Introduction
The stria vascularis maintains blood flow stability
in the inner ear and also the balanced state of the endocochlear
potential (endolymph positive potential), ion transport and
endolymph (1,2). Within the microcirculation of the
inner ear, the spiral artery of the cochlea has been extensively
investigated (3–11). In addition, the morphological
characteristics of the pericytes of the stria vascularis have also
been widely explored (12,13). However, further research
investigating potassium channels (K+ channels) in the
capillary membranes of pericytes within the intrastria space (IS)
of the corpus striatum in the stria vascularis is required. These
channels may be closely associated with the formation of
endocochlear potential, particularly electrophysiological
properties.
The high potassium and endocochlear potentials in
the inner lymphatic fluid are established by the vascular lines of
the lateral wall of the cochlea (1,2).
Following entry into hair cells, K+ exits across the
basolateral membrane via K+ channels and reaches the
lateral cochlear wall either by a perilymphatic route or via the
gap-junctional network comprising Deiters' cells and epithelial
cells on the basilar membrane (2).
K+ is subsequently transported across the lateral
cochlear wall and finally returned back to endolymph (1,2,12,13).
Aspirin (acetylsalicylic acid) is commonly used to
treat pain, fever and inflammation (14); however, it is associated with a
number of side effects, including tinnitus and hearing loss
(15). As such, the toxicity and
effects of acetylsalicylic acid on the auditory system have been
evaluated previously, though definitive conclusions have yet to be
drawn. For instance, aspirin is thought to cause cochlear damage
(16,17). Blood flow volume is reduced by
sodium salicylate, which influences the metabolism of calcium ions
in hair cells, and alters their form and kinetic characteristics
(16,17). Sodium salicylate also causes neural
damage of the spiral ganglion. Cellular damage to hair cells and
spiral ganglion as a result of the ototoxicity of aminosalicylic
acid has also been described; however, the effects of aspirin on
capillary pericytes in the stria vascularis have yet to be
reported. The potential ototoxicity of aminosalicylic acid in the
auditory system, in terms of the alterations in the high potassium
potential of the endolymph, is also unknown.
In the present study, the function and associated
mechanism of the effects of aspirin on the cochlear pericytes of
the stria vascularis were observed and recorded using a whole-cell
patch-clamp technique. The aim was to evaluate the capillary
pericytes in the stria vascularis in order to present experimental
evidence regarding the mechanisms associated with maintaining the
stability of the internal environment of the stria vascularis and
the function of the cochlea, and also to provide a novel
theoretical basis for the causes of aspirin toxicity.
Materials and methods
Animals
A total of 100 Dunkin-Hartley guinea pigs (weight,
250–350 g; Animal Testing Center of Xinjiang Medical University,
Xinjiang, China) were selected regardless of sex. The use of
animals in the present study was approved by the Committee of
Animal Experimental Ethics of The First Affiliated Hospital of
Medical College, Shihezi University (Shihezi, China) (animal use
certificate no. SCXK Xin 2003-0001). Guinea pigs were housed in
separate cages in a specific pathogen-free environment at 24±3°C
with a relative humidity of 40–70% under a 12 h light/dark cycle,
and were provided with free access to food and water. All protocols
were approved by the Institutional Animal Care and Use Committee
(IACUC) at the Medical College of Shihezi University and consistent
with the Guidelines for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (18).
Reagents and instruments
Collagenase, trypsin, tetraethylammonium (TEA),
4-aminopyridine (4-AP, cat. no. 275875), propidium iodide (PI),
Triton X-100, aspirin and iberiotoxin (IBTX, cat. no. 56718) were
all obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Antibodies against desmin (cat. no. ab32362; Abcam, Cambridge, MA,
USA) and α-smooth muscle actin (SMA, cat. no. 6198; Sigma-Aldrich;
Merck KGaA) were also used. The remaining reagents were of
analytical grade and were locally procured. An Axon 700B amplifier
(Axon Instruments; Molecular Devices, LLC, Sunnyvale, CA, USA) and
a laser scanning confocal microscope (Zeiss LSM 510; Zeiss AG,
Oberkochen, Germany) were also used in the following
experiments.
Immunofluorescence identification of
pericytes in the stria vascularis
Guinea pigs were randomly divided into the
experimental and control groups (n=5/group) in order to identify
the pericytes of the stria vascularis. The guinea pigs were
intramuscularly injected with 10% chloral hydrate (0.35 ml/100 g
body weight, solution of 10 g chloral hydrate and 100 ml of normal
saline) and then sacrificed by bloodletting under anesthesia. All
protocols were approved by the IACUC at the Medical College of
Shihezi University and consistent with the Guidelines for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (18). The
cochlea was removed and placed in 4% paraformaldehyde at 4°C for 4
h. The stria vascularis and the spiral ligament were removed from
the cochlea with self-made injector-bent needles under a dissecting
microscope. The stria vascularis was obtained by separating the
stria vascularis and spiral ligaments using microforceps, which
were then placed into Eppendorf (EP) tubes containing PBS solution
and processed by centrifugation at 111.8 × g for 6 min at room
temperature; the supernatant was then discarded. The specimens were
rinsed thrice, and the immunostaining blocking liquid (5% PBS) with
0.1% Triton X-100 was added to the stria vascularis. Each sample
was incubated for 1 h at room temperature and then rinsed with PBS.
Samples in the experimental group were treated with anti-desmin
diluents (1:100; Abcam) or anti-α-SMA (1:100; Sigma-Aldrich; Merck
KGaA), and the control group was treated with PBS only. The samples
were placed in a wet box at 4°C for 12 h. Rewarming was performed
the following day for 1 h at 37°C. The stria vascularis in the EP
tube was rinsed three times in PBS solution and the extra liquid
was absorbed with filter paper strips. The samples were treated
with secondary antibody (1:100; fluorescein isothiocyanate
(FITC)-labeled second antibody, ZF-0311; OriGene Technologies,
Inc., Beijing, China), placed in a wet box and further incubated
for 1 h at room temperature. Each sample was rinsed for 30 sec with
PBS at room temperature prior to staining with PI for 30 sec at
room temperature. The stria vascularis was rinsed three times with
PBS; during the first two washes, the tubes were centrifuged at
111.8 × g for 6 min at room temperature and the supernatant was
discarded. Finally, the stria vascularis and PBS were aspirated
with a 1 ml pipette, and then transferred onto microscope slides.
