Introduction
With the rapid development of surgical technology,
laparoscopic surgery has gradually replaced the traditional open
operation owing to its improved cosmetic results, shorter
post-operative hospital stays, reduced pain and faster return to
preoperative condition (1–3). Despite these benefits, laparoscopic
procedure can produce adverse effects secondary to intraabdominal
pressure and an increasing number of studies have demonstrated that
high intraabdominal pressure caused by carbon dioxide during
laparoscopic surgery may have adverse effects on splanchnic organs
(4,5). Clinical and experimental studies have
established that the increase in intraabdominal pressure that
develops depending on the degree of pneumoperitoneum during
laparoscopic surgery may cause hypoperfusion of intraabdominal
organs (6,7). Increases in ischemia and the
oxidative stress response have been observed with
pneumoperitoneum-dependent impairment of organ perfusion. Following
desufflation, reperfusion injury occurred with a decrease in
intraabdominal pressure (8,9).
Kidneys, as important splanchnic organs, are
inevitably affected by intraabdominal pressure. Some animal
experiments have demonstrated that high and erratic elevations of
intraabdominal pressure can decrease venous return, compress the
renal vasculature and cause systemic hormonal changes, which
eventually decrease renal blood flow, urinary output and glomerular
filtration rate significantly (10). Other studies have observed
increases in renal ischemia and oxidative stress response with
increased intraabdominal pressure (11,12).
Although abdominal deflation at the end of laparoscopic procedures
reduces intraabdominal pressure and increases renal perfusion,
damage from the ischemic injury remains.
However, the majority of studies concerning
pneumoperitoneum pressure damage are based on normal kidneys,
whereas certain patients who undergo laparoscopic surgery may also
exhibit a certain degree of kidney obstruction (13,14).
The influence of intraabdominal pressure on hydronephrosis kidneys,
caused by stones, tumors or congenital anomalies, cannot be
ignored. A kidney with hydronephrosis exhibits a thinner renal
cortex, its blood perfusion is already subnormal and hydronephrosis
itself has adverse effects on renal tubule function. Therefore, it
was hypothesized that hydronephrotic kidneys may have an increased
susceptibility to injury as a result of increased kidney pressure
during endourological procedures. The present study investigated
whether tolerance to pneumoperitoneum pressure differs in rabbit
models of no, mild and severe hydronephrosis by evaluating
oxidative damage and mitochondrial injuries.
Materials and methods
Animals and groups
A total of 72 adolescent male New Zealand rabbits (6
months old, weighing 2.0–2.5 kg) were purchased from the Wuhan
Institute of Biological Products Co., Ltd. (Wuhan, China). Rabbits
were allowed to adapt to the laboratory environment for one week
prior to the beginning of the experiment. The rabbits were housed
in standard cages with free access to tap water and food, at a
temperature of 18–25°C and relative humidity of 45–55%. The entire
procedure complied with the guidelines for the Care and Use of
Laboratory Animals (15) and the
Ethical and Research Committee of Wuhan University Medical School
(Wuhan, China) approved the animal study.
The rabbits were randomly divided into three groups
consisting of 24 rabbits each: Normal (N), mild (M) and severe (S)
hydronephrosis groups. For the M and S groups, rabbits underwent
surgical procedures to induce mild or severe hydronephrosis. For
the N group, rabbits received a sham surgical procedure and no
hydronephrosis was induced. Following surgery, the rabbits were
randomly assigned to 4 subgroups (N0-N3, M0-M3 and S0-S3)
consisting of 6 rabbits each. Rabbits in groups 0–3 were
insufflated with carbon dioxide in their abdomens to maintain
intraabdominal pressures of 0, 5, 10 and 15 mmHg, respectively.
Surgical manipulation
The surgical model by Wen et al (16) was employed. Briefly, the rabbits
were anesthetized with 40 mg/kg intraperitoneal sodium
pentobarbital at room temperature. The left ureter, left lumbar
vein and psoas muscle were exposed through a midline abdominal
incision. Separately, for the mild and severe hydronephrosis
groups, the proximal ureter was buried in a 2- and 4-cm notch
within the psoas muscle. For the normal group, only a midline
abdominal incision was performed, and the abdomen was then closed
(sham procedure). After 2 weeks, B-ultrasonography was used to
confirm hydronephrosis. In the M and S groups, respectively, pyelic
distention levels of 0.95±0.27 and 1.69±0.34 cm, and parenchymal
thicknesses of 0.33±0.09 and 0.22±0.05 cm, were observed. A second
laparotomy was then performed where, following the anesthetization,
a 0.5-cm-long incision was made in the left abdomen. A 10-gauge
Veress needle was inserted into the peritoneal cavity through the
incision and the other side of the Veress needle was connected to a
CO2 insufflator (Stryker Endoscopy, Kalamazoo, MI, USA).
Subsequently, the incision was sutured to prevent CO2
leakage from the abdomen. The pressure for the 0–3 subgroups was
set at 0, 5, 10 and 15 mmHg, respectively, for the N, M and S
groups. After 1 h of insufflation, the pneumoperitoneum was
released, the psoas muscle obstruction was relieved and the abdomen
was sutured closed. Rabbits were sacrificed using 150 mg/kg
pentobarbital (20%) through the ear marginal vein injection after
24 h, and the left kidneys were collected for biochemical and
histological evaluations.
Determination of reactive oxygen
species (ROS)
Kidney tissue samples were initially homogenized
using a T25 digital Ultra-Turrax® disperser
(IKAH-Labortechnik, Staufen, Germany) in 100 mmol/l PBS and
centrifuged at 13,000 × g and 4°C for 10 min (Heraeus Biofuge Primo
R centrifuge), after which the supernatants were collected for
detection. The homogenized supernatants were incubated with
4-amino-5-methylamino-2′,7′-difluorofluorescein (1 mmol/l; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) for 30 min at
37°C. The absorbance was detected at 500 nm using an automatic
microplate reader (Multiskan MK3; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The results are expressed as fluorescence
intensity/mg protein (A.U./mg prot).
Detection of superoxide dismutase
(SOD)
Tissues were homogenized using a T25 digital
Ultra-Turrax disperser in Tris buffer (pH 7.4) containing butylated
hydroxytoluene to prevent new lipid peroxidation that may occur
during homogenization. Samples were centrifuged at 13,000 × g and
4°C for 20 min (Heraeus Biofuge Primo R centrifuge), after which
the supernatants were collected. Total Superoxide Dismutase (T-SOD)
assay kit (A001-1-1; Nanjing Jiancheng Bioengineering Institute)
was used for determining SOD levels. The xanthine oxidase method
(17) was used for detection and
the absorbance was detected at a wavelength of 550 nm using an
automatic microplate reader (Multiskan MK3). The results are
expressed as units/mg protein (U/mg prot).
