Introduction
The heterodimeric cytokine of interleukin (IL)-35,
as a newly identified member of the IL-12 family, is likely to
contribute to the development of autoimmune diseases. It is formed
by Epstein-Barr virus induced 3 (EBI3) associated with IL-12p35.
IL-35 was demonstrated to be expressed in mouse
CD+CD25+Foxp3+ regulatory T cells
(Tregs) (1) and was then
demonstrated to be expressed by naturally occurring
CD4+CD25+ Tregs isolated from human
peripheral blood mononuclear cells instead of
CD4+CD25−conventional responder T cells
(Tres) (2). It can downregulate
the development of T helper type 17 (Th17) cells and then inhibit
autoimmune inflammation in other autoimmune diseases (2,3) and
may occur in type 1 cytokine [interferon-γ (IFN-γ), tumor necrosis
factor-a (TNF-α) and IL-2 mediated]/type 17 (IL-17A and IL-17F
mediated) and type 2 [IL-4, transforming growth factor (TGF)-β
mediated] immune-inflammatory diseases.
Bone marrow mononuclear cells (BMMNC)-Coombs
test-positive hemocytopenia, also termed immunorelated
hemocytopenia (IRH) is a type of hemocytopenia exhibiting these
following features: i) Hemocytopenia or pancytopenia with a normal
or higher percentage of reticulocytes and/or neutrophils; ii)
hyperplasia-bone marrow (BM) with a higher percentage of nucleated
erythroid cells in sternum, with erythroblastic islands (EIs) that
were easily observed; iii) good responses to corticosteroids or
high-dose intravenous immunoglobulin (IVIg); iv) exclusion of other
primary and secondary hemocytopenia disorders; and v) positive
result by BMMNC-Coombs test (4,5).
This disease is an immuno-associated hemocytopenia and the
production of the autoantibodies may be due to the abnormal number
and function of B lymphocytes (6),
regulatory T cells (7) and Th17
(8).
To investigate its interrelationship and
immunopathological role in IRH, the present study determined the
level of IL-35 and its Treg cells in relation to the progression of
this disease. The present study detected the serum IL-17
concentration on this autoimmune disease to test the connection
between IL-35 and Th17 cells. Additionally, CD4+ T
lymphocytes were sorted to culture with IL-35 and determine its
effect on Th17 cell differentiation.
Patients and methods
Patients
A total of 43 (25 females and 18 males; median age,
36 years; range, 11–69 years) patients with IRH were enrolled in
the current study, including 18 untreated patients (11 females and
7 males; median age, 44.5 years; range, 16–68 years) and 25 in
remission (14 females and 11 males; median age, 31 years; range,
11–69 years). All were inpatients in the Department of Hematology,
Tianjin Medical University General Hospital (Tianjin, China)
between August 2015 and September 2016 and diagnosed according to
Fu (5). Patients were given
corticosteroids (prednisone, 0.5 mg/kg/day) and cyclosporine (CsA)
(3 mg/kg/day) as immunosuppressive therapy and some received
high-dose IVIg (0.4 g/kg/day for 5 days; Chengdu Institute of
Biological Products, Sichuan, China) if they depend on blood
transfusion. Complete blood count (CBC) and bone marrow (BM)
examination were performed regularly. The response criteria were
measured according to those of aplastic anemia (AA) (9) and the median follow-up time was 12
months (range, 3–21 months). A total of 19 healthy volunteers with
normal blood picture and immune parameters (9 females and 10 males;
median age, 32 years; range, 22–48 years) were selected as normal
controls. The current study was approved by the Ethical Committee
of the Tianjin Medical University and written informed consent was
obtained from the patients for the publication of the current
study.
Enzyme-linked immunosorbent assay
(ELISA)
Serum levels of IL-35 and IL-17 in patients with IRH
and normal control individuals were measured using ELISA reagent
kits (cat. nos. SEC008Hu and SEA063Hu; USCN LIFE, Wuhan, China)
according to the manufacturer's protocol. Diluted standards and
patient serum (100 µl) were added in duplicate and incubated at
37°C for 2 h. After washing the plate 5 times, 100 µl of antibody
was added to each well and incubated at room temperature for 90 min
and horseradish peroxidase was added to each well. Following
incubation at 37°C for 30 min, the wells were washed 5 times.
Subsequently, tetramethylbenzidine solution was added to each well
and the samples were incubated in the dark at room temperature for
20 min. Finally, a stop solution was added, and the optical density
was read at 450 nm within 15 min.
Purification of T lymphocyte subsets
using MACS micro bead technology
Using Ficoll-Hypaque density gradient centrifugation
to isolate peripheral blood mononuclear cells (PBMCs) from heparin
anticoagulant venous blood of IRH and normal controls, diluted
blood was diluted 1:1 in Ficoll-Hypaque fluid and then centrifuged
at 400 × g for 20 min at 4°C. The interface was collected and
washed with PBS at 300 × g for 10 min. CD4+ T
lymphocytes were purified using CD4+ T cell isolation
kit (130096533; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
according to the manufacturer's protocol. Every 107
PBMCs were resuspended in 40 µl of buffer. Then 10 µl CD4
Biotin-Antibody cocktail (Miltenyi Biotec GmbH) was added and
incubated at 4°C in the dark for 5 min. After that, 30 µl buffer
was added and 20 µl of Anti-Biotin micro beads. Finally, cells were
resuspended up in 500 µl of buffer. The LS column was placed in the
magnetic field of a suitable MACS separator (Miltenyi Biotec GmbH).
After preparing the column by rinsing with 3 ml buffer, the cells
were added into the column. The column was washed with 3 ml buffer
and all unlabeled CD4+ T cells that passed through were
collected. Some of the isolated CD4+ T cells were
resuspended in 90 µl per 107 cells and using anti-CD25
mAb-conjugated microbeads (130092983; Miltenyi Biotec GmbH) to
isolate the CD4+CD25+ Tregs and 10 µl CD25
MicroBeads were added in the sorting system. Following incubation
for an additional 15 min at 4°C in the dark, cells were washed by
adding 2 ml buffer and resuspended up to 500 µl every
108. The MS column was placed in the magnetic field of a
suitable MACS separator. After preparing the column by rinsing with
1.5 ml buffer, the cells were applied into the column. The column
was washed with 1.5 ml buffer and all flow-through containing
unlabeled cells was collected. Magnetically labeled cells were
immediately flushed out by firmly pushing the plunger into the
column and finally the CD4+CD25+ Tregs were
collected. Sorted collection was incubated with CD4-FITC
(130092358; 1:10) and CD25-APC (130092858; 1:10; Miltenyi Biotec
GmbH) in the dark for 5 min and mouse IgG1-FITC, IgG2b-APC
(130098847 and 130098890; 1:10; Miltenyi Biotec GmbH) were used to
stain the negative controls. Then the purity was tested by flow
cytometry (FCM).