The extra PBS was absorbed with filter paper prior to sealing the
slide with 85% glycerinum for fluorescence quenching. The
fluorescence was observed and recorded with a laser scanning
confocal microscope (Zeiss LSM 510; Zeiss AG, Oberkochen,
Germany).
Preparation of single pericytes from
the cochlear stria vascularis
As aforementioned, guinea pigs in the experimental
and control groups were given intramuscular injections of 10%
chloral hydrate (10 g of chloral hydrate in 100 ml of normal
saline) and sacrificed by bloodletting under anesthesia. The
cochlea was immediately removed and placed in a low-Ca2+
buffer solution containing: 142 mM NaCl, 5 mM KCl, 0.05 mM
CaCl2, 1 mM MgCl2, 5 mM Na-HEPES, 6 mM HEPES
(pH 7.2) and 7.5 mM glucose (pH was adjusted to 7.4). The stria
vascularis with spiral ligaments was removed from the cochlear
lateral wall under a dissecting microscope using self-made
injector-bent needles. The stria vascularis was obtained by
separating the stria vascularis and spiral ligament using
microforceps, and was then placed in a mixture containing digestive
enzymes including 0.1% collagenase and 0.1% trypsin for 10 min in a
water bath at 37°C. The sample was centrifuged at 111.8 × g at room
temperature for 6 min and the supernatant was discarded. A cell
suspension was manually cleaned in a Petri dish filled with an
aerated normal external solution composed of: 138 mM NaCl, 5 mM
KCl, 1.6 mM CaCl2, 1.2 mM MgCl2, 5 mM
Na-HEPES, 6 mM HEPES and 7.5 mM glucose (pH 7.4; osmolarity, 300
mOsm/l). The cell suspension was transferred into a clean and dry
Petri dish and left to stand for 30 min in the saline solution at
room temperature. The liquid in the Petri dish was substituted
twice with the saline solution to remove the other cells once the
pericytes became adherent. The whole-cell patch-clamp experiments
were then performed according to pericyte morphology following 10
min.
Whole-cell patch-clamp recording
The whole-cell patch-clamp experiment was conducted
at room temperature (20–25°C) with an Axon 700B amplifier. The
recorded diameter of the electrode tip was 1 µm, and the electrode
impedance was ~7-9 MW. The electrode contained 130 mmol/l
K-gluconate, 10 mmol/l NaCl, 2.0 mmol/l CaCl2, 1.2
mmol/l MgCl2, 10 mmol/l HEPES, 5 mmol/l EGTA and 7.5
mmol/l glucose; the pH was adjusted to 7.2, with an osmotic
pressure of 290 mOsm/l. Sealing-in was obtained with negative
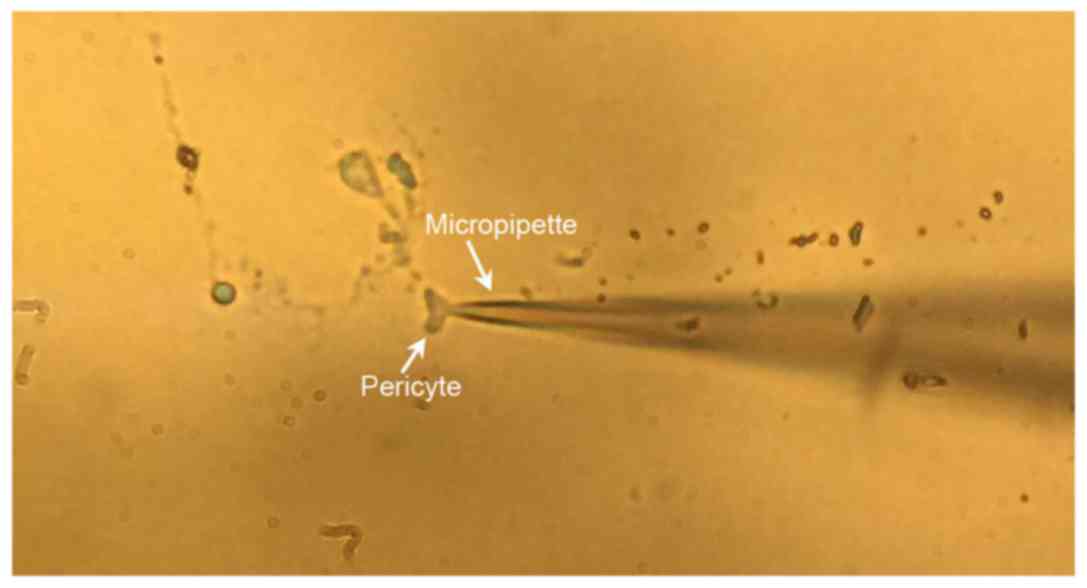
pressure once the micromanipulator came into contact with the cell;
the membrane was broken and a whole-cell patch-clamp was formed
with electrical stimulation or instant high negative pressure
following the compensation of capacitors and electrodes (Fig. 1). The compensation did not cover
the membrane capacitance current due to the online monitoring of
membrane parameters, as well as the calculation of membrane
capacitance (Cinput) and membrane resistance
(Rinput). Cinput was acquired with the
following formula: Cinput = Q/V, where Q, the electric
quantity, was acquired from the membrane capacitance
charge-discharge initiated by V, the command current. When
observing the differences in the outward current following the
application of 1 mmol/l TEA and 1 mmol/l 4-AP, the discontinuous
square-wave pulse stimulation proceeded as follows: Patch-clamp
potential of −40 mV, readings were recorded from −80 mV step
stimulation depolarization to +60 mV; time of 150 msec; sampling
interval of 10, 20 and 100 µsec; and the membrane current was
processed with 10 kHz (−3 dB) low-frequency filtration. The data
were analyzed with pClamp™ v10.2 software (Axon Instruments;
Molecular Devices, LLC).
Detection of the type of potassium
channel in the pericytes membrane
Step stimulation (from −80 mV to +60 mV, interval
was 20 mv) and ramp stimulation (from −80 mV to +60 mV; wave
width=150 ms) were applied. Clampfit v10.2 software (Molecular
Devices, LLC) was used to obtain the current-voltage (I/V)
curve.
