Introduction
Dry eye disease is an ocular surface disorder that
can affect quality of life by causing ocular discomfort and visual
disturbances. Historically, dry eye disease has been defined by
tear film abnormalities, ocular discomfort and potential damage to
the interpalpebral ocular surface. According to a previous study,
ocular disease affects ~20% of people globally depending on age and
sex (1), and specific geographic
locations, environments and lifestyles can increase the risk of
developing dry eye disease. A further study estimated that
7.4–33.4% of the global population experience ocular disease
throughout their lifetime, the highest proportion of which occurs
within Asian populations (2). The
treatment options for dry eye disease are limited to palliation
with artificial tears and tear conservation techniques. In the
majority of mild or temporary cases of dry eye disease, such as
response to seasonal changes, environmental factors, medication, or
illness, therapeutic palliation generally provides adequate relief;
however, patients with chronic, moderate, and severe cases may
continue to experience symptoms despite maximum use of palliative
treatment. In the most severe forms of dry eye disorders,
associated with corneal ulcers, treatment may enhance
endophthalmitis (3). Considering
the adverse symptoms experienced as a result of current treatment
options, herbal medicine has becoming increasingly proposed as an
attractive form of alternative therapy.
Lycium barbarum (Solanaceae) is a
particularly well-known traditional medicinal plant. Fruits of the
plant commonly termed goji berries (gou qi zi in Chinese)
have long been consumed for nutritional and medicinal purposes
throughout Asia. Goji berries are widely used in cooking due to
their sweet flavor and general health benefits (4). Furthermore, goji berries are often
processed into tinctures due to their anti-aging and antioxidant
effects (5). In addition, goji
berries have been reported to have various pharmaceutical
properties, including immune enhancement, reducing the risks of
hypoglycemia, hypolipidemia and metabolic syndromes; as well as
antioxidative, antitumor, anti-inflammatory, hepatoprotective and
renal protective activities (5–7).
Furthermore, goji berries have been demonstrated to ameliorate the
adverse side effects of chemotherapy and radiotherapy, and to
improve the general wellbeing of patients with various stages of
cancer (8–15). According to ancient Chinese materia
medica, goji berry can be consumed to nourish the liver and
kidneys, and to improve vision. Scientific research has since
revealed that the consumption of goji berries facilitates retinal
and macular functioning, enhances the protective effects exerted by
ganglion cells on to the retina, and decreases retinal ischemia
injury (16,17). Thus, goji berry has been considered
to improve the pathogenesis of glaucoma in clinical applications.
Additionally, goji berries decrease the risk of cataracts, prevent
irreversible loss of central vision in older people and ameliorate
diabetic retinopathy (8,9,18–20).
Despite the substantial literature, relatively little has been
established regarding the implications of goji berry consumption on
the pathogenesis of dry eye disease. Therefore, the present study
aimed to determine the beneficial effects of goji berry consumption
in rats with a model of dry eye disease.
Materials and methods
Materials
Lycium barbarum was purchased from a herb
store in Pingtung (Taiwan), and was botanically identified and
verified at the Medicinal Plant Research Laboratory at Tajen
University (Pingtung, Taiwan). Aqueous goji berry extract (GBE) was
prepared in accordance with the previous study (21). Betaine
(C5H11NO2, ≥98% perchloric acid
titration) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) and liquid chromatography-grade acetonitrile was purchased
from Tedia Co. (Fairfield, OH, USA). The Cosmosil 5 NH2-MS
high-performance liquid chromatography (HPLC) column (250×4.6 mm,
internal diameter of 5 µm; Nakalai Tesque, Inc., Kyoto, Japan) was
employed for subsequent analysis. All other chemicals utilized were
of an analytical reagent grade.
Determination of polysaccharides and
betaine in GBE
The polysaccharide content in GBE was measured using
a phenol-sulfuric acid method (22). An aliquot of 1 ml GBE solution (40
µg/ml) was briefly mixed with 98% concentrated sulfuric acid (pH
0.3) (5 ml) and 5% phenol solution (1 ml). The mixture was then
agitated in a water bath at 30°C for 30 min and following this, the
absorbance was determined at 490 mm using a microplate reader
(SpectraMax 190; Molecular Devices, LLC, Sunnyvale, CA, USA). The
polysaccharide concentration in GBE was then calculated via
reference to a calibration curve generated using galactose standard
solutions. Following this, the betaine content in the GBE was then
determined using a modified version of a previously described
method (23). A pump system
(Hitachi L-2130; Hitachi, Ltd., Tokyo, Japan) equipped with an
L-2450 diode array detector and L-2200 autosampler was used to
measure the betaine content via HPLC using the Cosmosil 5 NH2-MS
HPLC column (250×4.6 mm, internal diameter of 5 µm, Nakalai Tesque,
Inc.) at 195 nm. A mixture of water and acetonitrile (15:85, v/v)
was used as the mobile phase, and the flow rate and injection
volume were set to 1.0 ml/min and 10 µl, respectively.
Test animals
A total of 45 male Sprague-Dawley (SD) rats (340–350
g) were obtained from BioLASCO Taiwan Co. (Taipei, Taiwan) and
housed under standard laboratory conditions (12 h light/dark
regular cycle and room temperature of 22±2°C) in 1 atmospheric
pressure (20.9% oxygen, normobaric conditions). Standard chow
(content: >25% crude protein, >4.5% crude fat, <12% water,
and <9% ash; Fwusow Industry Co., Ltd., Taichung, Taiwan) and
sterilized water were available ad libitum. A week was
allotted for the rats to become acclimatized to the laboratory
environment and diet. Approval for this study was obtained from the
Animal Care and Use Committee of the Kaohsiung Armed Forces General
Hospital (no. A105-10).
Effect of GBE on dry eye disease in
animal model
Five rats were initially used for validation of the
results of the Schirmer's test, the tear break-up time (BUT), and
the grading of corneal and conjunctival fluorescein staining
post-nerve blockage (24,25). Schirmer's test is commonly used for
the measurement of tear production, and was an indispensable
component of this examination. A low Schirmer's test score,
denoting a decrease in lacrimal glad output and potential damage to
the ocular surface, is an indicator of dry eye disease (26). Initially, proparacaine (0.5%), a
topical anesthetic eye drop, was injected into the inferior
conjunctival cul-de-sac of the rats. A sterile Schirmer's strip was
then immediately placed in the lateral canthus for 5 min. Following
this, the length of the moistened strip was measured to determine
tear production. Wetting length measurements <5 mm on the paper
was considered to correspond with low Schirmer's test scores, and
indicated a severe lack of tear production.
The tear BUT is a crucial metric of dry eye severity
and reflects the overall tear quality throughout all layers of the
tear film (27,28). Specifically, the tear BUT of an
individual eye is the interval between a complete blink and the
first appearance of a dry spot on the precorneal surface of the
tear film. In the present study, following the application of the
paper fluorescein strips (Haag-Streit AG, Koeniz, Switzerland) to
the inferior conjunctival fornix, the rats resumed normal blinking.