Measurement of malondialdehyde
(MDA)
First, kidney tissue samples were homogenized using
a T25 digital Ultra-Turrax® disperser
(IKAH-Labortechnik) in normal saline and centrifuged at 13,000 × g
and 4°C for 10 min (Heraeus Biofuge Primo R centrifuge), then the
concentration of malondialdehyde (MDA) was measured using an assay
kit (A003-1; Nanjing Jiancheng Bioengineering Institute). Briefly,
MDA reacts with thiobarbituric acid to form a stable chromophoric
product, which was subsequently detected with an automatic
microplate reader (Multiskan MK3) at a wavelength of 532 nm. The
difference in absorption reflects different MDA concentration in
each sample. Results are expressed as units/ml (U/ml).
Detection of catalase (CAT)
activity
Tissues were homogenized and centrifuged at 13,000 ×
g and 4°C for 10 min. Catalase (CAT) assay kit (A007-1; Nanjing
Jiancheng Bioengineering Institute) was used for determining CAT
levels. The CAT levels of the homogenates were assayed at 520 and
535 nm using an automatic microplate reader (Multiskan MK3). The
results were expressed as U/ml.
Glutathione peroxidase (GSH-Px)
assay
Tissues were homogenized and centrifuged at 13,000 ×
g and 4°C for 10 min. A Glutathione Peroxidase assay kit (A006;
Nanjing Jiancheng Bioengineering Institute) was used. According to
the manufacturer's protocol, GSH reacts with
5,5′-dithiobis-2-nitrobenzoic acid and the absorbance spectrum of
the product has a maximum absorbance at a wavelength of 410 nm. The
results were expressed as units/g protein (U/g prot).
Lactate (LD) levels
Lactate (LD) is the product of anaerobic respiration
and LD levels indicate the extent of hypoxia. Homogenates were
prepared after homogenation and centrifugation at 10,000 × g and
4°C for 10 min. Then a lactate assay kit (A018; Nanjing Jiancheng
Bioengineering Institute) was used for LD detection. The results
were expressed as nanomoles/g protein (nmol/g prot).
Mitochondrial membrane potential (MMP)
detection
JC-1, a cationic dye, is used as an indicator of
mitochondrial potential. It represents mitochondrial
potential-dependent accumulation, which is detected based on a
fluorescence emission shift from green to red. Briefly, fresh renal
tissue was cleaned with 0.9% normal saline and was subsequently
digested in trypsin solution (Beyotime Institute of Biotechnology,
Haimen, China) at 37°C for ~20 min. The digestion was terminated by
the addition of 30% bovine serum (Hangzhou Sijiqing Biological
Engineering Materials Company, Hangzhou, China). Suspension cells
were centrifuged at 2,000 × g and 4°C for 4 min and washed with PBS
three times. For JC-1 staining, the cells (~3×105/ml)
were loaded with 1X JC-1 (Beyotime Institute of Biotechnology) at
37°C for 20 min and then washed and analyzed via flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) and related
software (Flowjo version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Mitochondrial structure by electron
microscopy
Minute pieces of renal cortex were sectioned and
fixed in 2.5% glutaraldehyde at 4°C overnight, washed with 0.1 M
PBS (pH 7.2) and subsequently fixed in 2% osmium tetroxide at 4°C
for 2 h. The tissues were dehydrated in graded alcohol and then
embedded in epoxy resin at 45°C for 12 h. All tissues samples were
sectioned at 50 nm and washed again with distilled water and prior
to being stained with uranyl acetate (2%) and lead citrate (10%)
for 30 min at 25°C, respectively. Then samples were visualized
under a transmission electron microscope (H-600; Hitachi, Ltd.,
Tokyo, Japan). The mitochondrial ultramicrostructure changes were
observed in five random fields of view for each section.
Western blotting
Cytochrome c (cytc) expression levels in left
rabbit kidney tissues were detected using western blotting.
Briefly, tissues were homogenized with a T25 digital Ultra-Turrax
disperser in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology) containing phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology) and were centrifuged
at 14,000 × g and 4°C for 20 min. The bicinchoninic acid method was
used to detect the protein concentration. Then ~50 µg proteins in
each group were added onto the gels per lane for detection, then
samples were subjected to 12% SDS-PAGE and were transferred onto
polyvinylidene difluoride membranes for 1 h at 200 mA. The
membranes were blocked with 5% dried skimmed milk at room
temperature (~25°C) for 1 h and were subsequently incubated with
mouse primary antibodies against cytc (NB100-56503; 1:5,000; Novus
Biologicals, LLC, Littleton, CO, USA) and β-actin (ab28052,
1:5,000; Abcam, Cambridge, UK) overnight at 4°C. After washing, a
goat anti-mouse secondary antibody (P/N 925-32210; 1:10,000; LI-COR
Biosciences, Lincoln, NE, USA) conjugated to IRDye 800CW was added
and incubated for 1 h at room temperature. The signal was
quantified using a western blot detection system (Odyssey Infrared
Imaging; LI-COR Biosciences). Semi-quantitative analysis was
conducted (Image Studio version 5.2.5; LI-COR Biosciences) for the
corresponding protein expression levels.
Statistical analysis
Data are presented as the mean + standard deviation.
All analyses were performed in duplicate. The statistical software
package SPSS version 19 (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. One-way analysis of variance and Tukey's post
hoc test were used for statistical comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Levels of ROS, SOD, MDA, GSH-Px, CAT
and LD in hydronephrotic kidney tissues following
pneumoperitoneum
In group N, the ROS, SOD, MDA, GSH-PX, CAT and LD
levels were comparable when subjected to intraabdominal pressures
of 0, 5 and 10 mmHg (N0, N1 and N2 groups, respectively; P>0.05;
Fig. 1A). However, when the
pressure reached 15 mmHg (N3 group), the ROS, MDA and LD levels
significantly increased, and the SOD GSH-Px and CAT levels
decreased, compared with the N0 group (P<0.05; Fig. 1A). In group M, similar results were
observed (Fig. 1B). In group S,
the ROS, SOD, MDA, GSH-Px, CAT and LD levels were comparable at
pressures of 0 and 5 mmHg (S0 and S1 groups, respectively);
however, at 10 and 15 mmHg (S2 and S3 groups, respectively), the
ROS, MDA and LD levels significantly increased and the SOD, GSH-Px
and CAT levels significantly decreased compared with the S0 group
(P<0.05; Fig. 1C). Furthermore,
marginal increases in the ROS, MDA and LD levels and decreases in
the SOD, GSH-Px and CAT levels were observed with the increasing
degree of hydronephrosis in groups that suffered no intraabdominal
pressure (N0, M0 and S0 groups; Fig.
