Introduction
Spinal cord injury (SCI), usually resulting in
severe sensory and motor deficits, is a major public health concern
due to high disability rate and low curable rate (1). In the past decades, though a number
of therapeutic strategies have been developed, such as medication,
surgical intervention and even gene therapy, they provide little
benefits for patients with SCI (2). To date, tissue engineering sheds
light on a promising therapeutic approach for treating SCI
resulting from priority to provide a suitable niche for survival
and functional exertion of local residued neural cell types in
injuried spinal cord.
Especially, transplantation of tissue engineering
materials seeded with stem cells has proven to benefit functional
recovery through promoting axonal extension, immunosuppression
and/or neurotrophic support (3–5). Our
colleagues have developed a method to obtain modified acellular
spinal cord scaffolds (ASCs), which is natural, soft, flexible and
stable under physiological conditions. Meanwhile, it contains
linear guidance pores extending through their full Iength, which
could serve as a support for axon regeneration. In addition, we
have seeded bone marrow mesenchymal stem cells (BMSCs) in ASCs to
certify their biocompatibility in vitro (6,7).
Furthermore, studies have indicated that engraftment of ASCs seeded
with umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs)
help enhance functional recovery via bridging spinal cord cavity
and promoting long-distance axon regeneration in SCI rats (8). In addition, the lack of integrality
of umbilical cord database and long-time storage under frozen
condition might be limitations to restrict the autologous
transplantation of UCB-MSCs in ready-to-use clinical application.
Hence, it is significant to find out a ready-to-use autologous stem
cell resource for engraftment.
Adipose-derived stem cells (ADSCs), identified as a
source of multipotent cells (9),
possess the potential to protect neural cells from glutamate
excitotoxicity-induced apoptosis (10), differentiate into glial cells,
secrete neurotrophic factors (11,12)
and suppress local inflammation after central nervous system (CNS)
injury (13). Meanwhile,
transplantation of ADSCs, which could be easily isolated from
abundant adipose tissue in the same subject with less damage, helps
improve mechanical allodynia and functional recovery in rats with
injured sciatic nerve (14). In
addition, recent studies have focused on transplantation of ADSCs
with other effective therapies to ensure its application in
therapeutic strategy for SCI (15,16).
ASC, a candidate for treatment of SCI, is worth illustrating its
application combined with ASDCs and possible underlying
mechnism.
In the present study, we hypothesized that
transplantation of ASCs seeded with rat ADSCs (rADSCs) would
facilitate functional recovery via harnessing axon regeneration and
reducing reactive gliosis in injuried rat spinal cord. To test our
hypothesis, we cultured rADSCs and assessed their characteristics.
Meanwhile, the biocompatibility between rADSCs and ASCs was
evaluated using co-culture pattern and Brdu assays in vitro
and in vivo. In addition, the beneficial ability to restore
injuried spinal cord of ASCs seeded with rADSCs was examined
through histopathological assessment using HE, immunofluorescence
staining, and behavioral test. The aim of this study is to provide
suitable strategy for SCI treatment based on tissue engineering, at
the same time, to probably enlarge the application scope of ADSCs
from bench to bed in the future research.
Materials and methods
Cell culture
rADSCs were obtained according to the previous
protocols (11,17,18).
Briefly, the adipose tissues were dissected from subcutaneous fat
in the inguinal region of Sprague-Dawley male rats. The tissues
were washed three times with phosphate-buffered saline (PBS) and
minced with scissors. Then, tissues were digested in PBS containing
2 mg/ml collagenase type II (Sigma-Aldrich, St. Louis, MO) for 40
min at 37°C with constant agitation. Then, tissues was mixed with
an equal volume of Dulbecco's Modified Eagle's Medium (DMEM, Gibco,
Grand Island, NY) supplemented with 10% fetal bovine serum (FBS,
Gibco, Grand Island, NY), passed through a 40-µm Nylon cell
strainer (BD Falcon, San Jose, CA) and centrifuged for 10 min at
1,500 g. Afterward, floating adipocytes were removed from the
stromal vascular fraction. Finally, the obtained cells were seeded
on cell culture flasks (Thermo Fisher Scientific, Waltham, MA)
supplemented with α-modified Eagle's medium (Gibco, Grand Island,
NY), 10% FBS, and 1% penicillin-streptomycin (Gibco, Grand Island,
NY). They were incubated at 37°C in a humidified atmosphere under
5% CO2. The culture medium was replaced every 2–3 days.
Cells were trypsinized with 0.25% trypsin-EDTA (Gibco, Grand
Island, NY) and expanded in new flasks when they reached 70–80%
confluence. Cells used for transplantation in ASCs were from
passages 3 to 6.
Differentiation assay
For osteogenic differentiation, rADSCs at passage 3
were inoculated at 4×103 cells/cm2 and
cultured for 2 weeks in osteogenic induction medium [MEM containing
10% FBS, 100 µg/ml ascorbate, 0.1 µM dexamethasone and 10 mM
β-glycerophosphate (All from Cyagen Biosciences, Suzhou, Jiangsu,
China)]. rADSCs were then fixed with 4% paraformaldehyde (PFA,
Boster Biosciences, Beijing, China) for 20 min, washed with PBS for
3 times, afterward, incubated with 1 mg/ml Alizarin Red
(Sigma-Aldrich, St. Louis, MO) solution for 30 min to stain for
calcium deposition. For adipogenic differentiation, rADSCs at
passage 3 were cultured for 2 weeks in adipogenic induction medium
[high glucose-DMEM containing 10% FBS, 1 µM dexamethasone, 0.5 mM
methyl-isobutylxanthine, 10 µg/ml insulin, and 100 µM indomethacin
(All from Cyagen Biosciences, Suzhou, Jiangsu, China)]. Then, cells
were fixed with 4% PFA for 20 min, washed with PBS for 3 times,
afterward, immersed in 0.3% Oil Red O solution (Sigma-Aldrich, St.