The effect of aspirin on the outward
current of pericytes
Following the rupture of the membrane formed the
whole-cell configuration, 3, 10, 30, 300, 1,000 µmol/l aspirin were
successively injected in to each cell. Using Clampfit v10.2
software (Molecular Devices, LLC), the I/V curve of the net current
of the diastolic blood vessel was obtained, and the ion mechanism
of the outgoing current induced by aspirin was analyzed according
to the I/V curve.
Determination of the type of channel
that mediates the outward current that is inhibited by aspirin
Following the rupture of the membrane formed the
whole-cell configuration; aspirin (300 µmol/l), IBTX (1 nmol/l) +
4-AP (300 µmol/l), Aspirin (300 µmol/l) + IBTX (1 nmol/l) + 4-AP
(300 µmol/l) were successively injected in to each cell.
Immunofluorescence identification was conducted once
the whole-cell patch-clamp experiments were performed to ensure
that the experimental objects were the pericytes of the stria
vascularis. Then, the identified pericyte of the experimental and
control groups was gently sucked into a glass pipette (tip, ~40
µm), and transferred onto a gelatine-smeared slide. Then, the cells
were immobilized in acetone at 4°C for 15 min, prior to washing
thrice with PBS (pH 7.4), for 5 min per wash. The immunostaining
blocking liquid (5% PBS) with 0.1% Triton X-100 was applied for 1 h
incubation at room temperature. The anti-desmin diluent (1:100,
rabbit anti-guinea pig monoclonal antibody; Abcam) was applied
prior to overnight incubation at 4°C. Following 1 h of rewarming at
37°C, the stria vascularis was rinsed thrice with PBS solution (5
min for each rinse), and excess liquid was absorbed using filter
paper. The sample was treated with the secondary antibody (1:100,
FITC-labeled second antibody, ZF-0311; OriGene Technologies, Inc.)
and incubated in a wet box for 1 h at room temperature, rinsed with
PBS and stained with PI for 30 sec at room temperature. The excess
fluid at the edge of the sample was absorbed with filter paper
following each rinse. The slide was sealed with 85% glycerinum to
prevent fluorescence quenching. Fluorescence was observed and
recorded with a laser scanning confocal microscope (FITC and PI
staining was detected at 488 and 536 nm, respectively; Zeiss LSM
510; Zeiss AG).
Data analysis
Current intensity was measured as the difference
between the initial current and the maximum activating current
during activation. Current density was determined as the ratio
between current density and membrane capacitance (pA/pF). Data were
analyzed using KaleidaGraph v3.6 (Synergy Software, Inc., Reading,
PA, USA), FreeHand MX11, Adobe Photoshop CS5 (both from Adobe
Systems, Inc., San Jose, CA, USA), and simplified and localized CAD
software (Autodesk, Inc., San Rafael, CA, USA).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean (n=6). Statistical analysis was performed using
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). A
homogeneity test for variance was performed followed by one-way
analysis of variance, and two-group comparisons were conducted
using the least significant difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
KaleidaGraph v3.6 (Synergy Software, Inc.) and Adobe Photoshop CS5
(Adobe Systems Europe, Ltd., Maidenhead, UK) were used for graphics
processing.
Results
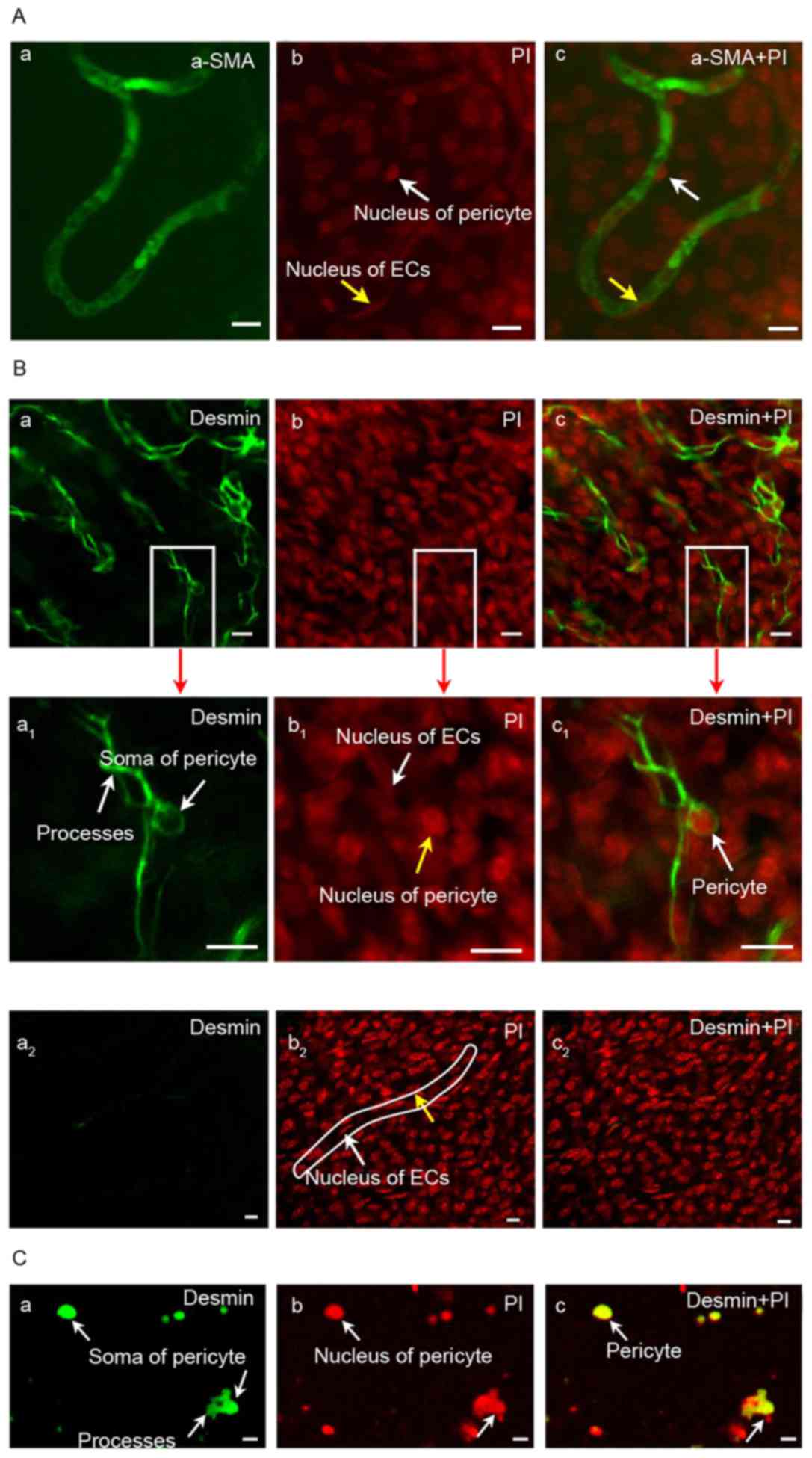
Identification of pericytes with the
marker protein desmin
As shown in Fig.