1).
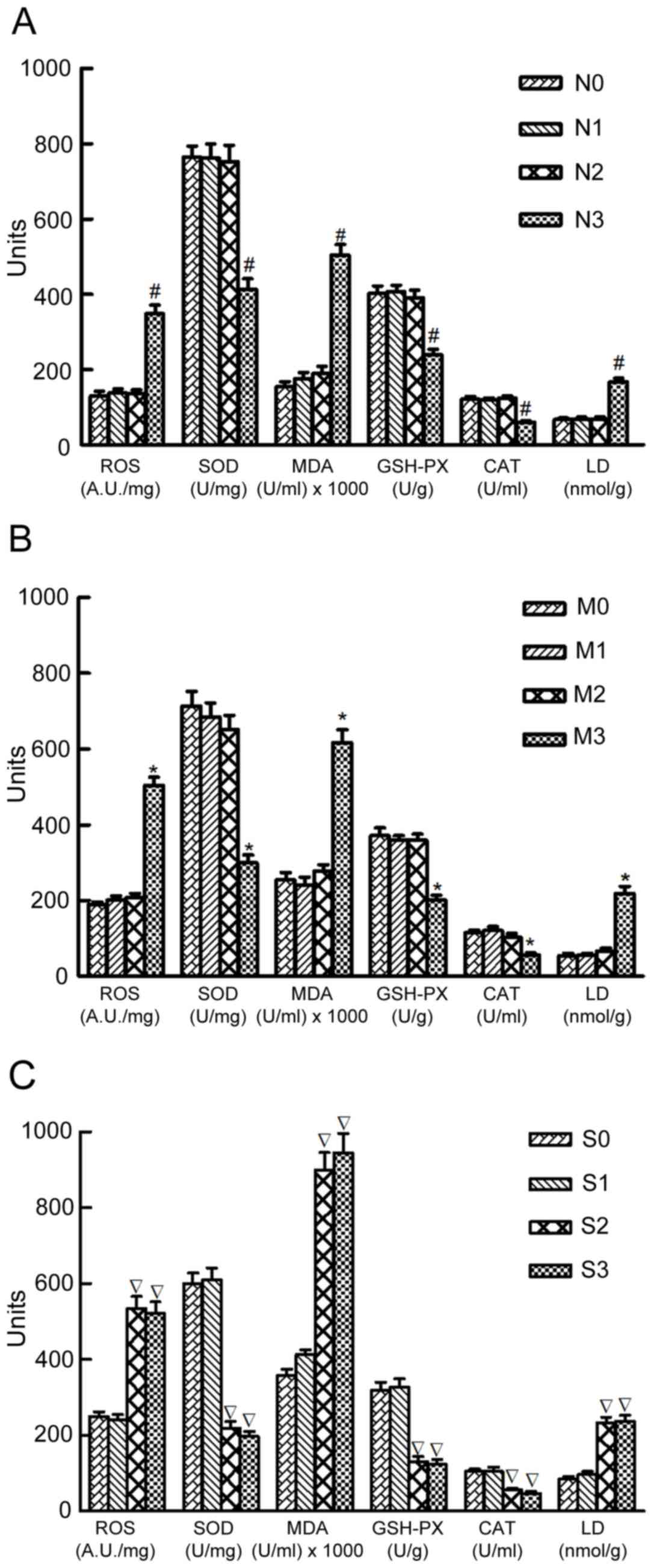 | Figure 1.Levels of ROS, SOD, MDA, GSH-Px, CAT
and LD in normal kidneys and kidneys with mild and severe
hydronephrosis under different intraabdominal pressures. (A) Normal
rabbit kidneys were represented by group N. N0, N1, N2 and N3
represented normal kidneys subjected to intraabdominal pressures of
0, 5, 10 and 15 mmHg, respectively. (B) Rabbit kidneys with mild
hydronephrosis were represented by group M. M0, M1, M2 and M3
represented rabbits with mild hydronephrosis subjected to
intraabdominal pressures of 0, 5, 10 and 15 mmHg, respectively. (C)
Rabbit kidneys with severe hydronephrosis were represented by group
S. S0, S1, S2 and S3 represented rabbits with severe hydronephrosis
subjected to intraabdominal pressures of 0, 5, 10 and 15 mmHg,
respectively. #P<0.05 vs. N0 group; *P<0.05 vs. M0
group; P<0.05 vs. S0 group. ROS, reactive oxygen species; SOD,
superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione
peroxidase; CAT, catalase; LD, lactate. |
Alterations in MMP levels of
hydronephrotic kidneys following pneumoperitoneum
The present study measured MMP to determine
mitochondrial injuries with JC-1. When MMP levels are high, JC-1
primarily exists in the mitochondrial matrix as a polymer, which
emits red fluorescence (excitation wavelength of 525 nm and
emission wavelength of 590 nm). When the MMP levels are low, JC-1
primarily exists in the cytoplasm as monomers, which emits green
fluorescence (excitation wavelength of 490 nm and emission
wavelength of 540 nm). Thus, alterations in the ratio of red to
green fluorescence intensity represents the alterations in MMP
levels; a decrease in MMP levels is considered to be an early
apoptotic event (18).
MMP values were expressed as the ratio of red
fluorescence intensity to the green fluorescence intensity,
indicated in Q2 and Q4, respectively. In groups N and M, the MMP
levels were similar in both groups at 0, 5 and 10 mmHg, but
decreased when the pressure reached 15 mmHg. In group S, the MMP
levels were similar when the pressure was 0 and 5 mmHg, but
significantly decreased when the pressure was 10 and 15 mmHg.
Furthermore, no significant differences were observed between the
S2 and S3 groups (Fig. 2).
Furthermore, a marginal decrease in MMP levels was observed with
the increased extent of hydronephrosis in groups that suffered no
intraabdominal pressure (N0, M0 and S0 groups; Fig. 2).
