Introduction
Spinal disorders, including traumatic vertebral
fracture, spinal tumors and spinal deformities, are a leading cause
of morbidity in orthopedics (1–3).
Spinal fusion is an accepted treatment approach for patients with
spinal disorders. Numerous techniques and biomaterials have been
developed to promote spinal fusion; however, the mechanisms
involved in spinal fusion remain to be investigated (4,5). The
role of neuronal mediators in fracture healing and bone
regeneration has been previously highlighted, and neuropeptides
have been reported to regulate fracture healing and local bone
turnover (6,7).
Substance P (SP) is an 11-amino acid neuropeptide
richly distributed in the peripheral and central nervous systems
(8). In a variety of chronic
pains, substance P acts as a pain neurotransmitter via the sensory
nerve afferent fibers up to the spinal cord, and is involved in the
conduction and modulation of pain. In addition to transmitting
nociceptive information, SP also serves a role in the analgesic
effect. The receptors of SP are known as neurokinin receptors
(NKRs) (9), and the use of
specific NKR antagonists may decrease postoperative pain (10–13).
During the present study SP, NKRs were detected in operative areas,
including bone fracture sites and inflammatory sites.
Evidence has demonstrated that SP-positive nerve
fibers were active in osteogenic areas, including the bone marrow,
periosteum and growth plate, in bone fractures (14,15).
NKRs were also observed on endothelial cells during the process of
angiogenesis (16). SP-positive
nerve fibers and NKRs accompany the fracture process and
angiogenesis; however, at present, studies mainly focus on their
roles in pain sensation, with little investigation into the
associations between SP, NKRs and spinal fusion. Therefore, the
present study aimed to investigate the effects of alterations in
the quantity of SP and NKRs during the spinal fusion process.
Materials and methods
Animals and surgery procedure
The Subcommittee on Animal Studies of The Second
Military Medical University approved all experiments. A total of 20
adult male Sprague-Dawley rats (~16-weeks-old, ~400 g) were used in
the present study. The rats were housed and maintained in a 26°C
constant temperature environment with filtered air and 60% relative
humidity under a 12-h light/dark cycle. Rats had free access to
food and water, and a pair of rats was placed in an isolator
cage.
Anaesthesia was induced and maintained with
isoflurane (xw266754671; Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China; 0.5–2%) via inhalational oxygen. The back lumbar
region and the hind limbs above the iliac crest were shaved. Rats
were disinfected and a 3-cm dorsolateral incision was created over
the lumbar 4/5 (L4/5) area, followed by blunt dissection of the
longitudinal back muscles. Once the transverse process of the L4
vertebra was exposed, the transverse process was decorticated with
an electric bur until shallow bleeding was observed. Then, a
demineralized freeze-dried bone allograft (Aorui Biological
Material Co., Ltd., Shanxi, China) was implanted in the
decorticated fusion beds of the transverse process. Finally, fascia
and skin underwent interrupted suturing layer by layer.
Postoperative antibiotics were administered intramuscularly for 2
consecutive days (cefuroxime; cat. no. YB-8342; Shanghai Yu Bo
Biological Technology Co., Ltd., Shanghai, China; 0.5 mg/kg).
Animals were sacrificed by excessive anaesthesia and euthanized at
1, 2, 3 and 4 weeks post-surgery (n=5/week). The specimens,
allograft and the fused transverse process were obtained and frozen
at −80°C until further analysis.
Histological analysis
Harvested specimens were fixed in 4%
paraformaldehyde for 24 h at 26°C, and then decalcified with 5%
nitric acid at room temperature for 72 h. Subsequently, the
specimens were washed in distilled water three times and then
embedded in paraffin. A series of sections (5-µm thickness) were
obtained from the midline of the transverse processes. The sections
were stained with hematoxylin & eosin (hematoxylin for 5 min
and with eosin for 3 min, both at 26°C) and viewed under the bright
field of an Eclipse 80i microscopy (Nikon Corporation, Shanghai,
China).
