Introduction
Dickkopf Wnt signaling pathway inhibitor 3 (Dkk-3)
is a member of the dickkopf (Dkk) family, and is also known as REIC
due to its reduced expression in immortalized cells (1). Overexpression of REIC/Dkk-3 using an
adenovirus vector has been demonstrated to induce growth
suppression and/or apoptosis in a variety of cancer cells (2,3).
Other Dkk family members, including Dkk-1, Dkk-2 and Dkk-4,
interfere with the Wnt signaling pathway (4); however, the physiological function of
REIC/Dkk-3 remains unclear. Previous studies investigating the
expression pattern of REIC/Dkk-3 in normal and pathological skin
tissues have demonstrated that the expression levels of REIC/Dkk-3
were evidently reduced, not only in skin cancer cells, but also in
the normal skin keratinocytes surrounding cancer nodules (5,6). In
addition, the level of REIC/Dkk-3 expression was reduced in normal
skin keratinocytes under inflammatory conditions (5). Other researchers also reported that
negative or very low expression of REIC/Dkk-3 was observed in
cutaneous squamous cell carcinoma tissues (7). However, the role of REIC/Dkk-3 in
normal and/or cancer skin tissues is still unclear. Furthermore,
cornified skin tissues were observed to express REIC/Dkk-3 at
varying levels (6). These previous
findings indicate that normal and/or cancer cells secrete a
factor(s) that regulates REIC/Dkk-3. These unknown regulators of
REIC/Dkk-3 expression may be potential therpeutic targets for skin
cancer. Therefore, the aim of the present study was to identify the
factors involved in the regulation of REIC/Dkk-3 in normal skin
keratinocytes.
Materials and methods
Reagents
Recombinant human epidermal growth factor (EGF),
transforming growth factor-β (TGF-β), tumor necrosis factor-α
(TNF-α) and interleukin (IL)-6 were purchased from Peprotech, Inc.
(Rocky Hill, NJ, USA). Recombinant IL-1F9 and IL-8 were purchased
from R&D Systems, Inc. (Minneapolis, MN, USA).
Animals
A total of 12 female C57BL/6 mice (age, 6–8 weeks;
body weight, 16–22 g) were purchased from Clea Japan, Inc.
(Hamamatsu, Japan) and maintained at 18–23°C with 40% humidity and
a 12 h light/12 h dark cycle. Mice were fed with a normal mouse
diet supplied by Clea Japan, Inc. and sacrified using excess amount
of the anesthetic drug. For anesthesia, 2,2,2-tribromoethanol (cat.
no. T1420; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was
injected intraperitoneally at a dose of 200 mg/kg body weight.
Animal experiments were approved and performed in accordance with
the guidelines of Okayama University (Okayama, Japan; permit no.
OKU-2011105).
Cell culture
Normal human keratinocytes (NHKs) were purchased
from Kurabo Industries, Ltd. (cat. no. KK-4009; Osaka, Japan) and
cultured in HuMedia-KG2 (Kurabo Industries, Ltd.). NHKs were
maintained at 37°C with 5% CO2 and incubated with 10
ng/ml of a specific neutralizing antibody against TNF-α (cat. no.
D2H4; monoclonal rabbit antibody; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 24 h to abrogate TNF-α activity. Treatment of
NHKs with the aforementioned recombinant protein factors was
achieved by culturing NHKs with various concentrations of EGF (0,
10, 50 and 100 ng/ml), TGF-β (0, 1, 5, 10 ng/ml), TNF-α (0, 10, 50
and 100 ng/ml), IL-6 (0, 10, 50 and 100 ng/ml), IL-8 (0, 10, 50 and
100 ng/ml), IL-1F9 (0, 10, 50 and 100 ng/ml) and Ca2+
(0, 0.5, 1.5 and 5 mM) for 24 h. The concentrations used in the
experiments were determined by previous studies (8,9).