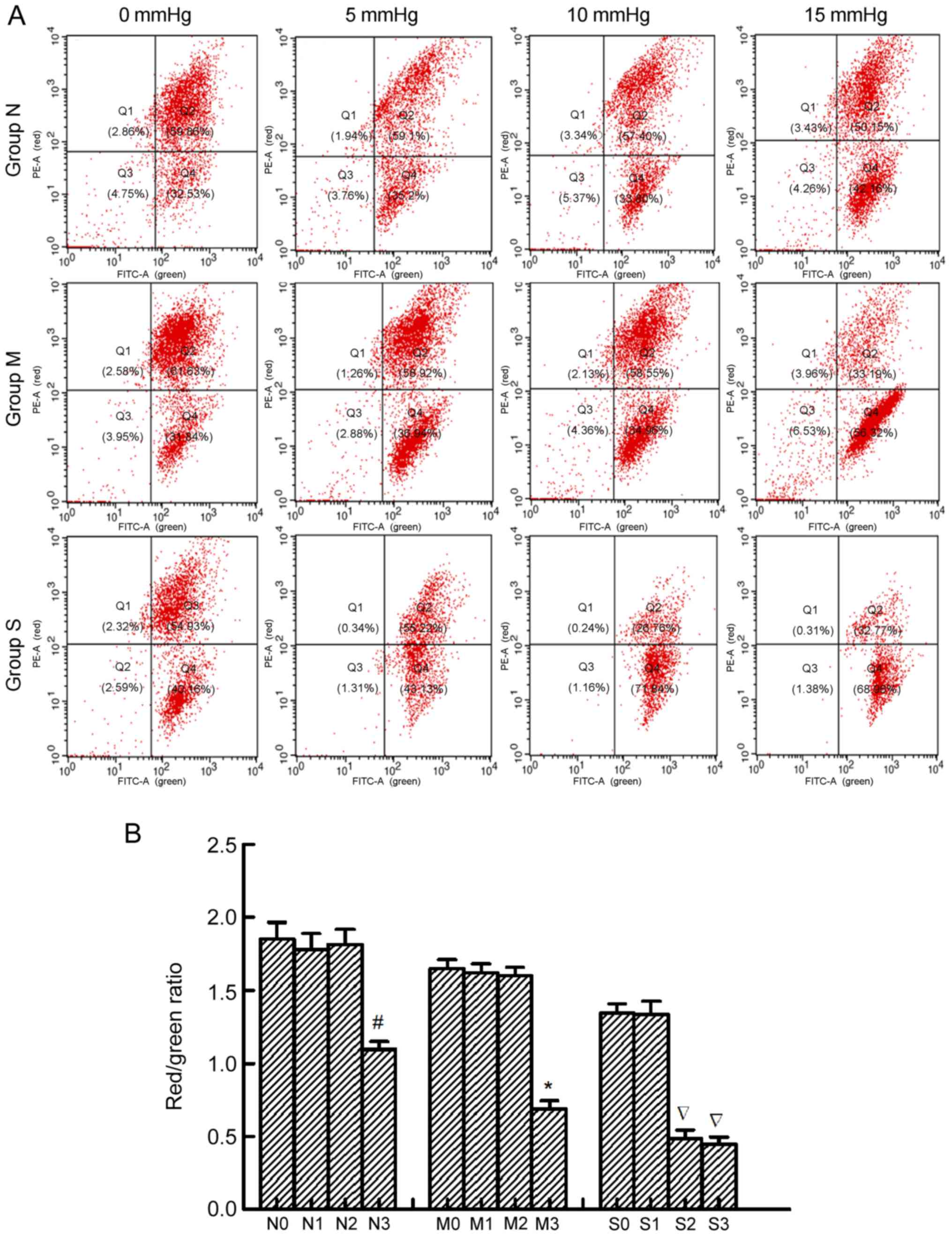 | Figure 2.MMP of renal cells in normal kidneys
and kidneys with mild and severe hydronephrosis under different
intraabdominal pressures. (A) MMP analysis by flow cytometry. PE-A
represented red fluorescence and FITC-A represented green
fluorescence. MMP values were expressed as the ratio of red
fluorescence intensity to the green fluorescence intensity,
indicated in Q2 and Q4, respectively. (B) Ratios of red
fluorescence intensity to the green fluorescence intensity in
rabbits with no, mild and severe hydronephrosis under different
intraabdominal pressures. Normal rabbit kidneys were represented by
group N. N0, N1, N2 and N3 represented normal kidneys subjected to
intraabdominal pressures of 0, 5, 10 and 15 mmHg, respectively.
Rabbit kidneys with mild hydronephrosis were represented by group
M. M0, M1, M2 and M3 represented rabbits with mild hydronephrosis
subjected to intraabdominal pressures of 0, 5, 10 and 15 mmHg,
respectively. Rabbit kidneys with severe hydronephrosis were
represented by group S. S0, S1, S2 and S3 represented rabbits with
severe hydronephrosis subjected to intraabdominal pressures of 0,
5, 10 and 15 mmHg, respectively. #P<0.05 vs. N0
group; *P<0.05 vs. M0 group; P<0.05 vs. S0 group. MMP,
mitochondrial membrane potential; PE, phycoerythrin; FITC,
fluorescein isothiocyanate; Q, quadrant. |
Mitochondrial ultramicrostructure
changes
Transmission electron microscopy was used to detect
the mitochondrial ultramicrostructure in renal cells. The present
study investigated the mitochondrial damage by counting the
percentages of swollen and vacuolar mitochondria in the different
groups. In group N, no swollen and vacuolar mitochondria were
observed at pressures of 0, 5 and 10 mmHg. However, when the
pressure increased to 15 mmHg, the percentage of swollen and
vacuolar mitochondria increased. In group M, the percentage of
swollen and vacuolar mitochondria were comparable at 0, 5 and 10
mmHg, but increased significantly when the pressure was 15 mmHg. In
group S, the percentage of swollen and vacuolar mitochondria were
similar at 0 and 5 mmHg, but significantly increased at pressures
of 10 and 15 mmHg. No significant differences were observed between
S2 and S3 groups (Fig. 3).
Furthermore, the percentage of swollen and vacuolar mitochondria
increased relatively with the increase in the extent of
hydronephrosis in rabbits suffering no intraabdominal pressure (N0,
M0 and S0 groups; Fig. 3).