Louis, MO) in 60% isopropanol for 30 min to assess lipid droplet
formation.
Flow cytometry analysis
Surface antigens of the undifferentiated rADSCs were
identified by flow cytometry using anti-CD29 (1:200, Biolegend, San
Diego, CA), CD90 (1:100, Biolegend, San Diego, CA), CD44 (1:400,
Abcam, Cambridge, UK) and CD45 (1:200, Biolegend, San Diego, CA)
antibodies. rADSCs were resuspended in buffer containing PBS
supplemented with 1% bovine serum albumin (BSA, Gibco, Grand
Island, NY) to reach a concentration of 1×107 cells/ml
after trypsinizing with 0.25% trypsin-EDTA, and incubated with
fluorescence-conjugated primary antibodies for 30 min on ice. Flow
cytometry analysis was performed on the BD Accuri™ C6 Flow
Cytometer System (BD Biosciences, NJ, USA) and data were analyzed
using Cflow Plus software (Becton-Dickinson, NJ, USA).
Preparation of rat acellular spinal
cord scaffolds
The rat ASCs were harvested as previously described
according to standard procedures developed by our colleagues
(6,7). In short, SD rats were anesthetized
with intraperitoneal injection of sodium pentobarbital (45 mg/kg
body weight). Thoracic spinal cord samples (~20 mm) were obtained
and then rinsed in PBS to remove blood. Obtained tissues were
incubated in distilled water (changed per 2 h) at room temperature
for 6 h. Then, samples were serially immersed in 1% Triton X-100
solution (3 h), PBS (3 h), 1% sodium deoxycholate solution (3 h),
and PBS (3 h), respectively. Meanwhile, specimens were oscillated
at 100 rpm throughout the immersion process. Afterward, samples
were lyophilized to obtain the acellular spinal cord scaffolds
after serial immersions were repeated once. The lyophilized
scaffolds were incubated in genipin and glutaraldehyde solutions
(both 5 mg/ml in 0.01 M PBS, pH 7.4), respectively, for chemical
crosslinking for 24 h at 37°C. All specimens were immediately
freeze-dried for 24 h in freeze drier and sterilized by irradiation
(16 kGy) with Cobalt-60 gamma rays before engraftment. Before
transplantation, rADSCs were cultured in 10 µM 5-bromodeoxyuridine
(BrdU, Sigma-Aldrich, St. Louis, MO) for 48 h. Subsequently, they
were injected into ASCs (0.2×0.1 cm) at a concentration of
5×106 cells/ml. The engraftments were then incubated at
37°C in a humidified atmosphere under 5% CO2 in rADSCs
culture medium for 2–3 days, stained with hematoxylin and eosin
(HE), and examined by light microscopy. The ultrastructure of the
engraftment were visualized by scanning electron microscopy (SEM,
S-3400 N, Hitachi, Japan) (7).
Rat spinal cord hemisection and
transplantation
All animal experiments were approved and supervised
by the ethics committee of The Third Military Medical University
according to international standards for animal welfare. Adult male
SD rats (body weight, 200–250 g) were purchased from animal center
of The Third Military Medical University and anesthetized with
intraperitoneal injection of sodium pentobarbital (45 mg/kg body
weight). Thoracic spinal cord segments were exposed after
laminectomy at the T9-10 level under aseptic condition. Two
right-sided hemisections of spinal cord were performed by
microdissection scissor between T9 and T10 level under surgical
microscope. A 2 mm-width gap was created with a 22-gauge syringe
needle (8). Then, muscle layers
and skin were sutured separately. To prevent infection, rats were
subcutaneously injected with ampicillin (100 mg/kg) and gentamicin
(12 mg/kg) following 3 days after surgery (once a day). Their
bladders were emptied twice daily until they regained bladder
control. Rats were randomly divided into 3 groups after hemisected
SCI: (1) without any intervention
(SCI only, n=15), (2) with ASC
scaffolds implantation (ASC only, n=15), (3) with ASC scaffolds seeded with rADSCs
transplantation (ASC + ADSCs, n=15). Behavioral tests (including
BBB locomotor rating scale, hypersensitivity to mechanical and
thermal stimulation) were assessed weekly before and after surgery.
Histological examinations and immunohistochemistry were performed
on all animals. BDA anterograde tracing were used to detect nerve
fibers in injuried rat spinal cord on day 14 post-SCI.
Behavioral tests after SCI
For bsehavioral tests, two independent investigators
blinded to the experimental design separately assessed and scored
behavioral recovery. The locomotor recovery after SCI was evaluated
in view of the BBB score as previously described (19) on day 1, 7, 14, 21 and 28
post-SCI.
BDA anterograde tracing
Rats were anesthetized and placed on a stereotaxic
frame and biotinylated dextran amines (BDA, Invitrogen, Breda,
Netherlands) was injected in view of previous study before
conducting surgery at spinal cord (20).
Hematoxylin and eosin (H&E)
staining
Scaffolds and tissue sections were fixed in 4%
paraformaldehyde, paraffin-embedded, sliced, and stained with
hematoxylin and eosin (H&E, Beyotime C0105, Beijing, China)
according to the manufacture's instructions (21). The stained samples were visualized
using a light microscopy (Leica, Wetzlar, Germany).
Histological analysis
The histological analysis was employed according to
standard procedures (8) and the
antibodies were as follows: Mouse anti-Brdu (1:200, Abcam,
Cambridge, UK), rabbit anti-glial fibrillary acidic protein (GFAP,
1:300, Abcam, Cambridge, UK).