Their eye openings were observed until the first defect of the tear
film was detectable using portable slit-lamp microscopy. The
measurement was conducted in a quiet, enclosed room without
ventilation currents. Ambient humidity and temperature were
monitored, with a relative humidity of 40–50%. Values <10 sec
were considered abnormal. Subsequently, the time period required
for the dye to disappear was recorded and the average time
durations of the trials were calculated. The procedure was repeated
three times for each eye tested.
Fluorescein staining, using the Oxford grading
scheme (Fig. 1), is the standard
method used for the diagnosis dry eye disease and was hereby used
to carry out daily keratoconjunctival staining for 21 days.
Severity of staining was quantified using a chart comprising a
series of panels, labeled A-E, of increasing severity. In each
panel, fluorescein staining is represented by punctate dots. To
grade the staining, comparisons were made between the panels and
the appearance of staining on the exposed interpalpebral
conjunctivas and corneas of the rats. The six Oxford scheme grades
(0–5), which denote the severity of dry eye, were used to record
the results. Specifically, the keratoconjunctival staining was
rated mild (stage 0 or 1), moderate (stage 2 or 3), or severe
(stage 4 or 5) (24). Significant
changes within stages 2–5 post-GBE treatment were considered to be
relevant.
For Experiment 1, atropine solution (1 mg/kg) was
injected into the right lacrimal gland of each rat (n=5) to induce
the formation of dry eye via the blocking of relevant nerves and
causing direct damage to the lacrimal glands (29). The Schirmer's test score, tear BUT
and keratoconjunctival fluorescein staining of the 5 rats were
recorded daily to determine the development of dry eye disease. In
Experiment 2, 40 rats were randomly divided into four groups:
Vehicle (control), low-dose GBE (LGBE; 250 mg/kg/bw), median-dose
GBE (MGBE; 350 mg/kg/bw) and high-dose GBE (HGBE; 500 mg/kg/bw).
All 40 rats were experimentally induced to develop dry eye disease,
and the LGBE, MGBE and HGBE groups were orally administered varying
amounts of GBE at 7 days post-atropine injection to evaluate the
therapeutic effects of goji berries. Schirmer's test. The tear BUT
and keratoconjunctival staining were then performed daily for 21
days to evaluate the condition and severity of dry eye
post-treatment as previously described (30,31).
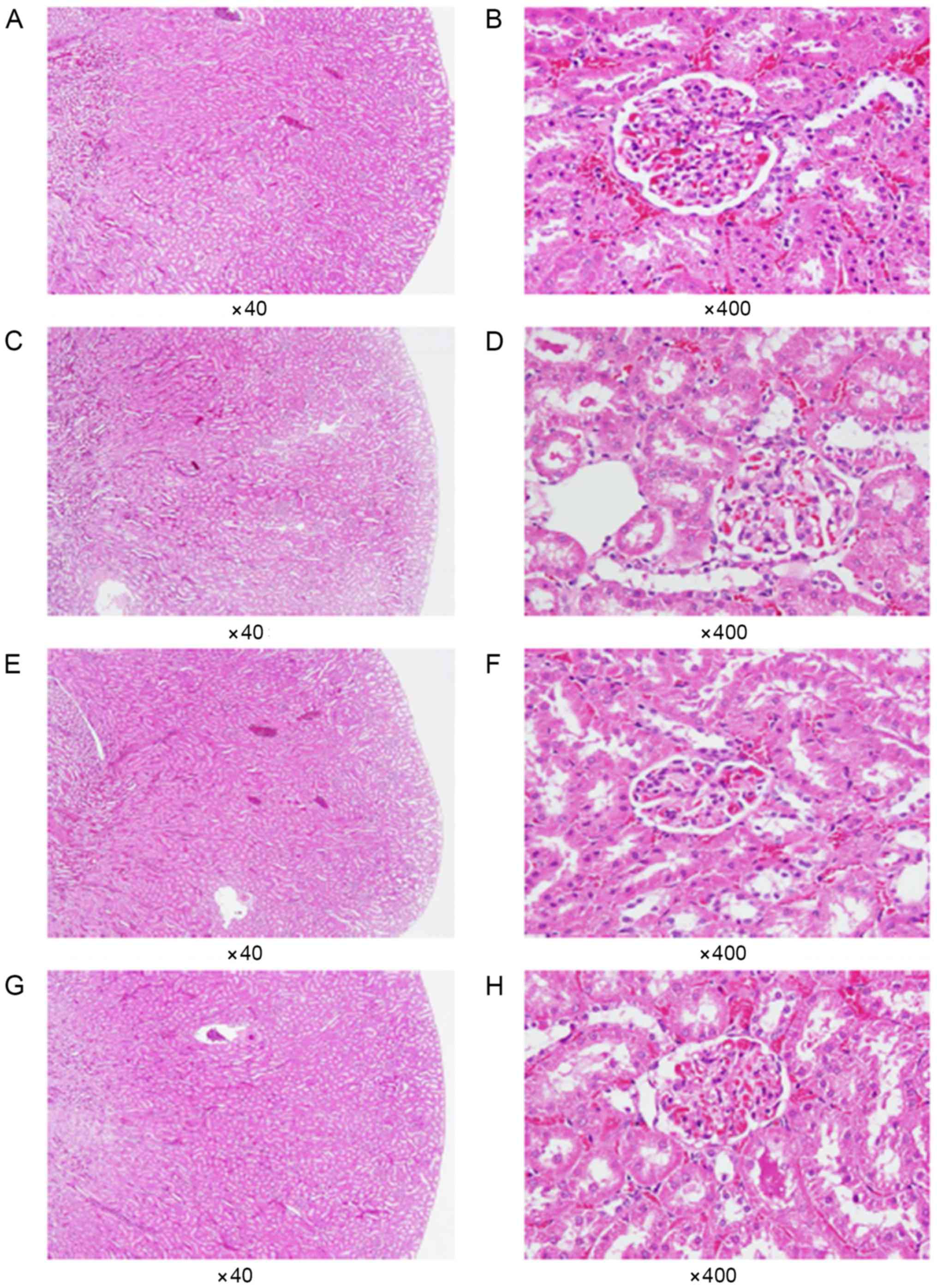
Histopathological examination
Following experimentation, the rats were sacrificed
using CO2. The kidneys and livers of all rats were then
carefully removed, and the excised specimens were soaked in 10%
formaldehyde for 1 week and then cut into 1 µm sections. The
specimens were then stained with 0.4% hematoxylin and eosin for
examination for 30 min at room temperature, and the tissues were
reviewed under light microscopy. Subsequently, the difference in
staining results between each group were compared for further
evaluation of the safety of dietary GBE, and the morphometric data
of the samples' associated corneas and conjunctivas were recorded
in detail.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
software (version 11.5; SPSS, Inc., Chicago, IL, USA). Intergroup
comparisons were performed using one-way analysis of variance,
followed by a Duncan's test. **P<0.01 was considered to indicate
a statistically significant difference.
