Introduction
Rheumatoid arthritis (RA) is a chronic and
debilitating systemic autoimmune disease, characterized by
inflammation and the destruction of the joints. It is reported that
~1% of the population suffers from RA worldwide, and a substantial
number of patients develop long-term joint damage, severe illness
and disability (1). The mechanisms
underlying RA are complex, including genetic and environmental
factors, and abnormalities of the innate and adaptive immune
systems. The activation and recruitment of immune cells,
particularly lymphocytes, neutrophils and monocytes, into joints
are major characteristics of RA (2).
Several studies have shown that T cells are involved
in the pathogenesis of RA at multiple levels (3–5).
Pivotal in the pathogenesis of RA is the production of pathogenic
autoantibodies, including rheumatoid factor (RF) and
anticitrullinated protein antibodies (ACPAs) (6,7).
Evidence from human studies and animal models indicate that
autoantibody production and the pathogenesis of RA are dependent
upon T cells (8–10). Previous studies have demonstrated
that T cells with abnormal costimulatory molecules can activate
autoantibody-producing B cells (11–13),
which suggests that costimulatory molecules are important in the
pathogenesis of RA. Therefore, determining abnormalities in
costimulatory molecule expression on immune cells is crucial for
understanding the mechanisms of RA (14–18).
Costimulatory molecules regulate the functional
outcome of T cell activation, and disturbance of the balance
between activating and inhibitory signals results in increased
susceptibility to the induction of autoimmunity. Programmed cell
death-1 (PD-1) is a 55-kDa transmembrane protein with 24% amino
acid homology to cytotoxic T lymphocyte antigen 4, a member of the
CD28 family (19,20). Upon binding with its physical
ligand, programmed death ligand 1 (PD-L1), PD-1 transmits an
inhibitory signal, which suppresses the activation and
proliferation of these PD-1-expressing cells, and protects tissues
from inflammatory or autoimmune attack (21).
Studies have shown that the PD-1 may be involved in
the pathogenesis of RA, however, this remain controversial
(14,22–24).
In the present study, the effect of PD-1 on RA was investigated by
comparing the expression of PD-1 on CD4+ T cells and
CD8+ T cells in the peripheral blood (PB) between
patients with RA and healthy controls. In addition, the expression
levels of PD-1 on CD4+ T cells and CD8+ T
cells were determined in the synovial fluid (SF) of patients with
RA. The correlation between PD-1 and the disease activity score 28
(DAS28) of the patients was also evaluated.
Subjects and methods
Subjects
Fresh PB was harvested by venipuncture from 81
patients with RA who fulfilled the American College of Rheumatology
criteria for RA (25), and from 30
healthy controls (HCs). Controls and RA patients were recruited
from The First Affiliated Hospital of Nanchang University
(Nanchang, China) from July 2015 to May 2016 The controls who were
unrelated to the patients and had no inflammatory or autoimmune
diseases. Among the RA patients, from the time at which the patient
complained of joint pain to the time of sample collection, a
duration of <6 months was defined as new onset rheumatoid
arthritis. SF was obtained from 33 of the 81 patients with RA. The
RA disease activity was measured using the DAS28 system (26). Patient details are exhibited in
Table I. The present study was
approved by the Ethics Committee of the First Affiliated Hospital
of Nanchang University and Jiujiang First People's Hospital (019;
Jiujiang, China), and was performed in compliance with the Helsinki
Declaration. Informed consent was obtained from all participants
prior to commencement of the study.
 | Table I.Baseline characteristics of patients
with RA and healthy controls. |
Table I.
Baseline characteristics of patients
with RA and healthy controls.
| Characteristic | Patients with RA
(n=81) | Healthy controls
(n=30) |
|---|
| Sex (female,
%) | 82.7 | 79 |
| Age (years) | 53.9±12.3 | 51±11 |
| DAS28 | 3.9±1.7 | – |
| RF (IU/ml) | 426.6±794.8 | – |
| ACPA (RU/ml) | 488.9±842.3 | – |
| CRP (mg/l) | 17.1±26.3 | – |
| ESR (mm/h) | 39.4±33.1 | – |
Flow cytometric analysis
The PB and SF samples were collected and analyzed
immediately to determine the molecular phenotypes of T lymphocytes
using flow cytometry. Prior to flow cytometry, the SF samples were
washed twice with D-HANKs. The following antibodies were used:
ECD-conjugated anti-CD3, PC5-conjugated anti-CD8, FITC-conjugated
anti-CD4 (Beckman Coulter, Inc., Brea, CA, USA), PE-conjugated
anti-PD1, and FITC-conjugated anti-PD1 (MIH clones; eBioscience,
San Diego, CA, USA). Briefly, 50 µl fresh, heparinized whole blood
were incubated simultaneously with 5 µl ECD-conjugated anti-CD3
(cat. no. IM2705U; undiluted), FITC-conjugated anti-CD4 (cat. no.
IM0448U; undiluted), 5 µl PC5-conjugated anti-CD8 (cat. no.
IM2638U; undiluted) and 5 µl PE-conjugated anti-PD1 (cat. no.
85-12-2799-42; undiluted) on ice in the dark for 30 min at room
temperature. Cells incubated with PE-conjugated mouse IgG (cat. no.
IM0670U; undiluted) were used as isotype controls. The red blood
cells were lysed with ammonium-chloride-potassium lysing buffer.
The lymphocytes were gated by forward scatter/side scatter, and the
T cell subsets were differentiated by CD3+ staining in
lymphocytes, following which CD4+ T cells were
identified based on CD4+ staining in T cells. The
CD8+ T cells were identified by CD8+ staining
in T cells. The samples were analyzed on a CYTOMICS FC 500 flow
cytometer (Beckman Coulter, Inc.) and associated software programs
(CXP 2.0).
Measurement of erythrocyte
sedimentation rate (ESR), C-reactive protein (CRP) and
autoantibodies
The ESR was determined according to the
manufacturer's instructions. CRP and RF were measured using
nephelometry with the IMMAGE 800 system (Beckman Coulter, Inc.).
The ACPAs of IgG in the serum were measured using commercially
available ELISA kits (Shanghai Kexin Biotech Co., Ltd., Shanghai,
China).
Statistical analysis
Statistical analysis and graphic presentation were
performed using GraphPad Prism, version 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). A Student's t-test was used for normally
distributed data; otherwise, the non-parametric Mann-Whitney test
was used to analyze the data. For evaluation of changes between the
PB and SF in the same patients, paired t-tests were performed.
