Introduction
In a broad sense, cardiovascular diseases (CVD) are
diseases associated with aging (1). A number of studies have indicated
that a dysfunction in vascular endothelial cells is a key
antecedent of vascular-associated diseases (2,3).
Aging or cell senescence is partly caused by the dysfunction of
vascular endothelial cells (4).
The loss of replicative capacity in senescent endothelial cells
destroys their cellular integrity and damages angiogenesis
(5,6).
Kinase non-catalytic C-lobe domain containing 1
(KNDC1) exists in dendrites, guanine nucleotide exchange factor
complexes and neuronal cell bodies as a putative protein-protein
interaction module that regulates a signaling pathway in dendrites,
which is suggested to serve an important role in the process of
cell senescence (7). KNDC1 serves
a key role in a number of signal transduction pathways that help in
protein recognition and functional regulation. Previous studies
demonstrated that the KNDC1 activity of Ras GEF by c-Jun N-terminal
kinase 1 (JNK1) and/or extracellular signal regulated kinase (ERK)
via the Ras-Raf-mitogen-activated protein (MAP) kinase pathway
induced MAP2 phosphorylation and microtubule binding activity,
thereby promoting an increase in the length of nerve cells
(8,9). A previous study also demonstrated
that the inhibition or knockdown of KNDC1 possibly delayed neuronal
cell senescence and promoted dendritic growth. This suggests that
it serves an important role in regulating neuronal dendrite
development (10). Other results
also suggest that KNDC1 regulates the development of neuronal
dendrites via the Ras-Raf-MAP kinase signaling pathway (11). However, only a few reports
regarding the regulatory functions and mechanisms of vascular
endothelial cells exist.
Previous experiments demonstrated that the knockdown
of KNDC1 may promote the proliferation of endothelial cells and
delay their aging. However, the effect of KNDC1 overexpression
remains unclear. Therefore, a KNDC1-adenovirus vector was
constructed and an endothelial cell model that expressed KNDC1 was
designed to elucidate its role in HUVECs.
Materials and methods
Chemicals and reagents
Enriched Culture Medium (ECM; cat. no. 1001;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA);
KNDC1-adenovirus vector was constructed by GeneWay Co., Ltd.,
(Shanghai China); Senescence-associated β-galactosidase (SA-β-gal)
staining kit (cat. no. C0602; Beyotime Institute of Biotechnology,
Shanghai, China); TRIzol™ reagent (cat. no. 15596018;
Thermo Fisher Scientific, Inc.); KNDC1 (cat. no. SC-90387; Santa
Cruz Biotechnology Inc., Dallas, TX, USA); PrimeScript™
RT Master Mix kit (cat. no. D3720; Takara Bio Inc., Otsu, Japan);
polyvinylidene difluoride (PVDF) membranes (cat. no. IPVH00010.
Immobilon-P; EMD Millipore, Billerica, MA, USA); anti-p53,
anti-phospho (p)-p53 and anti-GAPDH (cat. nos. 9282T, 9284T and
5174T, respectively. Cell Signaling Technology Inc., Danvers, MA,
USA); superoxide dismutase (SOD) and glutathione peroxidase (GPx)
assay kits (cat. no. A001-3, A006; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China).
Cells and culture
Endothelial cells were isolated from umbilical cord
tissue obtained from women who underwent a full-term normal
pregnancy, which was supplied by Beijing Hospital (Beijing, China).
The umbilical cord samples for the present study were taken on 1st
August 2013 (Beijing Hospital) from pregnant women without maternal
hypertensive disorders, diabetes mellitus, or any other contagious
diseases (n=5; aged 23–28-years-old). The umbilical cord of the
newborn was cut under aseptic conditions and preserved in normal
saline at 4°C. As the primary culture is directly from the tissue
or organ part of the organism, in vitro time is short; the
genetic characteristics and functional structure of the primary
culture are similar to the body. Therefore, the primary culture is
suitable for the study of cell morphology, function and
differentiation. Umbilical vein endothelial cells were isolated and
cultured within 12 h. Endothelial cells were isolated using an
enzyme perfusion digestion method (0.1% type I collagenase at 37°C
for 10–12 min) following primary passage (P0), inoculated in ECM
containing 100 mg/ml streptomycin, 100 IU/ml penicillin, 40 µg/ml
endothelial cell growth supplement and 20% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a
humidified atmosphere of 95% air and 5% CO2. Endothelial
cells were identified based on two aspects, morphology and
immunohistochemistry, after they were grown to the second or third
generation.