Mixed-culture of IL-35 and
CD4+ T lymphocytes
CD4+ T lymphocytes from 5 untreated IRH
patients were seeded at 2×106 cell/ml in the 24 well
plates in cell medium RPMI 1640 supplemented (Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China) with 15% fetal
bovine serum (FBS). Each patient's CD4+ T lymphocytes
were divided into 3 groups and cultured with: i) Blank control,
group (a); ii) 1 µg/ml anti-CD3 MAbs (BioLegend, Inc., San Diego,
CA, USA), 10 µg/ml anti-CD28 MAbs (BioLegend, Inc.), 20 ng/ml TGF-β
(Peprotech, Rocky Hill, CT, USA), 50 ng/ml IL-6 (Peprotech) to
promote differentiation to Th17 cells, group (b); and iii) 1 µg/ml
anti-CD3 MAbs (BioLegend, Inc.), 10 µg/ml anti-CD28 MAbs
(Biolegeng), 20 ng/ml TGF-β (Peprotech), 50 ng/ml IL-6 (Peprotech),
50 ng/ml IL-35 (Peprotech) to find out whether IL-35 may inhibit
the differentiation of CD4+ T lymphocytes to Th17 cells,
group (c), then incubated at 37°C, 5% CO2 for 3
days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to investigate IL-35 mRNA in Tregs
and gp130, IL-12R-β2, RAR-related orphan receptor (ROR)γt and
IL-17a mRNA in mix-cultured CD4+ T lymphocytes. Total
RNA was isolated from Tregs/cultured CD4+ T lymphocytes
using TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian,
China) from 1×105 isolated cells. A total 1 µg RNA was
converted to cDNA using reverse transcription with PrimeScript RT
reagent kit at 37°C for 15 min then 5 sec at 85°C for 1 cycle
(Takara Biotechnology Co., Ltd.). qPCR was performed in a 25 µl
reaction volume containing 12.5 µl of SYBR-Green (Takara
Biotechnology Co., Ltd.). All primer sequences were presented in
Table I. The thermocycling profile
was as follows: 95°C 5 sec and 60°C 45 sec for 45 cycles. The
relative quantity of target mRNA expression was calculates using
the quantification cycle (Cq) method using the equation: Relative
quantity=2−∆∆Cq (10).
 | Table I.All primer sequences used in this
research. |
Table I.
All primer sequences used in this
research.
| Gene | Sense (5′-3′) | Anti-sense
(5′-3′) |
|---|
| Ebi3 |
TCCTTCATTGCCACGTACAG |
GCTCTGTTATGAAAGGCACG |
| IL-12p35 |
AAACCTCCCCGTGGCCACTCC |
GAAGCATTCAGATAGCTCATCACT |
| gp130 |
GCTGCGAGTTATTACTGGAG |
TTATCATGGCACTCTGTTGAG |
| IL-12R-β2 |
AAAGAGGACGAGACACCCAC |
GAAGAGGAGCTTCCAAGACT |
| RORγt |
CTCCATCTTTGACTTCTCCCACTCCCTA |
CACATGCTGGCTACACAGGCTC |
|
IL-17a |
CAGATTACTACAACCGAT |
CATGTGGTAGTCCACGTTCC |
| β-actin |
TGGACATCCGCAAAGACCTGT |
CACACGGAGTACTTGCGCTCA |
FCM
In order to determine the
CD4+CD25+Foxp3+ cell level, fresh
200 µl PB samples were lysed with hemolysin (BD Pharmingen,
Franklin Lakes, NJ, USA) and erythrocytes washed with PBS.
CD4-fluorescein isothiocyanate (FITC) and CD25-APC (1:20; cat. nos.
130092358 and 130092858; Miltenyi Biotec GmbH) were used as the
fluorophore-conjugated monoclonal antibodies and IgG1-FITC,
IgG2b-APC (1:20; cat nos. 130098847 and 130098890; Miltenyi Biotec
GmbH) were used to stain the negative controls. The forkhead box P3
(FoxP3) Staining Buffer set (Miltenyi Biotec GmbH) was used for
cell fixation and permeabilization. After that, cells were stained
with FoxP3-phycoerythrin (PE; 1:2; cat. no. 130093014; Miltenyi
Biotec GmbH) or IgG1-PE (1:2; cat. no. 130098845; Miltenyi Biotec
GmbH) as negative control. Regulatory T cells were identified as
CD4+CD25+Foxp3+ and the
frequencies were determined.
To determine the Th17 cell level,
PBMCs/mixed-culture CD4+ T lymphocytes were incubated
with 25 ng/ml phorbol ester (Beyotime Institute of Biotechnology,
Shanghai, China), 1 µg/ml Brefeldin A (Beyotime Institute of
Biotechnology) and 1 µg/ml Ionomycin (Beyotime Institute of
Biotechnology) at 37°C for 5 h. Next, CD4-FITC (2:5; cat. no.
130092358; Miltenyi Biotec GmbH) was used as the fluorophore
conjugated monoclonal antibodies and mouse IgG1-FITC (1:5; cat. no.
130098847; Miltenyi Biotec GmbH) were used to stain the negative
controls. Following fixation and permeabilization with
Cytofix/Cytoperm Buffer kit (BD Pharmingen, San Diego, CA, USA),
cells were stained with IL-17A-PE (1:10; cat. no. 130094521) or
IgG1-PE (1:10; cat. no. 130098845; both from Miltenyi Biotec GmbH)
as negative control. Th17 cells were identified as CD4+
IL-17+ and the frequencies were determined. At least
104-105 cells were acquired and analyzed by
FACS Calibur flow cytometer (BD Biosciences) and CellQuest software
version 6.0.
Autoantibodies on the membrane of BM
hematopoietic cells by FCM analysis
The positive rates of autoantibodies were tested by
FCM on granulocytes (CD15+), stem cells
(CD34+), nucleated erythrocytes (GlyCoA+)
using antibodies against CD15-FITC, CD34-FITC, GlycoA-FITC
(5233893, 6082713 and 5288793; 1:10; BD Biosciences) and anti-human
IgG-PE, IgM-APC antibodies (6144699 and 6042621; 1:10; BD
Biosciences). As described in our previous study (5) a value of >4.0% was defined as
positive. From these findings, all the enrolled patients with IRH
were divided into three groups: i) Untreated patients; ii)
remission patients with positive FCM results
(remission+); and iii) remission patients with negative
FCM result (remission−).
Statistical analysis
The SPSS version 21.0 (IBM Co., Armonk, NY, USA) was
used for statistical analysis. Data are presented as mean ±
standard deviation. The significance of the differences was
assessed by one-way analysis of variance, followed by multiple post
hoc comparisons using the least significant difference test for
homogeneous variances or Tamhane test for non-homogeneous
variances. Correlation between patient characteristics was tested
using Spearman's rank correlation test. P<0.05 was considered to
indicate statistically significant difference.
Results
Decreased serum level of IL-35 in
peripheral blood samples from patients with IRH
All enrolled patients with IRH and normal controls
serum levels of IL-35 were detected using a standard ELISA. As
presented in Fig. 1A, untreated
patients had significantly lower serum level of IL-35 (20.59±6.047
pg/ml) than the remission patients (30.737±10.6 pg/ml; P<0.01)
and normal controls (98.45±57.016 pg/ml; P<0.01). Furthermore,
serum levels of IL-35 in remission patients were lower than normal
controls (P<0.01).