Tissue culture
Mice were clipped and skin tissue was collected from
the back using an 8 mm biopsy punch (Maruho, Co., Ltd., Osaka,
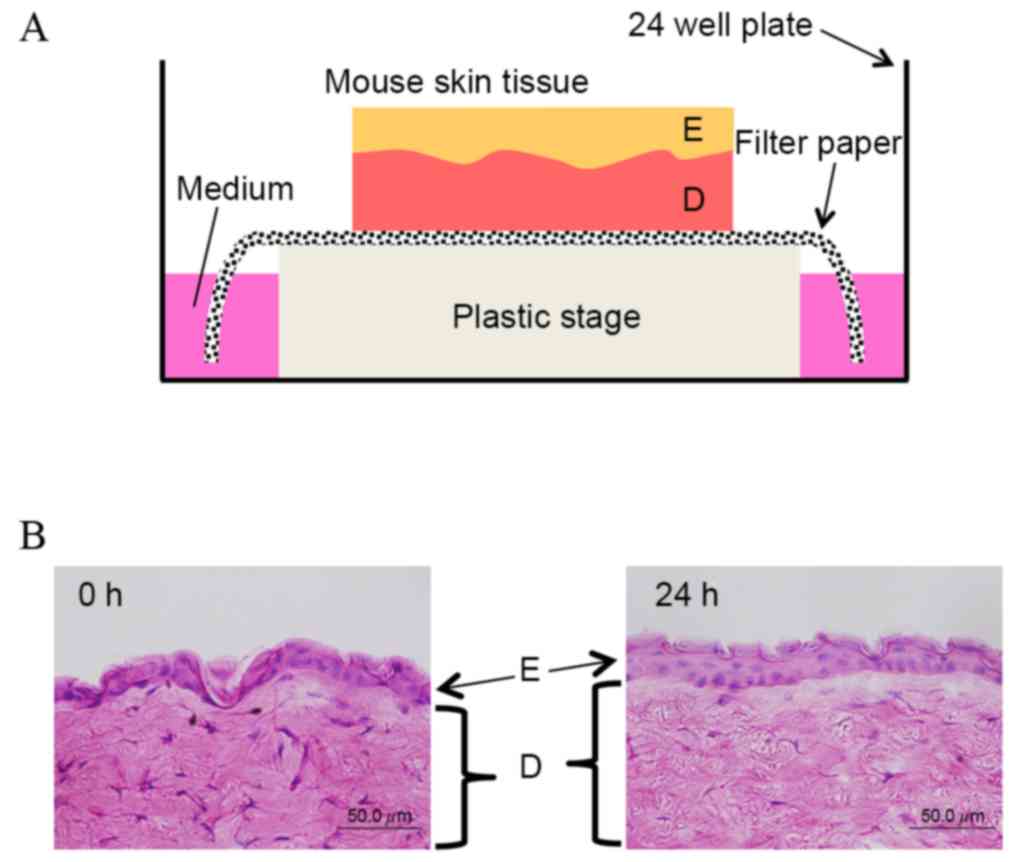
Japan). As shown in Fig. 1A, the
mouse skin tissue was then placed on a piece of filter paper
(Advantec MFS, Inc., Tokyo, Japan), and both edges of the paper
were immersed in Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum, 100 µg/ml kanamycin (Meiji Seika Pharma Co., Ltd.,
Tokyo, Japan) and 0.5 µg/ml amphotericin B (Gibco;Thermo Fisher
Scientific, Inc.). Hematoxylin and eosin staining of frozen tissue
sections was performed using conventional methods (5,6).
Preliminary experiments demonstrated that the structure of skin
tissue on the filter paper was maintained for 24 h (Fig. 1B). Skin tissue extracts were
treated without or with 100 ng/ml recombinant TNF-α. Hair follicles
were plucked from the mouse upper lip with tweezers and incubated
in the ø 35 mm culture dish (Corning Incorporated, Corning, NY,
USA) without or with 100 ng/ml TNF-α in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum, 100 µg/ml
kanamycin and 0.5 µg/ml amphotericin B for 24 h.
Immunocytochemical and
immunohistochemical analyses
Immunocytochemical detection of REIC/Dkk-3 in NHKs
cultured on glass slide culture vessels (Thermo Fisher Scientific,
Inc.) and tissue culture specimens was conducted as described
previously (5,6). Briefly, samples were fixed in cold
acetone for 10 min, washed with phosphate-buffered saline
containing 0.05% Tween 20 (PBST), and incubated with a blocking
solution (10% skim milk, 6% glycine and 0.01 N KOH in PBST) at room
temperature for 1 h. Subsequently, the slides were incubated with
goat polyclonal antibody against REIC/Dkk-3 (cat. no. AF1118;
R&D Systems, Inc.) at a 1:50 dilution at room temperature for 1
h. Subsequent to washing with PBST, the tissue sections were again
incubated with the blocking solution, followed by probing with
polyclonal donkey antibody against goat IgG (H+L) labeled with
Alexa Fluor 488 dye (cat. no. A11055; Thermo Fisher Scientific,
Inc.) at a 1:500 dilution. After washing with PBST, the tissue
sections were mounted using VECTASHIELD with DAPI (Vector
Laboratories, Inc., Burlingame, CA, USA). Normal goat IgG was used
as a negative control for the primary antibody.
Preparation of protein lysates and
western blot analysis
Protein extracts (10 µg) were obtained by lysing
cells in mammalian protein extraction reagent (M-PER; Thermo Fisher
Scientific, Inc.), and were subsequently used for western blot
analysis. Protein from the tissue culture medium was prepared as
described previously (10).