Pearson's test was used for correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of study subjects
The characteristics of the patients with RA and HCs
enrolled in the present study are listed in Table I. No significant differences were
observed between the patients and HCs in terms of age or sex. The
patients with RA were classified into a remission group (DAS28
<2.6) and active group (DAS28 >2.6) according to the DAS28
system (26). Overall, 74.1% of
the patients with RA were in the active group. Among these, 13
cases were classified as new-onset RA (<6 months of disease
duration) (13). According to the
degree of disease activity, all patients received therapy with one
of or more disease-modifying antirheumatic drugs (DMARDs),
including hydroxychloroquine, sulphasalazine, methotrexate and
leflunomide.
Expression of PD-1 on CD4+
and CD8+ T cells is elevated in patients with RA
To understand the effect of PD-1 on RA, flow
cytometry was used to assess the expression of PD-1 on T cells,
including CD4+ and CD8+ T cells in the PB.
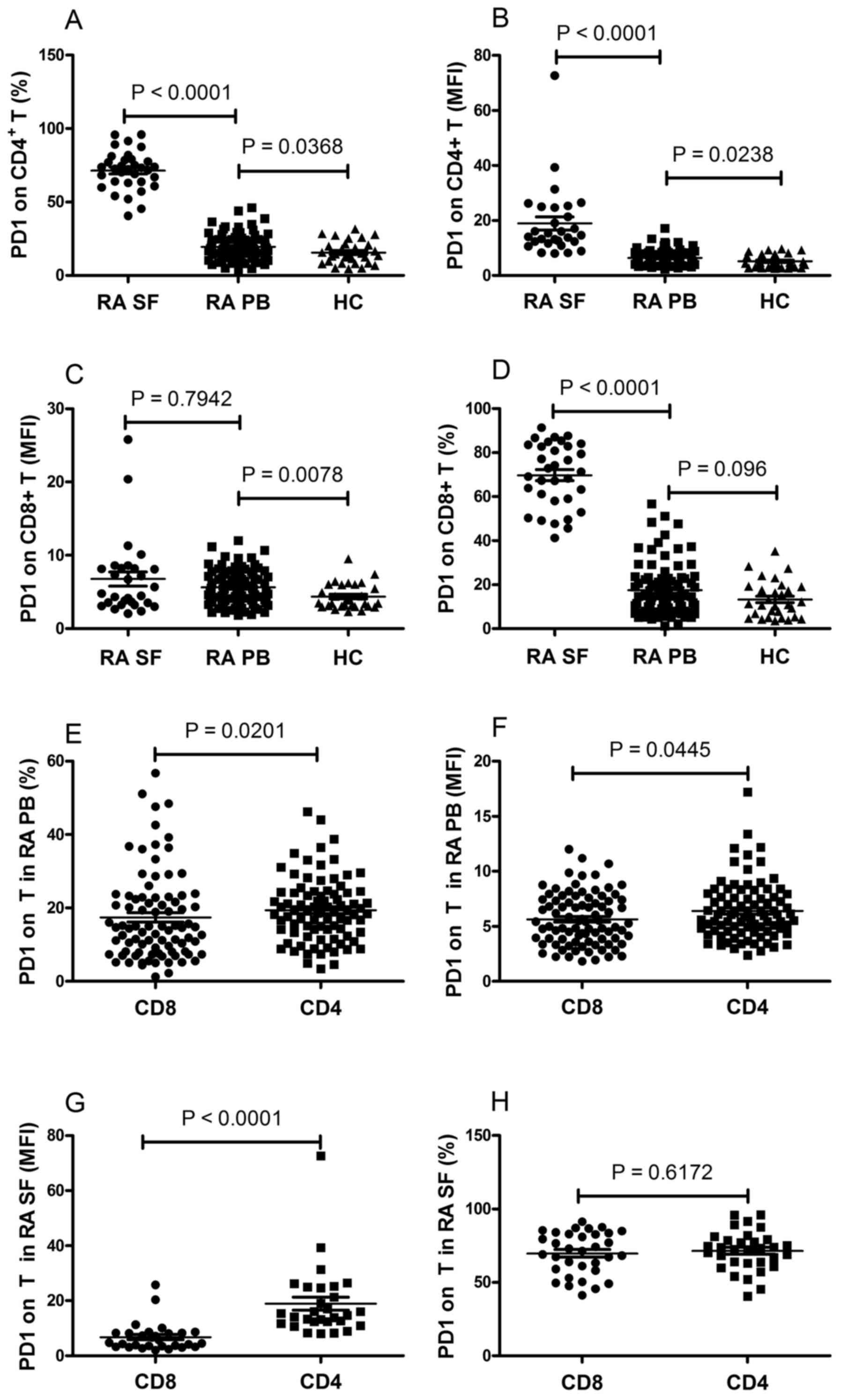
The resulting data showed that the frequency of PD-1-expressing
CD4+ T cells and the mean fluorescence intensity (MFI)
of PD-1 on CD4+ T cells were significantly elevated in
patients with RA, compared with those in the HCs (P<0.05;
Fig. 1A and B). The MFI of PD-1 on
CD8+ T cells were significantly elevated in patients
with RA, compared with HCs (P=0.0078; Fig. 1C). No significant differences were
observed in the frequency of PD-1-expressing CD8+ T
cells between the RA and HC groups (P=0.096; Fig. 1D). In addition, the frequency of
PD-1-expressing CD4+ T cells and the MFI of PD-1 on
CD4+ T cells were significantly elevated, compared with
CD8+ T cells in the PB of patients with RA (P<0.05;
Fig. 1E and F), whereas no
statistically significant differences were found between the
expression of PD-1 on CD4+ T cells and CD8+ T
cells in the PB of the HCs (data not shown).
Lymphocytes can be activated and recruited into
joints, and studies have reported that changes in the SF may
reflect the development and progression of RA more directly and
clearly (27,28). Therefore, the present study
investigated the expression of PD-1 on CD4+ and
CD8+ T cells in the SF of patients with RA. As shown in
Fig. 1A and B, the frequency of
PD-1-expressing CD4+ T cells and the MFI of PD-1 on
CD4+ T cells in the SF were significantly increased,
compared with those in the PB of patients with RA (P<0.0001).
The frequency of PD-1-expressing CD8+ T cells in the SF
was significantly increased, compared with that in the PB of the
patients with RA (P<0.0001; Fig.