Results
Determination of polysaccharides and
betaine in GBE
A total of 42.2% of GBE was recovered from goji
berries following aqueous extraction and lyophilization.
Subsequently, the concentration of polysaccharide content within
GBE was revealed to be 843.5 mg/g, indicating a high concentration
of polysaccharides. Betaine is another component of goji berries.
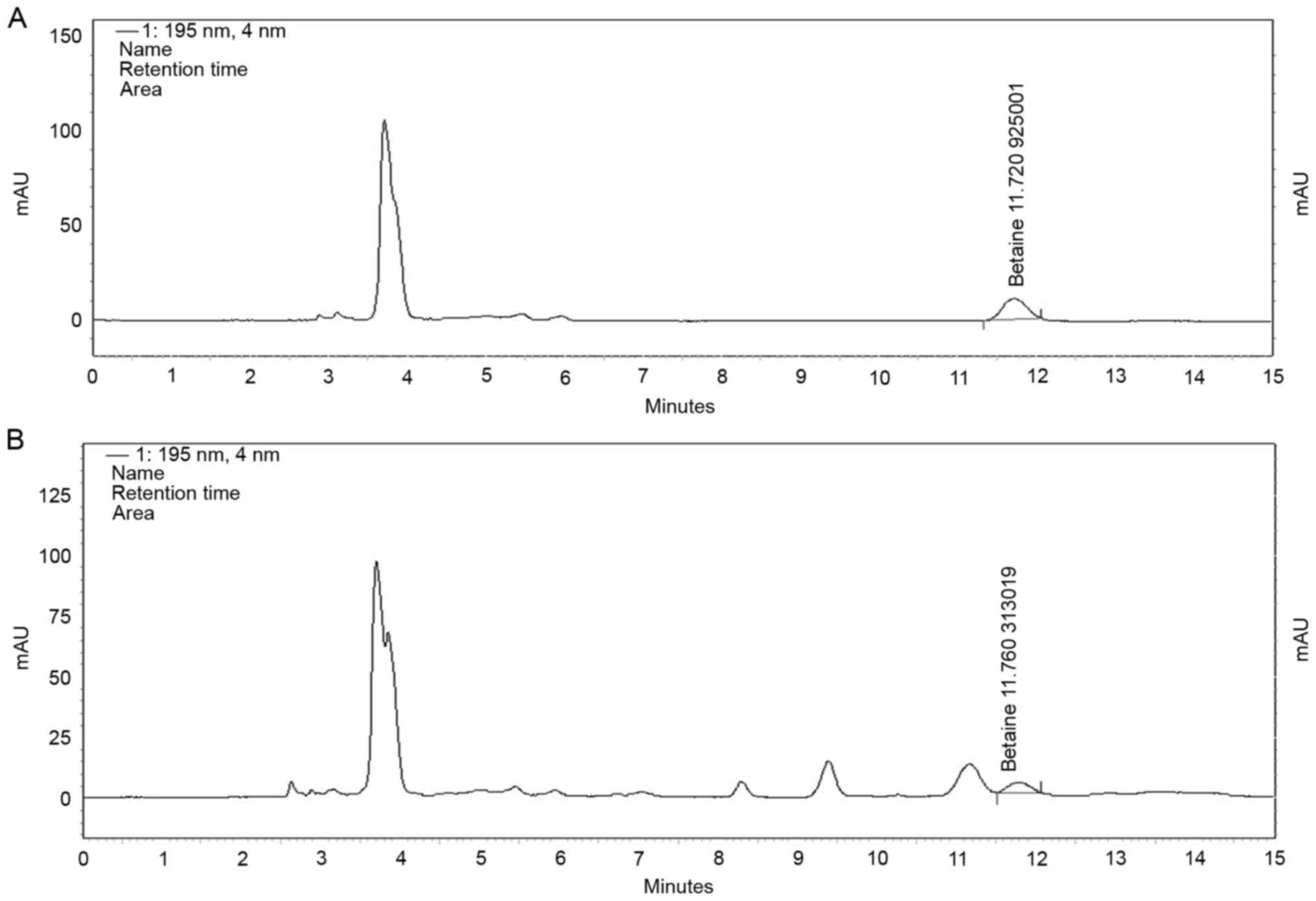
Fig. 2A and B depict the HPLC
chromatograms of a betaine standard and GBE, respectively. From
this analysis, the concentration of betaine in GBE was revealed to
be 9.1 mg/g.
Effect of GBE on dry eye disease in an
animal model
The results of Experiment 1 revealed significantly
decreased tear production within 7 days after atropine injection,
which validated the animal model for further study of dry eye
disease. Following this, the results between eyes (right, dry eye;
left, normal eye) in the rats were compared, and reductions in both
the Schirmer's test scores (1.2±0.3 mm) and tear BUTs (1.5±0.5 sec)
were demonstrated in the right eyes of the rats. By contrast, the
Schirmer's test scores and tear BUTs were >10 mm/5 min and
>10 sec, respectively, for the left eyes. Experiment 2 was then
performed to investigate the influence of GBE on a set of objective
parameters in the four groups (n=10). At 7 days after atropine
injection, GBE at different doses was administered to the rats with
the exception of the control group. Changes of the Schirmer's test
score, tear BUT, and keratoconjunctival staining were measured
daily.
