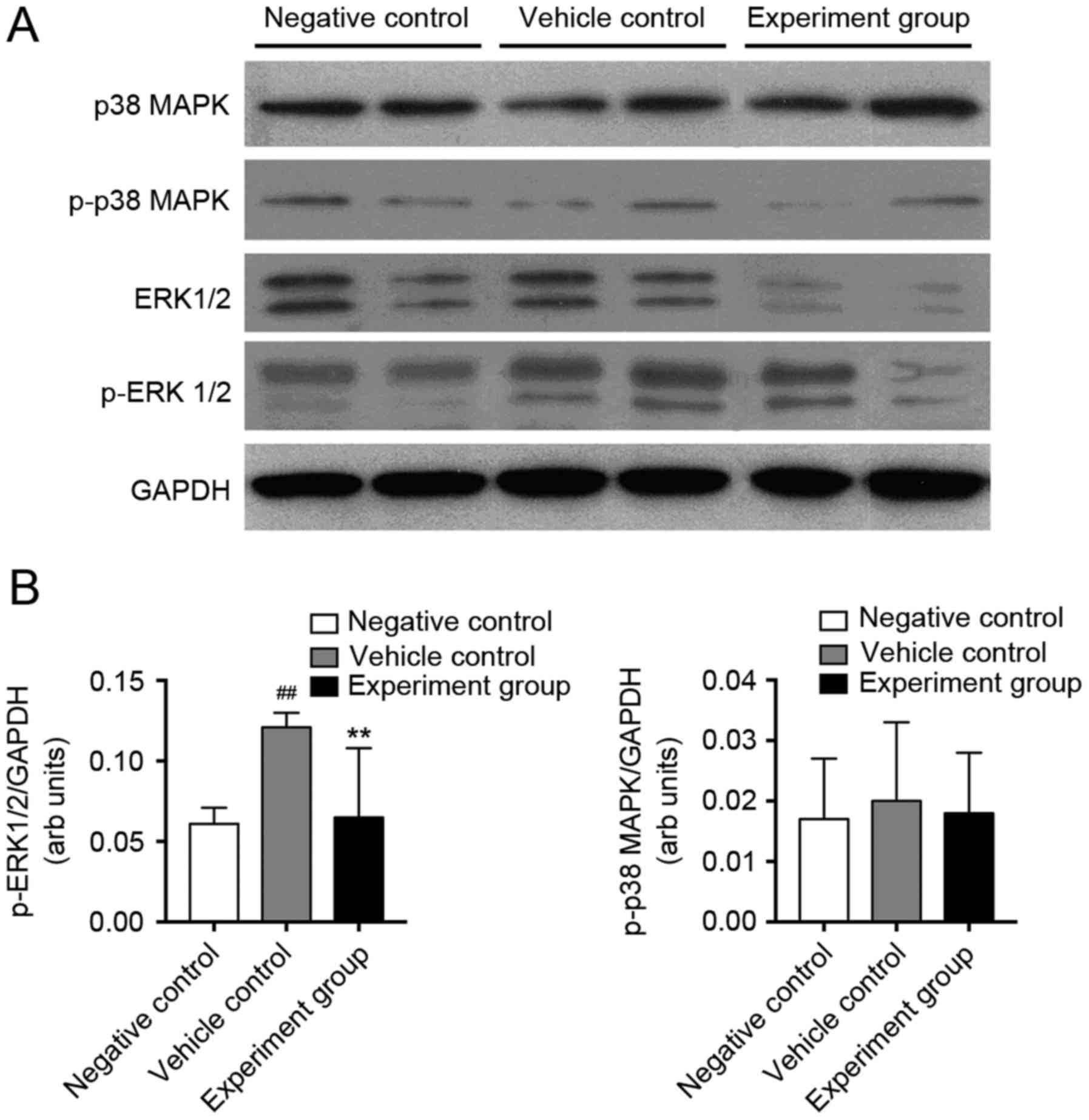 | Figure 4.Western blot analysis of p38 MAPK and
ERK1/2 phosphorylation in negative control, vehicle control, and
experimental rats. (A) Representative western blotting showing
immunolabeled bands for p38-MAPK, p-p38 MAPK, ERK1/2, p-ERK1/2, and
GAPDH was used as an internal control. (B) Mean density values of
p-ERK1/2 and p-p38 MAPK expressed as the ratio relative to GAPDH
expression. GAPDH used as an internal control.