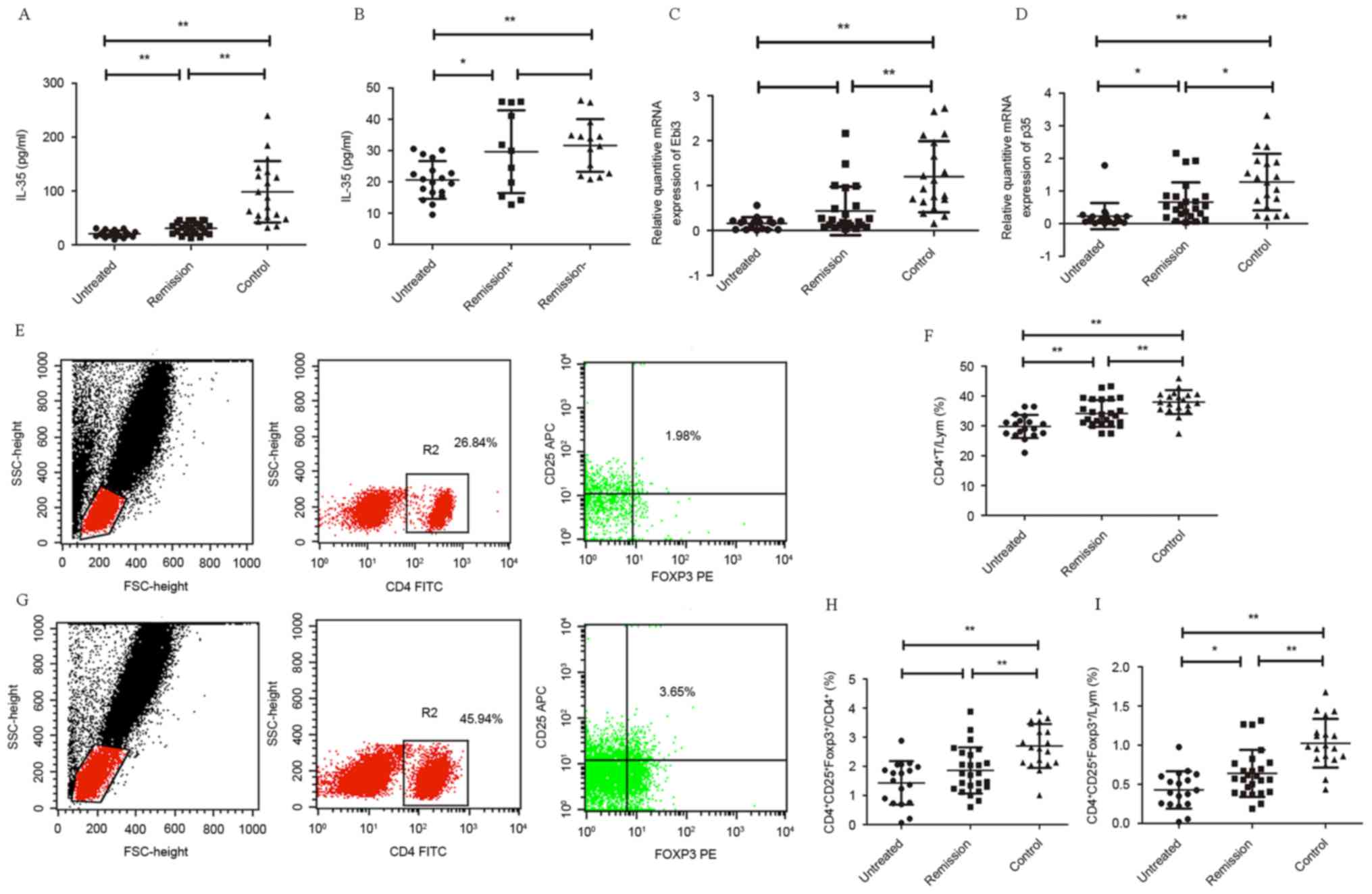 | Figure 1.(A) Serum level of IL-35 was from
untreated IRH patients (n=18), remission patients (n=25) and normal
controls (n=19). (B) Serum level IL-35 was from IRH patients
(n=18), remission patients with positive cell membrane antibodies
(remission+, n=11) and negative cell membrane antibodies
(remission−, n=14) according to the cell membrane
antibody results. Relative mRNA expression of (C) EBI3 and (D) p35
in isolated CD4+CD25+ Tregs from untreated
patients (n=18), remission patients (n=21) and normal controls
(n=19) detected by reverse transcription-quantitative polymerase
chain reaction. Level of Tregs
(CD4+CD25+Foxp3+) in peripheral
blood in untreated patients (n=18), remission patients (n=21) of
IRH and normal controls (n=19). (E) Level of Tregs in IRH patients
detected by flow cytometry. (F) Levels of CD4+ T cells
in lymphocyte population (G) Level of Tregs in normal control
detected by flow cytometry. (H) Level of Tregs in CD4+ T
lymphocytes. (I) Level of Tregs in lymphocytes. Data were expressed
as median and range. *P<0.05, **P<0.01. IL-35,
interleukin-35; IRH, immune-related hemocytopenia; EBI3,
Epstein-Barr virus induced 3; Tregs, regulatory T cells; FOXP3,
forkhead box P3. |
Correlation between IL-35 and clinical
data of patients with IRH
In order to assess the correlation between serum
level of IL-35 and clinical data of the patients, the Spearman's
correlation coefficient was performed (Table II). These correlations were made
only among IRH patients' clinical data and serum levels of IL-35.
It is evident that the IL-35 level was significantly positively
correlated with hemoglobin concentration, white blood cell counts
and platelet counts (P<0.01, r=0.620; P<0.01, r=0.429).
However, it seemed that the IL-35 concentration was not associated
with neutrocyte count and reticulocyte proportion (P=0.92, r=0.055;
P=0.632, r=0.045). FCM analysis was used to identify the
frequencies of CD5+CD19+ B cell gating on
CD19+ B cell population and lymphocyte population.
Negative correlation was identified between the serum IL-35
concentration and the frequencies of
CD5+CD19+ B cell gating on CD19+ B
lymphocyte population and gating on lymphocyte population and the
trend had statistical significance (P<0.01, r=−0.308; P=0.028,
r=−0.295).
 | Table II.Serum level of IL-35 correlations
with clinical blood picture. |
Table II.