2A, the expression of α-SMA in guinea pig cochlear pericytes
from the stria vascularis was negative; however, nonspecific
staining of blood vessels was observed. The expression of desmin
was positive based on the results of microscopy; enlarged pericytes
and large nuclei were observed. Pericytes were detected in the
negative control (Fig. 2B and C).
The nuclei of pericytes were large and surrounded by less
cytoplasm; the cytoplasmic processes gradually became smaller as
branches grew parallel with the capillary axis, thereby surrounding
the capillaries with its tips (Fig.
2B). This result demonstrated that the identification of
pericytes with marker protein desmin may be feasible.
Identification experiments were also conducted following the
whole-cell patch-clamp experiment to ensure that the experimental
objects were the pericyte.
Electrophysiological properties of
guinea pig cochlear pericytes
The electrophysiological properties of single
pericytes were recorded using the whole-cell patch-clamp recording
technique. The membrane capacitance was 5.9±0.3 pF, the membrane
resistance was 2.18±0.3 GW and the membrane resting potential was
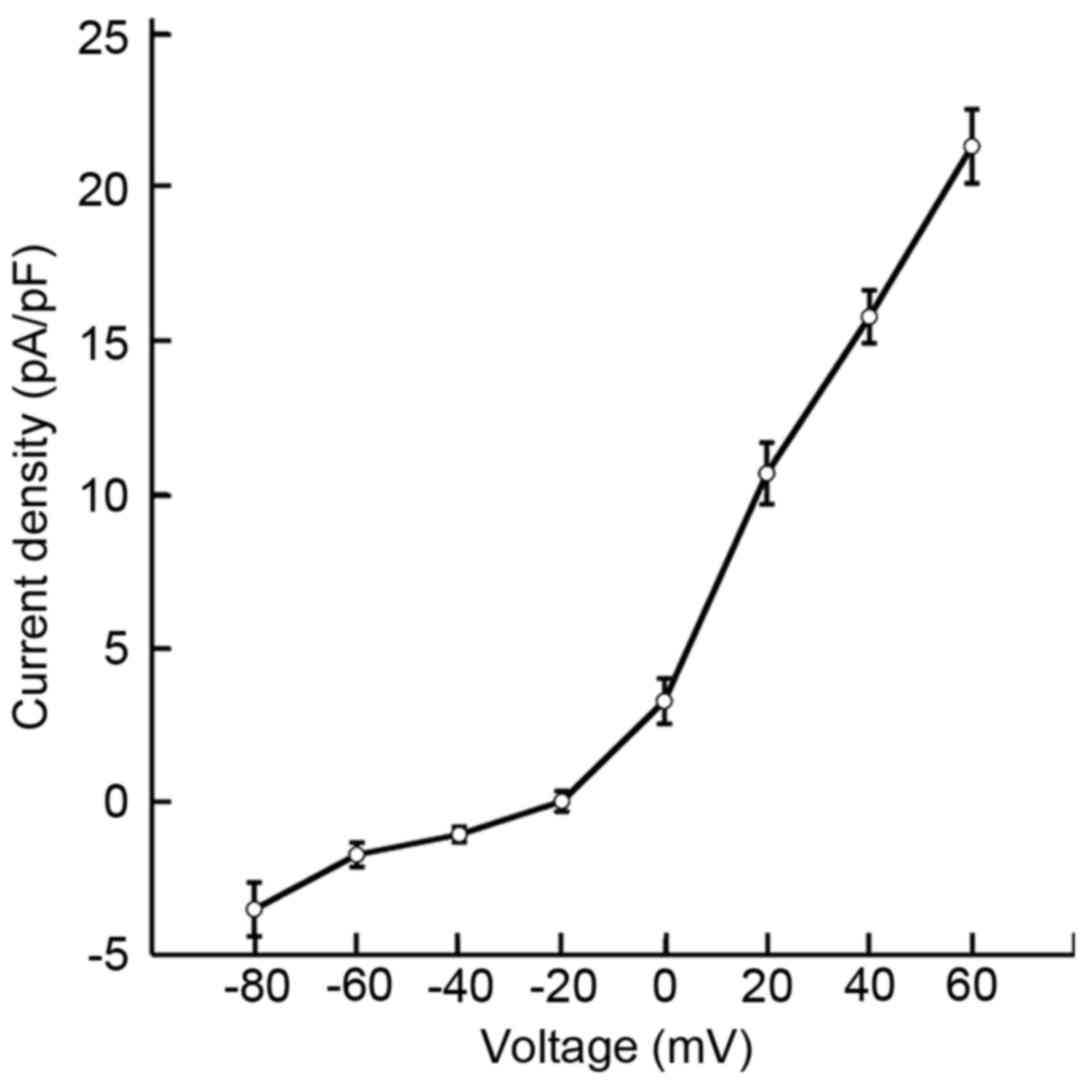
−30.9±1.2 mV. Fig. 3 reveals the
current densities of single pericytes. At command voltages of 0,
+20, +40 and +60 mV, the pericyte current densities were 3.2±0.7,
10.6±0.9, 15.7±0.8 and 21.2±1.2 pA/pF, respectively. These results
indicated that the current density of cochlear pericytes may be
voltage dependent.