##P<0.01 vs. negative control; **P<0.01 vs.
vehicle control, n=8. p38 MAPK, p38 mitogen-activated protein
kinase; p-p38 MAPK, phosphorylated-p38 MAPK; ERK1/2,
extracellular-signal regulated kinase 1/2; p-ERK1/2,
phosphorylated-ERK1/2. |
Discussion
In the present study, reduced cardiac function in a
DCM rat model was associated with LV remodeling, as well as
increased myocardial collagen deposition and upregulated myocardial
type I and III collagen expression. Treatment with HuMSCs
significantly improved LV remodeling, LV systolic function and
reduced collagen deposition, likely by inhibiting the TGF-β/TNF-α,
ERK1/2 signaling pathways.
A loss of cardiomyocytes and an increase in
interstitial fibrosis is characteristic for DCM (27). The degree of cardiac fibrosis,
leading to passive ventricular stiffness and reduced cardiac
function, may be determined by measuring the myocardial collagen
volume fraction. Masson's staining revealed a significant increase
in collagen deposition in DCM rats. The abnormal deposition of
collagen within myocardial tissues was significantly reduced
following treatment with HuMSCs. Cardiac fibroblasts, the cells
that form the interstitial tissue within the healthy myocardium,
are considered to be the major source of collagen and fibrosis
after cardiac injury (28). The
major fibrillar collagens of the adult heart are type I and III
collagens (29,30) and if the level of collagen within
the myocardium increases, ventricular compliance may be reduced.
The data of the present study indicated that type I and III
collagen was upregulated in myocardial tissues of DCM rats;
however, their expression levels were significantly reduced
following treatment with HuMSCs.
CTGF is another fibrosis-associated molecule that
may induce fibrocyte differentiation into a myofibroblast phenotype
and increase ECM deposition in the myocardium (31). The data of the present study
indicated that CTGF was upregulated in myocardial tissues of DCM
rats; however, the expression level was significantly reduced
following treatment with HuMSCs.
A previous study determined that TGF-β is
upregulated in several models of myocardial infarction and DCM
(32). It has been previously
suggested that TGF-β1 is a master switch for inducing myocardial
fibrosis and may upregulate the expression of procollagen genes to
promote synthesis of ECM components, subsequently leading to
fibrosis (32).
TGF-β1-overexpressing mice had significant cardiac hypertrophy
along with interstitial fibrosis (33). TGF-β1 levels in dilated and
infarcted hearts were reduced following treatment with
angiotensin-converting enzyme inhibitors or angiotensin II receptor
blockers (34,35). A previous study suggested that the
antifibrotic mechanism of HuMSCs in lung fibrogenesis may be
associated with TGF-β1 downregulation, which may lead to
suppression of fibrosis following lung injury (36). The present study determined that
HuMSCs downregulated myocardial TGF-β1 mRNA expression and led to
the suppression of myocardial fibrosis in DCM rats.
TGF-β1 activates various non-canonical signaling
pathways, including the MAPK and phosphatidylinositol-3 kinase/Akt
pathways (37,38). The progression of FasL-induced DCM
and congestive heart failure was prevented by blocking the ERK1/2
pathway, with reductions in fibrosis, inflammation and apoptosis
(39). Isoproterenol and cAMP
signaling attenuated the profibrotic effect of TGF-β1 in cardiac
fibroblasts by suppressing ERK1/2 phosphorylation. Additionally,
the MEK/ERK pathway is involved in interleukin-17-mediated cardiac
fibrosis in EAM (40). In the
present study, HuMSC administration significantly reduced ERK1/2
phosphorylation in DCM rats. However, phosphorylation of p38-MAPK
was not altered in the three experimental groups. These findings
support previous observations (37,38),
that the TGF-β/ERK1/2 pathway is an important mediator in cardiac
fibrosis. The data of the present study revealed that ERK1/2, not
p38-MAPK signaling, was involved with fibrosis in DCM and
identified that HuMSCs treatment may effectively protect against
cardiac fibrosis by attenuating the activation of the ERK1/2
pathway.