1D). No significant difference was observed in the MFI of PD-1
on CD8+ T cells between the SF and PB of the patients
with RA (P=0.7942; Fig. 1C). It
was also found that the MFI of PD-1 on CD4+ T cells was
significantly elevated, compared with that of CD8+ T
cells in the SF of patients with RA (P<0.0001; Fig. 1G). However, no statistically
significant difference was found between the frequency of
PD-1-expressing CD4+ and CD8+ T cells in the
SF of the patients with RA (P=0.6172; Fig. 1H). The expression of PD-1 was also
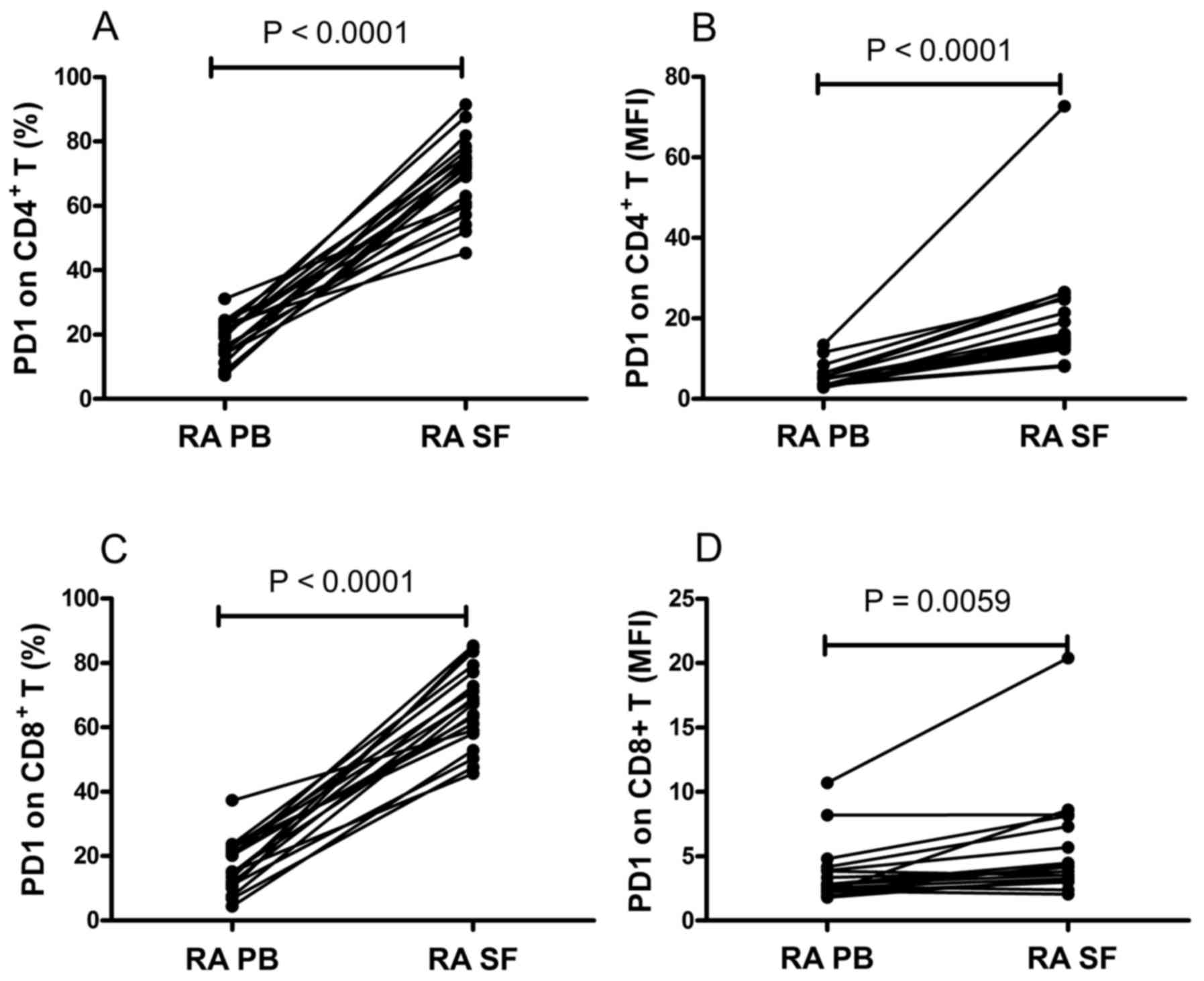
compared between the PB and SF from the same patients. It was found
that the frequency of PD-1-expressing CD4+ T cells and
the MFI of PD-1 on CD4+ T cells were significantly
increased in the SF, compared with those in the PB (P<0.0001;
Fig. 2A and B). Similarly, the
frequency of PD-1-expressing CD8+ T cells and the MFI of
PD-1 on CD8+ T cells in SF was significantly higher,
compared with those in the PB (P<0.01; Fig. 2C and D). These data suggested that
the expression of PD-1 was upregulated in the local environments of
RA.