Serum level of IL-35 correlations
with clinical blood picture.
|
| n | Median | Range | +/− | P-value | r |
|---|
| Hb (g/l) | 43 | 89 | 41-149 | + | 0.01 | 0.620 |
| WBC
(×109/l) | 43 | 4 | 1.12–14.96 | + | 0.01 | 0.429 |
| N (%) | 43 | 51.2 | 12.3–89 | N | 0.92 | 0.055 |
| Plt
(×109/l) | 43 | 40 | 6–149 | + | 0.01 | 0.558 |
| Ret (%) | 43 | 1.91 | 0.4–3.76 | N |
0.632 | 0.045 |
|
CD5+CD19+ (%) | 37 | 0.77 | 0.11–5.53 | − |
0.028 | −0.295 |
|
CD5+CD19+/CD19+
(%) | 37 | 12.04 | 3.29–38.74 | − |
0.005 | −0.308 |
From the 43 IRH patients, it is evident in Fig. 1B that the untreated group had the
lowest IL-35 concentration (20.59±6.047 pg/ml) and there was a
significant difference when compared with the remission−
group (31.6352±8.4148 pg/ml; P<0.01) than with the
remission+ group (29.6624±8.4148 pg/ml; P=0.013).
Additionally, remission+ group had a lower serum level
of IL-35 compared with the remission− group but no
significant difference was identified (P=0.592).
mRNA expression levels of EBI3 and
p35
CD4+CD25+ Tregs were sorted
from 41 IRH patients and 19 normal control PBMC. The expression of
IL-35 subsets in Tregs was assayed separately. Data are presented
as the fold-change of gene expression normalized to the endogenous
reference gene. As presented in Fig.
1C, the mRNA expression of EBI3 in patients with IRH, untreated
(n=18) and remission (n=23), were lower compared with the normal
control (P<0.01). The EBI3 mRNA expression in untreated patients
was lower than the patients in remission; however, this was not
statistically significant (P=0.124). The p35 mRNA expression in the
untreated group was evidently the lowest of all the 3 groups
(P=0.027 vs. remission patients; P<0.01 vs. normal control;
Fig. 1D). Additionally, expression
level of p35 in the remission patients was also lower than the
normal control (P=0.042).
Tregs by FCM analysis in PB
Circulating CD4+ T lymphocytes and
regulatory T cells were identified by flow cytometric analysis of
the IRH patients (Fig. 1E) and
normal controls (Fig. 1G). The
level of CD4+ T cells in the lymphocyte population of
the untreated IRH, remission IRH and normal control group was
29.8039±3.8688, 34.1536±4.4754, and 37.9705±6.047%, respectively
(Fig. 1F). All of the IRH patients
had a significantly reduced level of CD4+ T cells
compared with normal controls (P<0.01) and the untreated
patients was even lower than that of the remission ones
(P<0.01). Level of
CD4+CD25+Foexp3+ Tregs in
CD4+ T lymphocytes of untreated and remission patients
was 1.4333±0.7465 and 1.8592±0.7935%, were lower than that of the
normal control group (2.7032±0.7539%; P<0.01; Fig. 1H). Additionally, level of Tregs in
lymphocytes in untreated patients (0.4269±0.2382%) and remission
patents (0.6389±0.3%) were lower than the of normal controls
(1.0245±0.3111%; P<0.01; Fig.
1I) and the untreated group was lower compared with the
remission group (P=0.014).
Level of IL-35 and reduced inhibition
on Th17 cells in patients with IRH
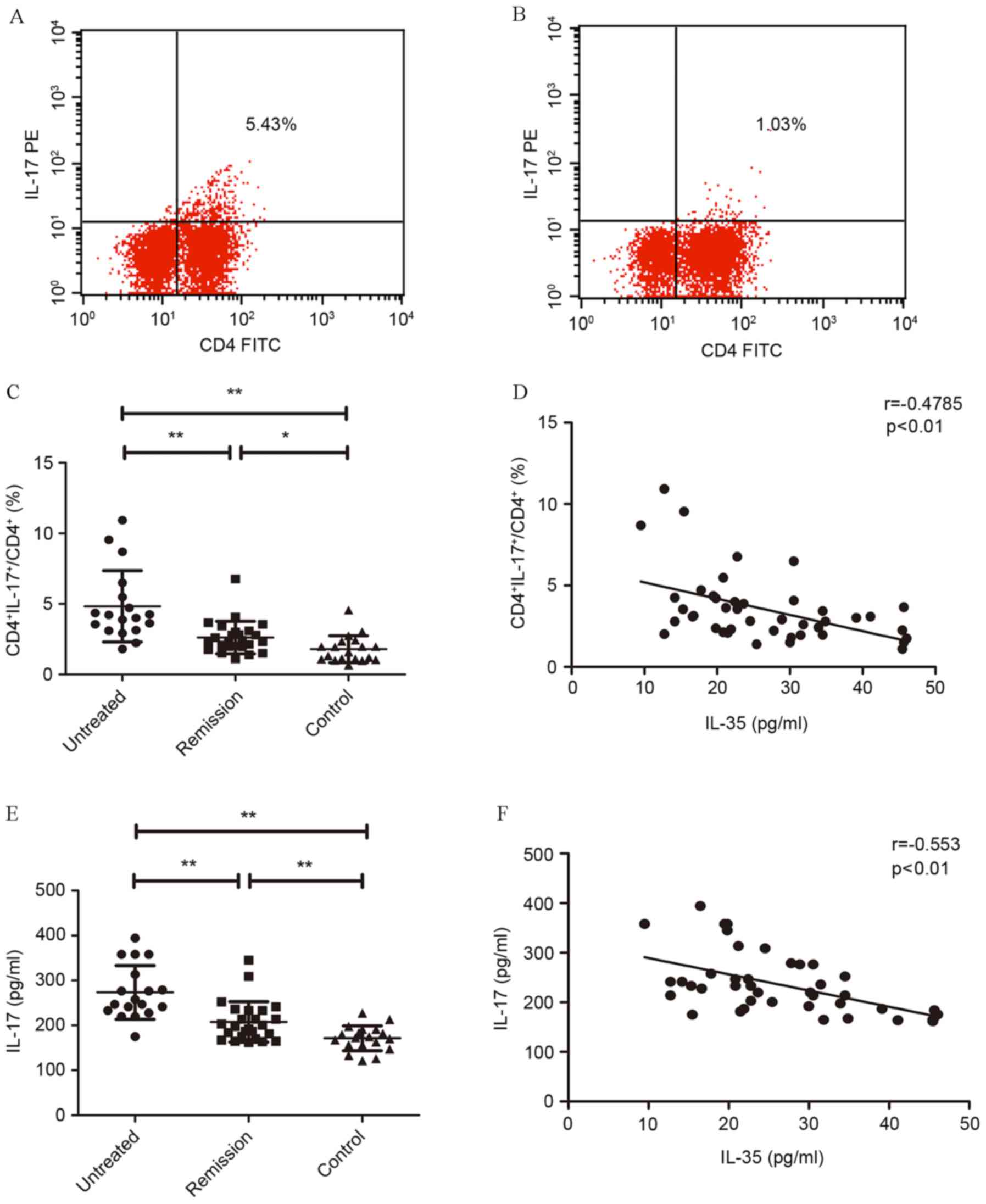
Th17 cells increased in untreated patients with IRH
(Fig. 2A and B). The percentage of
Th17 (CD4+IL-17+) significantly increased in
untreated patients (4.83±2.53%) with IRH compared with normal
controls (1.8±0.92%; P<0.01). After the treatment, the
percentage of Th17 significantly decreased (P<0.01), whereas it
was still higher than normal controls (P=0.036; Fig. 2C). Correlation analysis between the
percentage of Th17 cells in CD4+ T lymphocytes and serum
level of IL-35 revealed a significant negative correlation
(r=−0.4785; P<0.01; Fig.