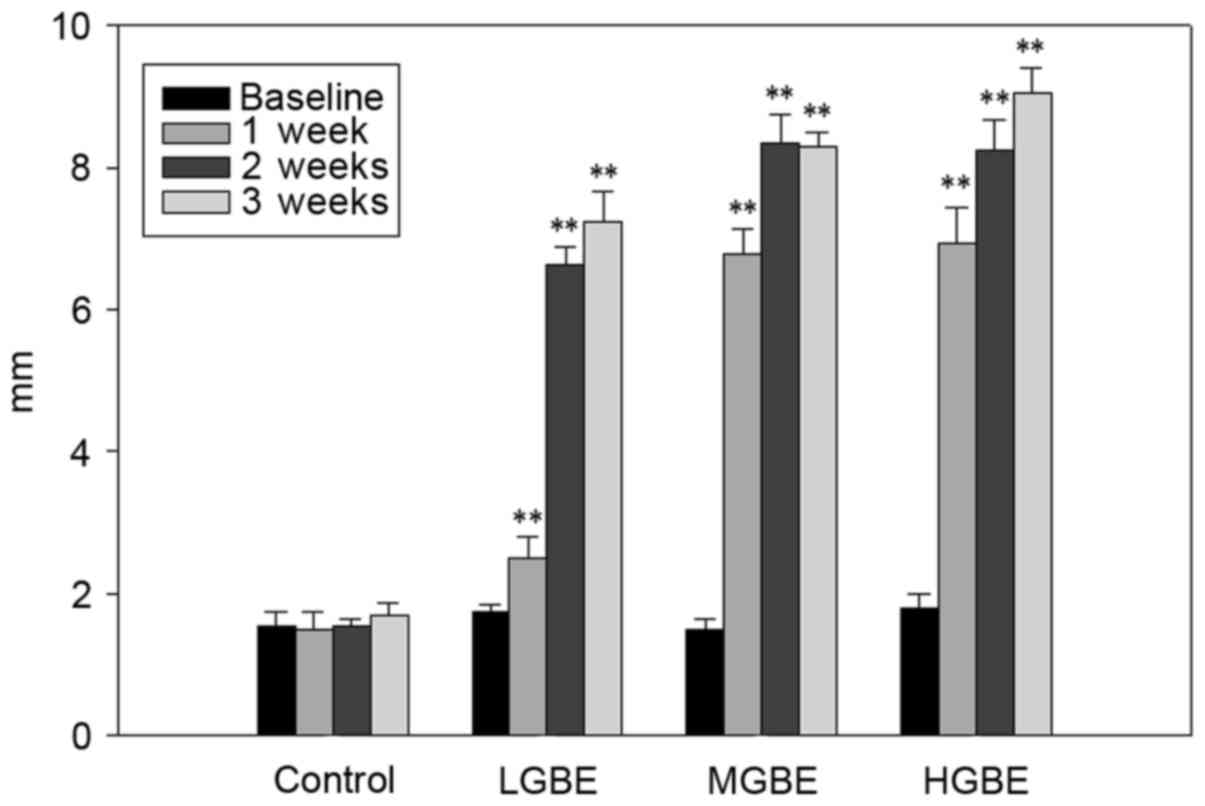
The Schirmer's test scores (mm) are presented in
Fig. 3. The range score of normal
mice was obtained in the first experiment; the Schirmer test score
was ~10 mm and the normal tear BUT in rats without dry eye disease
was ~10 sec. The scores for the control group were 1.2±0.1,
1.3±0.5, 1.2±0.3 and 1.3±0.4 mm, at weeks 0 (baseline), 1, 2 and 3,
respectively. Notably, the dry eye condition remained unchanged
over the 3 weeks (<5 mm) in the absence of GBE treatment. For
the LGBE group, the scores were 1.2±0.4, 2.3±0.8, 6.8±1.5, and
7.4±1.8 mm, at weeks 0, 1, 2 and 3, respectively. These scores
suggest that the adverse symptoms of dry eye can be significantly
alleviated following 1 week of LGBE therapy (P<0.01). Similarly,
the scores for the MGBE group were 1.1±0.8, 6.2±1.5, 7.4±2.1, and
8.2±1.5 mm, at weeks 0, 1, 2 and 3, respectively; thus, significant
suppression of dry eye disease symptoms was apparent following 1
week of GBE administration (P<0.01). A similar pattern was
observed in the HGBE group, the scores of which were 1.3±7.1,
8.2±1.1, and 9.4±0.5, at weeks 0, 1, 2 and 3, respectively. A
normal Schirmer's test score in rats with no dry eye disease was
~10 mm. In conclusion, the reduction in Schirmer's test score
caused by dry eye disease was normalized (9.4±0.5 mm) following 3
weeks of HGBE treatment, indicating that administration of GBE
significantly increased tear volume and enhanced the secretion of
tears.
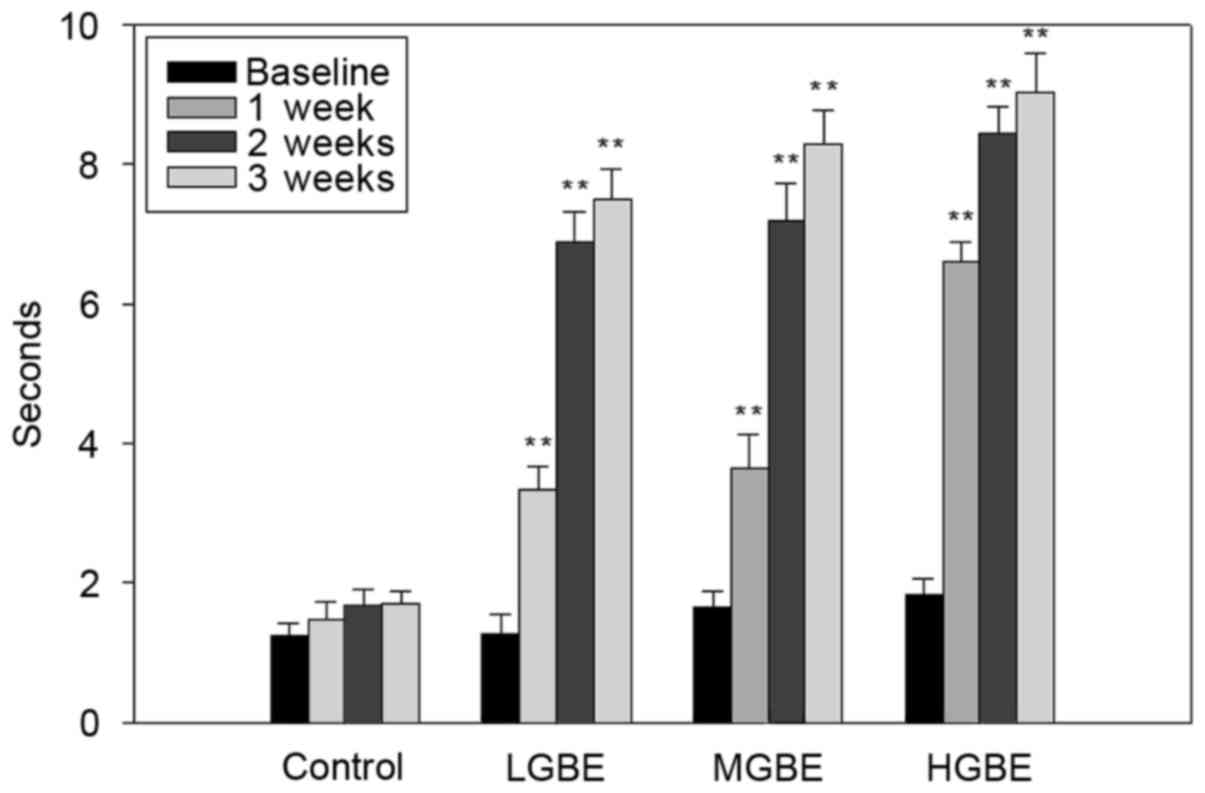
Fig. 4 presents the
results of tear BUTs analysis. The tear BUTs in the control group
were all <5 sec. The tear BUTs results for the LGBE group were:
1.2±0.4, 3.4±1.4, 6.7±1.5 and 7.2±2.4 sec for weeks 0, 1, 2 and 3,
respectively. These results therefore demonstrate that the
precorneal tear film was stabilized after 1 week of LGBE therapy
(P<0.01). The tear BUTs in the MGBE group were 1.4±0.8, 5.8±1.2,
7.5±2.1 and 7.9±2.4 sec, at weeks 0, 1, 2 and 3, respectively.