Briefly, the culture medium was mixed with an equal volume of
acetone and incubated at −20°C for 24 h. Protein in the medium was
precipitated by centrifugation at 12,000 × g for 20 min at 4°C and
the pellet was reconstituted into the same volume (~50 µl) of M-PER
as that of the cell extracts. The concentration of protein extracts
from the cell lysates and the tissue culture medium was determined
using a Bio-Rad protein assay (cat. no. 500-0006JA; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the same volume of
protein extract (10 µg protein extract) obtained from both samples
was used for western blot analysis. Western blot analysis was
performed using 10 µg of protein extracts and the procedures
described previously (5). Briefly,
extracted protein samples were separated by 4–20% polyacrylamide
gel electrophoresis and transferred onto polyvinylidene difluoride
membranes (GE Healthcare Life Sciences, Chalfont, UK). After
blocking in 10% skim milk, the membranes were incubated with
primary antibodies at a 1:500 dilution at 4°C overnight. The
membranes were then washed with PBS, incubated with secondary
antibodies at a 1:1,000 dilution at room temperature for 1 h and
rinsed with PBS. The antibodies used were as follows: Rabbit
anti-REIC/Dkk-3 antibody raised in our laboratory (Okayama
University of Science, Okayama, Japan), monoclonal mouse
anti-tubulin antibody (cat. no. T5168; Sigma-Aldrich, St Louis, MO,
USA), and horseradish peroxidase-linked anti-rabbit IgG secondary
antibody (cat. no. 7074; Cell Signaling Technology, Inc.) or
anti-mouse IgG secondary antibody (cat. no. 7076; Cell Signaling
Technology, Inc.). Signals were visualized using the Enhanced
Chemiluminescence Plus detection reagent (GE Healthcare Life
Sciences).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from cultured NHKs using the
SV Total RNA Isolation system (Promega Corporation, Madison, WI,
USA) and pretreated with DNase I according to the manufacturer's
instructions. RT to generate cDNA was performed using the
SuperScript II First-Strand Synthesis system (Thermo Fisher
Scientific, Inc.). Total RNA was incubated with oligo dT primer,
dNTP mixture and reverse transcriptase at 42°C for 50 min. PCR
analysis of human REIC/Dkk-3 mRNA expression levels was conducted
using isolated RNA (10 µg) as described previously (11). Briefly, cDNA was amplified by ExTaq
(cat. no. RR001; Takara Bio, Inc., Otsu, Japan) under the following
conditions: Initial incubation at 94°C for 4 min followed by 30
cycles at 94°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec, and
then a final step at 72°C for 5 min. GAPDH was used as an internal
control. The primers used for PCR analysis were as follows: Human
REIC/Dkk-3, forward 5′-CAGTTATCACATCTGTGGGAGACGAA-3′ and reverse
5′-AACTTCATACTCATCGGGGACCTCT-3′; GAPDH, forward
5′-GGGTGTGAACCATGAGAAGTATGA-3′ and reverse
5′-TGCTAAGCAGTTGGTGGTGC-3′. The PCR products were examined for
specificity via 1.5% agarose gel electrophoresis and visualized by
ethidium bromide.
Results
Screening of factors regulating
REIC/Dkk-3 expression in human keratinocytes
In order to identify factors that regulate
REIC/Dkk-3 expression in normal human skin keratinocytes, NHK cells
were treated with growth factors and cytokines that are reportedly
involved in keratinocyte growth and differentiation, including EGF,
TGF-β, TNF-α, IL-1F9, IL-6, IL-8 and Ca2+ (8,9). The
protein expression levels were then determined by western blot
analysis (12,13). As shown in Fig. 2, among these seven factors, only
TNF-α was observed to downregulate REIC/Dkk-3 protein expression
levels in NHKs. Downregulation of REIC/Dkk-3 by TNF-α was observed
in both the cell extracts and tissue culture medium. IL-8 treatment
appeared to increase REIC/Dkk-3 expression in the cell extract, but
not in the culture medium.