2D).
Upregulated serum level of IL-17 in
untreated patients with IRH
Serum level of IL-17 was determined in all enrolled
patients. It was determined that untreated patients had
significantly higher serum level of IL-17 (273.479±59.7449 pg/ml)
than the remission patients (206.5586±45.0886 pg/ml; P<0.01;
Fig. 2E) and normal controls
(171.2928±27.6907 pg/ml; P<0.01). Additionally, serum level of
IL-17 in remission patients was higher than the normal controls
(P<0.01). Significant negative correlation was identified
between the serum level of IL-17 and IL-35 in patients with IRH
(r=−0.553; P<0.01; Fig.
2F).
Mixed-culture of IL-35 and
CD4+ T lymphocytes
After a 3-day incubation in RPMI 1640 medium
supplemented with 15% FBS with group: i) Blank control, group (a);
ii) anti-CD3 MAbs, anti-CD28 MAbs, TGF-β, IL-6, group (b); and iii)
anti-CD3 MAbs, anti-CD28 MAbs, TGF-β, IL-6, IL-35, group (c). The
Th17 cells in the three groups were separately identified using FCM
(Fig. 3A) and the percentage of
Th17 cells in CD4+ T lymphocytes population was
presented in Fig. 3B. In group (b)
CD4+ T lymphocytes were cultured with MAbs and cytokines
to promote its differentiation to Th17 cells. In group (c) culture
medium also contained IL-35, unlike group (b). It is evident that
the level of Th17 cells in CD4+ T lymphocyte population
was the highest in group (b) [P=0.044 vs. group (a); P=0.048 vs.
group (c)], whereas there was no statistical significance between
groups (a) and (c) (P=0.946).
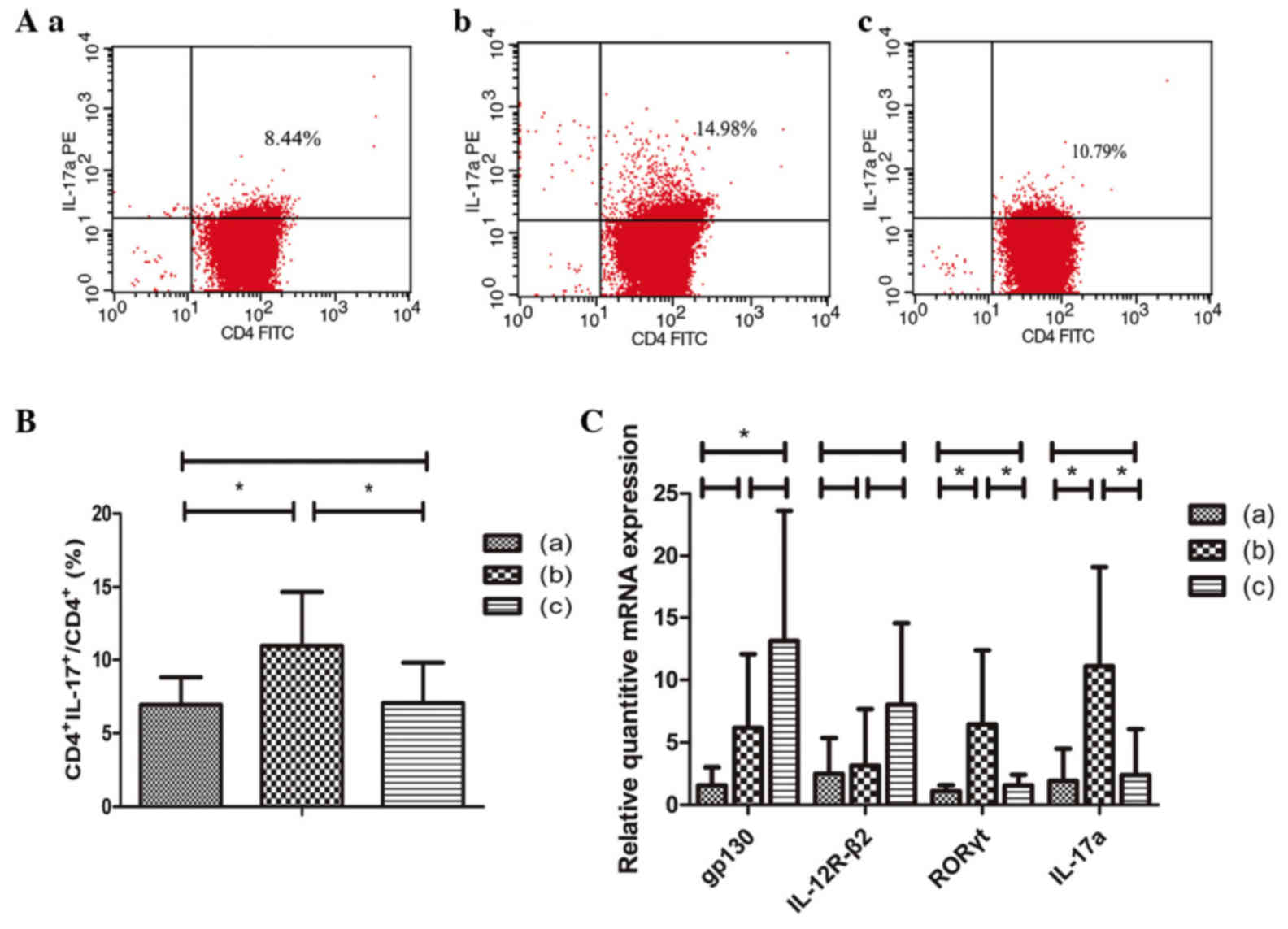 | Figure 3.(A) Levels of Th17 cells in the 3
groups; (B) level of Th17 cells in CD4+ T cells
population. (C) mRNA expression levels of gp130, IL-12R-β2, RORγt,
and IL-17a obtained using reverse transcription-quantitative
polymerase chain reaction. *P<0.05. group (a), blank control;
group (b), anti-CD3 MAbs, anti-CD28 MAbs, TGF-β, IL-6; group (c),
anti-CD3 MAbs, anti-CD28 MAbs, TGF-β, IL-6, IL-35. Th17, T helper
17; IL, interleukin; ROR, RAR-related orphan receptor; TGF,
transforming growth factor. |
The mRNA expression levels of IL-35 receptor
subunits, including gp130 and IL-12R-β2 were separately assayed in
all 3 groups. Data were presented as the fold change of gene
expression normalized to the endogenous reference gene. As
presented in Fig. 3C, the mRNA
expression of gp130 was the higher in group (c) compared with group
(a) (P=0.023). The IL-12R-β2 mRNA expression was the highest in
group (c), although there was no statistical significance
(P>0.05). The mRNA levels of RORγt (an important transcription
factor of Th17) and IL-17a were also quantified and were the
highest in group (b) when compared with the other 2 groups [P=0.032
vs. group (a); P=0.046 vs. group (c) in RORγt, and P=0.018 vs.
group (a) and P=0.023 vs. group (c) in IL-17a].