Similarly to the results obtained by the LGBE group, the results of
the MGBE group demonstrate that the viscosity of the tear film was
stable following 1 week of GBE administration (P<0.01).
Furthermore, the same pattern was observed in the HGBE group; the
tear BUTs were 1.3±0.5, 6.7±2.5, 8.4±0.4 and 8.8±1.2 sec, at weeks
0, 1, 2 and 3, respectively (P<0.01). The normal tear BUT in
rats without dry eye disease was ~10 sec. In conclusion, the tear
BUTs were significantly normalized (8.8±1.2 sec) following 3 weeks
of HGBE treatment, demonstrating that administration of GBE
significantly stabilizes the tear film and decreases the rate of
tear evaporation.
Finally, the results of keratoconjunctival staining
suggested that dry eye disease may result in keratopathy and
conjunctival epithelial damage due to a lack of protection
otherwise provided by naturally produced tears. Microscopic
analysis of the keratoconjunctival-stained tissue revealed that
37.5, 37.5, and 25.0% of the rats exhibited mild, moderate or
severe morphological changes following induction of dry eye prior
to GBE administration (Table I).
However, the rats morphologies improved following GBE treatment:
The proportion of rats post-GBE administration with mild, moderate,
and severe changes were 82.5, 12.5, and 5.0%, respectively. The
majority (82.5%) of mild changes were observed after 3 weeks of
treatment (P<0.05). Furthermore, the corneal and conjunctival
lesions of the dry-eyed rats were ameliorated by GBE
administration. Thus, the results suggest that administration of
GBE enhances tear flow and has a protective effect on the ocular
surface of the eye.
 | Table I.Morphological changes visualized
following 21 days of GBE treatment (n=40). |
Table I.
Morphological changes visualized
following 21 days of GBE treatment (n=40).
| Morphological
change | Baseline (dry
eye) | 21 days
post-therapy |
P-valuea |
|---|
| Mild | 37.5% (15/40) | 82.5%
(33/40) | <0.05 |
| Moderate | 37.5% (15/40) | 12.5% (5/40) | <0.05 |
| Severe | 25%
(10/40) | 5% (2/40) | <0.05 |
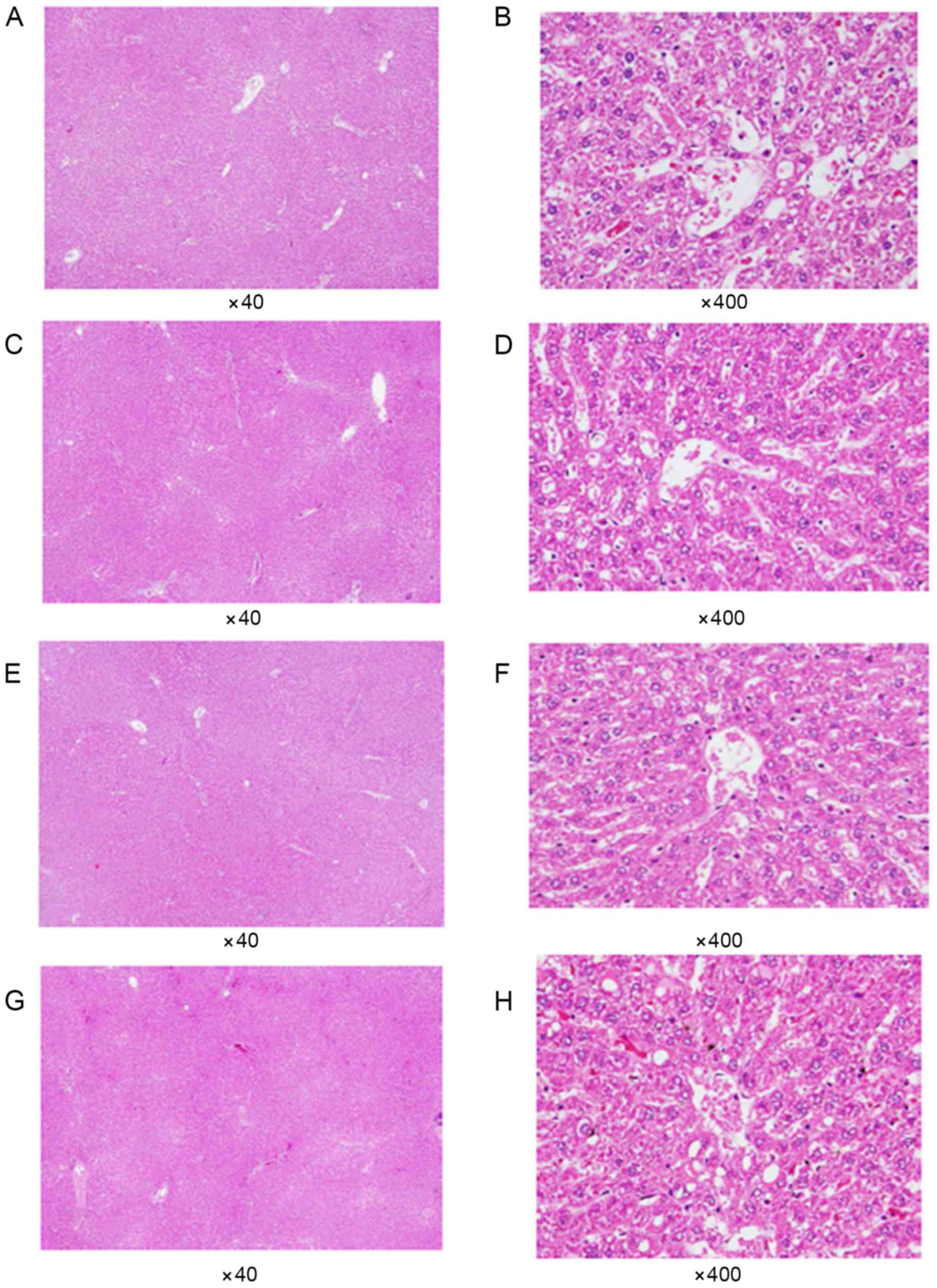
Histopathological examination
All rats (except those belonging to the control
group) received daily administration of GBE via oral gavage for 21
days consecutively. Subsequently, each group was
histopathologically examined using light microscopy to observe the
morphological differences in the kidney and liver tissues of the
rats. As shown in Figs. 5 and
6, no abnormal histopathological
changes were observed in any of the liver or kidney tissues from
the rats that were administered doses of GBE.
Discussion
Dry eye disease is the most common complaint
reported to ophthalmological clinical practices, with 68% of people
aged ≥60 years old presenting associated symptoms (32,33).
Only 20% of patients with dry eye disease experiencing mild
symptoms seek medical assistance, compared with 50% of patients
experiencing moderate symptoms, and virtually all patients
experiencing severe symptoms (34). The two predominant therapeutic
approaches for the treatment of dry eye disease currently used in
clinical practice are artificial tears, ointments and gels for mild
to moderate cases, and anti-inflammatory drugs for severe cases to
reduce ocular surface inflammation. In addition to clinical
medication, herbal medicine may provide alternative treatment
options for sufferers. Herbal medicine has attracted considerable
attention for >5,000 years, particularly in China. To the best
of our knowledge, this study was the first to investigate the
therapeutic potential of goji berries for the treatment of dry eye
disease in rats.