Expression of PD-1 on T cells is
correlated with markers of the autoimmune response
RA is characterized by the overproduction of
auto-antibodies, including RF and ACPA. Therefore, the hallmark
antibodies of RA, RF and ACPA, were determined and analyzed in the
present study for their correlation with the expression of PD-1 on
CD4+ T and CD8+ T cells. The data showed that
62 patients were positive for RF and 60 patients were positive for
ACPA in the patients who received auto-antibody detection. As shown
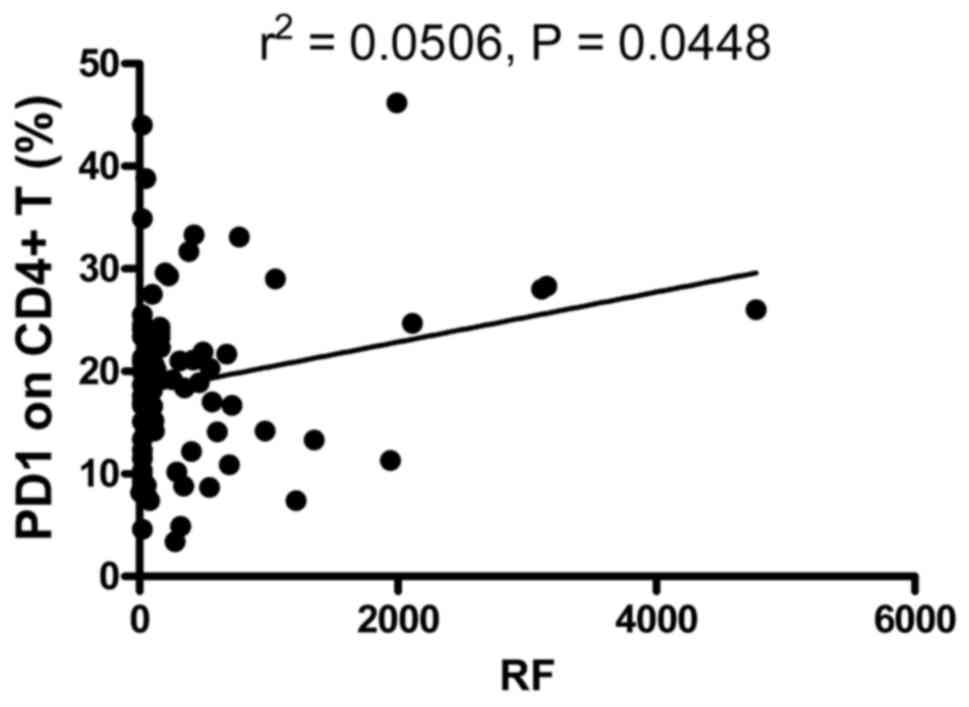
in Fig. 3, the frequency of
PD-1-expressing CD4+ T cells was positively correlated
with RF (r2=0.0506; P=0.0448). However, the frequency of
PD-1-expressing CD8+ T cells, and the MFI of PD-1 on
CD4+ T and CD8+ T cells were not correlated
with RF (data not shown). The expression levels of PD-1 on
CD4+ T and CD8+ T cells were elevated in the
patients with positive ACPA, however, this was not a significant
difference (data not shown). The correlations between the
expression of PD-1 on CD4+ T and CD8+ T cells
in the SF and the hallmark antibodies in the serum of RA, were
investigated, however, no correlation was found (data not shown).
These results showed that the elevated frequency of PD-1-expressing
CD4+ T cells was correlated with markers of the
autoimmune response, suggesting that the expression of PD-1 on
CD4+ T cells may be associated with the pathogenesis of
RA.
Correlation between the expression of
PD-1 on T cells and markers of inflammation
RA is characterized by synovial hyperplasia and
inflammation. Patients with RA are frequently found to have
elevated levels of inflammatory markers. In order to investigate
the correlation between the expression of PD-1 on T cells and
inflammatory markers, the present study analyzed the markers of
inflammation, ESR and CRP, for their correlation with the
expression of PD-1 on CD4+ T and CD8+ T cells
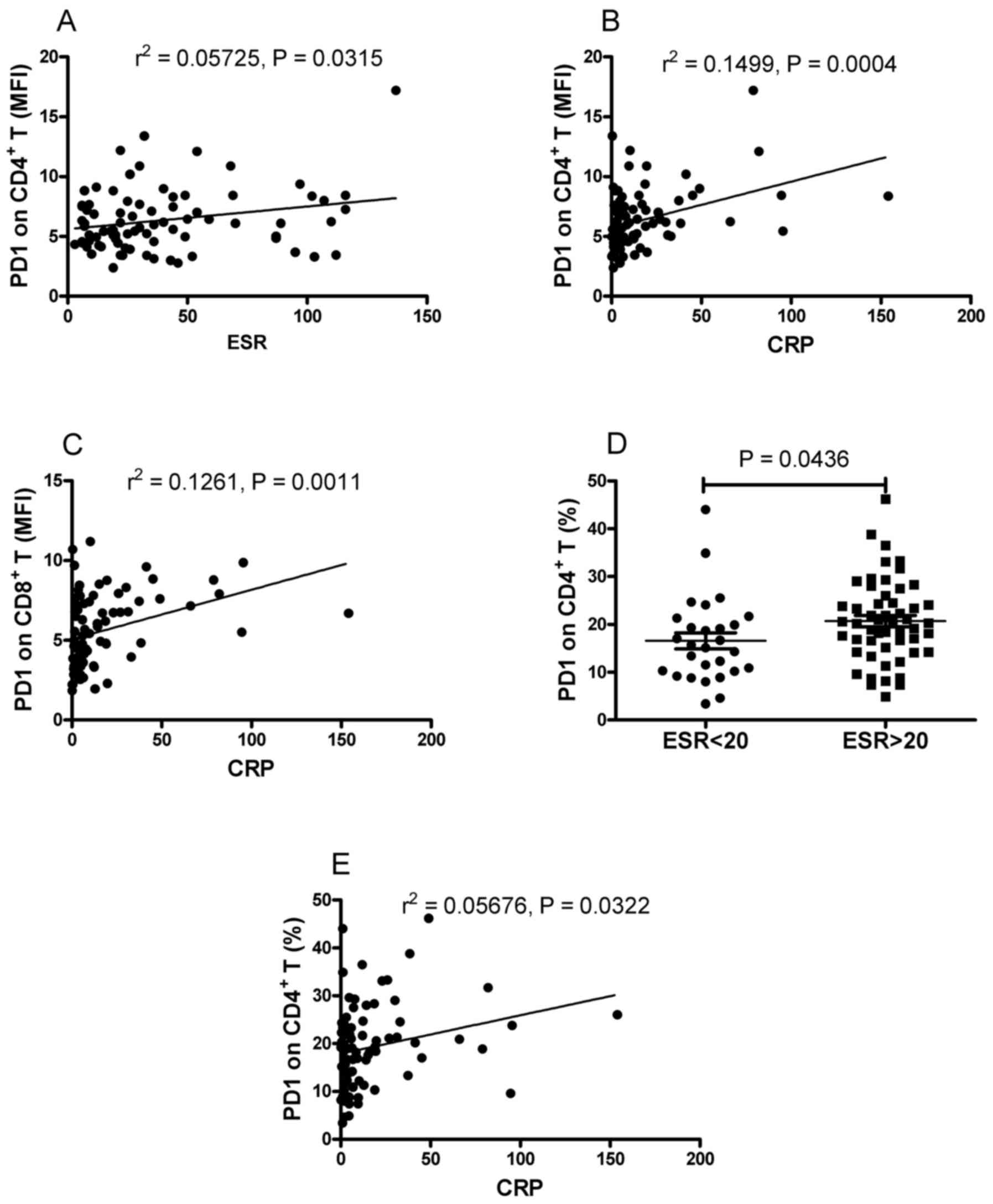
in the patients with RA. As shown in Fig. 4A and B, positive correlations were
found between the MFI of PD-1 on CD4+ T cells and the
ESR and CRP. It was also found that the MFI of PD-1 on
CD8+ T cells was positively correlated with CRP
(r2=0.1261; P=0.0011; Fig.