Discussion
The World Health Organization classification defined
idiopathic cytopenia of undetermined significance (ICUS) as a
condition with dysplastic cells of <10% and blasts of <5% in
BM, which do not fulfill the minimal criteria for myelodysplastic
syndromes (MDS) (11). After a
period of follow-up, some patients with ICUS were diagnosed as MDS.
However, there is still a subset of patients who do not fulfill the
criteria for MDS or other diseases even after a thorough follow-up
time (12). Our previous study
(5) tested IgM and IgG antibodies
on various bone marrow cells membrane of various bone marrow cells
of patients with ICUS by BMMNC-Coombs test, FCM and
immunofluorescence analysis and determined that some patients had
autoantibodies that lead to the immune dysfunction resulting in the
destruction of BM hematopoietic cells and leading to hemocytopenia,
which we termed as IRH. It is a type of hemocytopenia regarded as
an autoimmune disease caused by unknown autoantibodies, which may
suppress bone marrow hematopoietic cells, finally lead to the
clinical manifestation of different degrees of anemia, bleeding and
infection (4,5).
The IL-12 family is important in autoimmune
diseases. IL-12 and IL-23 are involved in pro-inflammatory
response. The two occur primarily via IFN-γ and IL-17 production
that have a role in several autoimmune diseases such as type 1
diabetes (13,14) autoimmune hepatitis (15,16),
autoimmune thyroiditis (17,18)
rheumatoid arthritis (19,20) ulcerative colitis (21) and CNS inflammatory demyelination
(22). IL-27 contributes to
anti-inflammatory activities and pro-inflammatory responses.
Therefore, these diseases may represent potential targets for
immunotherapeutic approaches based on IL-23, IFN-γ or IL-17
antagonists or an IL-27 agonist. Anti-IL-12/IL-23 monoclonal
antibody, such as ustekinumab has already been used for the
treatment of inflammatory bowel disease (23). Additionally, a previous study has
been extensively researched in animal and clinical trials that
monoclonal antibody targeting IL-17A may be used for the treatment
of autoimmune diseases (24). For
example, secukinumab, which has already been used for managing
plaque psoriasis in clinical practice, may selectively bind to
IL-17A molecule and prevents the interaction with target receptors
(25).
IL-35 is the newest member of IL-12 family. It is a
heterodimeric cytokine that involves two subunits which can be also
seen in IL-12 (p35) and IL-27 (EBI3) and is termed a definite
immunosuppressor with a high potent of suppression (26,27).
T cells (Th1, Th17) may be suppressed via cell cycle termination in
the G1 phase. Th1 and Th17 cells may be suppressed via cell cycle
termination, not apoptosis (28).
A previous study also used an intravitreal injection of
pcDNA3.1-IL-35 plasmid into the vitreous cavity of BALB/c mice that
boosted the proliferation of Tregs by increasing the expression of
IL-10 and TGF-β (29).
Furthermore, recombinant IL-35 facilitated the function of natural
Treg in vitro and reduced the levels of proinflammatory
cytokines, such as IL-17 and IFN-γ (30,31).
Therefore, IL-35 may occur in type1 cytokine/type 17 and type 2
immune-inflammatory diseases.
The present study assessed the serum levels of IL-35
in patients with IRH and normal controls. Although some of the
findings were close to the detectable dose of the ELISA kit used
and results may be influenced by the blocking of the tested antigen
binding site or other cytokines, due to the limitations of this
method, it is evident that the IL-35 level was significantly
reduced in patients with IRH compared to healthy controls.
Additionally, IL-35 level in the untreated group was lower than the
remission group. It is of note that the serum level of IL-35 was
positively correlated with hemoglobin concentration, white blood
cell and platelet counts. FCM was used to detect the level of
CD5+CD19+ B cell gating on CD19+ B
lymphocyte population and lymphocyte population. There was a
negative correlation between IL-35 level and level of
CD5+CD19+ B cell and BMMC autoantibodies have
been identified to be produced by CD5+CD19+ B
cell. As all the clinical data and hematological parameters are
associated with the progression of IRH (6), IL-35 may be a biomarker reflecting
the activity of IRH and involved in the pathogenesis of IRH.
Patients with positive BMMC membrane autoantibodies had lower
levels of IL-35 than remission patients with negative BMMC membrane
autoantibodies. These findings suggested that IL-35 may be involved
in the pathogenesis of IRH and could be used to predict factors for
response of treatment with corticosteroids or high-dose IVIG
treatment in IRH.
The cause of the decrease of IL-35 level in IRH may
be the lower level of Tregs in the patients. Foxp3 has a central
role in the differentiation and maintenance of Treg cells. It been
previously established that IL-35 is produced primarily by Treg
(32). As FoxP3 is a nuclear
protein, assessment of its expression in T cells requires fixation
and permeabilization of the cells. Using FCM, the present study
determined that the level of Treg was significantly reduced in
patients with IRH.
A previous study revealed that the mRNA expressions
of the IL-35 subunits (EBI3 and IL-12p35) were reduced in
CD4+ T cells in allergic asthmatics (33) and increased in chronic hepatitis B
virus-infected patients (34,35)
when compared with normal controls. Conversely, using phased joint
embolization in patients with portal hypertension caused by liver
cirrhosis may reduce the protein and mRNA expression levels of
IL-35 (36). Using cell sorting
techniques and RT-qPCR the present study determined the mRNA levels
of IL-35 subunits (EBIi3, p35) in CD4+CD25+ T
cells, finding them both decreased in IRH patients compared with
the normal controls. This indicated the low expression of IL-35 in
CD4+CD25+ T cells. However, as Foxp3 was not
the biomarker used while sorting Tregs, the lower mRNA expression
of IL-35 subunits (EBI3, p35) may be associated with the lower
level of CD4+CD25+ that Foxp3 cells.
Foxp3−/−Tconv(conventional
CD4+Foxp3− T cells) cells have already been
identified to be converted to IL-35 iTR which express
IL-35 and mediate suppression in a manner indistinguishable from
their wild type counterparts (37). In addition, iTR35 cells
do not express Foxp3 following in vivo inoculation (38). The iTR35 cell may be
suppressive and stable without the expression of Treg transcription
factor Foxp3. These types of cells have a positive feedback
association with IL-35, as IL-35 suppresses T cell proliferation
and converts naïve T cells into IL-35-producing iTR35
(37,38). The lack of IL-35 may lead to the
lack of iTR35, which may also lead to the low expression
of EBI3 and p35 mRNA in CD4+CD25+ Tregs.
It has been previously reported that IL-35 may
inhibit the differentiation of CD4+ T lymphocytes to
Th17 cells (1). The present study
determined that the IL-17 serum level in patients with IRH was
higher than that in normal control patients, and it had a negative
correlation with the serum level of IL-35. Additionally, the
CD4+ T lymphocytes were sorted and cultured with
anti-CD3 MAbs, anti-CD28 MAbs, TGF-β, IL-6 to promote Th17 cell
differentiation (39–41) and with IL-35 to determine its
effect on Th17 cell differentiation. The findings revealed a lower
Th17 level and reduced mRNA expression of RORγt, IL-17A when IL-35
was added to the mixed-culture system. This demonstrated that IL-35
may inhibit Th17 cell differentiation.