TNF-α, a proinflammatory cytokine with a wide range
of biological effects, is involved in the pathophysiology of
various cardiovascular diseases (41,42).
TNF-α overexpression in transgenic mice may lead to adverse cardiac
remodeling, characterized by increased total matrix
metalloproteinase (MMP) activity and increased fibrosis (43). Inhibition of TNF-α may protect from
myocardial inflammation and fibrosis through reduced ERK
phosphorylation in experimental diabetic cardiomyopathy (44). In the present study, administration
of HuMSCs effectively reduced myocardial mRNA expression of TNF-α
in DCM rats. Therefore, it is possible that similar to the
TGF-β/ERK1/2 pathway, HuMSCs alter TNF-α/ERK1/2 signaling and may
alleviate cardiac fibrosis, which subsequently improves ventricular
compliance and cardiac function.
In conclusion, the present findings demonstrated
that injected HuMSCs improve cardiac function by attenuating
myocardial collagen network remodeling via downregulation of TGF-β1
and TNF-α expression and activation of ERK1/2 signaling in DCM
rats. Therefore, HuMSC treatment may be a potential therapeutic
avenue for treatment of DCM.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81671525
and 81070478), the Department of Health of Guangdong Province
(grant no. B2013276), Medical Science and Technology Research
Foundation of Guangdong Province (grant no. A2015247), the Science
and Technology Program Project of Shantou (grant nos. 2012165 and
2013), the Basic and Clinical Scientific Research Foundation of
Shantou University Medical College (grant no. 201410), the Science
and Technology Program of Shenzhen (grant no.
JCYJ20150402092905162) and the Research Project of Health and
Family Planning Commission of Shenzhen Municipality (grant no.
201501053).
References
|
1
|
Krejci J, Mlejnek D, Sochorova D and Nemec
P: Inflammatory Cardiomyopathy: A current view on the
pathophysiology, diagnosis, and treatment. Biomed Res Int.
2016:40876322016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lauer B, Schannwell M, Kühl U, Strauer BE
and Schultheiss HP: Antimyosin autoantibodies are associated with
deterioration of systolic and diastolic left ventricular function
in patients with chronic myocarditis. J Am Coll Cardiol. 35:11–18.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Binah O: Pharmacologic modulation of the
immune interaction between cytotoxic lymphocytes and ventricular
myocytes. J Cardiovasc Pharmacol. 38:298–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodama M, Matsumoto Y, Fujiwara M, Masani
F, Izumi T and Shibata A: A novel experimental model of giant cell
myocarditis induced in rats by immunization with cardiac myosin
fraction. Clin Immunol Immunopathol. 57:250–262. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spezzacatene A, Sinagra G, Merlo M,
Barbati G, Graw SL, Brun F, Slavov D, Di Lenarda A, Salcedo EE,
Towbin JA, et al: Arrhythmogenic phenotype in dilated
cardiomyopathy: Natural history and predictors of life-threatening
arrhythmias. J Am Heart Assoc. 4:e0021492015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanganalmath SK and Bolli R: Cell therapy
for heart failure: A comprehensive overview of experimental and
clinical studies, current challenges, and future directions. Circ
Res. 113:810–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelkar AA, Butler J, Schelbert EB, Greene
SJ, Quyyumi AA, Bonow RO, Cohen I, Gheorghiade M, Lipinski MJ, Sun
W, et al: Mechanisms contributing to the progression of ischemic
and nonischemic dilated cardiomyopathy: Possible modulating effects
of paracrine activities of stem cells. J Am Coll Cardiol.
66:2038–2047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mancini Kizilay O, Shum-Tim D, Stochaj U,
Correa JA and Colmegna I: Age, atherosclerosis and type 2 diabetes
reduce human mesenchymal stromal cell-mediated T-cell suppression.
Stem Cell Res Ther. 6:1402015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hare JM, Fishman JE, Gerstenblith G,
Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker
JA, et al: Comparison of allogeneic vs. autologous bone
marrow-derived mesenchymal stem cells delivered by transendocardial
injection in patients with ischemic cardiomyopathy: The POSEIDON
randomized trial. Jama. 308:2369–2379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karantalis V and Hare JM: Use of
mesenchymal stem cells for therapy of cardiac disease. Circ Res.