4C). However, no significant correlation was found between the
MFI of PD-1 on CD8+ T cells and ESR (data not shown).
The present study also examined the correlation between the
frequency of PD-1-expressing T cells and inflammatory markers in
RA. As shown in Fig. 4D, the
frequency of PD-1-expressing CD4+ T cells was
significantly increased in the patients with elevated ESR, compared
with that in patients with normal ESR (P=0.0436). It was also found
that the frequency of PD-1-expressing CD4+ T cells was
positively correlated with CRP (r2=0.05676, P=0.0322;
Fig. 4E). No correlation was found
between the frequency of PD-1-expressing CD8+ T cells
and ESR or CRP (data not shown). The present study investigated the
correlation between the expression of PD-1 on T cells in the SF and
inflammatory markers in the serum of patients with RA. No
significant correlation was found (data not shown). These results
indicated that the expression of PD-1 on T cells was associated
with markers of inflammation.
Correlation between the expression of
PD-1 on T cells and with disease activity of RA
The results described above indicated that the
expression of PD-1 on T cells was correlated with markers of the
autoimmune response and inflammation. A number of these markers,
including RF and ACPA, are reported to correlate with disease
activity and the severity of joint destruction in RA (29). ESR and CRP are valuable for
calculating DAS28, a scoring system used for assessing disease
severity in patients with RA. Therefore, patients with RA were
further classified into active and remission groups according to
the DAS28, and were analyzed for their correlation with the
expression of PD-1 on T cells and DAS28. The resulting data showed
that the MFI of PD-1 on CD4+ T cells in patients with
active RA was significantly higher, compared with that in patients
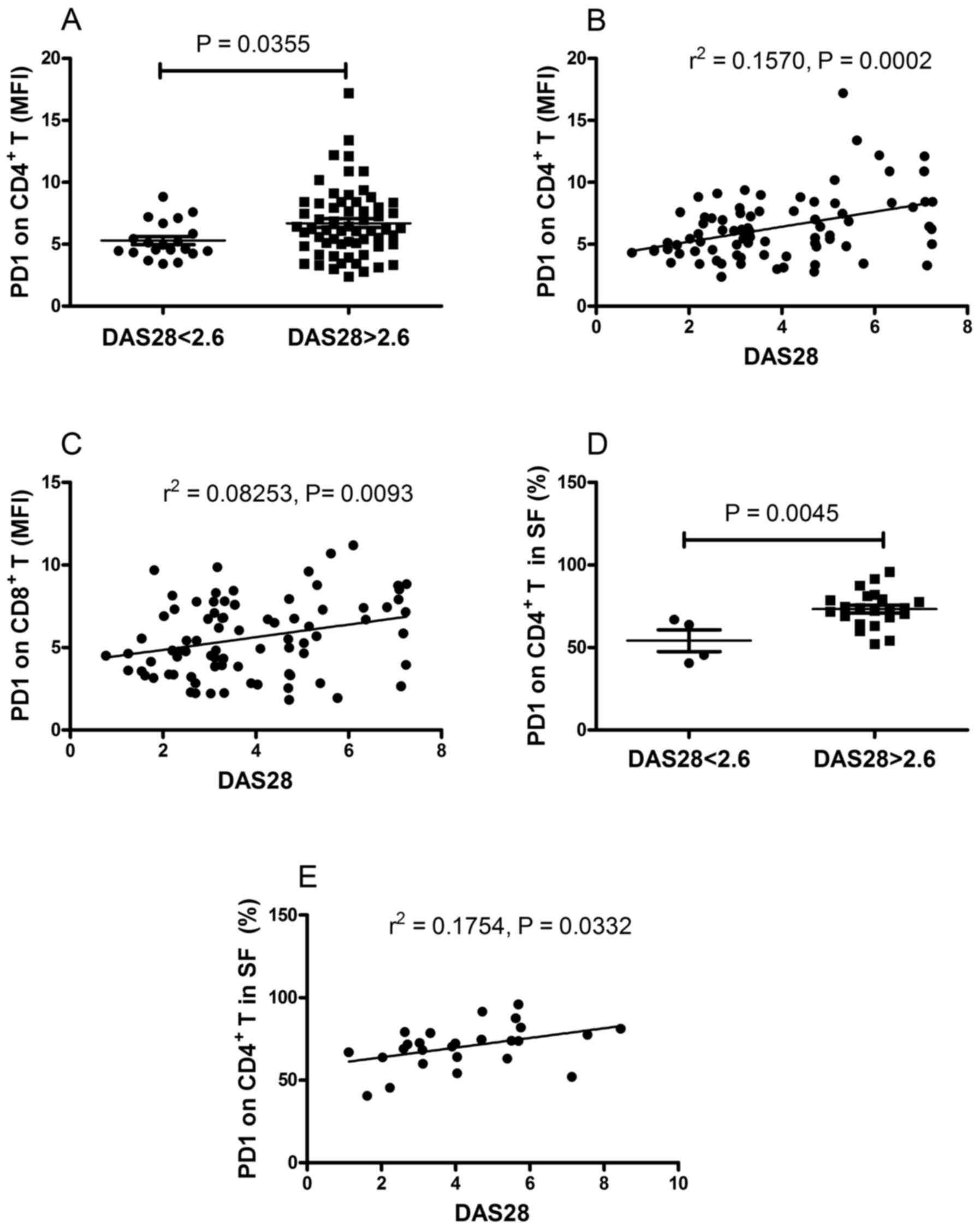
in the remission group (P=0.0355; Fig.
5A). Furthermore, it was found that there was a positive
correlation between the DAS28 score and the MFI of PD-1 on
CD4+ T cells (r2=0.1570, P=0.0002; Fig. 5B) and the MFI of PD-1 on
CD8+ T cells (r2=0.08253, P=0.0093; Fig. 5C). The present study also
investigated the correlation between the frequency of
PD-1-expressing T cells and DAS28, however, no significant
correlation was found (data not shown). The correlation between the
expression of PD-1 on T cells in the SF and DAS28 was also
examined. As shown in Fig. 5D, the
frequency of PD-1-expressing CD4+ T cells in patients
with active RA was significantly higher, compared with that in
patients in the remission group (P=0.0045). Furthermore, a positive
correlation was found between the frequency of PD-1-expressing
CD4+ T cells and DAS28 (r2=0.1754, P=0.0332;
Fig. 5E). No correlations were
found between DAS28 and the MFI of PD-1 on CD4+ T cells,
the MFI of PD-1 on CD8+ T cells, or the frequency of
PD-1-expressing CD8+ T cells (data not shown). These
results demonstrated that the expression of PD-1 on T cells
correlated with the disease activity of RA.