A previous study demonstrated that the hyperfunction
of Th17 cells may lead to IRH (8).
However, the present study may have elucidated an additional reason
for Th17 cell hyperfunction: That decreased level of IL-35 can lead
to the hyperfunction of Th17 and this decrease may be the
pathogenesis of IRH. A previous study had determined that Listeria
monocytogenes use IL-27/EBI3 to escape Th17-mediated immune
surveillance in IL-12p35-deficient mice (42). IL-35 signals have been identified
to encompass three receptor subunits comprising
IL-12R-β2-IL-12R-β2, IL-12R-β2-gp130, and gp130-gp130 which
activate STAT1 and STAT4 molecules (43–45).
Previous studies have revealed that IL-27-mediated suppression of
human Th17 cells was associated with the activation of STAT1
(46,47) and IL-27 shares the same subunit
EBI3 with IL-35. However, whether IL-35 used the same pathway for
suppression of Th17 cell differentiation remains to be elucidated
and the mechanism of IL-35 inhibiting Th17 cell differentiation
requires further investigation. In conclusion, the present study
primarily determined that IL-35 may be a monitoring indicator of
progression of IRH and a new therapeutic target for IRH in the
future.
Due to its immunosuppressive roles in autoimmunity
and inflammation, IL-35 has a pivotal role in controlling effector
immunity and may constitute a treatment target in autoimmune
diseases. In previous studies on several autoimmune diseases have
yielded encouraging results upon IL-35 treatment in animal models.
For example, significant remissions of histopathological
characteristics of lupus flare and nephritis were observed in
MRL/lpr mice (48), alleviations
in synovial hyperplasia, cartilage and bone erosion, and
fibroblast-like synoviocytes growth were observed in collagen
induced arthritis mice (2,49). However, its signaling pathway and
further clinical use remain to be fully elucidated.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570106, 81570111,
81600093, 81600088 and 81500101) and the Tianjin Municipal Natural
Science Foundation (grant nos. 14JCYBJC25400 and
15JCYBJC24300).
References
|
1
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS and Vignali DA:
The inhibitory cytokine IL-35 contributes to regulatory T-cell
function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niedbala W, Wei XQ, Cai B, Hueber AJ,
Leung BP, McInnes IB and Liew FY: IL-35 is a novel cytokine with
therapeutic effects against collagen-induced arthritis through the
expansion of regulatory T cells and suppression of Th17 cells. Eur
J Immunol. 37:3021–3029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kochetkova I, Golden S, Holderness K,
Callis G and Pascual DW: IL-35 stimulation of CD39+ regulatory T
cells confers protection against collagen II-induced arthritis via
the production of IL-10. J Immunol. 184:7144–7153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao Y, Fu R, Liu H, Wang Y, Ding S, Wang
H, Li L and Shao Z: IgG autoantibody subclasses altered in
immuno-related hemocytopenia. Cell Immunol. 294:13–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu R, Liu H, Wang Y, Liu H, He H, Chen J,
Wang H, Yu H, Ding K, Huang L, et al: Distinguishing immunorelated
haemocytopenia from idiopathic cytopenia of undetermined
significance (ICUS): A bone marrow abnormality mediated by
autoantibodies. Clin Exp Immunol. 177:412–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu R, Shao Z, He H, Liu H, Jia H, Sun J,
Zhao M, He G, Shi J, Bai J, et al: Quantity and apoptosis-related
protein level of B lymphocyte in patients with immunorelated
pancytopenia. Zhonghua Xue Ye Xue Za Zhi. 23:236–238. 2002.(In
Chinese). PubMed/NCBI
|
|
7
|
Fu R, Chen J, Wang HL, Wang J, Li LJ, Liu
H, Wang YH, Ren Y and Shao ZH: Quantity and function of regulatory
T cells in hemocytopenic patients with positive BMMNC-Coombs test.
Zhonghua Yi Xue Za Zhi. 90:2989–2993. 2010.(In Chinese). PubMed/NCBI
|
|
8
|
Fu R, Wang HL, Chen J, Wang J, Li LJ, Liu
H, Wang YH, Ren Y and Shao ZH: Study of the quantity and function
of Th17 cells in the blood cytopenic patients with positive
BMMNC-Coombs test. Zhonghua Xue Ye Xue Za Zhi. 31:684–687. 2010.(In
Chinese). PubMed/NCBI
|
|
9
|
Zhu X, Guan J, Xu J, Wei J, Jiang L, Yin
J, Zhao L and Zhang Y: Pilot study using tacrolimus rather than
cyclosporine plus antithymocyte globulin as an immunosuppressive
therapy regimen option for severe aplastic anemia in adults. Blood
Cells Mol Dis. 53:157–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valent P, Horny HP, Bennett JM, Fonatsch
C, Germing U, Greenberg P, Haferlach T, Haase D, Kolb HJ, Krieger
O, et al: Definitions and standards in the diagnosis and treatment
of the myelodysplastic syndromes: Consensus statements and report
from a working conference. Leuk Res. 31:727–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ando K, Kodama A, Iwabuchi T, Ohyashiki JH
and Ohyashiki K: Idiopathic neutropenia with fewer than 5%
dysplasia may be a distinct entity of idiopathic cytopenia of
undetermined significance. Ann Hematol. 89:733–735. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicoletti F, Zaccone P, Di Marco R,
Lunetta M, Magro G, Grasso S, Meroni P and Garotta G: Prevention of
spontaneous autoimmune diabetes in diabetes-prone BB rats by
prophylactic treatment with antirat interferon-gamma antibody.
Endocrinology. 138:281–288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicoletti F, Di Marco R, Zaccone P, Magro
G, Di Mauro M, Grasso S and Meroni PL: Endogenous interleukin-12
only plays a key pathogenetic role in non-obese diabetic mouse
diabetes during the very early stages of the disease. Immunology.
97:367–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicoletti F, Zaccone P, Xiang M, Magro G,
Di Mauro M, Di Marco R, Garotta G and Meroni P: Essential
pathogenetic role for interferon (IFN-)gamma in concanavalin
A-induced T cell-dependent hepatitis: Exacerbation by exogenous
IFN-gamma and prevention by IFN-gamma receptor-immunoglobulin
fusion protein. Cytokine. 12:315–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nicoletti F, Di Marco R, Zaccone P,
Salvaggio A, Magro G, Bendtzen K and Meroni P: Murine concanavalin
A-induced hepatitis is prevented by interleukin 12 (IL-12) antibody
and exacerbated by exogenous IL-12 through an
interferon-gamma-dependent mechanism. Hepatology. 32:728–733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang H, Mignon-Godefroy K, Meroni PL,
Garotta G, Charreire J and Nicoletti F: The effects of a monoclonal
antibody to interferon-gamma on experimental autoimmune thyroiditis
(EAT): Prevention of disease and decrease of EAT-specific T cells.