For Brdu staining, sections were firstly incubated
in 2 M HCL for 30 min, then neutralized with 0.1 M sodium borate
buffer (pH 8.5) for 30 min at room temperature for DNA hydrolysis
(22). Slices were visualized by a
light microscopy (Leica, Wetzlar, Germany).
For BDA tracing, three sagittal slices were selected
from each group and rinsed with PBS, then treated with avidin-Cy3
containing 0.25% TritonX-100 in PBS at room temperature for 2 h and
rinsed in PBS for three times for analysis.
All sections with immunofluorescence were examined
by a confocal microscope (Carl Zeiss, LSM780, Weimar, Germany) and
analyzed using Zen 2011 software (Carl Zeiss, Weimar, Germany).
Statistical analysis
Data were represented as the mean ± SEM and data
analysis were conducted using SPSS V18.0 (SPSS Inc, Chicago, IL).
Comparisons among multiple sets were performed using One-way ANOVA
followed by Tukey's post hoc test and a P<0.05 was
considered as significance.
Results
Characterization of rADSCs and
biocompatibility of ASCs
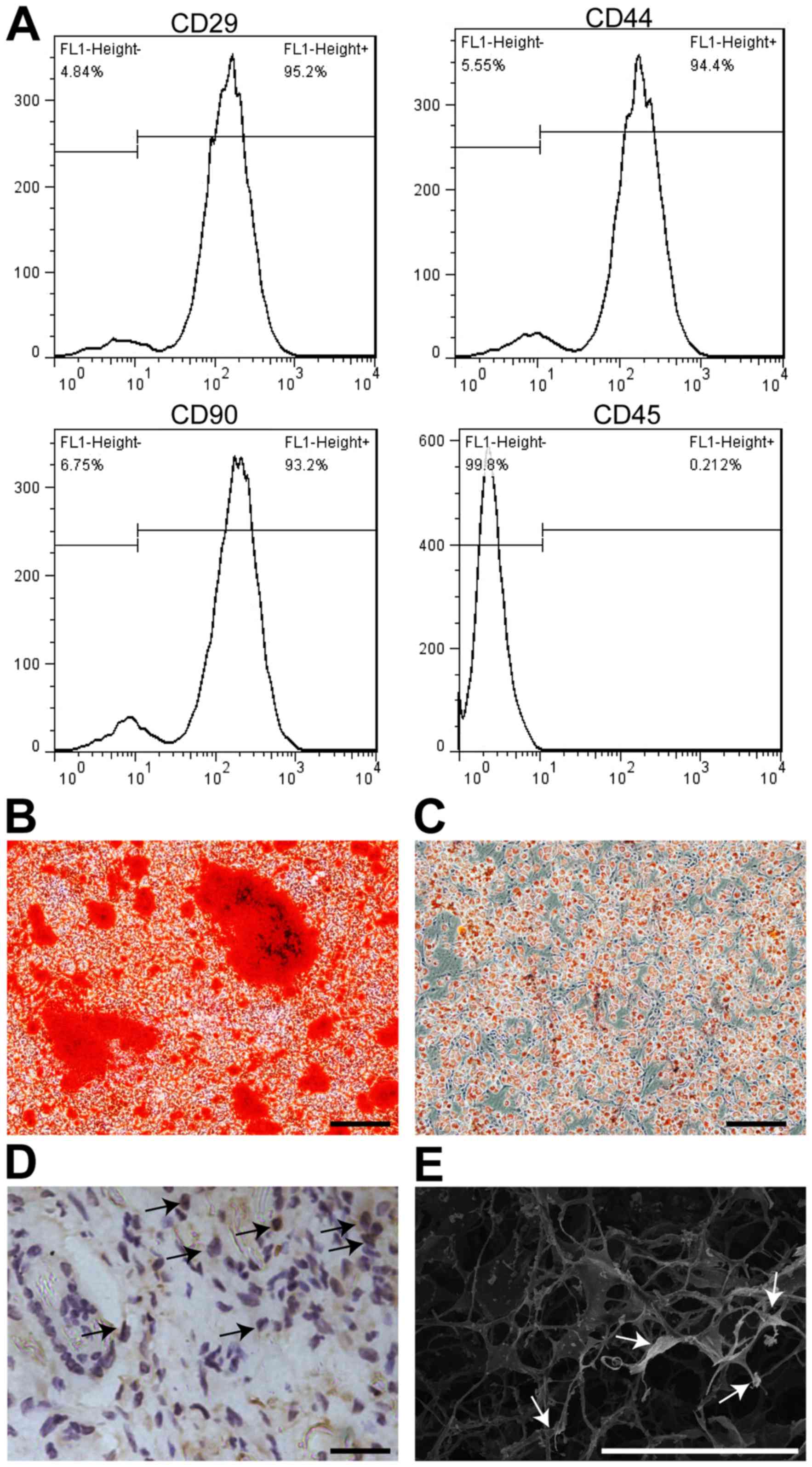
rADSCs expressed cell surface antigen CD29, CD44 and
CD90 after 3 passages, but negative for CD45 by flow cytometry
analysis (Fig. 1A). To assess the
differentiation potential of cultured cells, they were incubated in
induction media for 14 day to evaluate their differentition into
osteocytes and adipocytes, respectively. The results indicated that
cultured cells held the capability of osteogenesis (Fig. 1B) and adipogenesis (Fig. 1C). To test the biocompatibility of
ASCs, rADSCs pre-cultured in 10 µM BrdU were seeded into ASCs,
which appeared to be white color and became soft and flexible two
days latter. Brdu+ cells were interspersed in the
network of ASCs before transplantation (Fig. 1D). Meanwhile, rADSCs with thin
fibers forming uniform mesh pores were observed using SEM (Fig. 1E). In short, these results
indicated rADSCs were able to survive and exert biological function
in ASCs, which were safe and biocompatible, according to our
established procedures.