Immunohistochemical staining
SP, NK1R, and NK2R were immunostained. The area of
interest included regions around the transverse process and the
allograft. The 5-µm thin sections were blocked at 4°C for 12 h with
5% bovine serum (cat. no. E661003; Sangon Biotech Co., Ltd.,
Shanghai, China) and 0.3% Triton X-100 (cat. no. P0096; Beyotime
Institute of Biotechnology, Shanghai, China). Then, the specimens
were incubated with a rabbit anti-rat polyclonal primary antibody
(1:1,000; Substance P antibody: Cat. no. sc-58591; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; NK-1R antibody: Cat. no.
sc-365091; Santa Cruz; Biotechnology, Inc.; NK-2R antibody: Cat.
no. 25270-1-AP; Wuhan Sanying Biotechnology, Wuhan, China) at 4°C
for 24 h, followed by a fluorescein isothiocyanate-conjugated
donkey anti-rabbit secondary antibody (1:200; cat. no. sc-2090;
Santa Cruz Biotechnology, Inc.) at room temperature for 2 h. Images
were captured with an Eclipse 80i fluorescent microscope (Nikon
Corporation). ImageJ software v.1.51 (National Institutes of
Health, Bethesda, MD, USA) was used to calculate the content of the
immunostained areas; the densities of SP, NK1R and NK2R were
measured as follows: (Positive area/total image area) × 100%.
Statistical analysis
The statistical data were analysed using GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS
v.22.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference. The densities of
SP, NK1R, and NK2R at different stages post-surgery were repeatedly
measured and compared with one-way with analysis of variance
(ANOVA) and SNK was used as a post hoc test. All
immunohistochemical staining results are presented as the mean ±
standard deviation.
Results
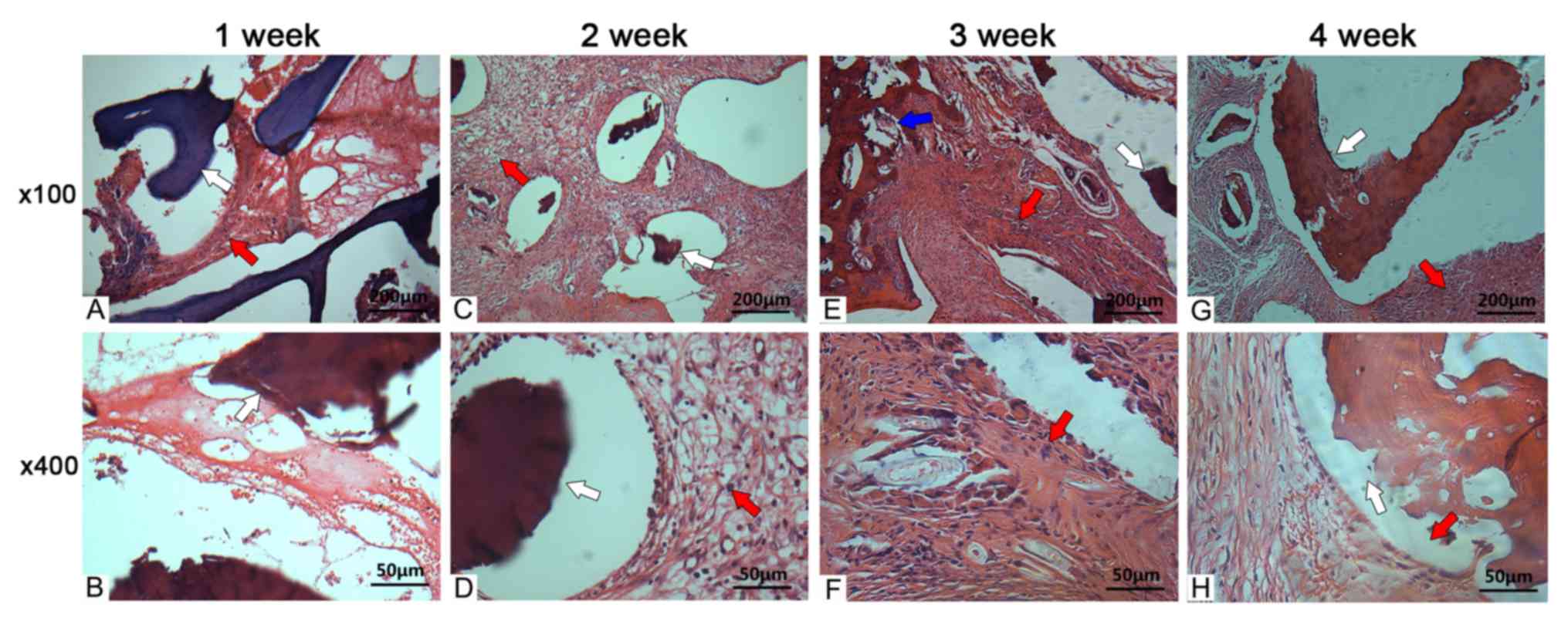
Histological osteogenesis
At 1 week post-surgery, a spot of deeply stained
cells were detected in the fusion site, and few fibrous tissues
were observed in the gap between the allograft and transverse
process. At 2 weeks post-surgery, chondrocytes were detected at the
fusion site. The quantity of deeply stained cells increased
compared with the deeply stained cells at 1 week post-surgery and a
layer of osteoblasts spread along the interlayer of the allograft
and the newly formed fibrous tissues. In comparison with those at 2
weeks post-surgery, at 3 weeks post-surgery, the number of
chondrocytes and fibrous tissues increased continuously, while the
number of deeply stained cells decreased. Chondrocytes were mainly
located on the allograft meshwork; novel cartilage formed
surrounding the allograft. Additionally, osteoblasts were observed
in the interface of the allograft. At 4 weeks post-surgery, the
deeply stained cells were detected at a normal level; more
osteoblasts in the in the interface of the allograft, and of
several layers were also observed (Fig. 1).