Under an inverted phase contrast microscope
(magnification, ×200), the morphology of endothelial cells
following passage were typically spherical, spindle or cobblestone;
HUVECs were fixed with 4% paraformaldehyde for 15 min at room
temperature, washed with PBS three times and blocked with 3% bovine
serum albumin (BSA) in PBS (Shanghai Qiao Yu Biological Technology
Co., Ltd., Shanghai, China) for 15 min at 37°C. Subsequently,
overnight incubation at 4°C was conducted with primary antibodies
diluted in 1% BSA PBS [cluster of differentiation (CD)31, CD34, von
Willebrand factor, vascular endothelial growth factor (VEGF)
receptor 1, VEGF receptor 2 (cat. nos. 3528S, 3569S, 65707S, 2893S,
9698S, respectively, Cell Signaling Technology Inc.), rewarmed 30
min at 37°C and rinsed with PBS three times (5 min per wash). Then
a fluorescein isothiocyanate-labeled rabbit secondary antibody
(1:50; cat. no. sc-2359, Santa Cruz Biotechnology Inc.) was added
and HUVECs were incubated for 1 h at room temperature, washed with
PBS 3 times (3 min per wash); propidium iodide dye (Beijing
Zhongsheng Ruitai Technology Co., Ltd. Beijing, China) was added
for incubation for 5 min at room temperature. HUVECs were washed
with PBS 3 times (3 min per wash), then immediately observed under
a fluorescence microscope (535 nm excitation wavelength,
magnification, ×200). The cytoplasm of endothelial cells exhibited
yellow-green fluorescent staining and the nucleus was dark green;
the nucleus and the cell outline were clear. The purity of
endothelial cells was close to 100% and expressed VEGF. Based on
the data from the author's previous study (7), the purity of endothelial cells at the
passage 6 (P6) generation was close to 100%. Endothelial cells at
P6 were used for further experiments and were seeded into 6-well
plates.
Transfections
KNDC1-adenovirus vector was constructed by GeneWay
Co., Ltd. P6 endothelial cells (7.5×105) were selected
to be transfected with the KNDC1-adenovirus vector and cultured for
24 h at 37°C. A non-targeting control vector (NT-adenovirus vector;
GeneWay Co., Ltd.) was also used [60 plaque forming units
(pfu)/cell]. They were grouped as follows: i) Blank control (no
treatment; C) group; ii) negative control group (A); HUVECs were
transfected with NT-adenovirus vector; iii) HUVECs were transfected
with 30/60/90 pfu/cell KNDC1-adenovirus vector (K30/60/90
experimental group, respectively). Finally, they were all cultured
in ECM for 24 h as described above.
SA-β-gal staining
For anti-senescence experiments, human endothelial
cells (7.0×105) were cultured in ECM using the
aforementioned procedure. The endothelial cells were transfected
with different doses of the KNDC1-adenovirus in the experimental
group. A blank control group and a negative control group was also
used.
Following a 2-day culture, the cell culture medium
was removed and the cells were washed twice with PBS. They were
then fixed for 15 min with PBS containing 2% formaldehyde and 0.2%
glutaraldehyde at room temperature. Following removal of the
fixative solution, the cells were washed three times with PBS and
then dyeing liquid was added. The cells were then incubated at 37°C
for 10 h in a staining solution of 40 mM citric acid, sodium
phosphate, (pH 6.0), 1 mg/ml
5-bromo-4-chloro-3-isolyl-β-d-galactoside (X-gal; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), 5 mM potassium ferrocyanide, 5 mM
potassium ferricyanide, 150 mM NaCl and 2 mM MgCl2.
Finally, they were observed under an inverted microscope (CKX31;
Olympus Corporation, Beijing, China) at a magnification of ×40. A
blue stained cytoplasm indicated that the cells were aged. A total
of five visual fields were randomly selected. A positive incidence
of SA-β-gal was the percentage of positive cells in the total cell
count. The experiment was repeated in triplicate.