Transplantation of ASCs seeded with
rADSCs promoted functional recovery after SCI in rats
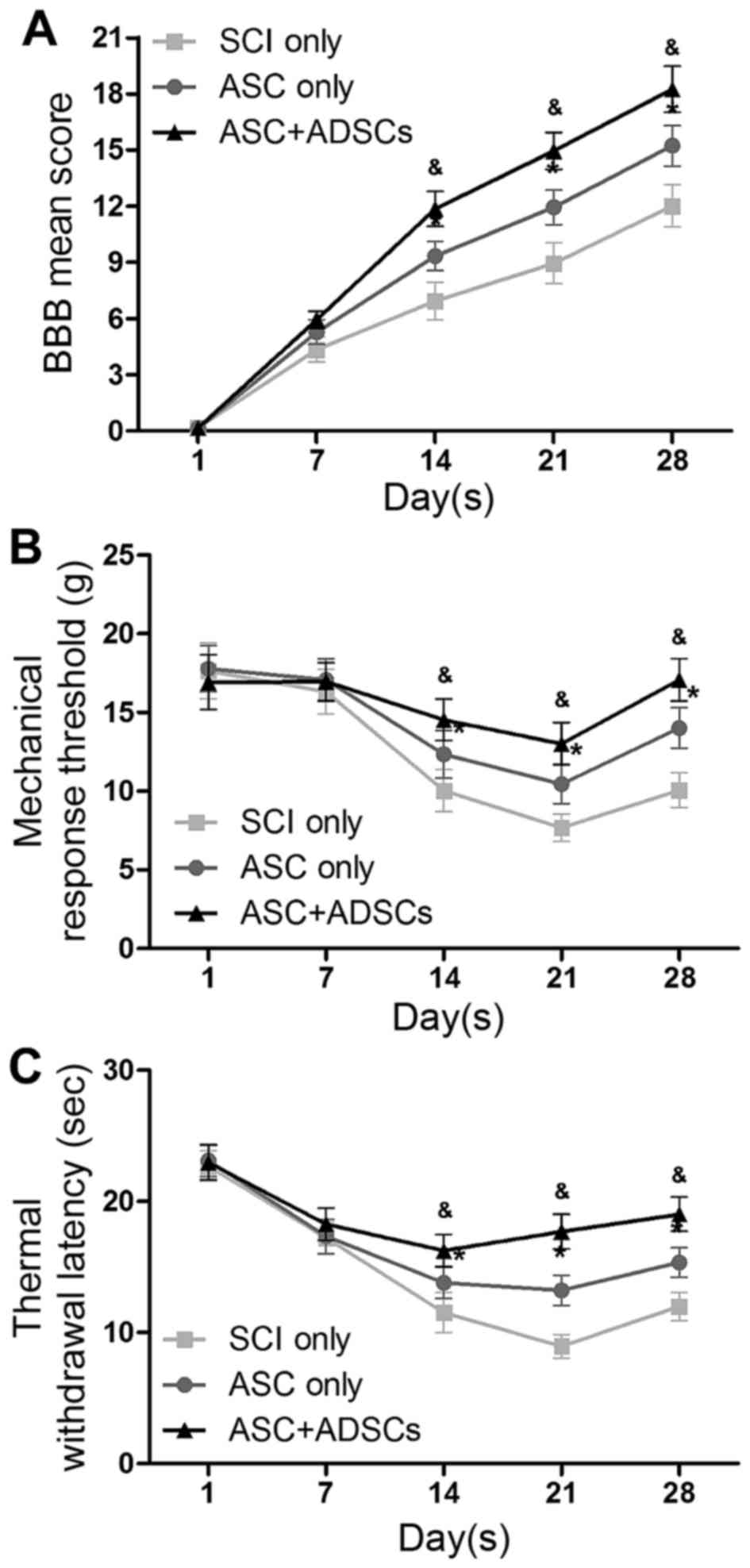
To explore the functional benefits with ASCs seeded
with rADSCs, BBB scores, hypersensitivity to mechanical and thermal
stimulation assays were performed on day 1, 7, 14, 21 and 28
post-SCI in three groups: SCI only, ASC only and ASC + ADSCs. The
data represented that rats in ASC + ADSCs group showed the best
improvement in locomotor recovery. Meanwhile, rats in ASC only
group represented better than those in SCI only group from day 14
after SCI (Fig. 2A). Moreover,
rats in ASC + ADSCs group depicted the same trend as locomotor
renovation in the other two functional tests of hypersensitivity to
mechanical and thermal stimulation assays (Fig. 2B and C). Together, the functional
tests data demonstrated that implantation of ASCs seeded with
rADSCs was able to enhance functional recovery after SCI in
rats.