Occurrence of SP in the fusion
site
The immunohistochemical analysis quantified and
demonstrated the alterations and specific localizations of
SP-positive nerves (Fig. 2). At 1
week post-surgery, few SP-positive nerve fibers were detected at
the fusion site. The number of SP-positive nerve fibers increased
continuously at week 2 post-surgery. The peak in SP abundance was
observed at 3 weeks post-surgery, which was as high as 10.33±1.23%
of the fusion site (Fig. 2;
Table I). At 4 weeks post-surgery,
the density of SP decreased to a lower level of 7.87±0.35%. One-way
ANOVA statistical analysis revealed that the density of SP at 3
weeks post-surgery was higher than at all other weeks
(*P<0.01).
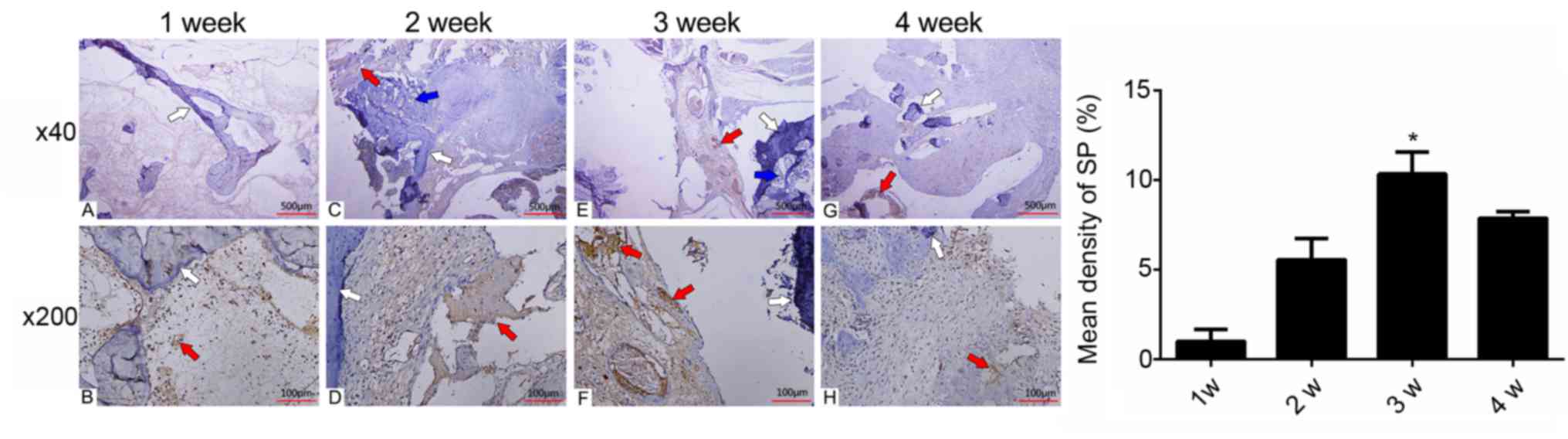 | Figure 2.Expression of SP at the (A and B) 1
week, (C and D) 2 weeks, (E and F) 3 weeks and (G and H) 4 weeks
post-surgery. At 1 week post-surgery, few SPs were detected in the
visual fields. SP abundance increased at 2 weeks post-surgery. SP
reached a peak at 3 weeks post-surgery temporally, and was mainly
distributed in the newly formed microvessels and the surrounding
chondrocytes spatially. At 4 weeks post-surgery, the density of SP
decreased, but remained higher than that at 2 weeks post-surgery.