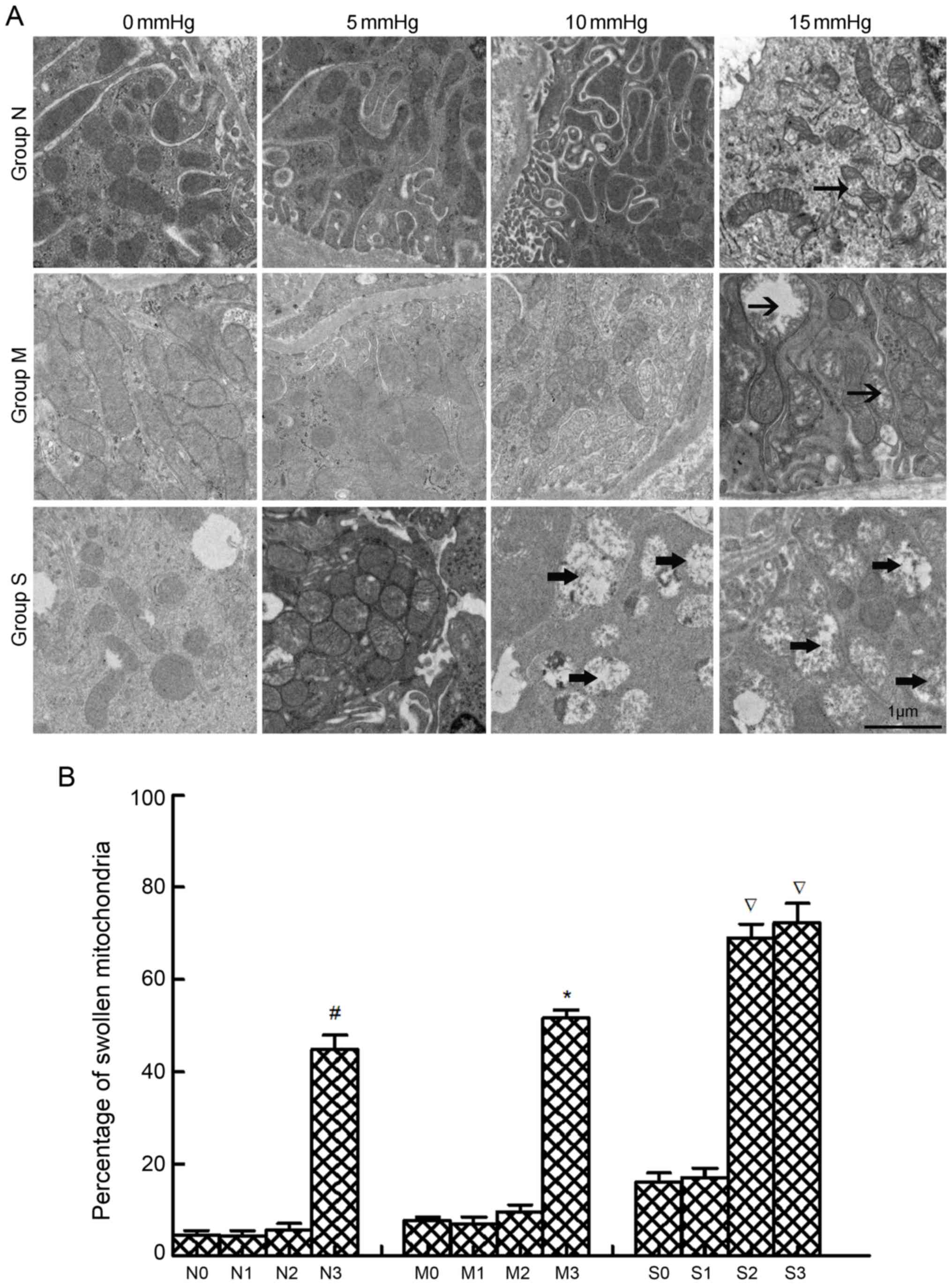 | Figure 3.Ultrastructural alterations of
mitochondria. (A) Swollen and vacuolar mitochondria in rabbit
kidneys with no, mild and severe hydronephrosis, represented by
groups N, M and S, respectively, were subjected to different
intraabdominal pressures and examined by transmission electron
microscopy. The arrows in this figure showed examples of swollen
and vacuolar mitochondria in different groups. Magnification,
×5,000. (B) Percentages of swollen and vacuolar mitochondria in
each group. The 0–3 subgroups for groups N, M and S represented
kidneys subjected to intraabdominal pressures of 0, 5, 10 and 15
mmHg, respectively. #P<0.05 vs. N0 group; *P<0.05
vs. M0 group; P<0.05 vs. S0 group. |
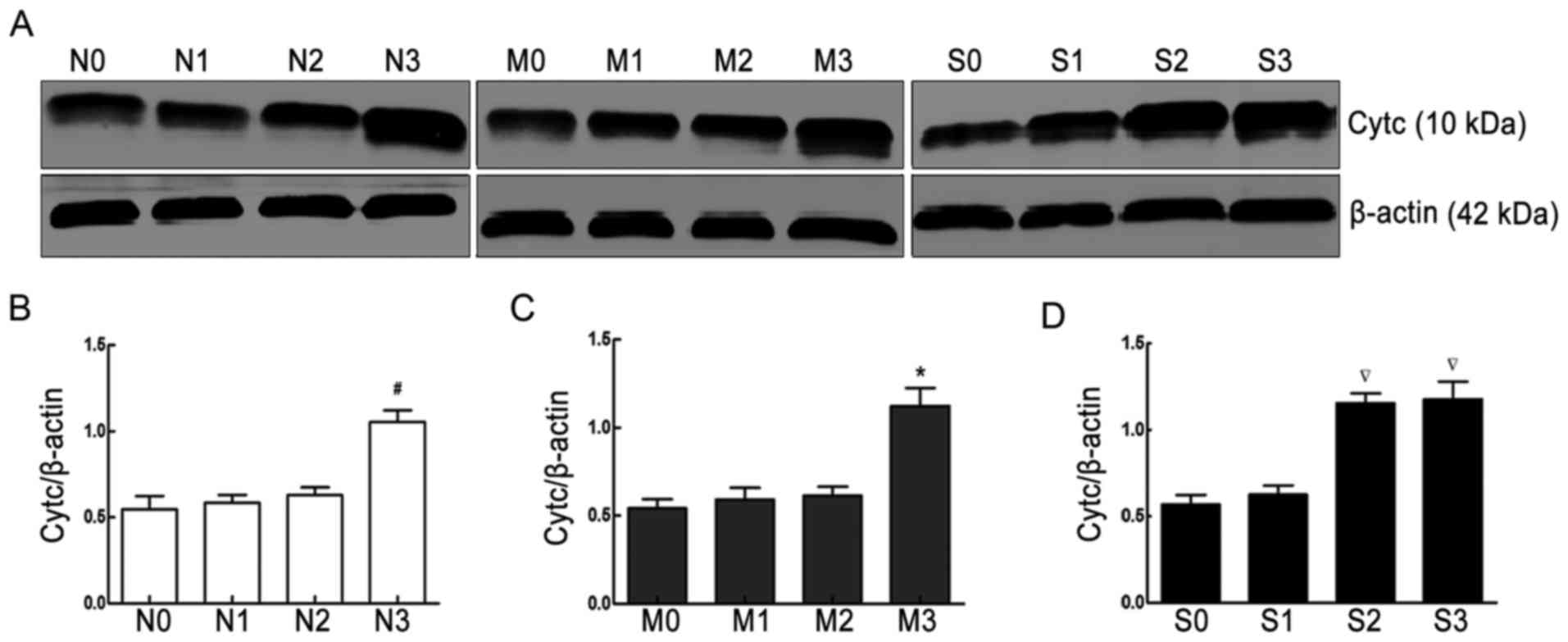
Expression of cytc
Western blot analysis demonstrated that cytc protein
expression in group N was comparable when subjected to
intraabdominal pressures of 0, 5 and 10 mmHg, but significantly
increased when the pressure was 15 mmHg. A similar result was
observed in group M. In group S, cytc protein expression was
comparable at intraabdominal pressures of 0 and 5 mmHg, and
significantly increased at pressures of 10 and 15 mmHg (Fig. 4).