116:1413–1430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang HS, Hung SC, Peng ST, Huang CC, Wei
HM, Guo YJ, Fu YS, Lai MC and Chen CC: Mesenchymal stem cells in
the Wharton's jelly of the human umbilical cord. Stem Cells.
22:1330–1337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho PS, Messina DJ, Hirsh EL, Chi N,
Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH and Huang CA:
Immunogenicity of umbilical cord tissue derived cells. Blood.
111:430–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batsali AK, Kastrinaki MC, Papadaki HA and
Pontikoglou C: Mesenchymal stem cells derived from Wharton's Jelly
of the umbilical cord: Biological properties and emerging clinical
applications. Curr Stem Cell Res Ther. 8:144–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun L, Wang D, Liang J, Zhang H, Feng X,
Wang H, Hua B, Liu B, Ye S, Hu X, et al: Umbilical cord mesenchymal
stem cell transplantation in severe and refractory systemic lupus
erythematosus. Arthritis Rheum. 62:2467–2475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Wang L, Cong X, Liu G, Zhou J, Bai
B, Li Y, Bai W, Li M, Ji H, et al: Human umbilical cord mesenchymal
stem cell therapy for patients with active rheumatoid arthritis:
Safety and efficacy. Stem Cells Dev. 22:3192–3202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Qiu X, Ni P, Qiu X, Lin X, Wu W,
Xie L, Lin L, Min J, Lai X, et al: Immunological characteristics of
human umbilical cord mesenchymal stem cells and the therapeutic
effects of their transplantion on hyperglycemia in diabetic rats.
Int J Mol Med. 33:263–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Can A, Ulus AT, Cinar O, Celikkan Topal F,
Simsek E, Akyol M, Canpolat U, Erturk M, Kara F and Ilhan O: Human
umbilical cord mesenchymal stromal cell transplantation in
myocardial ischemia (HUC-HEART Trial). A study protocol of a Phase
1/2, controlled and randomized trial in combination with coronary
artery bypass grafting. Stem Cell Rev. 11:752–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu CB, Huang H, Sun P, Ma SZ, Liu AH, Xue
J, Fu JH, Liang YQ, Liu B, Wu DY, et al: Human umbilical
cord-derived mesenchymal stromal cells improve left ventricular
function, perfusion, and remodeling in a porcine model of chronic
myocardial ischemia. Stem Cells Transl Med. 5:1004–1013. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng M, Wu G, Song Y, Wang L, Tu L, Zhang
L and Zhang C: Celastrol-induced suppression of the MiR-21/ERK
signalling pathway attenuates cardiac fibrosis and dysfunction.
Cell Physiol Biochem. 38:1928–1938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Li GN, Xie J, Li R, Chen QH, Chen
JZ, Wei ZH, Kang LN and Xu B: Resveratrol ameliorates myocardial
fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in
STZ-induced diabetic mice. BMC Cardiovasc Disord. 16:52016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soetikno V, Sari FR, Sukumaran V,
Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata
M, Takagi R and Watanabe K: Curcumin prevents diabetic
cardiomyopathy in streptozotocin-induced diabetic Rats: Possible
involvement of PKC-MAPK signaling pathway. Eur J Pharm Sci.
47:604–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang HW, Lin LM, He HY, You F, Li WZ,
Huang TH, Ma GX and Ma L: Human umbilical cord mesenchymal stem
cells derived from Wharton's jelly differentiate into
insulin-producing cells in vitro. Chinese Med J (Engl).
124:1534–1539. 2011.
|
|
25
|
Zhang C, Zhou G, Cai C, Li J, Chen F, Xie
L, Wang W, Zhang Y, Lai X and Ma L: Human umbilical cord
mesenchymal stem cells alleviate acute myocarditis by modulating
endoplasmic reticulum stress and extracellular signal regulated
1/2-mediated apoptosis. Mol Med Rep. 15:3515–3520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dec GW and Fuster V: Idiopathic dilated
cardiomyopathy. N Engl J Med. 331:1564–1575. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kania G, Blyszczuk P and Eriksson U:
Mechanisms of cardiac fibrosis in inflammatory heart disease.