Current clinical diagnosis of dry eye disease uses
slit-lamp examination (with and without staining, including
fluorescein, rose bengal and lissamine green), Schirmer's test,
tear BUT measurement, tear pH measurement, corneal smoothness
evaluation, the cotton thread test, blinking rate calculation, tear
clearance evaluation, meniscus height measurement, ocular
protection index calculation, blinking reflex assessment,
epithelial thickness measurement, corneal epithelial glycogen level
measurement, tear film osmolality evaluation, tear volume
measurement, impression cytology, corneal sensitivity evaluation
and lid margin redness examination (35). In the present study, the Schirmer's
test, tear BUT measurement and keratoconjunctival fluorescein
staining were utilized to evaluate the effects of GBE
administration on dry eye disease in rats. The results revealed
that dry eye symptoms were successfully induced at 7 days after
injection of atropine solution into the lacrimal glands of rats and
dry eye symptoms were significantly alleviated (P<0.01)
following 1 week of GBE administration at low, median and high
doses. Furthermore, symptoms of dry eye disease were almost
entirely eliminated by 3 weeks post-HGBE treatment. In addition,
according to the keratoconjunctival staining results, the corneal
and conjunctival lesions in dry-eye-afflicted rats were
significantly ameliorated after 3 weeks of GBE treatment.
Therefore, dry eye symptoms may be alleviated within a short
treatment period of GBE administration. Schirmer's test and tear
BUT measurement are the most common tests for the examination of
tear physiology associated with dry eye disease. In the present
study, it was determined that administration of GBE may enhance
tear formation and outflow, stabilize the tear film shape in
situ and decrease the possibility of tear evaporation.
The molecular mechanisms and various therapies
associated with dry eye disease have become increasingly clinically
relevant. Dry eye disease is a multifactorial disease with several
implicated pathological mechanisms, including instability of the
tear film, tear hyperosmolarity, oxidative stress and inflammation
of the ocular surface (2,36). Tear hyperosmolarity has previously
been revealed to initiate dry eye inflammation via the activation
of epithelial and stromal cells on the ocular surface, which
increase the presence of pro-inflammatory cytokines and various
chemokines (37). Furthermore,
hyperosmolarity can affect corneal epithelial barrier function,
which may lead to the pathogenic infiltration of immune and stromal
cells or cytokine release (38).
Furthermore, dry eye disease can result in discomfort and visual
disturbance (39–41). Finally, allergies and other
inflammatory conditions of the ocular surface can destabilize the
tear film.
Artificial tears, eye drops and anti-inflammatory
drugs are clinically prescribed to reduce ocular surface
inflammation. Recently, topical use of cyclosporine A, combined
with epithelial keratopathy, has become a popular treatment option
for patients with severe dry eye disease (42). Donnenfeld and Pfugfelder (43) demonstrated that topical
cyclosporine A administration alleviated adverse symptoms
experienced by patients with dry eye disease, evidenced by an
increase in tear BUTs and a decrease in keratoconjunctival
staining, following three months of treatment; however,
nephrotoxicity and hypertension side effects of cyclosporine A
administration were observed. In addition to this finding, goji
berry with its inherent antihypertensive activity was suggested to
act as an alternative therapeutic for patients with dry eye disease
to avoid the contraindications induced by cyclosporin A
administration (44). In a further
study, symptoms of dry eye disease associated with ocular
inflammation were reduced following administration of
corticosteroids and tetracyclines (45). Furthermore, goji berries have
previously been reported to exhibit anti-inflammatory and
antimicrobial activity, which may also provide partial relief of
the symptoms experienced by patients with dry eye disease (46).
Recently, a newly established model of dry eye
disease, according to which oxidative stress induces the functional
decline of lacrimal glands, was proposed; Uchino et al
(47) revealed that free radicals
were associated with ocular surface epithelial damage and a
decrease in lacrimal gland secretory function. Other studies have
demonstrated that oxidative stress reduces aqueous tear production
and that antioxidants increase Schirmer's test scores and tear
stability (48,49). Higuchi et al (50) revealed that excessive exposure to
oxidative stress induced lacrimal gland pathophysiology; therefore
suggesting that increases in reactive oxidative species (ROS)
underpin the inflammatory mechanisms associated with dry eye
disease. A further study demonstrated that radical scavengers and
antioxidants were prominent components of the goji berry, both of
which are beneficial for humans (51). Furthermore, antioxidant enzyme
activity has been revealed to occur naturally in the eye, with the
highest levels detected in the retina, lower levels detected in the
sclera and cornea, and minimal levels detected in tears (52). In addition, antioxidant biomarkers,
including glutathione reductase, superoxide dismutase and
malondialdehyde (MDA), were present at elevated levels in the serum
following long-term (i.e., >30 days) consumption of goji berries
juice at the dose of 120 ml/day (8). Furthermore, Lycium barbarum
polysaccharides (LBPs) have been suggested to significantly inhibit
the generation of ROS, reduce the level of MDA and increase the
functional abilities of antioxidants (51). The present study demonstrated that
abundant polysaccharides were present in the GBE, therefore
suggesting that LBPs exhibit an antioxidant activity in GBE and
thus are implicated in relieving dry eye symptoms associated with
oxidative stress.
In addition to LBPs, the present study revealed the
presence of betaine in the extract. Betaine primarily functions as
an osmolyte and methyl donor for transmethylation, however, it also
exerts a protective function to cells, proteins and enzymes against
environmental stressors. Betaine has also been demonstrated to
regulate cellular functioning and survival under various stressful
conditions (53), and to inhibit
the expression of pro-inflammatory cytokines [tumor necrosis
factor-α, interleukin (IL)-1β, IL-6, IL-8 and chemokine ligand 2]
and chemokines (54). Hua et
al (55) determined that
betaine could be utilized as a therapeutic treatment for dry eye
disease, by increasing the levels of pro-inflammatory cytokines and
chemokines in the tear fluid, increasing the expression of immune
activation and adhesion molecules by the conjunctival epithelium,
and increasing the number of T-lymphocytes in the conjunctiva.
Furthermore, betaine may provide technical effects on the
improvement of moisture control and nutritional benefits (56). Elevated tear osmolality is one of
the key pathological factors in dry eye disease, leading to ocular
discomfort, and is associated with damage to the ocular surface and
inflammation. Garrett et al (57) demonstrated that the betaine in GBE
may stabilize epithelial cell volume under hyperosmotic
stress-induced apoptosis conditions. Therefore, administration of
GBE betaine may relieve symptoms of dry eye disease via a mechanism
associated with the therapeutic administration of artificial tears
to refresh the precorneal surface in moist environments.