Eur J Immunol. 23:275–278. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaccone P, Hutchings P, Nicoletti F, Penna
G, Adorini L and Cooke A: The involvement of IL-12 in murine
experimentally induced autoimmune thyroid disease. Eur J Immunol.
29:1933–1942. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li R, Zheng X, Popov I, Zhang X, Wang H,
Suzuki M, Necochea-Campion RD, French PW, Chen D, Siu L, et al:
Gene silencing of IL-12 in dendritic cells inhibits autoimmune
arthritis. J Transl Med. 10:192012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karri SK and Sheela A: Potential route of
Th17/Treg cell dynamics in targeting type 1 diabetes and rheumatoid
arthritis: An autoimmune disorder perspective. Br J Biomed Sci.
74:8–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong Z, Du L, Xu X, Yang Y, Wang H, Qu A,
Qu X and Wang C: Aberrant expression of circulating Th17, Th1 and
Tc1 cells in patients with active and inactive ulcerative colitis.
Int J Mol Med. 31:989–997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rostami A and Ciric B: Role of Th17 cells
in the pathogenesis of CNS inflammatory demyelination. J Neurol
Sci. 333:76–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deepak P and Loftus EV Jr: Ustekinumab in
treatment of Crohn's disease: Design, development, and potential
place in therapy. Drug Des Devel Ther. 10:3685–3698. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai F, Tian H, Niu Z, Liu M, Ren G, Yu Y,
Sun T, Li S and Li D: Chimeric anti-IL-17 full-length monoclonal
antibody is a novel potential candidate for the treatment of
rheumatoid arthritis. Int J Mol Med. 33:711–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reszke R and Szepietowski JC: Secukinumab
in the treatment of psoriasis: An update. Immunotherapy. 9:229–238.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vignali DA and Kuchroo VK: IL-12 family
cytokines: Immunological playmakers. Nat Immunol. 13:722–728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun L, He C, Nair L, Yeung J and Egwuagu
CE: Interleukin 12 (IL-12) family cytokines: Role in immune
pathogenesis and treatment of CNS autoimmune disease. Cytokine.
75:249–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olson BM, Sullivan JA and Burlingham WJ:
Interleukin 35: A key mediator of suppression and the propagation
of infectious tolerance. Front Immunol. 4:3152013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou C, Wu Q, Ouyang C and Huang T: Effects
of an intravitreal injection of interleukin-35-expressing plasmid
on pro-inflammatory and anti-inflammatory cytokines. Int J Mol Med.
38:713–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan SY, Leng RX, Khan MI, Qureshi H, Li
XP, Ye DQ and Pan HF: Interleukin-35: A potential therapeutic agent
for autoimmune diseases. Inflammation. 40:303–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Egwuagu CE, Yu CR, Sun L and Wang R:
Interleukin 35: Critical regulator of immunity and
lymphocyte-mediated diseases. Cytokine Growth Factor Rev.
26:587–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Li P, Chen YF and Yang J: A
potential immunopathogenic role for reduced IL-35 expression in
allergic asthma. J Asthma. 52:763–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zhang H and Li Y: IL-35 expression
in peripheral blood CD4(+) T cells from chronic hepatitis B
virus-infected patients directly correlates with virus load.
Cytokine. 73:169–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi M, Wei J, Dong J, Meng W, Ma J, Wang
T, Wang N and Wang Y: Function of interleukin-17 and −35 in the
blood of patients with hepatitis B-related liver cirrhosis. Mol Med
Rep. 11:121–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Dong J, Meng W, Ma J, Wang N, Wei
J and Shi M: Effects of phased joint intervention on IL-35 and
IL-17 expression levels in patients with portal hypertension. Int J
Mol Med. 33:1131–1139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wong CK, Leung TF, Chu IM, Dong J, Lam YY
and Lam CW: Aberrant expression of regulatory cytokine IL-35 and
pattern recognition receptor NOD2 in patients with allergic asthma.
Inflammation. 38:348–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghaedi M, Namdari H, Rahimzadeh P, Morteza
Gholi S, Azimi Mohamadabadi M and Salehi E: Different doses of
transforming growth factor-b on in vitro differentiation of human
naive CD4+ T cells to T helper 17. Iran J Allergy Asthma Immunol.
14:633–637. 2015.PubMed/NCBI
|
|
40
|
Ghoreschi K, Laurence A, Yang XP, Tato CM,
McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N,
et al: Generation of pathogenic T(H)17 cells in the absence of
TGF-β signalling. Nature. 467:967–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Volpe E, Servant N, Zollinger R, Bogiatzi
SI, Hupé P, Barillot E and Soumelis V: A critical function for
transforming growth factor-beta, interleukin 23 and proinflammatory
cytokines in driving and modulating human T(H)-17 responses. Nat
Immunol. 9:650–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chung Y, Yamazaki T, Kim BS, Zhang Y,
Reynolds JM, Martinez GJ, Chang SH, Lim H, Birkenbach M and Dong C:
Epstein Barr virus-induced 3 (EBI3) together with IL-12 negatively
regulates T helper 17-mediated immunity to Listeria monocytogenes
infection. PLoS Pathog. 9:e10036282013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Presky DH, Yang H, Minetti LJ, Chua AO,
Nabavi N, Wu CY, Gately MK and Gubler U: A functional interleukin
12 receptor complex is composed of two beta-type cytokine receptor
subunits. Proc Natl Acad Sci USA. 93:pp. 14002–14007. 1996;
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Collison LW, Delgoffe GM, Guy CS, Vignali
KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA,
Drake CG, et al: The composition and signaling of the IL-35
receptor are unconventional. Nat Immunol. 13:290–299. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Behzadi P, Behzadi E and Ranjbar R: IL-12
family cytokines: General characteristics, pathogenic
microorganisms, receptors, and signalling pathways. Acta Microbiol
Immunol Hung. 63:1–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu H and Rohowsky-Kochan C:
Interleukin-27-mediated suppression of human Th17 cells is
associated with activation of STAT1 and suppressor of cytokine
signaling protein 1. J Interferon Cytokine Res. 31:459–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peters A, Fowler KD, Chalmin F, Merkler D,
Kuchroo VK and Pot C: IL-27 induces Th17 differentiation in the
absence of STAT1 signaling. J Immunol. 195:4144–4153. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cai Z, Wong CK, Dong J, Chu M, Jiao D, Kam
NW, Lam CW and Tam LS: Remission of systemic lupus erythematosus
disease activity with regulatory cytokine interleukin (IL)-35 in
Murphy Roths Large (MRL)/lpr mice. Clin Exp Immunol. 181:253–266.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Wu S, Li Y, Jiang S, Lin T, Xia L,
Shen H and Lu J: Interleukin-35 (IL-35) inhibits proliferation and
promotes apoptosis of fibroblast-like synoviocytes isolated from
mice with collagen-induced arthritis. Mol Biol Rep. 43:947–956.
2016. View Article : Google Scholar : PubMed/NCBI
|