Red arrows, SP; white arrows, allograft; and blue arrows, newly
formed bone. *P<0.01. SP, substance P. |
 | Table I.Mean density of SP in the fusion
site. |
Table I.
Mean density of SP in the fusion
site.
| Variable (%) | Week 1 | Week 2 | Week 3 | Week 4 |
|---|
| MD of SP |
0.99±0.65 |
5.54±1.18 |
10.32±1.23a |
7.87±0.35 |
Occurrence of NKRs at the fusion
site
At 1 week post-surgery, there were only a few NKRs
at the fusion site. At 2 weeks post-surgery, the density of NKRs
had increased; some NKRs appeared surrounding the allograft and
microvessels. Additionally, NKRs were observed around the
chondrocytes in the cartilage areas. At 3 weeks post-surgery,
numerous NKRs were detected at the fusion site. Most NK1R and NK2R
were distributed within the endothelium of the microvessels and the
interface of the allograft. The density of NK1R and NK2R was as
high as 20.26±1.25 and 22.21±2.36%, respectively, and peaked at 3
weeks post-surgery. At 4 weeks post-surgery, NKRs numbers began to
decrease but were higher compared with in specimens analysed at 2
weeks post-surgery. NK2R numbers were lower than those of NK1R, but
their respective distributions were similar (Fig. 3; Table II).
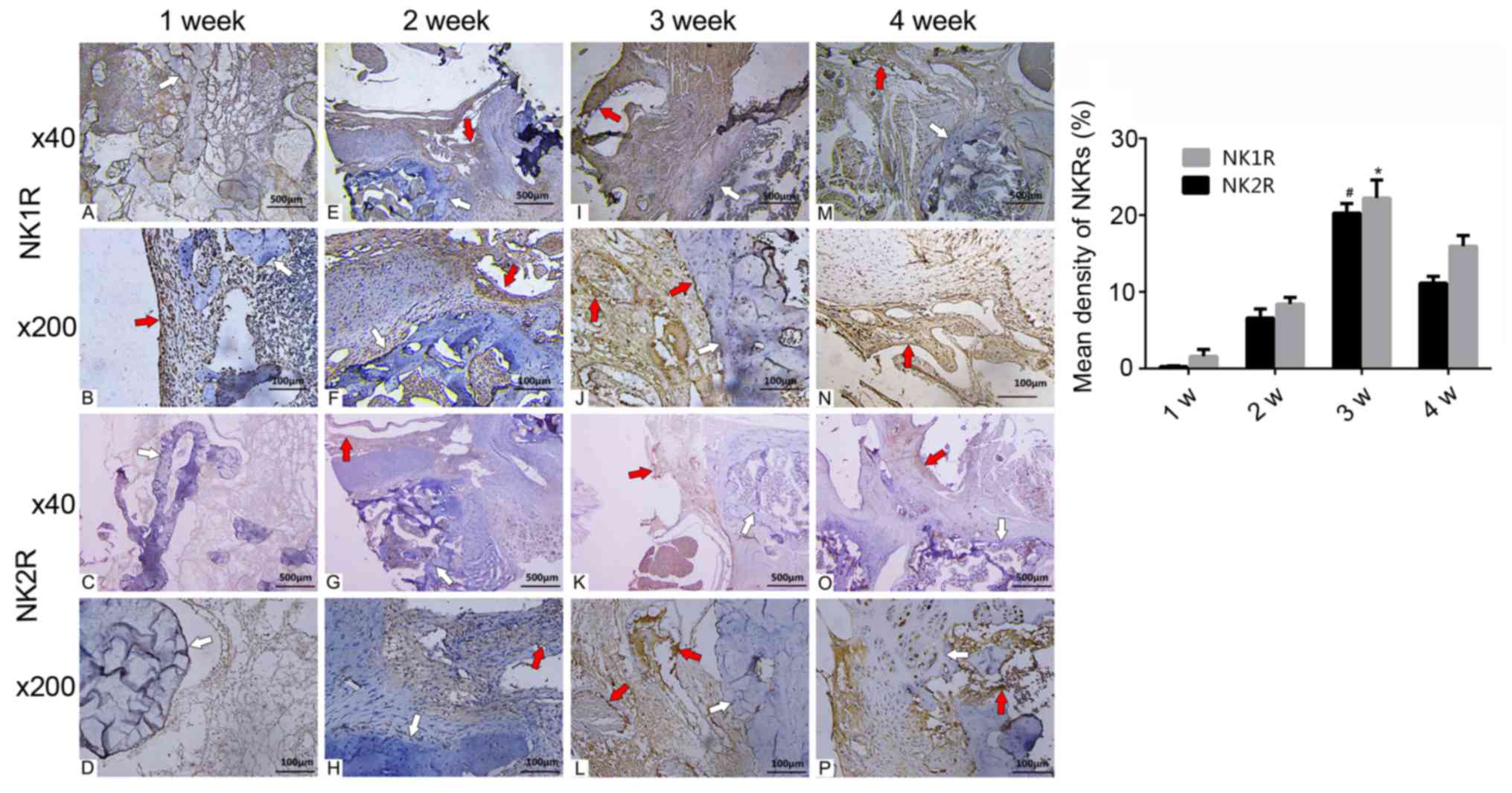 | Figure 3.Expression of NKRs at (A-D) 1, (E-H)
2, (I-L) 3 and (M-P) 4 weeks post-surgery. NKRs increased and
reached a peak at 3 weeks post-surgery with a distribution
surrounding the allograft and endothelial cells of the
microvessels. Fewer NK2Rs were present than NK1Rs, but were
similarly distributed. Density of NK1Rs and NK2Rs was significantly
higher at 3 weeks post-surgery compared to other time points (weeks
1, 2 and 3). Red arrows, NK1/2R; white arrows,
allograft.#P<0.01 vs. NK1R, *P<0.01 vs. NK2R. NKR,
neurokinin receptors; NK1R, neurokinin 1 receptor; NK2R, neurokinin
2 receptor. |
 | Table II.Mean density of NKRs in the fusion
site. |
Table II.
Mean density of NKRs in the fusion