Regarding the safety of GBE treatment, there were no
mortalities in the groups receiving GBE treatment, and
histopathological examinations of kidney and liver tissue samples
acquired from all animal participants revealed no apparent abnormal
histopathological changes. In addition, a previous toxicological
study on rats revealed no indication of toxicity following oral
intake of goji berry juice; even at the maximum dosage (10
ml/kg/day), the researchers observed no mortality or organ damage
and did not find a median lethal dose of GBE (58).
In conclusion, dry eye disease can reduce visual
functioning and quality of life, and is a common eye problem
presented to ophthalmologists. In this study, administration of GBE
significantly ameliorates the symptoms of dry eye in rats according
to three measures: The Schirmer's test score, tear BUT and
keratoconjunctival fluorescein staining. Furthermore, the
beneficial effects of goji berries may be associated with their
polysaccharide and betaine content, both of which promote
antioxidant and anti-inflammatory activity. Nutraceutical
approaches for both therapeutic and preventative treatments for dry
eye disease are currently under intensive investigation. Compared
with artificial tears or eye drops, GBE may be an excellent dietary
supplement for the relief of dry eye disease symptoms.
In conclusion, the current study indicates that goji
berries are a safe food supplement with substantial benefits that
ameliorate the symptoms of dry eye disease by enhancing the tear
volume and repairing the damaged ocular surface cells. Future
studies should focus on the investigation of the underlying
molecular mechanisms of the therapeutic effects of GBE associated
with dry eye disease.
Acknowledgements
This study was financially supported by clinical
research grants from the Kaohsiung Armed Forces General Hospital
(Kaohsiung, Taiwan).
References
|
1
|
Dogru M and Tsubota K: Pharmacotherapy of
dry eye. Expert Opin Pharmacother. 12:325–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P
and Hsu WM: Prevalence of dry eye among an elderly chinese
population in Taiwan: The Shihpai eye study. Ophthalmology.
110:1096–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klotz SA, Penn CC, Negvesky GJ and Butrus
SI: Fungal and parasitic infections of the eye. Clin Microbiol Rev.
13:662–685. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bo R, Ma X, Feng Y, Zhu Q, Huang Y, Liu Z,
Liu C, Gao Z, Hu Y and Wang D: Optimization on conditions of
Lycium barbarum polysaccharides liposome by RSM and its
effects on the peritoneal macrophages function. Carbohyd Polym.
117:215–222. 2015. View Article : Google Scholar
|
|
5
|
Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW,
He ZX, Sun T, Zhang X, Zhao RJ, Gu L, et al: An evidence-based
update on the pharmacological activities and possible molecular
targets of Lycium barbarum polysaccharides. Drug Des Devel
Ther. 9:33–78. 2014.PubMed/NCBI
|
|
6
|
Qian D, Zhao Y, Yang G and Huang L:
Systematic review of chemical constituents in the genus
Lycium (Solanaceae). Molecules. 22:pii: E911. 2017.
View Article : Google Scholar
|
|
7
|
Xing X, Liu F, Xiao J and So KF:
Neuro-protective mechanisms of Lycium barbarum.
Neuromolecular Med. 18:253–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amagase H, Sun BX and Borek C: Lycium
barbarum (goji) juice improves in vivo antioxidant biomarkers
in serum of healthy adults. Nutr Res. 29:19–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li SY, Yang D, Yeung CM, Yu WY, Chang RC,
So KF, Wong D and Lo AC: Lycium barbarum polysaccharides
reduce neuronal damage, blood-retinal barrier disruption and
oxidative stress in retinal ischemia/reperfusion injury. PLoS One.
6:e163802011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gan L, Zhang SH, Liu Q and Xu HB: A
polysaccharide-protein complex from Lycium barbarum
upregulates cytokine expression in human peripheral blood
mononuclear cells. Eur J Pharmacol. 471:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XM, Ma YL and Liu XJ: Effect of the
Lycium barbarum polysaccharides on age-related oxidative
stress in aged mice. J Ethnopharmacol. 111:504–511. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu MS, Lai CS, Ho YS, Zee SY, So KF, Yuen
WH and Chang RC: Characterization of the effects of anti-aging
medicine Fructus lycii on beta-amyloid peptide neurotoxicity. Int J
Mol Med. 20:261–268. 2007.PubMed/NCBI
|
|
13
|
Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK
and Kim CH: Protective effect of Lycium chinense fruit on
carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol.
96:529–535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo Q, Cai Y, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong H, Shen P, Jin L, Xing C and Tang F:
Therapeutic effects of Lycium barbarum polysaccharide (LBP)
on irradiation or chemotherapy-induced myelosuppressive mice.
Cancer Biother Radiopharm. 20:155–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He M, Pan H, Chang RC, So KF, Brecha NC
and Pu M: Activation of the Nrf2/HO-1 antioxidant pathway
contributes to the protective effects of Lycium barbarum
polysaccharides in the rodent retina after
ischemia-reperfusion-induced damage. PLoS One. 9:e848002014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mi XS, Feng Q, Lo AC, Chang RC, Lin B,
Chung SK and So KF: Protection of retinal ganglion cells and
retinal vasculature by Lycium barbarum polysaccharides in a
mouse model of acute ocular hypertension. PLoS One. 7:e454692012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bucheli P, Vidal K, Shen L, Gu Z, Zhang C,
Miller LE and Wang J: Goji berry effects on macular characteristics
and plasma antioxidant levels. Optom Vis Sci. 88:257–262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan HC, Chang RC, Koon-Ching Ip A, Chiu
K, Yuen WH, Zee SY and So KF: Neuroprotective effects of Lycium
barbarum Lynn on protecting retinal ganglion cells in an ocular
hypertension model of glaucoma. Exp Neurol. 203:269–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect
human lens epithelial cells against oxidative stress-induced
apoptosis and senescence. PLoS One. 9:e1102752014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horng CT, Huang JK, Wang HY, Huang CC and
Chen FA: Antioxidant and antifatigue activities of polygonatum
alte-lobatum hayata rhizomes in rats. Nutrients. 6:5327–5337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dubois M, Gilles KA, Hamilton JK, Rebers P
and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
23
|
Lee HW, Kim YH, Kim YH, Lee GH and Lee MY:
Discrimination of Lycium chinense and Lycium barbarum
by taste pattern and betaine analysis. Int J Clin Exp Med.
7:2053–2059. 2014.PubMed/NCBI
|
|
24
|
Bron AJ, Evans VE and Smith JA: Grading of
corneal and conjunctival staining in the context of other dry eye
tests. Cornea. 22:640–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG and Chung JG: Antitumor
effects of emodin on LS1034 human colon cancer cells in vitro and
in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts
model. Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kashkouli MB, Pakdel F, Amani A, Asefi M,
Aghai GH and Falavarjani KG: A modified Schirmer test in dry eye
and normal subjects: Open versus closed eye and 1-minute versus
5-minute tests. Cornea. 29:384–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lemp MA: Advances in understanding and
managing dry eye disease. Am J Ophthalmol. 146:350–356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Wood Gibson W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altinors DD, Bozbeyoglu S, Karabay G and
Akova YA: Evaluation of ocular surface changes in a rabbit dry eye
model using a modified impression cytology technique. Curr Eye Res.