Membranes of pericytes in the stria
vascularis contain high-conductance calcium-activated K+
(BKCa) and voltage-dependent K+
(KV) channels
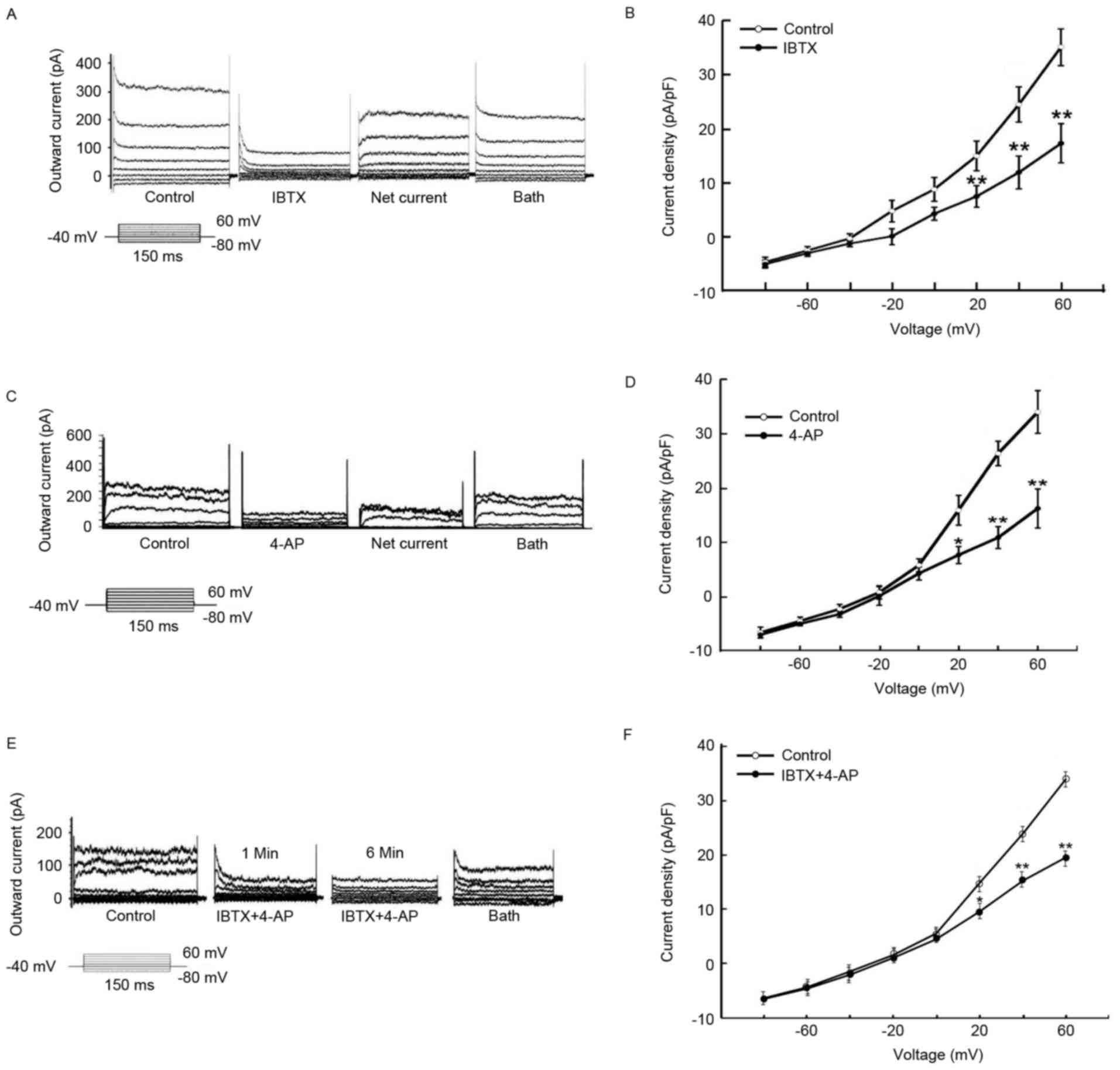
With the step stimulation of −80 mV to +60 mV, the
single pericyte current demonstrated marked outwardly rectifying
characteristics (Fig. 4A, C and
E). The outward current of the pericytes treated with the
K+ channel retardant became sensitive to the specific
retardant IBTX (1 nmol/l) in BKCa channels (Fig. 4A and B). The inhibiting current
exhibits a typical graph of voltage-dependent KV, as
displayed in Fig. 4A. At 1 mmol/l
4-AP (specific retardant of KV channels), the outward
current of the pericytes was partially inhibited compared with in
the control (Fig. 4C and D). At 1
mmol/l 4-AP and 1 nmol/l IBTX, the outward current of pericytes was
also inhibited (Fig. 4E and F).
These results demonstrated that the outward current of pericytes
may be initiated by activating BKCa and KV
channels.
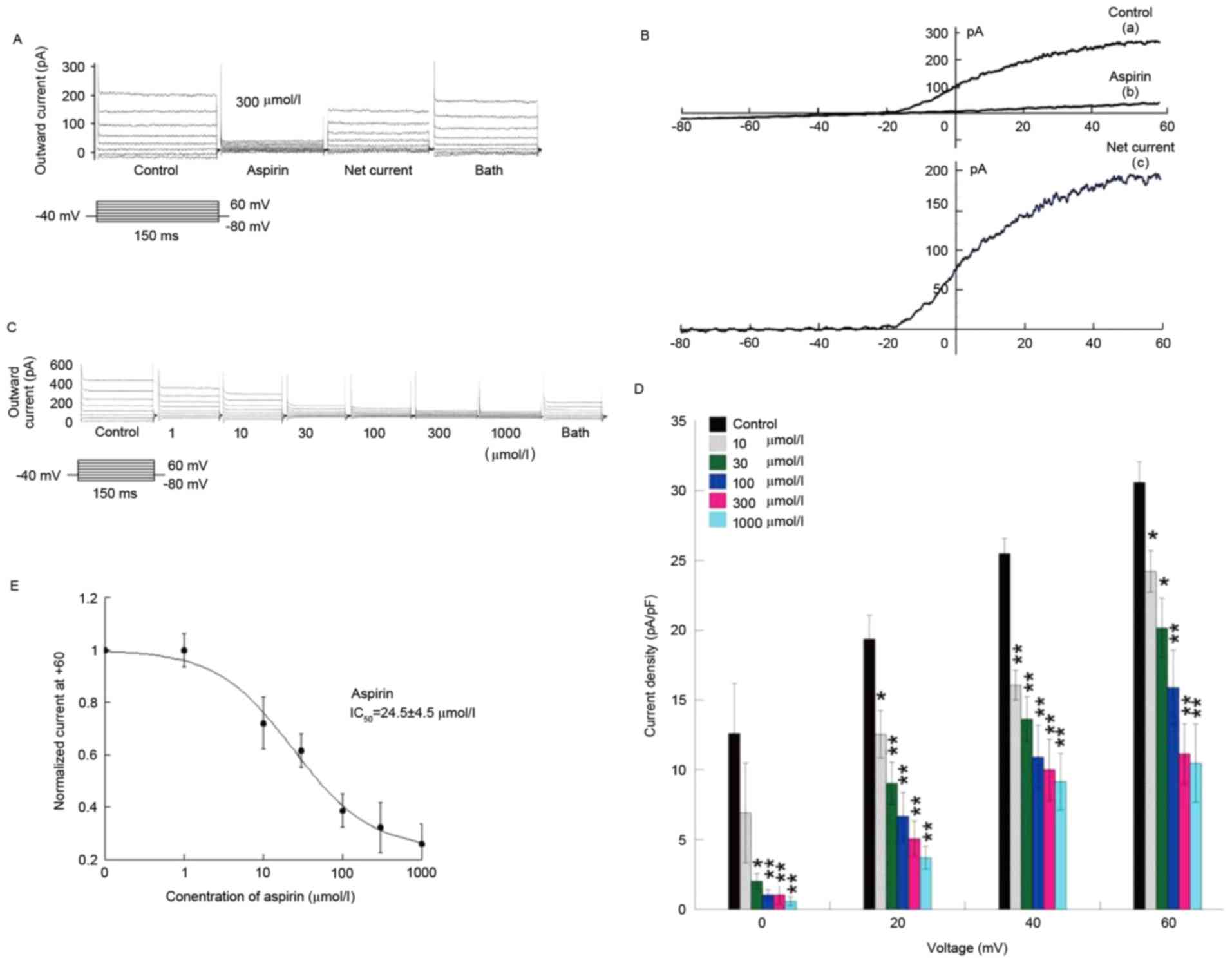
Inhibition of the outward current by
aspirin is concentration-dependent in guinea pig cochlear pericytes
from the stria vascularis
The inhibition of the outward current by aspirin in
the cochlear pericytes of the stria vascularis was marked and
concentration dependent (Fig. 5).
Aspirin exerted weak effects on the membrane current within the
voltage range of −80-0 mV in the pericyte I/V curve;
the drug primarily inhibited the activated current within the
voltage range of 0–60 mV (Fig.
5B).
With the perfusion of aspirin at different
concentrations in the experiment, the effect of aspirin was
concentration dependent for the pericyte outward current of stria
vascularis capillaries. Following removal of the background leakage
current, treatment with 3, 10, 30, 300 and 1,000 µmol/l aspirin
yielded the following inhibition ratios: 20.8±4.8, 34.1±6.9,
48.2±6.7, 63.6±7.1 and 65.7±8.1%, respectively (Fig. 5C and D). Under conditions with
background leakage current, the half maximal inhibitory
concentration (IC50) was 24.5±4.5 µmol/l in terms of the
inhibition of the outward current of cochlea stria vascularis
pericyte with aspirin (Fig. 5E).
These results revealed that inhibition of the outward current of
cochlea stria vascularis pericyte with aspirin is concentration
dependent.
BKCa and Kv
channels are inhibited by aspirin
To understand the type of mediation on the outward
current produced by aspirin, the following treatments were perfused