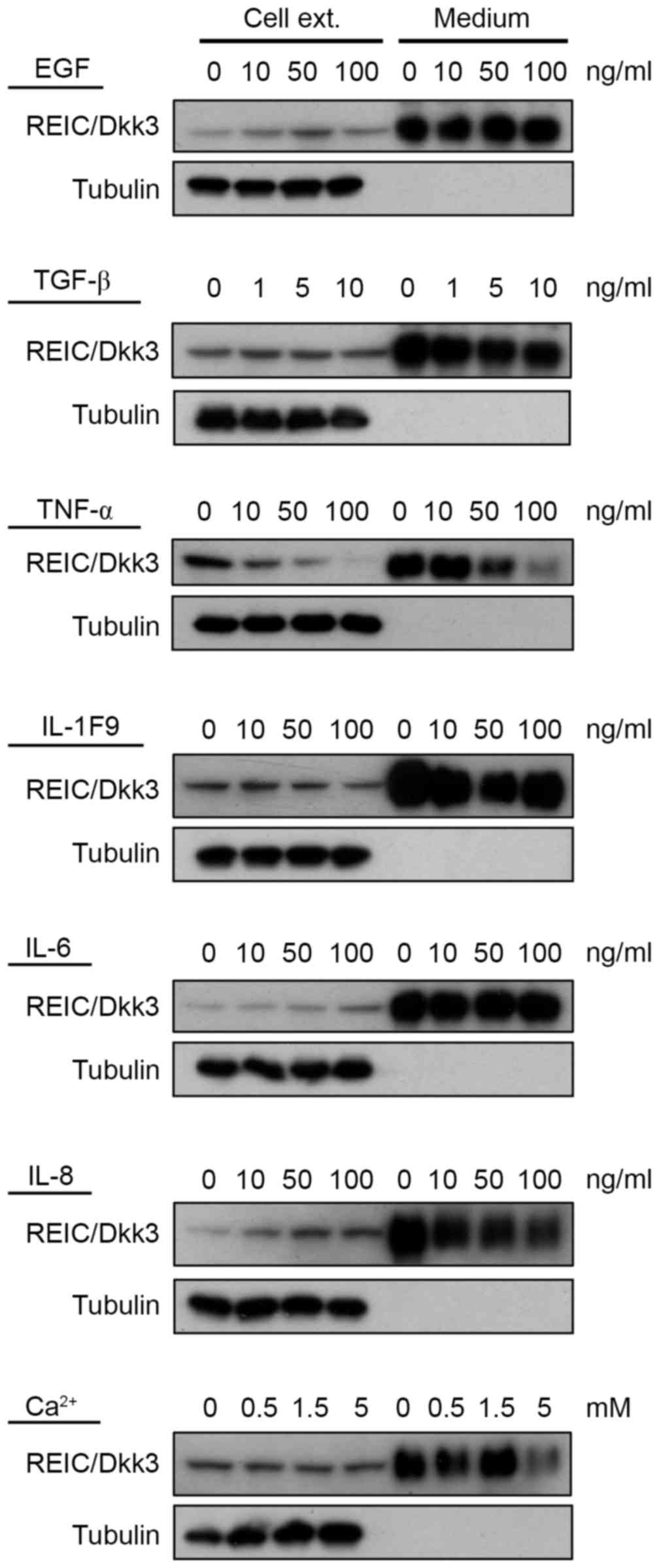 | Figure 2.Identification of factors that
regulate REIC/Dkk3 in NHKs and mouse skin tissue extracts. Western
blot analysis was used to determine the REIC/Dkk-3 protein
expression in cultured NHK cells following treatment with EGF,
TGF-β, TNF-α, IL-1F9, IL-6, IL-8 and Ca2+. Tubulin was
used as a control for the amount of protein in preparations. Cell
ext., culture cell extract; Medium, culture medium; REIC/Dkk-3,
dickkopf Wnt signaling pathway inhibitor 3; NHK, normal human
keratinocyte; EGF, epidermal growth factor; TGF-β, transforming
growth factor-β; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
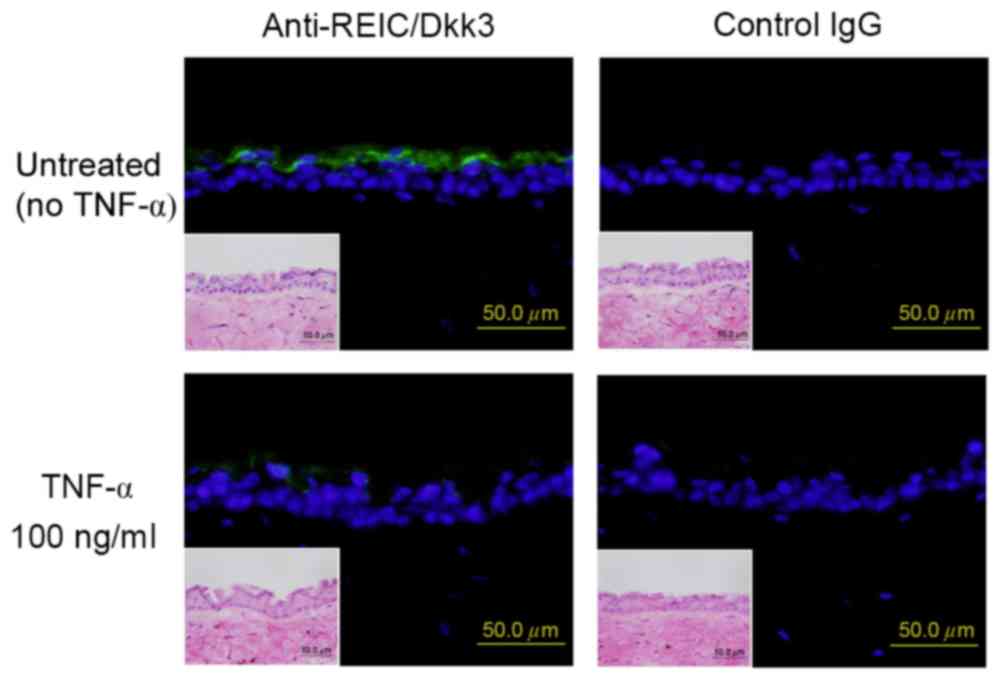