Discussion
The present study demonstrated that oxidative damage
and mitochondrial injuries occurred in obstructed kidneys during
pneumoperitoneum. It was also demonstrated that oxidative damage
and mitochondrial injuries were more severe with a greater extent
of obstruction, meaning that severely obstructed kidneys may
exhibit reduced cell tolerance to intraabdominal pressure and that
they are more likely to suffer oxidative damage and mitochondrial
injuries.
Pneumoperitoneum, although generally considered to
be essential for adequate exposure in laparoscopic surgery, has
been reported to exert adverse effects on renal physiology,
particularly under high intraabdominal pressure (>10 mmHg)
(19,20). Numerous factors contribute to these
adverse effects. Wiesenthal et al (21) emphasized that high intraabdominal
pressure may noticeably decrease renal blood flow. Borba et
al (22) demonstrated that the
renin-angiotensin-aldosterone system also affects renal blood flow.
These effects may eventually cause hypoxic-ischemic damage, and
when intraabdominal pressure is removed, ischemia/reperfusion
injury may occur. This type of injury has been associated with
oxidative damage and mitochondrial injuries (23), and apoptosis or death eventually
occurs (24,25). As mentioned previously, certain
patients undergoing surgery also present with a certain degree of
kidney hydronephrosis. It has been reported that kidneys with
hydronephrosis are more likely to suffer hypoxia problems (26,27).
According to our previous study, rabbit kidneys with severe
hydronephrosis suffered acute kidney injury more readily compared
with those with mildly nephrotic kidneys when exposed to
pneumoperitoneal pressure (28).
Another study demonstrated that severe hydronephrosis (≥grade 3)
led to prolonged pneumoperitoneum time and total operation time in
laparoscopic radical nephroureterectomy (29). The prolonged operation time may
lead to increased oxidative stress. Therefore, the present study
investigated the effect of intraabdominal pressure based on kidneys
with hydronephrosis.
The generation of ROS appears to be an important
factor in tissue injury. To an extent, ROS content represents the
degree of oxidative damage. Reperfusion reintroduces oxygen to the
previously ischemic tissue, which results in a sudden burst of ROS
(30). The primary mechanisms of
ROS generation include anaerobic mitochondrial respiration,
activated neutrophils, increased xanthine oxidase levels and other
factors (31). Mitochondrial
membrane lipid peroxidation is considered to be an effect of ROS,
and mitochondrial damage promotes the release of large amounts of
ROS, which leads to a vicious cycle (32). MDA is a mediator of mitochondrial
damage and leads to mitochondrial dysfunction through the
inhibition of respiration and the inactivation of important
mitochondrial enzymes (33).
Therefore, by measuring MDA levels, the damage that ROS incurs on
kidney tissue may be determined. SOD, CAT and GSH-Px enzymes
constitute the primary components of the enzymatic antioxidant
defense system against oxidative stress. SOD performs dismutation
that leads to the formation of H2O2, which is
subsequently removed by GSH-Px and CAT. GSH has also been reported
to serve an additional role against lipid peroxidation (34). LD is a product of anaerobic
respiration. When severe hypoxia occurs, tissues are not able to
generate sufficient ATP from aerobic respiration and pyruvic acid
is converted into LD by lactate dehydrogenase (35). In the present study, compared with
the respective 0 mmHg intraabdominal pressure groups, increased LD,
ROS and MDA levels, and decreased SOD, GSH-Px and CAT levels, were
observed in normal kidneys and kidneys with mild hydronephrosis
subjected to 15 mmHg intraabdominal pressure, and in rabbits with
severe hydronephrosis subjected to 10 and 15 mmHg pressure. The
results indicated that kidneys with severe hydronephrosis suffered
from oxidative damage more readily compared with normal kidneys and
kidneys with mild hydronephrosis after being subjected to
intraabdominal pressure.
A loss of MMP is reported to have an adverse effect
on mitochondrial function (36).
It is generally accepted that decreased MMP affects the opening of
the mitochondrial permeability transition pore, which controls the
release of apoptosis-activating factors, such as cytc. The release
of cytc eventually leads to mitochondrial-dependent cell death
(37). The current study observed
a loss of MMP and higher cytc expression in normal kidneys and
kidneys with mild hydronephrosis subjected to 15 mmHg
intraabdominal pressure, and in rabbits with severe hydronephrosis
subjected to 10 and 15 mmHg pressure, compared with the respective
0 mmHg intraabdominal pressure groups, which indicated an increased
severity of mitochondrial damage. This observation may be
substantiated by the detection of ultrastructural alterations in
the mitochondria. Chronic hypoxia has been reported to augment the
quantity and the superficial area of mitochondria, which is
conductive to oxygen diffusion. However, severe hypoxia may lead to
mitochondrial deformation and swelling, and potentially the rupture
of the outer membrane or spillover of the mitochondrial matrix
(38). In the present study, the
percentage of swollen and vacuolar mitochondria increased in the
mild hydronephrosis and normal groups upon exposure to a pressure
of 15 mmHg, and increased in the severe hydronephrosis group at
pressures of 10 and 15 mmHg, compared with the respective 0 mmHg
groups.