Trends Cardiovasc Med. 19:247–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eckhouse SR and Spinale FG: Changes in the
myocardial interstitium and contribution to the progression of
heart failure. Heart Fail Clin. 8:7–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Broberg CS and Burchill LJ: Myocardial
factor revisited: The importance of myocardial fibrosis in adults
with congenital heart disease. Int J Cardiol. 189:204–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosin NL, Falkenham A, Sopel MJ, Lee TD
and Legare JF: Regulation and role of connective tissue growth
factor in AngII-induced myocardial fibrosis. Am J Pathol.
182:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dobaczewski M, Chen W and Frangogiannis
NG: Transforming growth factor (TGF)-β signaling in cardiac
remodeling. J Mol Cell Cardiol. 51:600–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rosenkranz S, Flesch M, Amann K, Haeuseler
C, Kilter H, Seeland U, Schlüter KD and Böhm M: Alterations of
beta-adrenergic signaling and cardiac hypertrophy in transgenic
mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol.
283:H1253–H1262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sukumaran V, Watanabe K, Veeraveedu PT,
Thandavarayan RA, Gurusamy N, Ma M, Yamaguchi K, Suzuki K, Kodama M
and Aizawa Y: Telmisartan, an angiotensin-II receptor blocker
ameliorates cardiac remodeling in rats with dilated cardiomyopathy.
Hypertension Res. 33:695–702. 2010. View Article : Google Scholar
|
|
35
|
Yu CM, Tipoe GL, Lai Wing-Hon K and Lau
CP: Effects of combination of angiotensin-converting enzyme
inhibitor and angiotensin receptor antagonist on inflammatory
cellular infiltration and myocardial interstitial fibrosis after
acute myocardial infarction. J Am Coll Cardiol. 38:1207–1215. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moodley Y, Atienza D, Manuelpillai U,
Samuel CS, Tchongue J, Ilancheran S, Boyd R and Trounson A: Human
umbilical cord mesenchymal stem cells reduce fibrosis of
bleomycin-induced lung injury. Am J Pathol. 175:303–313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Massague J: Integration of Smad and MAPK
pathways: A link and a linker revisited. Genes Dev. 17:2993–2997.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo X and Wang XF: Signaling cross-talk
between TGF-beta/BMP and other pathways. Cell research. 19:71–88.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huby AC, Turdi S, James J, Towbin JA and
Purevjav E: FasL expression in cardiomyocytes activates the ERK1/2
pathway, leading to dilated cardiomyopathy and advanced heart
failure. Clin Sci (Lond). 130:289–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Zhu H, Su Z, Sun C, Yin J, Yuan H,
Sandoghchian S, Jiao Z, Wang S and Xu H: IL-17 contributes to
cardiac fibrosis following experimental autoimmune myocarditis by a
PKCβ/Erk1/2/NF-kappaB-dependent signaling pathway. Int Immunol.
24:605–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sriramula S, Haque M, Majid DS and Francis
J: Involvement of tumor necrosis factor-alpha in angiotensin
II-mediated effects on salt appetite, hypertension, and cardiac
hypertrophy. Hypertension. 51:1345–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun M, Chen M, Dawood F, Zurawska U, Li
JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R and Liu
PP: Tumor necrosis factor-alpha mediates cardiac remodeling and
ventricular dysfunction after pressure overload state. Circulation.
115:1398–1407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sivasubramanian N, Coker ML, Kurrelmeyer
KM, MacLellan WR, DeMayo FJ, Spinale FG and Mann DL: Left
ventricular remodeling in transgenic mice with cardiac restricted
overexpression of tumor necrosis factor. Circulation. 104:826–831.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Westermann D, Van Linthout S, Dhayat S,
Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A,
Schultheiss HP and Tschöpe C: Tumor necrosis factor-alpha
antagonism protects from myocardial inflammation and fibrosis in
experimental diabetic cardiomyopathy. Basic Res Cardiol.
102:500–507. 2007. View Article : Google Scholar : PubMed/NCBI
|