Downregulation of REIC/Dkk-3 in skin
tissues by TNF-α
Since the study identified that TNF-α treatment was
able to reduce the expression of REIC/Dkk-3, its effect in tissue
culture models of mouse skin were further investigated. Following
in vitro incubation with 100 ng/ml TNF-α for 24 h,
REIC/Dkk-3 expression in the mouse epidermis was downregulated when
compared with that in the untreated epidermis tissue extracts
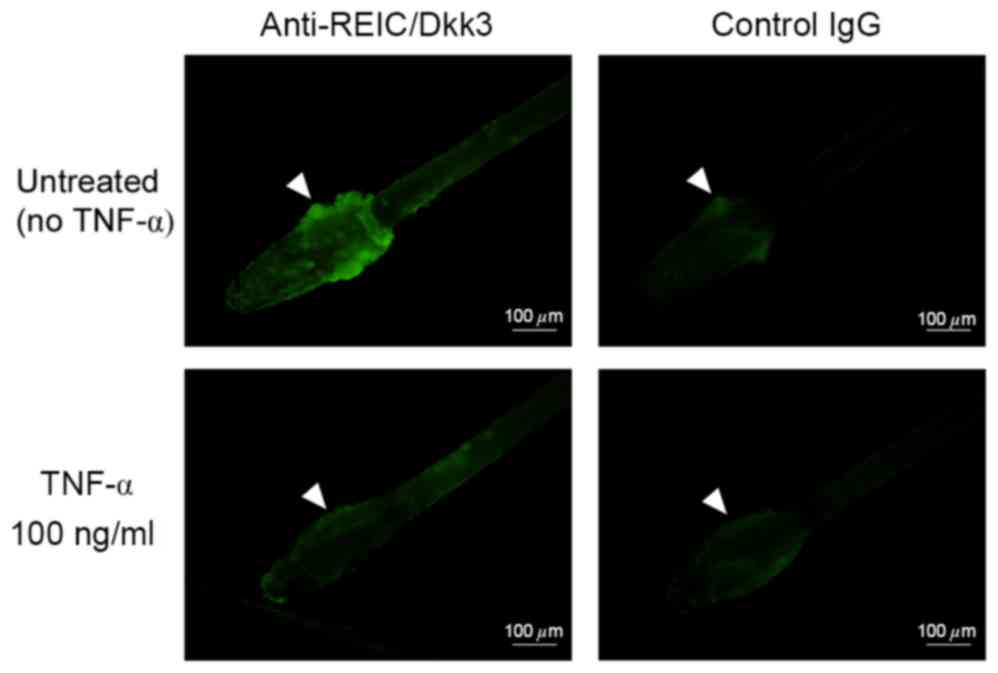
(Fig. 3). Consistent with these
observations, plucked hair follicles incubated with 100 ng/ml TNF-α
exhibited a reduction in REIC/Dkk-3 expression compared with the
untreated mouse hair follicles (Fig.
4).