In conclusion, the present study indicated that
kidneys with severe hydronephrosis may be more likely to suffer
mitochondrial injury than normal kidneys and kidneys with mild
hydronephrosis following subjection to intraabdominal pressure.
Additionally, marginal effects were identified with the increasing
extent of hydronephrosis, even without increased intraabdominal
pressure. This phenomenon may be explained as the effect
hydronephrosis itself has on the kidneys rather than
pneumoperitoneum.
However, if inherent limitations associated with
animal models do apply, it would be irrelevant to consider whether
similar intraabdominal pressures may be applied in humans as the
level of cell tolerance to intraabdominal pressure may differ
between humans and rabbits (39).
The difference in the size of kidney samples between two species
causes the pressure to be different per unit of kidney surface
area. The current study indicated that oxidative damage and
mitochondrial injuries are more likely to occur in obstructed
kidneys during pneumoperitoneum and that the pressure should be
kept lower when performing surgery. Based on the results of this
study, further studies regarding the exact underlying mechanisms
responsible for the decrease in tolerance to intraabdominal
pressure are required. Furthermore, the insufflation of
CO2 in experiments in the present study was performed at
room temperature (20–25°C) and dry (0–5% relative humidity)
conditions, and the intraabdominal pressures lasted for 1 h. A
retrospective analysis has provided evidence for the benefits of
using humidified, warm CO2 vs. dry, cool CO2
in surgery (40). However, Sammour
et al (41) revealed that
warming and humidification of insufflation gas had no effect on
measures of oxidative stress compared with unwarmed and
non-humidified controls. In another animal experiment, Akbulut
et al (42) demonstrated
that oxidative stress on kidneys increased with the prolonged
duration of pneumoperitoneal pressure from 120–240 min, and the
findings indicated that operating time should be limited to <120
min during laparoscopic surgery. The present study only determined
the effect that different intraabdominal pressures had on
obstructed kidneys. However, the duration of intraabdominal
pressures and the properties of gas should also be investigated as
other important factors.
In conclusion, the results of the current study
demonstrated that rabbit kidneys with severe hydronephrosis were
more likely to suffer oxidative damage and mitochondrial injuries
than mild hydronephrosis and normal kidneys when they were exposed
to pneumoperitoneal pressure. Therefore, intraabdominal pressure
should be appropriately controlled and reduced during laparoscopic
surgery in the context of kidney obstruction.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Nation Natural Science Fund Project of China (grant no.
81400698).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and SZ performed the experiment and were major
contributors in writing the manuscript. FC conceived and designed
the experiments. TR, WY and YR collected and analyzed the data. XY
and RY supplied materials and analyzed the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical and
Research Committee of Wuhan University Medical School (Wuhan,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van der Toorn F, van den Hoek J,
Wolffenbuttel KP and Scheepe JR: Laparoscopic transperitoneal
pyeloplasty in children from age of 3 years: Our clinical outcomes
compared with open surgery. J Pediatr Urol. 9:161–168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seims AD, VanHouwelingen L, Mead J, Mao S,
Loh A, Sandoval JA, Davidoff AM, Wu J, Wang WC and Fernandez-Pineda
I: Operative and immediate postoperative differences between
traditional multiport and reduced port laparoscopic total
splenectomy in pediatric patients. J Laparoendosc Adv Surg Tech A.
27:206–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartın MK, Kemik Ö, Çaparlar MA, Bostancı
MT and Öner MÖ: Evaluation of the open and laparoscopic
appendectomy operations with respect to their effect on serum IL-6
levels. Ulus Travma Acil Cerrahi Derg. 22:466–470. 2016.PubMed/NCBI
|
|
4
|
Wever KE, Bruintjes MH, Warlé MC and
Hooijmans CR: Renal perfusion and function during pneumoperitoneum:
A systematic review and meta-analysis of animal studies. PLoS One.
11:e01634192016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sodha S, Nazarian S, Adshead JM, Vasdev N
and Mohan-S G: Effect of pneumoperitoneum on renal function and
physiology in patients undergoing robotic renal surgery. Curr Urol.
9:1–4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Geus HR, Betjes MG, Schaick Rv and
Groeneveld JA: Plasma NGAL similarly predicts acute kidney injury
in sepsis and nonsepsis. Biomark Med. 7:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cekic B, Geze S, Ozkan G, Besir A, Sonmez
M, Karahan SC and Mentese A: The effect of dexmedetomidine on
oxidative stress during pneumoperitoneum. Biomed Res Int.
2014:7603232014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko KJ, Choi DK, Shin SJ, Ryoo HS, Kim TS,
Song W, Jeon HG, Jeong BC and Seo SI: Predictive factors of
prolonged warm ischemic time (≥30 minutes) during partial
nephrectomy under pneumoperitoneum. Korean J Urol. 56:742–748.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Barros RF, Miranda ML, de Mattos AC,
Gontijo JA, Silva VR, Iorio B and Bustorff-Silva JM: Kidney safety
during surgical pneumoperitoneum: An experimental study in rats.
Surg Endosc. 26:3195–3200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demyttenaere S, Feldman LS and Fried GM:
Effect of pneumoperitoneum on renal perfusion and function: A
systematic review. Surg Endosc. 21:152–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozmen MM, Zulfikaroglu B, Besler TH, Col
C, Cinel L and Cinel I: The correlation between reactive oxygen
species and histopathology of the liver, gut, and kidneys in
animals with elevated intra-abdominal pressure. J Laparoendosc Adv
Surg Tech A. 19:339–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sammour T, Mittal A, Loveday BP, Kahokehr
A, Phillips AR, Windsor JA and Hill AG: Systematic review of
oxidative stress associated with pneumoperitoneum. Br J Surg.
96:836–850. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Seigneux S, Klopfenstein CE, Iselin C
and Martin PY: The risk of acute kidney injury following
laparoscopic surgery in a chronic kidney disease patient. NDT Plus.