Transplantation of ASCs seeded with
rADSCs facilitated histopathological rehabilitation
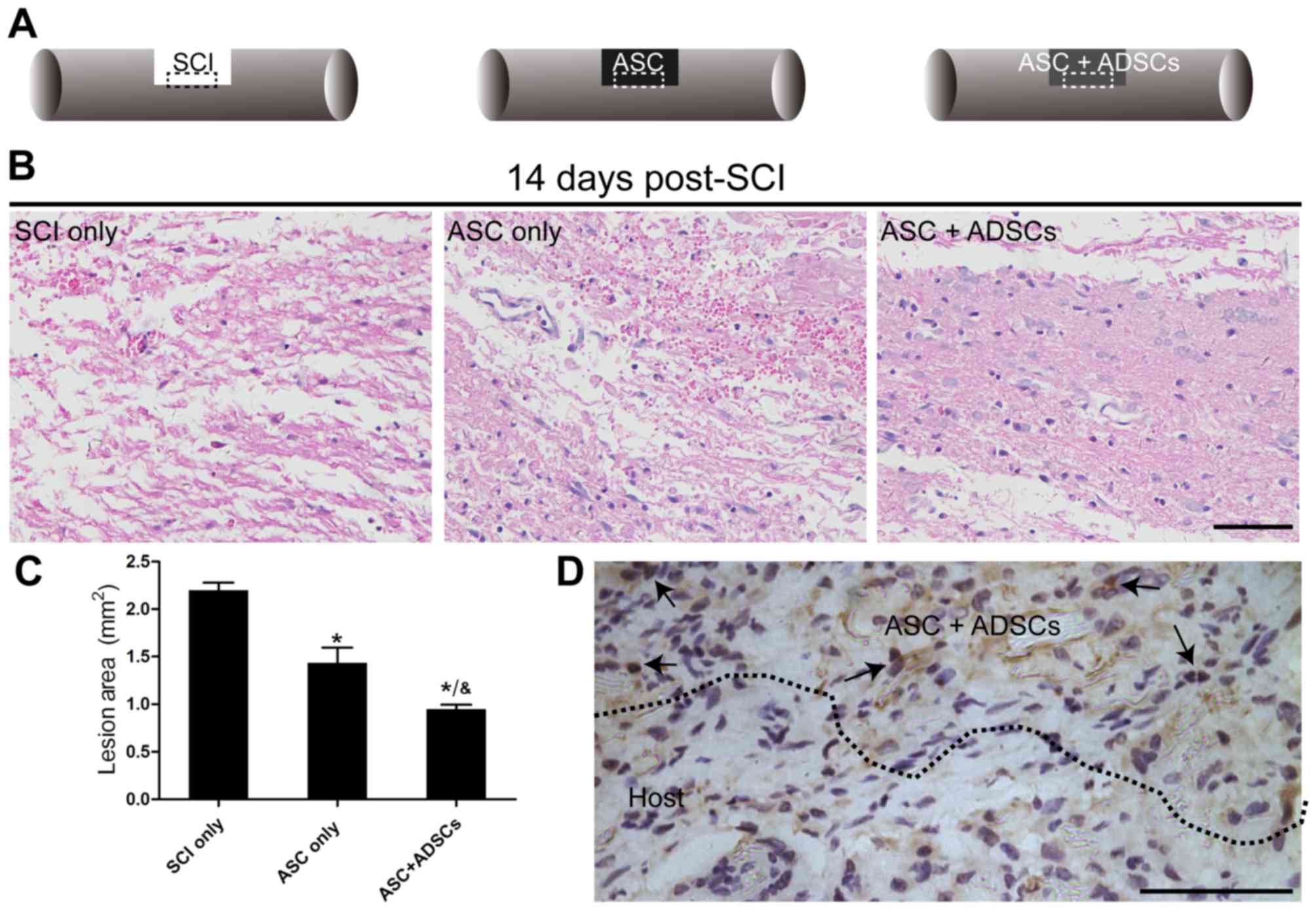
To illuminate the reason why functional recovery
improved greatly in rats receiving engraftment of ASCs seeded with
rADSCs, H&E staining was used to observe histopathological
changes on day 14 post-SCI. The images represented that rats in ASC
+ ADSCs group showed significant diminution and less atrophy of the
injured site than that in the ASConly and SCI only groups (Fig. 3B). Meanwhile, the semi-quantitative
data revealed the lesion area in ASC + ADSCs group decreased most
than that in the ASC only and SCI only groups. Furthermore, ASC
only group indicated better improvement than that in the SCI only
group (Fig. 3C). In addition, a
certain number of Brdu+ rADSCs survived in the grafted
ASCs and distributed over the host spinal cord in ASC + ADSCs group
on day 14 after SCI (Fig. 3D),
which was in line with previous study in hUCB-MSCs transplantation
(8).
Transplantation of ASCs seeded with
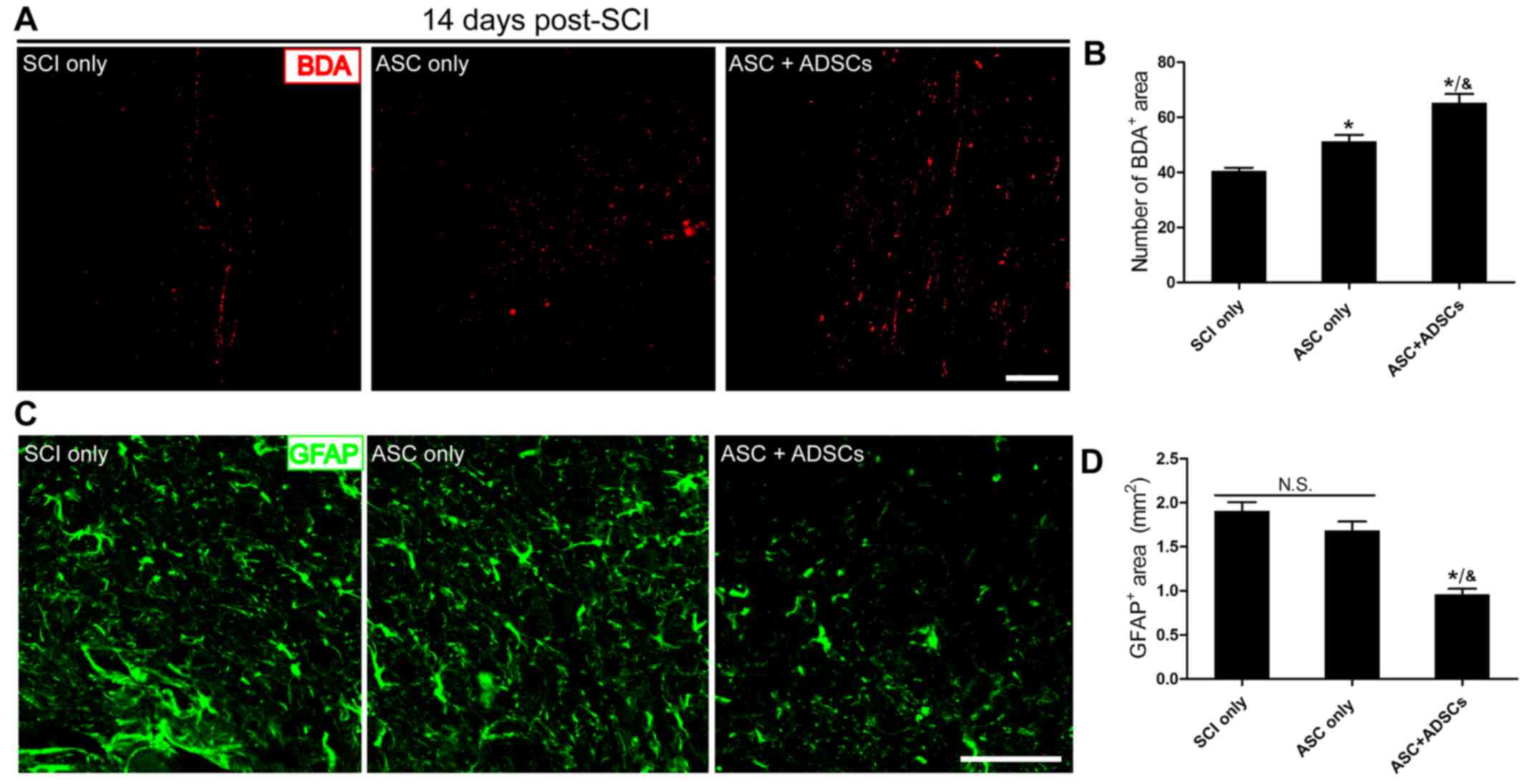
rADSCs promoted axon regeneration and reduced reactive gliosis
Our previous research implied that ASCs manufactured
by our colleagues might potentially support axon regeneration after
nervous system injury (6). Here,
we conducted BDA anterograde tracing with immunofluorescence
staining showing that transplantation of ASCs seeded with rADSCs
held the best improvement potential in harnessing axon regeneration
to bridge lesions in ASC + ADSCs group than that in the ASC only
and SCI only groups. BDA positive area in ASC only group was larger
than that in the SCI only group (Fig.
4A). Meanwhile, the semi-quantitative data indicated similar
tendency observed from Fig. 4A
(Fig. 4B). Moreover, we performed
GFAP staining to assess reactive gliosis in lesions. The results
indicated that GFAP+ area was much smaller in ASC +
ADSCs group than that in the ASC only and SCI only groups (Fig. 4C and D). Furthmore, there was no
obvious difference between ASC only group and SCI only group
(Fig. 4C and D). Taken together,
our data certified that ASCs manufactured by our colleagues could
support axon regeneration and implantation with ADSCs enhanced this
effectiveness after SCI. In addtion, it reduced reactive gliosis,
and might finally decreased glial scar formation when
transplantation ASCs seeded with ADSCs.