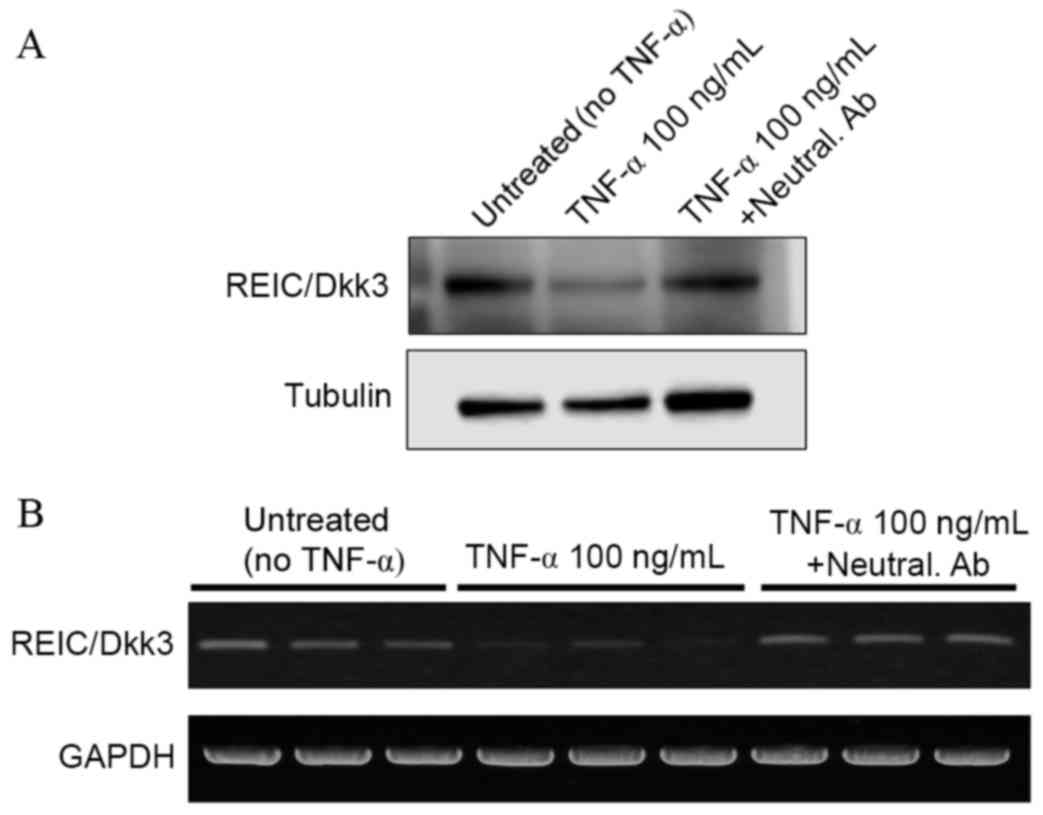
Abrogation of TNF-α-mediated
downregulation of REIC/Dkk-3 using a neutralizing anti-TNF-α
antibody
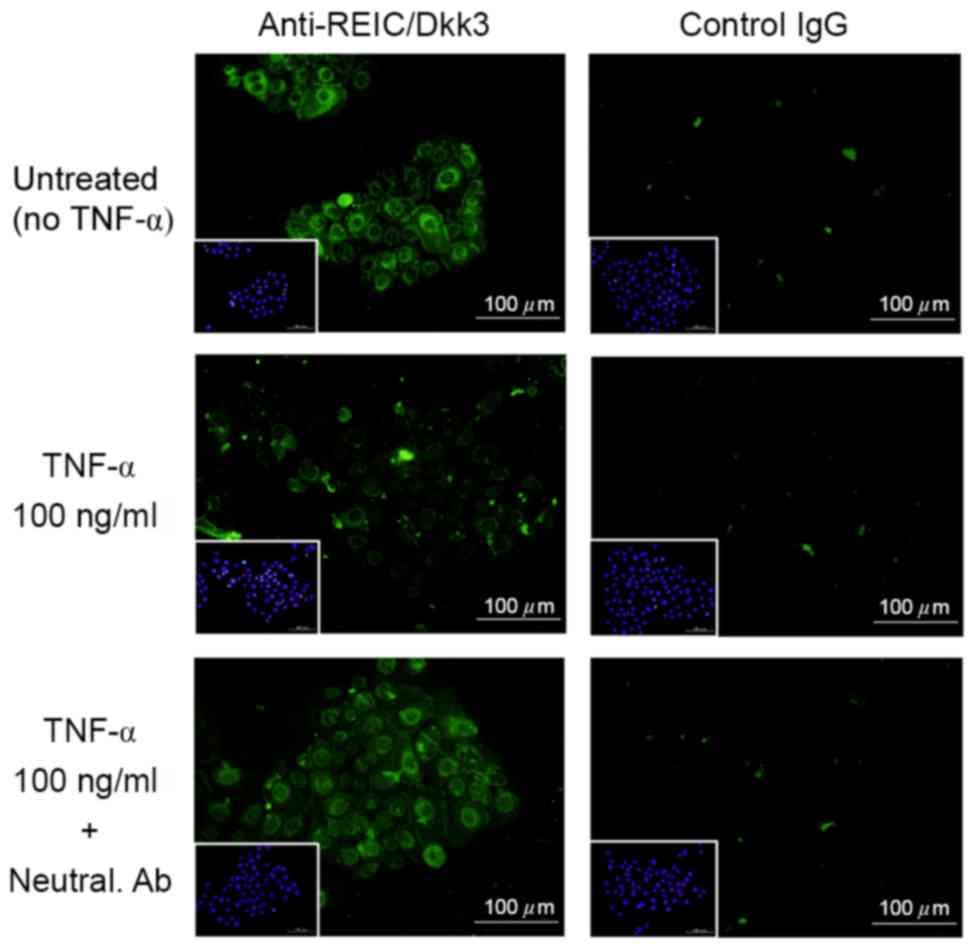
In order to verify the effect of TNF-α treatment on
the expression levels of REIC/Dkk-3, a competition assay was
performed in NHKs using a specific neutralizing antibody against
TNF-α. Immunocytochemical analysis demonstrated that treatment of
NHKs with the anti-TNF-α antibody abrogated the TNF-α-mediated
downregulation of REIC/Dkk-3 expression (Fig. 5). Similar results were obtained by
western blot (Fig. 6A) and RT-qPCR
analyses (Fig. 6B) of the
REIC/Dkk-3 protein and mRNA expression levels in NHK cells,
respectively.