32:301–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCarty CA, Bansal AK, Livingston PM,
Stanislavsky YL and Taylor HR: The epidemiology of dry eye in
Melbourne, Australia. Ophthalmology. 105:1114–1119. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl isothiocyanate (BITC) inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uchino M and Schaumberg DA: Dry eye
disease: Impact on quality of life and vision. Curr Ophthalmol Rep.
1:51–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhavsar AS, Bhavsar SG and Jain SM: A
review on recent advances in dry eye: Pathogenesis and management.
Oman J Ophthalmol. 4:50–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gayton JL: Etiology, prevalence, and
treatment of dry eye disease. Clin Ophthalmol. 3:405–412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson EJ, Chung HY, Caldarella SM and
Snodderly DM: The influence of supplemental lutein and
docosahexaenoic acid on serum, lipoproteins, and macular
pigmentation. Am J Clin Nutr. 87:1521–1529. 2008.PubMed/NCBI
|
|
36
|
Messmer EM: The pathophysiology,
diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int.
112:71–81. 2015.PubMed/NCBI
|
|
37
|
Stevenson W, Chauhan SK and Dana R: Dry
eye disease: An immune-mediated ocular surface disorder. Arch
Ophthalmol. 130:90–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng Q, Ren Y, Reinach PS, She Y, Xiao B,
Hua S, Qu J and Chen W: Reactive oxygen species activated NLRP3
inflammasomes prime environment-induced murine dry eye. Exp Eye
Res. 125:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Begley C, Chen M, Bradley A,
Bonanno J, McNamara NA, Nelson JD and Simpson T: A link between
tear instability and hyperosmolarity in dry eye. Invest Ophthalmol
Vis Sci. 50:3671–3679. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng R, Hua X, Li J, Chi W, Zhang Z, Lu F,
Zhang L, Pflugfelder SC and Li DQ: Oxidative stress markers induced
by hyperosmolarity in primary human corneal epithelial cells. PLoS
One. 10:e01265612015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao B, Wang Y, Reinach PS, Ren Y, Li J,
Hua S, Lu H and Chen W: Dynamic ocular surface and lacrimal gland
changes induced in experimental murine dry eye. PLoS One.
10:e01153332015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karn PR, Kim HD, Kang H, Sun BK, Jin SE
and Hwang SJ: Supercritical fluid-mediated liposomes containing
cyclosporin A for the treatment of dry eye syndrome in a rabbit
model: Comparative study with the conventional cyclosporin A
emulsion. Int J Nanomedicine. 9:3791–3800. 2014.PubMed/NCBI
|
|
43
|
Donnenfeld E and Pflugfelder SC: Topical
ophthalmic cyclosporine: Pharmacology and clinical uses. Surv
Ophthalmol. 54:321–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ciarcia R, Damiano S, Florio A, Spagnuolo
M, Zacchia E, Squillacioti C, Mirabella N, Florio S, Pagnini U,
Garofano T, et al: The protective effect of apocynin on
cyclosporine a-induced hypertension and nephrotoxicity in rats. J
Cell Biochem. 116:1848–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
De Paiva CS, Corrales RM, Villarreal AL,
Farley WJ, Li DQ, Stern ME and Pflugfelder SC: Corticosteroid and
doxycycline suppress MMP-9 and inflammatory cytokine expression,
MAPK activation in the corneal epithelium in experimental dry eye.
Exp Eye Res. 83:526–535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mocan A, Vlase L, Vodnar DC, Bischin C,
Hanganu D, Gheldiu AM, Oprean R, Silaghi-Dumitrescu R and Crișan G:
Polyphenolic content, antioxidant and antimicrobial activities of
Lycium barbarum L. and Lycium chinense Mill. leaves.
Molecules. 19:10056–10073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Uchino Y, Kawakita T, Miyazawa M, Ishii T,
Onouchi H, Yasuda K, Ogawa Y, Shimmura S, Ishii N and Tsubota K:
Oxidative stress induced inflammation initiates functional decline
of tear production. PLoS One. 7:e458052012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Drouault-Holowacz S, Bieuvelet S, Burckel
A, Rigal D, Dubray C, Lichon JL, Bringer P, Pilon F and
Chiambaretta FR: Antioxidants intake and dry eye syndrome: A
crossover, placebo-controlled, randomized trial. Eur J Ophthalmol.
19:337–342. 2009.PubMed/NCBI
|
|
49
|
Blades KJ, Patel S and Aidoo KE: Oral
antioxidant therapy for marginal dry eye. Eur J Clin Nutr.
55:589–597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Higuchi A, Inoue H, Kawakita T, Ogishima T
and Tsubota K: Selenium compound protects corneal epithelium
against oxidative stress. PLoS One. 7:e456122012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang JH, Wang HZ, Zhang M and Zhang SH:
Effect of anti-aging Lycium barbarum polysaccharide. Acta
Nutrimenta Sinica. 24:189–191. 2002.(In Chinese).
|
|
52
|
Petrov A, Perekhvatova N, Skulachev M,
Stein L and Ousler G: SkQ1 ophthalmic solution for dry eye
treatment: Results of a phase 2 safety and efficacy clinical study
in the environment and during challenge in the controlled adverse
environment model. Adv Ther. 33:96–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim YG, Lim HH, Lee SH, Shin MS, Kim CJ
and Yang HJ: Betaine inhibits vascularization via suppression of
Akt in the retinas of streptozotocin-induced hyperglycemic rats.
Mol Med Rep. 12:1639–1644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li JM, Ge CX, Xu MX, Wang W, Yu R, Fan CY
and Kong LD: Betaine recovers hypothalamic neural injury by
inhibiting astrogliosis and inflammation in fructose-fed rats. Mol
Nutr Food Res. 59:189–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hua X, Su Z, Deng R, Lin J, Li DQ and
Pflugfelder SC: Effects of L-carnitine, erythritol and betaine on
pro-inflammatory markers in primary human corneal epithelial cells
exposed to hyperosmotic stress. Curr Eye Res. 40:657–667. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schwab U, Törrönen A, Toppinen L, Alfthan
G, Saarinen M, Aro A and Uusitupa M: Betaine supplementation
decreases plasma homocysteine concentrations but does not affect
body weight, body composition, or resting energy expenditure in
human subjects. Am J Clin Nutr. 76:961–967. 2002.PubMed/NCBI
|
|
57
|
Garrett Q, Khandekar N, Shih S, Flanagan
JL, Simmons P, Vehige J and Willcox MD: Betaine stabilizes cell
volume and protects against apoptosis in human corneal epithelial
cells under hyperosmotic stress. Exp Eye Res. 108:33–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Amagase H: General toxicity and
histological analysis from acute toxicological study of a
standardized Lycium barbarum (Goji) juice (GoChiTM) in
rodents. Faseb J. 22:S7222008.
|