Discussion
In the present study, data indicated that ASCs
manufactured according to our procedures held the ability to
promote axon regeneratin in vivo, which enlarged the
application of ASCs based on our previous studies (6,7).
Here, we also introduced a new stem cell type, named ADSCs, for
transplantation in treatment of SCI, especially engraftment with
ASCs. Meanwhile, we preliminarily explored the underlying
mechanism, and results revealed that ASCs seeded with rADSCs
facilitated histopathological rehabilitation and axon regeneration,
while reduced reactive gliosis. Furthermore, our data indicated
that rADSCs were biocompatible with host spinal cord tissue, which
might be served as a cause resulting in functional improvement, and
survived rADSCs might guide axons sprouting into the distal lesions
in injuried spinal cord, therefore ultimately reinforced effective
reinnervation of target neurons.
ADSCs, one type of adult stem cells, are free from
ethical restriction associated with embryonic stem cells (ES) or
neural stem cells (NSCs) (23,24).
Furthermore, ADSCs are easily isolated and enriched from abundant
adipose tissue in the same subject with less damage, therefore,
ADSCs are more suitable for autologous transplantation (25). In addition, research has proved
that ADSCs easily escape from immune system surveillance like T
cells because of their surface antigens (14). As a result, ADSCs could serve as an
appropriate candidate for autografts, allografts, and even
xenografts (14,26). The beneficial effect resulting from
implantation of ASCs seeded with rADSCs needs to be explained.
Reasons why implantation of ASCs seeded with rADSCs
promote axon regeneration and reduce reactive gliosis can be
ascribed as follows: (1) rebalance
of local disturbed niche, (2)
physical support of allograft, (3)
neurotrophic factors secretion and (4) inflammation suppression. Excitotoxic
damage, high levels of the gelatinase and matrix
metalloproteinase-9 (MMP-9) often damages local microenvironment in
acute phase after SCI to elicit neuronal loss and severe locomotor
impairment (27,28). While, inflammation in subacute
phase usually increases vascular permeability and myeloid cell
infiltration to deteriorate locomotor networks (29–32).
Besides, glial scar formation is likely to jeopardize axon
outgrowth in chronic phase (33,34).