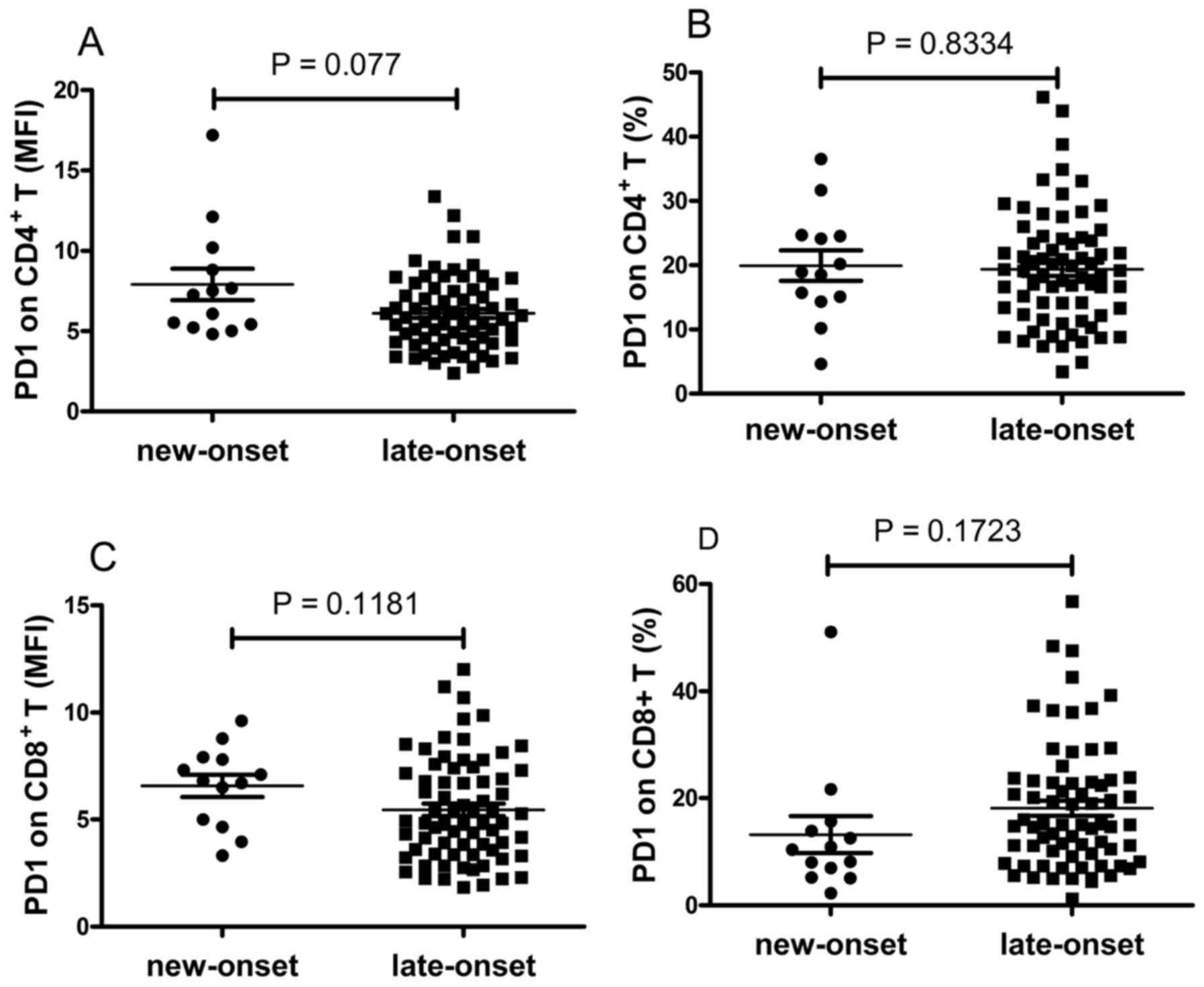
The expression levels of PD-1 on T cells were also
compared between the patients with new-onset and late-onset RA. The
data revealed that the expression of PD-1 on T cells was elevated
in patients with new-onset RA, however, this was not significant
(P>0.05; Fig. 6A-D).
Discussion
RA is a chronic debilitating systemic autoimmune
disease with unclear etiology. In the 2010 American College of
Rheumatology/European League Against Rheumatism classification
criteria (30), the ACPA, RF, CRP
and ESR are used to diagnose RA. However, these biomarkers show
weak correlation with disease activity in RA. Therefore, novel
biomarkers with improved correlation with the disease activity of
RA are required for the evaluation of curative effects or disease
development. It is well known that the expression of costimulatory
molecules is important in determining the activation status and
function of immune cells. Certain costimulatory molecules,
particularly immunosuppressive costimulatory molecules, including
PD1, PD-L1, T cell immunoglobulin and mucin domain-containing
molecule-3 (Tim-3) and T cell immunoreceptor with Ig and
immunoreceptor tyrosine-based inhibitory domains (TIGIT), have been
reported to be abnormally expressed on T cells, monocytes or
natural killer cells in patients with RA (14–18).
Several studies have investigated the role of the PD-1/PD-L1
pathway in human studies and animal models of RA. Studies have
shown that PD-1+ T cells are enriched in patients with
RA (14,22,23),
although another study reported conflicting results in which the
expression of PD-1 on T cells was significantly decreased in
patients with RA (24). The
present study investigated the expression of PD-1 on
CD4+ and CD8+ T cells in patients with RA,
and it was shown that the expression of PD-1 on T cells was
significantly increased in patients with RA, compared with that in
the healthy individuals. In addition, the expression levels of PD-1
on CD4+ and CD8+ T cells were examined in the
SF of patients with RA. It was shown that the expression of PD-1 on
T cells in the SF was significantly increased, compared with the
expression in the PB of the patients with RA or the healthy
controls. The present study also revealed that the expression of
PD-1 on T cells was associated with the disease activity of RA.
Lymphocytes are reported to be important in the
development and progression of RA (9,10).