at different time intervals in the experiment: 300 µmol/l aspirin,
1 nmol/l IBTX, or 300 µmol/l aspirin + 1 nmol/l IBTX (Fig. 6A and B); 300 µmol/l aspirin, 1
mmol/l 4-AP, or 300 µmol/l aspirin + 1 mmol/l 4-AP (Fig. 6C and D); 300 µmol/l aspirin, 1
nmol/l IBTX + 1 mmol/l 4-AP, or 300 µmol/l aspirin + 1 nmol/l IBTX
+ 1 mmol/l 4-AP (Fig. 6E and F).
Following flushing of the extracellular fluid, the current returned
to almost the original levels; however, the current reduced again
following treatment with 1 nmol/l IBTX. After 5 min, aspirin (300
µmol/l) + IBTX (1 nmol/l) were injected in the same cell and the
current reduced further. The current recovered after flushing.
These results indicated that the outward current may be inhibited
by aspirin, and mediated by the BKCa and KV
channels.
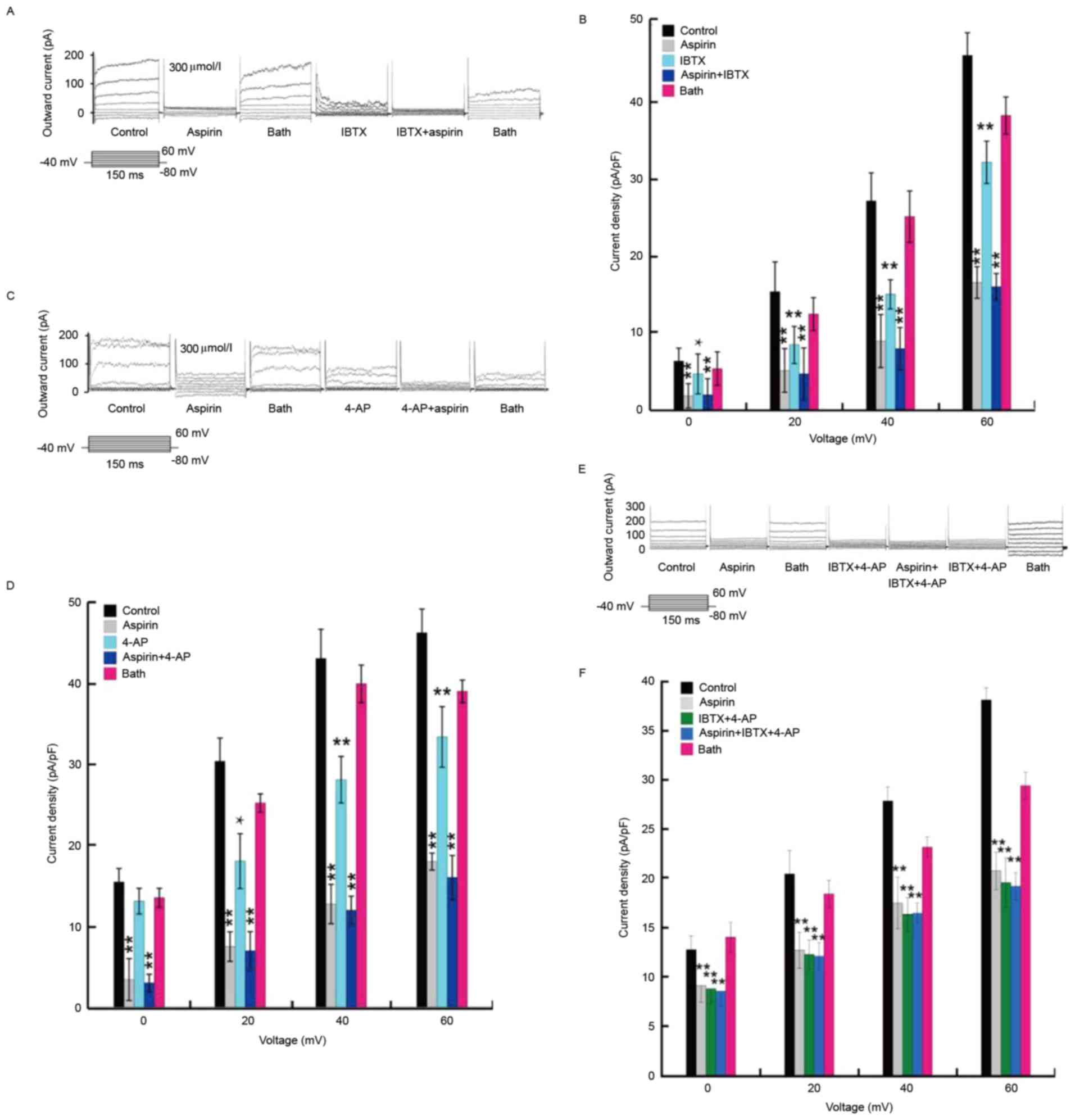 | Figure 6.BKCa and Kv
channels can be inhibited by aspirin. (A) Whole-cell current
initiated by step stimulation showing the influence of 300 µmol/l
aspirin, 1 nmol/l IBTX, and 300 µmol/l aspirin + 1 nmol/l IBTX, on
the outward current. (B) Pericyte current density following the
application of 300 µmol/l aspirin, 1 nmol/l IBTX, and 1 nmol/l IBTX
+ 300 µmol/l aspirin. (C) Whole-cell current initiated by step
stimulation showing the influence of 300 µmol/l aspirin, 1 mmol/l
4-AP, and 300 µmol/l aspirin + 1 mmol/l 4-AP on the outward
current. (D) Pericyte current density following the application of
300 µmol/l aspirin, 1 mmol/l 4-AP, and 300 µmol/l aspirin + 1
mmol/l 4-AP. (E) Whole-cell current initiated by step stimulation
showing the influence of IBTX + 1 mmol/l 4-AP, 300 µmol/l aspirin,
and IBTX + 1 mmol/l 4-AP + 300 µmol/l aspirin on the outward
current. (F) Pericyte current density following the application of
IBTX + 1 mmol/l 4-AP, 300 µmol/l aspirin and IBTX + 1 mmol/l 4-AP +
300 µmol/l aspirin. Data are presented as the mean ± standard error
of the mean of 6 independent experiments. *P<0.05 and
**P<0.01 vs. control. IBTX, iberiotoxin; 4-AP, 4-aminopyridine;
BKCa, high-conductance calcium-activated K+
channels; KV, voltage-dependent K+
channels. |
Discussion
It is generally believed that pericytes and smooth
muscle cells serve similar roles in adjusting blood flow volume and
vascular permeability (19–21).
Nicosia and Villaschi (19)
observed that pericytes in mice can be differentiated from the
intima of the mesentery artery and the vascular smooth muscle cell
subgroup below the intima during vasculogenesis in vitro.