4:339–341. 2011.PubMed/NCBI
|
|
14
|
Dolkart O, Khoury W, Amar E and Weinbroum
AA: Pneumoperitoneum in the presence of acute and chronic kidney
injury: An experimental model in rats. J Urol. 192:1266–1271. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. National Acadamies Press;
Washington, DC: 1996
|
|
16
|
Wen JG, Chen Y, Frøkiaer J, Jørgensen TM
and Djurhuus JC: Experimental partial unilateral ureter
obstruction. I. Pressure flow relationship in a rat model with mild
and severe acute ureter obstruction. J Urol. 160:1567–1571. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oyanagui Y: Reevaluation of assay methods
and establishment of kit for superoxide dismutase activity. Anal
Biochem. 142:290–296. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 3:e4302012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Makarov DV, Kainth D, Link RE and Kavoussi
LR: Physiologic changes during helium insufflation in high-risk
patients during laparoscopic renal procedures. Urology. 70:35–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Özdemir-van Brunschot DM, van Laarhoven
KC, Scheffer GJ, Pouwels S, Wever KE and Warlé MC: What is the
evidence for the use of low-pressure pneumoperitoneum? A systematic
review. Surg Endosc. 30:2049–2065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiesenthal JD, Fazio LM, Perks AE, Blew
BD, Mazer D, Hare G, Honey RJ and Pace KT: Effect of
pneumoperitoneum on renal tissue oxygenation and blood flow in a
rat model. Urology. 77:1508.e9–e15. 2011. View Article : Google Scholar
|
|
22
|
Borba M, Lopes R, Carmona M, Neto BM,
Nahas SC and Pereira PR: Effects of enalaprilat on the
renin-angiotensin-aldosterone system and on renal function during
CO2 pneumoperitoneum. J Endourol. 19:1026–1031. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cay A, Imamoğlu M, Unsal MA, Aydin S,
Alver A, Akyol A and Sarihan H: Does anti-oxidant prophylaxis with
melatonin prevent adverse outcomes related to increased oxidative
stress caused by laparoscopy in experimental rat model? J Surg Res.
135:2–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tosun M, Yucel M, Kucuk A and Sezen S: P53
related apoptosis in kidneys in CO2 pneumoperitoneum rat
model: An immunohistochemical study. Mol Biol Rep. 41:6391–6395.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong N, Zhang Y, Zhu HF and Zhou ZN:
Intermittent hypoxia exposure prevents mtDNA deletion and
mitochondrial structure damage produced by ischemia/reperfusion
injury. Sheng Li Xue Bao. 52:375–380. 2000.PubMed/NCBI
|
|
26
|
Li W, Cao Z, Xia Z, Meng Q, Yu WM, Yao X
and Cheng F: Acute kidney injury induced by various
pneumoperitoneum pressures in a rabbit model of mild and severe
hydronephrosis. Urol Int. 94:225–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Z, Yu W, Li W, Cheng F, Rao T, Yao X,
Zhang X and Larré S: Oxidative damage and mitochondrial injuries
are induced by various irrigation pressures in rabbit models of
mild and severe hydronephrosis. PLoS One. 10:e01271432015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Z, Yu W, Li W, Cheng F, Xia Y, Rao T,
Yao X, Zhang X and Larré S: Acute kidney injuries induced by
various irrigation pressures in rat models of mild and severe
hydronephrosis. Urology. 82:1453.e9–16. 2013. View Article : Google Scholar
|
|
29
|
Shigeta K, Kikuchi E, Hagiwara M, Hattori
S, Kaneko G, Hasegawa M, Takeda T, Jinzaki M, Akita H, Miyajima A,
et al: Visceral to total obesity ratio and severe hydronephrosis
are independently associated with prolonged pneumoperitoneum
operative time in patients undergoing laparoscopic radical
nephroureterectomy for upper tract urothelial carcinoma.
Springerplus. 4:2902015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwak GH, Kim TH and Kim HY:
Down-regulation of MsrB3 induces cancer cell apoptosis through
reactive oxygen species production and intrinsic mitochondrial
pathway activation. Biochem Biophys Res Commun. 483:468–474. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehaney DA, Darwish HA, Hegazy RA, Nooh
MM, Tawdy AM, Gawdat HI and El-Sawalhi MM: Analysis of oxidative
stress status, catalase and catechol-O-methyltransferase
polymorphisms in Egyptian vitiligo patients. PLoS One.
9:e992862014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tortora M, Corsini S and Nistri A:
Nicotinic receptors modulate the onset of reactive oxygen species
production and mitochondrial dysfunction evoked by glutamate uptake
block in the rat hypoglossal nucleus. Neurosci Lett. 639:43–48.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Gao H, Na XL, Dong SY, Dong HW, Yu
J, Jia L and Wu YH: Aniline induces oxidative stress and apoptosis
of primary cultured hepatocytes. Int J Environ Res Public Health.
13:E11882016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cigremis Y, Turel H, Adiguzel K, Akgoz M,
Kart A, Karaman M and Ozen H: The effects of acute acetaminophen
toxicity on hepatic mRNA expression of SOD, CAT, GSH-Px, and levels
of peroxynitrite, nitric oxide, reduced glutathione, and
malondialdehyde in rabbit. Mol Cell Biochem. 323:31–38. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mookerjee SA and Brand MD: Measurement and
analysis of extracellular acid production to determine glycolytic
rate. J Vis Exp: e53464. 2015. View
Article : Google Scholar
|
|
36
|
Sung DK, Chang YS, Kang S, Song HY, Park
WS and Lee BH: Comparative evaluation of hypoxic-ischemic brain
injury by flow cytometric analysis of mitochondrial membrane
potential with JC-1 in neonatal rats. J Neurosci Methods.
193:232–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang LR, Xue X, Hu XM, Wei MY, Zhang CQ,
Ge GL and Liang XJ: Structure-dependent mitochondrial dysfunction
and hypoxia induced with single-walled carbon nanotubes. Small.
10:2859–2869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Avital S, Itah R, Szomstein S, Rosenthal
R, Inbar R, Sckornik Y and Weinbroum A: Correlation of CO2
pneumoperitoneal pressures between rodents and humans. Surg Endosc.
23:50–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balayssac D, Pereira B, Bazin JE, Le Roy
B, Pezet D and Gagnière J: Warmed and humidified carbon dioxide for
abdominal laparoscopic surgery: Meta-analysis of the current
literature. Surg Endosc. 31:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sammour T, Mittal A, Delahunt B, Phillips
AR and Hill AG: Warming and humidification have no effect on
oxidative stress during pneumoperitoneum in rats. Minim Invasive
Ther Allied Technol. 20:329–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akbulut G, Polat C, Aktepe F, Yilmaz S,
Kahraman A, Serteser M, Gökçe C and Gökçe O: The oxidative effect
of prolonged CO2 pneumoperitoneum on renal tissue of
rats. Surg Endosc. 18:1384–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|