To conquer these factors above and look for a suitable candidate to
deal with them once for all, previous studies have revealed that
implantation of ADSCs rebuilds the local microenvironment by
secreting neurotrophins like brain derived neurotrophic factor
(BDNF), nerve growth factor (NGF), basic fibroblasts growth factor
(bFGF) and neurogenin 2 (35,36)
after CNS injury. Meanwhile, study also represents ADSCs
facilitates laminin secretion, which help restore local blood
vessels network (37). Moreover,
researchers have also discovered engraftment of ADSCs could
suppress local inflammation after stroke (38,39).
There must be some significant issues need to be
fully addressed in our future work. First, the mechanism of how
transplantation of ASCs seeded with ADSCs to sprout axon outgrowth
and the extent of axon outgrowth. Next, the function of ADSCs
implantation must be multifaceted, it is better to elucidate which
one is dominated in the recovery process. Third, to pursuit
excellent effectiveness, therapeutic window for engraftment needs
to be optimized as well. These are all vital questions need to be
issued in our future work.
In this study, we have demonstrated that
transplantation of ASCs seeded with ADSCs is a safe and feasible
strategy for cell replacement therapy in the treatment of SCI in
rats. The underlying mechanisms must be multifaceted, but at least
promoting axon outgrowth and reducing reactive gliosis are two main
factors to benefit functional renovation. Given that ADSCs are
easily harvested from abundant adipose tissue in the same subject
with less damage, so they hold a significant priority of ethical
restriction. Furthermore, our results indicate the use of ADSCs is
safe and effective, especially transplantation with ASCs, which
implies that the engraftment of ASCs seeded with ADSCs could be a
feasible therapeutic strategy for SCI.
Acknowledgements
This study was financed by grants from the National
Natural Science Foundation of China (81271362, 81471262).
References
|
1
|
Selvarajah S, Hammond ER and Schneider EB:
Trends in traumatic spinal cord injury. Jama. 314:16432015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubiano AM, Carney N, Chesnut R and Puyana
JC: Global neurotrauma research challenges and opportunities.
Nature. 527 Suppl:S193–S197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Requejo-Aguilar R, Alastrue-Agudo A,
Cases-Villar M, Lopez-Mocholi E, England R, Vicent MJ and
Moreno-Manzano V: Combined polymer-curcumin conjugate and ependymal
progenitor/stem cell treatment enhances spinal cord injury
functional recovery. Biomaterials. 113:18–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ban DX, Liu Y, Cao TW, Gao SJ and Feng SQ:
The preparation of rat's acellular spinal cord scaffold and
co-culture with rat's spinal cord neuron in vitro. Spinal cord.
55:411–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchida S, Hayakawa K, Ogata T, Tanaka S,
Kataoka K and Itaka K: Treatment of spinal cord injury by an
advanced cell transplantation technology using brain-derived
neurotrophic factor-transfected mesenchymal stem cell spheroids.
Biomaterials. 109:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo SZ, Ren XJ, Wu B and Jiang T:
Preparation of the acellular scaffold of the spinal cord and the
study of biocompatibility. Spinal Cord. 48:576–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang T, Ren XJ, Tang JL, Yin H, Wang KJ
and Zhou CL: Preparation and characterization of
genipin-crosslinked rat acellular spinal cord scaffolds. Mater Sci
Eng C Mater Biol Appl. 33:3514–3521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Chen J, Liu B, Yang C, Xie D, Zheng
X, Xu S, Chen T, Wang L, Zhang Z, et al: Acellular spinal cord
scaffold seeded with mesenchymal stem cells promotes long-distance
axon regeneration and functional recovery in spinal cord injured
rats. J Neurol Sci. 325:127–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu SH, Huang SH, Lo YC, Chai CY, Lee SS,
Chang KP, Lin SD, Lai CS, Yeh JL and Kwan AL: Autologous
adipose-derived stem cells attenuate muscular atrophy and protect
spinal cord ventral horn motor neurons in an animal model of burn
injury. Cytotherapy. 17:1066–1075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu S, Lu C, Han Q, Li J, Du Z, Liao L and
Zhao RC: Adipose-derived mesenchymal stem cells protect PC12 cells
from glutamate excitotoxicity-induced apoptosis by upregulation of
XIAP through PI3-K/Akt activation. Toxicology. 279:189–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomita K, Madura T, Sakai Y, Yano K,
Terenghi G and Hosokawa K: Glial differentiation of human
adipose-derived stem cells: Implications for cell-based
transplantation therapy. Neuroscience. 236:55–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lukovic D, Stojkovic M, Moreno-Manzano V,
Jendelova P, Sykova E, Bhattacharya SS and Erceg S: Concise review:
Reactive astrocytes and stem cells in spinal cord injury: Good guys
or bad guys? Stem Cells. 33:1036–1041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim Y, Jo SH, Kim WH and Kweon OK:
Antioxidant and anti-inflammatory effects of intravenously injected
adipose derived mesenchymal stem cells in dogs with acute spinal
cord injury. Stem Cell Res Ther. 6:2292015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HY, Lee HL, Yun Y, Kim JS, Ha Y, Yoon
DH, Lee SH and Shin DA: Human adipose stem cells improve mechanical
allodynia and enhance functional recovery in a rat model of
neuropathic pain. Tissue Eng Part A. 21:2044–2052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarveazad A, Babahajian A, Bakhtiari M,
Soleimani M, Behnam B, Yari A, Akbari A, Yousefifard M, Janzadeh A,
Amini N, et al: The combined application of human adipose derived
stem cells and chondroitinase ABC in treatment of a spinal cord
injury model. Neuropeptides. 61:39–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji WC, Zhang XW and Qiu YS: Selected
suitable seed cell, scaffold and growth factor could maximize the
repair effect using tissue engineering method in spinal cord
injury. World J Exp Med. 6:58–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komiyama S, Sakakura C, Murayama Y,
Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D,
Hujiwara H, et al: Adipose-derived stem cells enhance tissue
regeneration of gastrotomy closure. J Surg Res. 185:945–952. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choron RL, Chang S, Khan S, Villalobos MA,
Zhang P, Carpenter JP, Tulenko TN and Liu Y: Paclitaxel impairs
adipose stem cell proliferation and differentiation. J Surg Res.