Another previous study observed myogenic phenotypes when pericytes
were cultured in vitro (20). Pericytes can be distinguished from
smooth muscle cells under certain conditions (20). Comparisons between the results of
the present study and those of a previous study (21), in regard to membrane resistance,
membrane conductance and current density of the single smooth
muscle cells of the cochlear spiral artery, showed no significant
differences between the pericytes and smooth muscle cells in terms
of their electrophysiological properties. Therefore, pericytes and
smooth muscle cells may have similar electrophysiological
properties.
Aspirin is a widely used medicine, and its main side
effects include tinnitus and hearing loss. Considerable research
has been conducted on the toxicity and side effects of
acetylsalicylic acid to the auditory system (21,22).
A previous study in mice demonstrated that sodium salicylate
inhibits the KV channels of cochlear spiral ganglion
cells (SGNs) in a concentration-dependent manner, with an
IC50 of ~1.15 mmol/l (22). Sodium salicylate inhibits the
outward rectification of the potassium current in cochlear SGNs and
the tail current, as the inactivation of the tail current is
accelerated; these effects lead to the abnormal
electrophysiological activities of cochlear SGNs, thereby damaging
the auditory system (22). Sodium
salicylate inhibits the voltage-gated calcium channel current in a
concentration-dependent manner. The IC50 value of sodium
salicylate to hippocampal neurons in the voltage-gated
Ca2+ channel current was 1.64 mmol/l (21). Sodium salicylate has also been
observed to inhibit the K+ channels of temple cortex
neurons in a concentration-dependent manner (0.1–10 mmol/l) in mice
(22); the IC50 value
of sodium salicylate inhibition on the K+ channels was
2.13 mmol/l (22). This experiment
indicates that aspirin at 1–1,000 µmol/l can inhibit pericyte
BKCa and KV channels depending on its
concentration; the greater the concentration, the greater the
inhibition (22). At a
concentration of 300 and 1,000 M, the inhibitory effect of the
outward current on the cochlear pericytes was not marked in the
present; however, the inhibition rate of aspirin increased with the
increase in concentration. This observation demonstrated the
effects of various blood concentrations on hearing. According to
the results of the present, the IC50 value of aspirin
for inhibiting the outward current of cochlear pericytes of the
stria vascularis was 24.5±4.5 µmol/l.