site.
| Variable (%) | Week 1 | Week 2 | Week 3 | Week 4 |
|---|
| MD of NK1R |
0.26±0.10 |
6.58±1.18 |
20.26±1.25a |
11.13±0.87 |
| MD of NK2R |
1.57±0.91 |
8.39±0.87 |
22.21±2.36b |
15.95±1.38 |
Discussion
The present study investigated the occurrence and
enhancement of SP and NKR expression in allograft spinal fusion.
The results demonstrated that SP and NKRs were increased in the
early phase of spinal fusion; a close association was noted between
alterations in the quantity of SP and NKRs, and the various stages
of histological healing. Nerve fibers detected post-surgery may be
necessary for the transportation of various neuronal mediators,
such as SP, in order to regulate the fusion process. In addiition,
elevated levels of NKRs were determined to be essential for the
function of SP. It has also been reported that SP has a lower
affinity for NK2R than NK1R, but that it can stimulated NK2R in
some peripheral nerve fibers (17).
SP-positive nerve fibers and NKRs appeared at 1 week
post-surgery. Haematoma usually occurred at ~8 h following injury,
and was gradually replaced by fibrous tissue in the subsequent 2–3
weeks (18). Previous studies
demonstrated that SP-positive nerve fibers may be observed in the
first week post fracture healing; these peaked at day 21,
suggesting the possible roles of SP in hematoma absorption and in
the inflammatory process (19,20).
As a type of wound modulatory peptide of the tachykinin family,
circulatory SP was increased by 10-fold within 24 h of the bone
fracture, with a marked effect on microvessel dilation and
increasing vascular permeability (18,21,22).
Eglezos et al (23)
revealed that, via the activation of NK1R in endothelium cells, SP
increased the permeability of microvessels and further caused
plasma extravasation and oedema (23). Therefore, the ingrowth of SP at the
fusion sites may be involved in the inflammatory response at 1 week
post-surgery.