Discussion
REIC/Dkk-3 is considered to be a tumor suppressor
gene as its expression levels are reduced in numerous human
malignancies (1). Previous studies
have demonstrated that the REIC/Dkk-3 promoter region is frequently
methylated in several malignant tissues, particularly in breast
cancer tissues (14,15). However, Saeb-Parsy et al
(16), reported that knockdown of
the membrane type-1 matrix metalloproteinase induced upregulation
of REIC/Dkk-3 expression in human urothelial carcinoma cells. Thus,
the mechanisms by which REIC/Dkk-3 expression is regulated in
normal and cancer cells are not fully understood.
In the present study, a number of growth factors and
cytokines were screened as potential regulators of REIC/Dkk-3
expression in normal skin keratinocytes. Among the seven factors
screened, only TNF-α was observed to downregulate REIC/Dkk-3
expression in NHKs. The skin tissue culture model employed in the
present study maintained a high level of REIC/Dkk-3 expression for
24 h. A reduction in REIC/Dkk-3 expression following TNF-α
treatment was confirmed using this skin tissue culture model, as
well as the incubated hair follicles, via by immunohistochemistry
analysis (Figs. 3 and 4). In addition, TNF-α-mediated
downregulation of REIC/Dkk-3 in NHKs was abrogated by the treatment
of cells with a neutralizing TNF-α-specific antibody.
TNF-α is a proinflammatory cytokine that is involved
in the early-phase reaction of skin inflammation (12,17,18).
TNF-α is expressed in pathological skin tissues, including
hyperproliferative, ultraviolet-irradiated and wounded epidermis
(19–21). TNF-α inhibitors have been used
previously for the treatment of psoriasis and psoriatic arthritis
(22,23). In a previous study, enhanced
REIC/Dkk-3 expression was observed in hyperproliferative epidermal
tissues, such as tissues in psoriasis and other inflammatory
diseases (5). In addition, a
downregulation of REIC/Dkk-3 expression was observed in skin
tissues following wound healing. These results suggest that
REIC/Dkk-3 may serve a pivotal role in the regeneration of damaged
skin tissues.
Following the exposure of skin keratinocytes,
fibroblasts and other cells to TNF-α in vitro, keratinocytes
exhibited an upregulation in mesenchymal markers and demonstrated
an increased migration potential (24). These features are indicators of
epithelial-mesenchymal transition (EMT), which is observed during
wound healing. Treatment of dermal fibroblasts with TNF-α resulted
in increased matrix metalloproteinase activity and enhanced cell
migration capabilities in vitro (25). In addition, TNF-α induced the
production of adhesion molecules and cytokines that mobilize immune
cells into skin tissue (26).
Cytokines produced by immune cells induced the proliferation and
differentiation of keratinocytes, which led to skin tissue
remodeling. Furthermore, stimulated keratinocytes produced
cytokines to stimulate the surrounding keratinocytes and immune
cells (27). The sequential
stimulation of skin cells by secreted cytokines is an essential
event during skin inflammation and skin tissue remodeling.
Lee et al (28), reported that activated human
mesenchymal stem/stromal cells (hMSCs) secreted REIC/Dkk-3 to
suppress the cell cycle progression in MDA-MB-231 breast cancer
cells (28). In addition, it was
demonstrated that REIC/Dkk-3 protein expression levels were
upregulated in hMSCs following incubation of the cells with TNF-α
(28). However, these previous
observations contradict the results of the present study, where
TNF-α was demonstrated to decarease REIC/Dkk-3 protein expression
levels. Since these observations are contradictory, further studies
are required to understand REIC/Dkk-3 regulation in different cell
types.
In conclusion, the present study demonstrated that
TNF-α reduced the expression of REIC/Dkk-3 in mouse skin
keratinocytes and NHKs. This was confirmed by the observation that
TNF-α reduced the expression of REIC/Dkk-3 in tissue culture models
of mouse skin and hair. These results suggest that REIC/Dkk-3 may
serve a pivotal role in skin inflammation and tissue
remodeling.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the Japan
Society for the Promotion of Science, Grants-in-Aid for Scientific
Research KAKENHI (grant nos. 24591943, 26106725 and 15K01303;
awarded to KK).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
KK and NH conceived the study. KK, NM, YA, HM and MS
performed the analysis. KK and MS wrote the paper. All authors read
and approved the manuscript.
Ethics approval and consent to
participate
Ethical approval for the animal study was provided
by Okayama University Animal Care and Use Committee (Okayama,
Japan).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biophys Res Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe M, Nasu Y and Kumon H:
Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an
autologous cancer vaccination therapy (Review). Oncol Lett.