196:404–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan J, Zou M, Xiang X, Zhu H, Chu W, Liu
W, Chen F and Lin J: Curcumin improves neural function after spinal
cord injury by the joint inhibition of the intracellular and
extracellular components of glial scar. J Surg Res. 195:235–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu R, Zhou J, Luo C, Lin J, Wang X, Li X,
Bian X, Li Y, Wan Q, Yu Y and Feng H: Glial scar and
neuroregeneration: Histological, functional and magnetic resonance
imaging analysis in chronic spinal cord injury. J Neurosurg Spine.
13:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prodromidou K, Papastefanaki F, Sklaviadis
T and Matsas R: Functional cross-talk between the cellular prion
protein and the neural cell adhesion molecule is critical for
neuronal differentiation of neural stem/precursor cells. Stem
Cells. 32:1674–1687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ide C, Nakano N and Kanekiyo K: Cell
transplantation for the treatment of spinal cord injury-bone marrow
stromal cells and choroid plexus epithelial cells. Neural Regen
Res. 11:1385–1388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nandoe Tewarie RD, Hurtado A, Levi AD,
Grotenhuis JA and Oudega M: Bone marrow stromal cells for repair of
the spinal cord: Towards clinical application. Cell Transplantat.
15:563–577. 2006. View Article : Google Scholar
|
|
25
|
Frese L, Dijkman PE and Hoerstrup SP:
Adipose tissue-derived stem cells in regenerative medicine.
Transfus Med Hemother. 43:268–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai L, Lennon DP, Eaton V, Maier K, Caplan
AI, Miller SD and Miller RH: Human bone marrow-derived mesenchymal
stem cells induce Th2-polarized immune response and promote
endogenous repair in animal models of multiple sclerosis. Glia.
57:1192–1203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazzone GL, Veeraraghavan P,
Gonzalez-Inchauspe C, Nistri A and Uchitel OD: ASIC channel
inhibition enhances excitotoxic neuronal death in an in vitro model
of spinal cord injury. Neuroscience. 343:398–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hansen CN, Norden DM, Faw TD, Deibert R,
Wohleb ES, Sheridan JF, Godbout JP and Basso DM: Lumbar myeloid
cell trafficking into locomotor networks after thoracic spinal cord
injury. Exp Neurol. 282:86–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anwar MA and Eid AH: Determination of
vascular reactivity of middle cerebral arteries from stroke and
spinal cord injury animal models using pressure myography. Methods
Mol Biol. 1462:611–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Na WH, Choi HY, Lee KH, Ju BG and
Yune TY: Jmjd3 mediates blood-spinal cord barrier disruption after
spinal cord injury by regulating MMP-3 and MMP-9 expressions.
Neurobiol Dis. 95:66–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haan N, Zhu B, Wang J, Wei X and Song B:
Crosstalk between macrophages and astrocytes affects proliferation,
reactive phenotype and inflammatory response, suggesting a role
during reactive gliosis following spinal cord injury. J
Neuroinflammation. 12:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Impellizzeri D, Ahmad A, Di Paola R,
Campolo M, Navarra M, Esposito E and Cuzzocrea S: Role of Toll like
receptor 4 signaling pathway in the secondary damage induced by
experimental spinal cord injury. Immunobiology. 220:1039–1049.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishii T, Ueyama T, Shigyo M, Kohta M,
Kondoh T, Kuboyama T, Uebi T, Hamada T, Gutmann DH, Aiba A, et al:
A novel rac1-gspt1 signaling pathway controls astrogliosis
following central nervous system injury. J Biol Chem.
292:1240–1250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruzicka J, Machova-Urdzikova L, Gillick J,
Amemori T, Romanyuk N, Karova K, Zaviskova K, Dubisova J, Kubinova
S, Murali R, et al: A comparative study of three different types of
stem cells for treatment of rat spinal cord injury. Cell
Transplant. 26:585–603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang L, Lu X, Zhu R, Qian T, Tao Y, Li K,
Zheng J, Zhao P, Li S, Wang X and Li L: Adipose-derived stem cells
expressing the neurogenin-2 promote functional recovery after
spinal cord injury in rat. Cell Mol Neurobiol. 36:657–667. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu XL, Zhang W and Tang SJ: Intracranial
transplantation of human adipose-derived stem cells promotes the
expression of neurotrophic factors and nerve repair in rats of
cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol.
7:174–183. 2014.PubMed/NCBI
|
|
37
|
Menezes K, Nascimento MA, Goncalves JP,
Cruz AS, Lopes DV, Curzio B, Bonamino M, de Menezes JR, Borojevic
R, Rossi MI and Coelho-Sampaio T: Human mesenchymal cells from
adipose tissue deposit laminin and promote regeneration of injured
spinal cord in rats. PloS one. 9:e960202014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeh DC, Chan TM, Harn HJ, Chiou TW, Chen
HS, Lin ZS and Lin SZ: Adipose tissue-derived stem cells in neural
regenerative medicine. Cell Transplant. 24:487–492. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang YC, Liu BS, Shen CC, Lin CH, Chiao MT
and Cheng HC: Transplantation of adipose tissue-derived stem cells
for treatment of focal cerebral ischemia. Curr Neurovasc Res.
8:1–13. 2011. View Article : Google Scholar : PubMed/NCBI
|