At 2 weeks post-surgery, distinct SP and NKRs were
observed at the fusion site. Additionally, chondrocytes and newly
formed microvessels were detected at the fusion site. The
synchronization between SP and chondrocytes in the present study
reflected the inducible association between SP and bone formation
as previously reported (24). At 3
weeks post-surgery, the density of both SP and NKRs peaked with the
newly formed bone callus and fewer deeply stained cells were
observed. At 4 weeks post-surgery, the density of SP and NKRs both
began to decrease in the fusion site. It is possible that SP may
have promoted spinal fusion in the early bone formation phase via
NKRs, particularly in the endochondral ossification stage, but not
in the later bone mechanical modification stage. Spatially, the
distribution alterations of SP were observed surrounding the
allograft and in the fibrous tissues; previous evidence
demonstrated that SP may stimulate the proliferation of fibroblasts
(25). Furthermore, previous
studies reported that NK1R expression levels were increased
post-fracture, and that they began to return to normal levels
gradually at 4 weeks post-fracture (11). Located on chondrocytes, osteoblasts
and osteoclasts, NKRs were demonstrated to influence the bone
remodeling process in vivo (26,27).
In vitro experiments revealed that the increase in
SP-positive nerve fibers during fracture healing accelerated the
bone formation process compared with the control number of
SP-positive nerve fibres (9,28).
In addition, animals with neuropathies or peripheral nerve
resection exhibited reduced SP levels and decreased bone mechanical
characteristics (17). Similar to
the increase in SP abundance, NK1R was also increased in the early
phase of spinal fusion. Compared with NK2R, NK1R numbers were
relatively more selective for SP and were distributed on
osteoblasts, particularly in areas with active osteogenesis, such
as the interface of the allograft, as observed in the present
study. Thus, SP may exert effects on spinal fusion by promoting
bone formation via NKRs in bone.
As aforementioned, circulatory SP levels increased
≥10-fold within 24 h of the bone fracture (21). As one of the released angiogenic
factors post-fracture, SP served a significant role in the primary
process of angiogenesis (29). SP
may induce the migration of endothelium progenitor cells that
expressed NK1R and promote the proliferation of endothelial cells,
thus accelerating reparative angiogenesis (29,30).
Additionally, SP may recruit granulocytes to the injured site and
bind specific receptors on granulocytes, promoting these cells to
release angiogenic cytokines, including vascular endothelial growth
factor, basic fibroblast growth factor and angiopoietin-2 (31–33).
In vivo experiment results revealed that NK1R agonists
induced endothelial cell proliferation and enhanced angiogenesis
(34). However, NKR antagonists
inhibited SP-induced proliferation and angiogenesis (13,35).
In vitro studies indicated that angiogenesis was reduced at
both the arteriolar and capillary levels in NK1R-KO mice, compared
with in wild type mice (29). The
newly formed microvessels carry oxygen, stem cells and various
growth factors (36,37). In the present study, SP and NKRs
were distributed around the microvessels and peaked in abundance at
3 weeks post surgery. Previous studies also reported that SP
reached a peak at approximately 21 days post injury (19,38).
Collectively, the results of the present study and prior evidence
indicated that SP may be involved in the positive regulatory
process of angiogenesis during spinal fusion.
Numerous limitations existed in the present study.
The in vivo analysis merely provided a morphological and
immunohistochemical analysis of SP and NKR occurrence without
reporting a causal association with spinal fusion. Furthermore,
regarding morphological observations, the rat spines were limited
to a small size, thus the fusion mass was not measured. Therefore,
the regulatory roles of SP and NKRs during spinal fusion, and
interventional in vivo experiments permitting investigations
into specific cell types during a different phase of spinal fusion,
should be conducted in the future.
In summary, the present study explored the
alterations in the quantity of SP and NKRs during allograft spinal
fusion. SP and NKRs were detected 1 week post surgery in the
fibrous tissues; the majority of SP and NKRs surrounded the
allograft and the newly formed microvessels. These results
highlighted the role of SP and NKRs in the processes of bone
metabolism and new microvessel formation during the early phase of
spinal fusion, which may present a novel strategy for promoting
spinal fusion from a neurogenesis perspective.
Acknowledgements
Not applicable.
Funding
The work was supported by the National Natural
Science Foundation of China (No. 81702141)
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TX and JS designed the study, and SW, XX and YZ
performed the experiments. PL and KS assisted with surgical
operations, and YZ conducted the statistical analysis. SW and XX
wrote the manuscript, and TX and JS revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Subcommittee on Animal Studies of the Second
Military Medical University approved all experiments.