Pericytes serve an important role in adjusting blood
flow volume and vascular permeability (12). The results of the present study
indicated that the ototoxicity of aspirin may depend on two
aspects. Firstly, pericytes lose their timely repolarization once
the outward current of pericytes is inhibited by aspirin, which
affects the blood flow volume of the inner ear as the capillary
network contracts in the stria vascularis. Secondly, the
aspirin-induced inhibition of the outward current of pericytes
affects the K+ concentration and potential in the stria
vascularis. Therefore, two conditions are required to activate the
Na+/K+-ATP enzyme of border cells: i) An
adequate ATP supply; and ii) a suitable K+ concentration
and potential in the stria vascularis. However, aspirin influences
these conditions. Therefore, this drug may affect the normal
auditory system by terminating K+ transport from the
stria vascularis to border cells via the
Na+/K+-ATP enzyme, or by preventing
K+ from entering the endolymph via K+
channels in the membranes of border cells. The results of the
present study enhanced the current understanding of the
electrophysiological properties of pericytes, and the toxic and
general side effects of aspirin on the auditory system. Applying
the technologies of immunohistochemistry, western blotting and
RT-qPCR technology, detection of aspirin for the pericytes of the
stria vascularis, the effects of potassium ion channel expression
may provide a theoretical basis for the prevention of aspirin
ototoxicity.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81560175 and
81260159) and the High Level Talent Research Project of Shihezi
University (grant no. RCSX201705).
References
|
1
|
Arai S, Kinouchi H, Akabane A, Owada Y,
Kamii H, Kawase M and Yoshimoto T: Induction of brain-derived
neurotrophic factor (BDNF) and the receptor trk B mRNA following
middle cerebral artery occlusion in rat. Neurosci Lett. 211:57–60.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uetsuka S, Ogata G, Nagamori S, Isozumi N,
Nin F, Yoshida T, Komune S, Kitahara T, Kikkawa Y, Inohara H, et
al: Molecular architecture of the stria vascularis membrane
transport system, which is essential for physiological functions of
the mammalian cochlea. Eur J Neurosci. 42:1984–2002. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Ma KT, Zhao L, Si JQ, Zhang ZS, Zhu
H and Li J: Niflumic acid hyperpolarizes smooth muscle cells via
calcium-activated potassium channel in spiral modiolar artery of
guinea pigs. Acta Pharmacol Sin. 29:789–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li L, Ma KT, Zhao L, Shi WY, Li XZ, Zhang
ZS and Si JQ: The characteristics of resting membrane potential on
smooth muscle cells and endothelial cells in guinea pigs cochlea
spiral artery. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 28:128–132.
2012.(In Chinese). PubMed/NCBI
|
|
5
|
Li L, Ma KT, Zhao L and Si JQ: Niflumic
acid hyperpolarizes the smooth muscle cells by opening BK(Ca)
channels through ryanodine-sensitive Ca(2+) release in spiral
modiolar artery. Sheng Li Xue Bao. 60:743–750. 2008.PubMed/NCBI
|
|
6
|
Ma KT, Li XZ, Li L, Zhang ZP, Zhao L, Zhu
H and Si JQ: Comparison of electrophysiological properties of
vascular smooth muscle cells in different arterioles in guinea pig.
Sheng Li Xue Bao. 62:421–426. 2010.(In Chinese). PubMed/NCBI
|
|
7
|
Wang YZ, Liu ZJ, Li L, Fan P, Si JQ, Zhao
L, Ma KT, Zhu L and Gao WJ: Effects of chloride channel blockers on
excitatory junction potentials in smooth muscle cells of cochlear
spiral modiolar artery in guinea pigs. Sheng Li Xue Bao.
58:456–462. 2006.(In Chinese). PubMed/NCBI
|
|
8
|
Zhang ZP, Li XZ, Si JQ, et al: Effects of
acute hypoxia on electrophysiological properties of VSMCs in
guinea-pig spiral modiolar artery. Chin J Mod Med. 21:3979–3983.
2011.(In Chinese).
|
|
9
|
Fetoni AR, Ferraresi A, Picciotti P,
Gaetani E, Paludetti G and Troiani D: Noise induced hearing loss
and vestibular dysfunction in the guinea pig. Int J Audiol.
48:804–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spicer SS and Schulte BA: Pathologic
changes of presbycusis begin in secondary processes and spread to
primary processes of strial marginal cells. Hear Res. 205:225–240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fetoni AR, Picciotti PM, Paludetti G and
Troiani D: Pathogenesis of presbycusis in animal models: A review.
Exp Gerontol. 46:413–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai M, Nuttall A, Yang Y and Shi X:
Visualization and contractile activity of cochlear pericytes in the
capillaries of the spiral ligament. Hear Res. 254:100–107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi X: Physiopathology of the cochlear
microcirculation. Hear Res. 282:10–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki Y, Inoue T and Ra C: NSAIDs,
mitochondria and calcium signaling: Special focus on
aspirin/salicylates. Pharmaceuticals (Basel). 3:1594–1613. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheppard A, Hayes SH, Chen GD, Ralli M and
Salvi R: Review of salicylate-induced hearing loss, neurotoxicity,
tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital.
34:79–93. 2014.PubMed/NCBI
|
|
16
|
Swanson RA, Morton MT, Tsao-Wu G, Savalos
RA, Davidson C and Sharp FR: A semiautomated method for measuring
brain infarct volume. J Cereb Blood Flow Metab. 10:290–293. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas WE: Brain macrophages: On the role
of pericytes and perivascular cells. Brain Res Brain Res Rev.
31:42–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
U.S. National Institutes of Health, .
Laboratory animal welfare: Public health service policy on humane
care and use of laboratory animals by awardee institutions; notice.
Fed Regist. 50:19584–19585. 1985.PubMed/NCBI
|
|
19
|
Nicosia RF and Villaschi S: Rat aortic
smooth muscle cells become pericytes during angiogenesis in vitro.
Lab Invest. 73:658–666. 1995.PubMed/NCBI
|
|
20
|
Ma KT, Li XZ, Li L, Zhang ZP, Zhao L, Zhu
H and Si JQ: Comparison of electrophysiological properties of
vascular smooth muscle cells in different arterioles in guinea pig.
Sheng Li Xue Bao. 62:421–426. 2010.(In Chinese). PubMed/NCBI
|
|
21
|
Zhu XL, Liu YX, Zhong BG, Ma HF, Jiang P
and Zhang XY: Effects of salicylate on voltage-gated calcium
channels in rat hippocampal neurons. Chin Pharmacol Bull. 1–1270.
2014.
|
|
22
|
Liu YX, et al: Inhibition of sodium
salicylate on delayed rectifier potassium channels in rat auditory
cortex neurons. Chin Pharmacol Bull. 4:482–486. 2011.(In
Chinese).
|