In accordance with a previous study (23), the present study found that PD-1
was expressed at a higher level on CD4+ T cell than on
CD8+ T cells in the PB and SF of patients with RA. The
expression levels of PD-1 on CD4+ T and CD8+
T cells were significantly elevated in patients with RA, compared
with those in the healthy controls. Generally, the binding of PD-L1
to PD-1 transmits an inhibitory signal, which reduces the
proliferation of PD-1-expressing cells. Therefore, as with a
previous study (23), the results
of the present study suggested that CD4+ helper T cells
are the predominant targets of the PD-L1/PD-1 pathway. In addition,
the expression levels of PD-1 on CD4+ T and
CD8+ T cells in the SF were significantly increased
compared with those in the PB of patients with RA or healthy
controls. This supports observations that the abnormal expression
of key signaling molecules on T lymphocytes is important in the
pathogenesis of RA (31,32).
PD-1 is reported to be involved in interactions
between T cells and follicular dendritic cells to regulate B cell
responses and promote antibody production (8,33,34).
It is well known that RA is s systemic autoimmune disease
characterized by elevated autoimmune antibodies, including RF and
ACPA. In the present study, the serous levels of RF and ACPA were
determined and analyzed to examine their association with the
expression of PD-1 on T cells. The data revealed that the frequency
of PD-1-expressing CD4+ T cells was positively
correlated with RF in the serum of patients with RA, suggesting
that PD-1-expressing CD4+ T cells may be associated with
autoimmune responses in RA. However, although increased in patients
with positive ACPA, the correlation between the expression of PD-1
on T cells and ACPA titer was not statistically significant. As the
ACPA titer often correlates positively with disease activity and
the severity of joint destruction, and is decreased following
therapy with DMARDs, the poor correlation between the expression of
PD-1 on T cells and ACPA may be due to the fact that the majority
of patients with RA had received therapy prior to involvement in
the present study.
It is known that the autoimmune response is a type
of chronic inflammation against autoantigens. Therefore, the
correlations between the expression of PD-1 on T cells and
inflammatory markers were analyzed in the present study. The
results showed that the expression of PD-1 on T cells was
positively correlated with ESR and CRP. Considering ESR and CRP are
used for calculating the DAS28, the present study investigated the
correlation between the expression of PD-1 on T cells and DAS28,
and the results of the DAS28 scores of patients with RA were
concordant.
The present study also found that the expression
levels of PD-1 on T cells from the PB and SF of RA were
upregulated. In addition, the elevated expression of PD-1 on T
cells from the PB correlated with RF titer, inflammatory markers
and the disease activity of RA. However, the elevated expression of
PD-1 on T cells from the SF was not associated with RF titer or the
inflammatory markers of RA. It may be that the PB indicates the
systemic environment and the SF indicates the local environment in
RA.
PD-1 is an inhibitory costimulatory molecule, which
mediates inhibitory signals in immune cells (14). Consistent with its inhibitory
characteristics, the expression of PD-1 on T cells is decreased in
patients with RA, and negatively correlated with RA disease
activity (24). Evidence from
animal models has shown that PD-1−/− mice exhibit
increased incidence and severity of collagen-induced arthritis
(14). This suggests the increased
expression of PD-1 on T cells in RA appears controversial to its
function. However, there is also evidence suggesting that PD-1 may
be involved in regulating B cell responses and promoting antibody
production (33,34), which is consistent with the
observation that the levels of RA specific autoantibody, including
RF, were positively correlated with the frequency of
PD-1-expressing T cells. Therefore, in addition to its function as
an inhibitory costimulatory molecule, PD-1 may have other effects
in RA. Future investigations are required to clarify the roles and
mechanisms of PD-1-expressing T lymphocytes in the condition of
RA.
The present study had a number of limitations. For
example, the design of the study included a relatively small sample
size, and the PD-1 examined may have been affected by medications
and severe disease states. Therefore, certain critical values may
result from the small sample size (SF). Future investigations with
a larger sample size and using additional techniques may be
required to validate the results of this preliminary study.
In conclusion, the present study demonstrated that
the expression of PD-1 was upregulated on CD4+ T and
CD8+ T cells in RA systemically (PB) and locally (SF).
The results of the present study also established a correlation
between the expression of PD-1 on T cells and the disease activity
of RA, which may improve our understanding of the role of PD-1 in
the pathogenesis of RA.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360459) and the
Jiangxi Provincial Natural Science Foundation of China (grant no.
20151BAB215031). The authors would like to acknowledge the
assistance of Dr Rui Wu of the Department of Rheumatology, The
First Affiliated Hospital of Nanchang University.
Glossary
Abbreviations
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
RF
|
rheumatoid factor
|
|
anti-CCP
|
antibodies against cyclic
citrullinated peptides
|
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
programmed death ligand 1
|
|
SF
|
synovial fluid
|
|
PB
|
peripheral blood
|
|
DAS28
|
disease activity score 28
|
|
HCs
|
healthy controls
|
|
ESR
|
erythrocyte sedimentation rate
|
|
CRP
|
C-reactive protein
|
|
ACPAs
|
anticitrullinated protein
antibodies
|
|
DMARDs
|
disease modifying antirheumatic
drugs
|
|
MFI
|
mean fluorescence intensity
|
|
TIGIT
|
T cell immunoreceptor with Ig and
immunoreceptor tyrosine-based inhibitory domains
|
References
|
1
|
Goekoop-Ruiterman YP and Huizinga TW:
Rheumatoid arthritis: Can we achieve true drug-free remission in
patients with RA? Nat Rev Rheumatol. 6:68–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gravallese EM, Manning C, Tsay A, Naito A,
Pan C, Amento E and Goldring SR: Synovial tissue in rheumatoid
arthritis is a source of osteoclast differentiation factor.