Consent for publication
Not applicable.
Competing interests
All authors declared that they have no competing
interests.
References
|
1
|
Kreinest M, Rillig J, Grützner PA, Küffer
M, Tinelli M and Matschke S: Analysis of complications and
perioperative data after open or percutaneous dorsal
instrumentation following traumatic spinal fracture of the thoracic
and lumbar spine: A retrospective cohort study including 491
patients. Eur Spine J. 26:1535–1540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Liu Z, Zhu Z and Qiu Y: Spinal
epidural lipomatosis-an easily ignored secondary intraspinal
disorder in spinal kyphotic deformities. BMC Musculoskelet Disord.
18:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meshkini A, Salehpour F, Rezakhah A,
Mirzaei F, Kazemzadeh M and Naseri Alavi SA: Textiloma: A case of
foreign body mimicking a spinal tumor. Spine. 42:E1272–E1274. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bowles RD and Setton LA: Biomaterials for
intervertebral disc regeneration and repair. Biomaterials.
129:54–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel VV, Andersson GB, Garfin SR, Resnick
DL and Block JE: Utilization of CT scanning associated with complex
spine surgery. BMC Musculoskelet Disord. 18:522017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Xu J, Ruan YC, Yu MK, O'laughlin
M, Wise H, Chen D, Tian L, Shi D, Wang J, et al: Implant-derived
magnesium induces local neuronal production of CGRP to improve
bone-fracture healing in rats. Nat Med. 22:1160–1169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lenza M, Belloti JC, Andriolo RB and
Faloppa F: Conservative interventions for treating middle third
clavicle fractures in adolescents and adults. Cochrane Database
Syst Rev: CD007121. 2014. View Article : Google Scholar
|
|
8
|
Hrabovszky E, Borsay BÁ, Rácz K, Herczeg
L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS and Liposits Z:
Substance P immunoreactivity exhibits frequent colocalization with
kisspeptin and neurokinin B in the human infundibular region. PLoS
One. 8:e723692013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mistrova E, Kruzliak P and Chottova
Dvorakova M: Role of substance P in the cardiovascular system.
Neuropeptides. 58:41–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dionne RA, Max MB, Gordon SM, Parada S,
Sang C, Gracely RH, Sethna NF and Maclean DB: The substance P
receptor antagonist CP-99,994 reduces acute postoperative pain.
Clin Pharmacol Ther. 64:562–568. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei T, Guo TZ, Li WW, Kingery WS and Clark
JD: Acute versus chronic phase mechanisms in a rat model of CRPS. J
Neuroinflammation. 13:142016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mander K, Harford-Wright E, Lewis KM and
Vink R: Advancing drug therapy for brain tumours: A current review
of the pro-inflammatory peptide substance P and its antagonists as
anti-cancer agents. Recent Pat CNS Drug Discov. 9:110–121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan TP, Hu DE, Guard S, Gresham GA and
Watling KJ: Stimulation of angiogenesis by substance P and
interleukin-1 in the rat and its inhibition by NK1 or interleukin-1
receptor antagonists. Br J Pharmacol. 110:43–49. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn
W, Jiang MH, Kim JC and Son Y: A new role of substance P as an
injury-inducible messenger for mobilization of CD29+
stromal-like cells. Nat Med. 15:425–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grässel SG: The role of peripheral nerve
fibers and their neurotransmitters in cartilage and bone physiology
and pathophysiology. Arthritis Res Ther. 16:4852014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muñoz M and Coveñas R: Involvement of
substance P and the NK-1 receptor in human pathology. Amino Acids.
46:1727–1750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seegers HC, Hood VC, Kidd BL, Cruwys SC
and Walsh DA: Enhancement of angiogenesis by endogenous substance P
release and neurokinin-1 receptors during neurogenic inflammation.
J Pharmacol Exp Ther. 306:8–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szczesny G: Fracture healing and its
disturbances. A literature review. Ortop Traumatol Rehabil.