7:595–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du G, Kataoka K, Sakaguchi M, Abarzua F,
Than SS, Sonegawa H, Makino T, Shimizu T and Huh NH: Expression of
REIC/Dkk-3 in normal and hyperproliferative epidermis. Exp
Dermatol. 20:273–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kataoka K, Du G, Maehara N, Murata H,
Sakaguchi M and Huh N: Expression pattern of REIC/Dkk-3 in mouse
squamous epithelia. Clin Exp Dermatol. 37:428–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin JM, Choi DK, Kang HY, Sohn KC, Lee Y,
Kim CD, Lee JH and Park BC: The expression pattern and functional
role of REIC/Dkk-3 in the development of cutaneous squamous cell
carcinoma. J Dermatol Sci. 84:88–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakaguchi M, Sonegawa H, Murata H, Kitazoe
M, Futami J, Kataoka K, Yamada H and Huh NH: S100A11, an dual
mediator for growth regulation of human keratinocytes. Mol Biol
Cell. 19:78–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakaguchi M, Miyazaki M, Takaishi M,
Sakaguchi Y, Makino E, Kataoka N, Yamada H, Namba M and Huh NH:
S100C/A11 is a key mediator of Ca(2+)-induced growth inhibition of
human epidermal keratinocytes. J Cell Biol. 163:825–835. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nukui T, Ehama R, Sakaguchi M, Sonegawa H,
Katagiri C, Hibino T and Huh NH: S100A8/A9, a key mediator for
positive feedback growth stimulation of normal human keratinocytes.
J Cell Biochem. 104:453–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Than SS, Kataoka K, Sakaguchi M, Murata H,
Abarzua F, Taketa C, Du G, Yashiro M, Yanagihara K, Nasu Y, et al:
Intraperitoneal administration of an adenovirus vector carrying
REIC/Dkk-3 suppresses peritoneal dissemination of scirrhous gastric
carcinoma. Oncol Rep. 25:989–995. 2011.PubMed/NCBI
|
|
12
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuschil A, Lam C, Haslberger A and Lindley
I: Interleukin-8 stimulates calcium transients and promotes
epidermal cell proliferation. J Invest Dermatol. 99:294–298. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi T, Asano H, Toyooka S, Tsukuda K,
Soh J, Shien T, Taira N, Maki Y, Tanaka N, Doihara H, et al: DNA
methylation status of REIC/Dkk-3 gene in human malignancies. J
Cancer Res Clin Oncol. 138:799–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Veeck J and Dahl E: Targeting the Wnt
pathway in cancer: The emerging role of Dickkopf-3. Biochim Biophys
Acta. 1825:18–28. 2012.PubMed/NCBI
|
|
16
|
Saeb-Parsy K, Veerakumarasivam A, Wallard
MJ, Thorne N, Kawano Y, Murphy G, Neal DE, Mills IG and Kelly JD:
MT1-MMP regulates urothelial cell invasion via transcriptional
regulation of Dickkopf-3. Br J Cancer. 99:663–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bashir MM, Sharma MR and Werth VP:
TNF-alpha production in the skin. Arch Dermatol Res. 301:87–91.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tracey D, Klareskog L, Sasso EH, Salfeld
JG and Tak PP: Tumor necrosis factor antagonist mechanisms of
action: A comprehensive review. Pharmacol Ther. 117:244–279. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ettehadi P, Greaves MW, Wallach D, Aderka
D and Camp RD: Elevated tumour necrosis factor-alpha biological
activity in psoriatic skin lesions. Clin Exp Immunol. 96:146–151.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Köck A, Schwarz T, Kirnbauer R, Urbanski
A, Perry P, Ansel JC and Luger TA: Human keratinocytes are a source
for tumor necrosis factor alpha: Evidence for synthesis and release
upon stimulation with endotoxin or ultraviolet light. J Exp Med.
172:1609–1614. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palladino MA, Bahjat FR, Theodorakis EA
and Moldawer LL: Anti-TNF-alpha therapies: The next generation. Nat
Rev Drug Discov. 2:736–746. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lebrec H, Ponce R, Preston BD, Iles J,
Born TL and Hooper M: Tumor necrosis factor, tumor necrosis factor
inhibition, and cancer risk. Curr Med Res Opin. 31:557–574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan C, Grimm WA, Garner WL, Qin L, Travis
T, Tan N and Han YP: Epithelial to mesenchymal transition in human
skin wound healing is induced by tumor necrosis factor-alpha
through bone morphogenic protein-2. Am J Pathol. 176:2247–2258.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi JY, Piao MS, Lee JB, Oh JS, Kim IG
and Lee SC: Propionibacterium acnes stimulates pro-matrix
metalloproteinase-2 expression through tumor necrosis factor-alpha
in human dermal fibroblasts. J Invest Dermatol. 128:846–854. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Krueger JG and Bowcock AM:
Psoriasis: Genetic associations and immune system changes. Genes
Immun. 8:1–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bak RO and Mikkelsen JG: Regulation of
cytokines by small RNAs during skin inflammation. J Biomed Sci.
17:532010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee RH, Yoon N, Reneau JC and Prockop DJ:
Preactivation of human MSCs with TNF-α enhances tumor-suppressive
activity. Cell Stem Cell. 11:825–835. 2012. View Article : Google Scholar : PubMed/NCBI
|