Arthritis Rheum. 43:250–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shigeyama Y, Pap T, Kunzler P, Simmen BR,
Gay RE and Gay S: Expression of osteoclast differentiation factor
in rheumatoid arthritis. Arthritis Rheum. 43:2523–2530. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gracie JA, Forsey RJ, Chan WL, Gilmour A,
Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, et al: A
proinflammatory role for IL-18 in rheumatoid arthritis. J Clin
Invest. 104:1393–1401. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rech J, Hueber AJ, Finzel S, Englbrecht M,
Haschka J, Manger B, Kleyer A, Reiser M, Cobra JF, Figueiredo C, et
al: Prediction of disease relapses by multibiomarker disease
activity and autoantibody status in patients with rheumatoid
arthritis on tapering DMARD treatment. Ann Rheum Dis. 75:1637–1644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agrawal S, Misra R and Aggarwal A:
Autoantibodies in rheumatoid arthritis: Association with severity
of disease in established RA. Clin Rheumatol. 26:201–204. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He J, Tsai LM, Leong YA, Hu X, Ma CS,
Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al:
Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate
Tfh cell activity and promote antibody responses upon antigen
reexposure. Immunity. 39:770–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bennett JC: The role of T lymphocytes in
rheumatoid arthritis and other autoimmune diseases. Arthritis
Rheum. 58 Suppl 2:S53–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmdahl R, Klareskog L, Rubin K, Björk J,
Smedegård G, Jonsson R and Andersson M: Role of T lymphocytes in
murine collagen induced arthritis. Agents Actions. 19:295–305.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Godefroy E, Zhong H, Pham P, Friedman D
and Yazdanbakhsh K: TIGIT-positive circulating follicular helper T
cells display robust B-cell help functions: Potential role in
sickle cell alloimmunization. Haematologica. 100:1415–1425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao
C, Wu W, Chen J, Tong J, Yang M, et al: Increased frequency of
circulating follicular helper T cells in patients with rheumatoid
arthritis. Clin Dev Immunol. 2012:8274802012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma
L and Jiang Y: High frequencies of activated B cells and T
follicular helper cells are correlated with disease activity in
patients with new-onset rheumatoid arthritis. Clin Exp Immunol.
174:212–220. 2013.PubMed/NCBI
|
|
14
|
Raptopoulou AP, Bertsias G, Makrygiannakis
D, Verginis P, Kritikos I, Tzardi M, Klareskog L, Catrina AI,
Sidiropoulos P and Boumpas DT: The programmed death 1/programmed
death ligand 1 inhibitory pathway is up-regulated in rheumatoid
synovium and regulates peripheral T cell responses in human and
murine arthritis. Arthritis Rheum. 62:1870–1880. 2010.PubMed/NCBI
|
|
15
|
Moret FM, van der Wurff-Jacobs KM, Bijlsma
JW, Lafeber FP and van Roon JA: Synovial T cell hyporesponsiveness
to myeloid dendritic cells is reversed by preventing PD-1/PD-L1
interactions. Arthritis Res Ther. 16:4972014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Peng D, He Y, Zhang H, Sun H, Shan
S, Song Y, Zhang S, Xiao H, Song H and Zhang M: Expression of TIM-3
on CD4+ and CD8+ T cells in the peripheral blood and synovial fluid
of rheumatoid arthritis. APMIS. 122:899–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Hou H, Wu S, Tang Q, Liu W, Huang
M, Yin B, Huang J, Mao L, Lu Y and Sun Z: TIGIT expression levels
on human NK cells correlate with functional heterogeneity among
healthy individuals. Eur J Immunol. 45:2886–2897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flores-Borja F, Jury EC, Mauri C and
Ehrenstein MR: Defects in CTLA-4 are associated with abnormal
regulatory T cell function in rheumatoid arthritis. Proc Natl Acad
Sci USA. 105:pp. 19396–19401. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishimura H, Okazaki T, Tanaka Y, Nakatani
K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N
and Honjo T: Autoimmune dilated cardiomyopathy in PD-1
receptor-deficient mice. Science. 291:319–322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai S, Jia R, Zhang X, Fang Q and Huang L:
The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol.
290:72–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan B, Nie H, Liu A, Feng G, He D, Xu R,
Zhang Q, Dong C and Zhang JZ: Aberrant regulation of synovial T
cell activation by soluble costimulatory molecules in rheumatoid
arthritis. J Immunol. 177:8844–8850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatachi S, Iwai Y, Kawano S, Morinobu S,
Kobayashi M, Koshiba M, Saura R, Kurosaka M, Honjo T and Kumagai S:
CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid
arthritis synovial fluid. J Rheumatol. 30:1410–1419.
2003.PubMed/NCBI
|
|
24
|
Li S, Liao W, Chen M, Shan S, Song Y,
Zhang S, Song H and Yuan Z: Expression of programmed death-1 (PD-1)
on CD4+ and CD8+ T cells in rheumatoid arthritis. Inflammation.
37:116–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American rheumatism assocaition 1987 revised criteria
for the classification of rheumatoid arthritis. Arthr Rhuem.
31:315–324. 1988. View Article : Google Scholar
|
|
26
|
Prevoo ML, van't Hof MA, Kuper HH, van
Leeuwen MA, van de Putte LB and van Riel PL: Modified disease
activity scores that include twenty-eight-joint counts. Development
and validation in a prospective longitudinal study of patients with
rheumatoid arthritis. Arthritis Rheum. 38:44–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lajas C, Abasolo L, Bellajdel B,
Hernández-García C, Carmona L, Vargas E, Lázaro P and Jover JA:
Costs and predictors of costs in rheumatoid arthritis: A
prevalence-based study. Arthritis Rheum. 49:64–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan FL, Li X, Lu WG, Li CW, Xu RS and
Dong J: IL-33: A promising therapeutic target for rheumatoid
arthritis? Expert Opin Ther Targets. 15:529–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wright HL, Moots RJ and Edwards SW: The
multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev
Rheumatol. 10:593–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen S and Emery P: The American College
of Rheumatology/European League Against Rheumatism Criteria for the
classification of rheumatoid arthritis: A game changer. Ann Rheum
Dis. 69:1575–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carruthers DM, Arrol HP, Bacon PA and
Young SP: Dysregulated intracellular Ca2+ stores and
Ca2+ signaling in synovial fluid T lymphocytes from
patients with chronic inflammatory arthritis. Arthritis Rheum.
43:1257–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malemud CJ: Dysfunctional immune-mediated
inflammation in rheumatoid arthritis dictates that development of
anti-rheumatic disease drugs target multiple intracellular
signaling pathways. Antiinflamm Antiallergy Agents Med Chem.
10:78–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Good-Jacobson KL, Szumilas CG, Chen L,
Sharpe AH, Tomayko MM and Shlomchik MJ: PD-1 regulates germinal
center B cell survival and the formation and affinity of long-lived
plasma cells. Nat Immunol. 11:535–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu R, Wu Q, Su D, Che N, Chen H, Geng L,
Chen J, Chen W, Li X and Sun L: A regulatory effect of IL-21 on T
follicular helper-like cell and B cell in rheumatoid arthritis.
Arthritis Res Ther. 14:R2552012. View
Article : Google Scholar : PubMed/NCBI
|