17:437–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Ahmed M, Bergstrom J, Ackermann P,
Stark A and Kreicbergs A: Occurrence of substance P in bone repair
under different load comparison of straight and angulated fracture
in rat tibia. J Orthop Res. 28:1643–1650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pavlovic S, Daniltchenko M, Tobin DJ,
Hagen E, Hunt SP, Klapp BF, Arck PC and Peters EM: Further
exploring the brain-skin connection: Stress worsens dermatitis via
substance P-dependent neurogenic inflammation in mice. J Invest
Dermatol. 128:434–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onuoha GN: Circulating sensory peptide
levels within 24 h of human bone fracture. Peptides. 22:1107–1110.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei T, Li WW, Guo TZ, Zhao R, Wang L,
Clark DJ, Oaklander AL, Schmelz M and Kingery WS: Post-junctional
facilitation of Substance P signaling in a tibia fracture rat model
of complex regional pain syndrome type I. Pain. 144:278–286. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eglezos A, Giuliani S, Viti G and Maggi
CA: Direct evidence that capsaicin-induced plasma protein
extravasation is mediated through tachykinin NK1 receptors. Eur J
Pharmacol. 209:277–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu S, Jin D, Liu S, Wang L, Wang Z, Mei G,
Zou ZL, Wu JQ and Xu ZY: Protective effect of neuropeptide
substance P on bone marrow mesenchymal stem cells against apoptosis
induced by serum deprivation. Stem Cells Int. 2015:2703282015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El Karim IA, Linden GJ, Irwin CR and Lundy
FT: Neuropeptides regulate expression of angiogenic growth factors
in human dental pulp fibroblasts. J Endod. 35:829–833. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ytteborg E, Torgersen JS, Pedersen ME,
Helland SJ, Grisdale-Helland B and Takle H: Exercise induced
mechano-sensing and substance P mediated bone modeling in Atlantic
salmon. Bone. 53:259–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aro H: Effect of nerve injury on fracture
healing. Callus formation studied in the rat. Acta Orthop Scand.
56:233–237. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niedermair T, Kuhn V, Doranehgard F,
Stange R, Wieskötter B, Beckmann J, Salmen P, Springorum HR, Straub
RH, Zimmer A, et al: Absence of substance P and the sympathetic
nervous system impact on bone structure and chondrocyte
differentiation in an adult model of endochondral ossification.
Matrix Biol. 38:22–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amadesi S, Reni C, Katare R, Meloni M,
Oikawa A, Beltrami AP, Avolio E, Cesselli D, Fortunato O, Spinetti
G, et al: Role for substance p-based nociceptive signaling in
progenitor cell activation and angiogenesis during ischemia in mice
and in human subjects. Circulation. 125(1774–1786): S1–S19. 2012.
View Article : Google Scholar
|
|
30
|
Kohara H, Tajima S, Yamamoto M and Tabata
Y: Angiogenesis induced by controlled release of neuropeptide
substance P. Biomaterials. 31:8617–8625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Artese L, Rubini C, Ferrero G, Fioroni M,
Santinelli A and Piattelli A: Vascular endothelial growth factor
(VEGF) expression in healthy and inflamed human dental pulps. J
Endod. 28:20–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tran-Hung L, Laurent P, Camps J and About
I: Quantification of angiogenic growth factors released by human
dental cells after injury. Arch Oral Biol. 53:9–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krishnan V and Davidovitch Z: On a path to
unfolding the biological mechanisms of orthodontic tooth movement.
J Dent Res. 88:597–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ziche M, Morbidelli L, Pacini M, Geppetti
P, Alessandri G and Maggi CA: Substance P stimulates
neovascularization in vivo and proliferation of cultured
endothelial cells. Microvasc Res. 40:264–278. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ziche M, Morbidelli L, Masini E, Amerini
S, Granger HJ, Maggi CA, Geppetti P and Ledda F: Nitric oxide
mediates angiogenesis in vivo and endothelial cell growth and
migration in vitro promoted by substance P. J Clin Invest.
94:2036–2044. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu X, Wang F, Yang Y, Zhou X, Cheng Y, Wei
X and Li M: LIPUS promotes spinal fusion coupling proliferation of
type H microvessels in bone. Sci Rep. 6:201162016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chandrasekhar KS, Zhou H, Zeng P, Alge D,
Li W, Finney BA, Yoder MC and Li J: Blood vessel wall-derived
endothelial colony-forming cells enhance fracture repair and bone
regeneration. Calcif Tissue Int. 89:347–357. 2011. View Article : Google Scholar : PubMed/NCBI
|