RNA expression analysis
After the human endothelial cells
(7.0×105) were treated with or without the
KNDC1-adenovirus vector using the aforementioned procedure, total
cellular RNA was extracted with TRIzol reagent according to the
manufacturer's protocol. A 2 µl RNA sample was taken to determine
its concentration and purity. The total RNA sample was reverse
transcribed to cDNA according to the manufacturer's protocol of the
PrimeScript™ RT Master Mix kit. The synthesized cDNA
samples were subjected to reverse transcription (RT)-qPCR according
to the protocol provided by the AceQ™ qPCR SYBR Green
Master Mix kit (Vazyme, Piscataway, NJ, USA). GAPDH was used as a
reference gene. The primer pairs used in the study are as follows:
KNDC1, forward (FW): 5′-CAGGCTTCTTTCCCTACTGTTCGT-3′, reverse (RV):
5′-CCGCTGCTTGTTTTGATAGTTCTC-3′; GAPDH (Shanghai Bioengineering
Inc., Shanghai, China), FW: 5′-CGCTGAGTACGTCGTGGAGTC-3′, RV:
5′-GCTGATGATCTTGAGGCTGTTGTC-3′. The PCR was run on an iCycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction
conditions were as follows: 95°C for 5 min initial denaturation,
followed by 40 cycles of 95°C for 10 sec and finally 60°C for 20
sec. The experimental results were analyzed by 2−ΔΔCq
method (12).
Western blotting
Endothelial cells (1.0×106) were
collected with a cell scraper and 120 µl radio immunoprecipitation
assay buffer was added to lyse the cells. Following lysis, the
supernatant was collected and the sample buffer was added. Protein
samples were prepared by boiling in water for 5–10 min. The
supernatant collected following cell lysis was used to quantify
protein concentration in the endothelial cells using standardized
bicinchoninic acid protein. Based on the quantitative results, the
protein samples (30 µg) were subjected to SDS-PAGE (10%)
electrophoretic separation and the membrane reaction was carried
out on the PVDF membrane (Immobilon-P; EMD Millipore). The PVDF
membranes were then blocked with 5% non-fat milk for 30 min at room
temperature, followed by diluted rabbit primary antibodies
(1:1,000) as follows: Anti-p53, anti-phospho-p53 (Cell Signaling
Technology Inc.). The membranes were incubated in diluted rabbit
primary antibodies overnight at 4°C. Then diluted corresponding
horseradish peroxidase-conjugated secondary antibodies (1:4,000;
Cell Signaling Technology Inc.) were added for incubation for 1 h
at room temperature. Chemiluminescence was performed in a gel
imaging system and protein bands were visualized by incubating the
membranes with high-performance autoradiography film (Fuji Film
Co., Tokyo, Japan). The experimental results i.e., gray band
analysis of protein bands with β-actin (cat. no. SC-8432, Santa
Cruz Biotechnology Inc.) or GAPDH (cat. no. sc-47724, Santa Cruz
Biotechnology Inc.) was carried out by using the ImageJ 2.1
software (National Institutes of Health, Bethesda, MD, USA). Values
were calculated in terms of integrated optical density and
expressed in arbitrary units.
Oxidative stress
The KNDC1-adenovirus vector was added to cells in
the logarithmic growth phase followed by incubation for 24 h.
Endothelial cells (5×105) from the KNDC1-adenovirus
vector group, negative control group A and control group C were
washed three times with PBS followed by digestion with 0.25%
trypsin for 30 sec at room temperature. ECM containing FBS was
added to terminate the digestion process. Next, the cell
suspensions were collected, centrifuged at 1,000 × g for 5 min at
room temperature and counted under an inverted phase contrast
microscope (magnification, ×200). Following cell density
calculation and adjustment, the cells were seeded into Eppendorf
tubes, each containing 1×104 cells, in 200 µl cell
lysate solution [0.05 mmol/l EDTA, 1% Triton-X 100, (pH 8.0)]. The
tubes were agitated to allow the lysate solution to completely lyse
the cells, following which, the tubes were placed on ice for 20 min
and finally centrifuged at 12,000 × g for 20 min at 4°C to isolate
the supernatant. The SOD and GPx activities were detected by an
ultraviolet spectrophotometer (excitation wavelength, 450 nm)
(Bio-Rad Laboratories Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 11.0
software for Windows (SPSS Inc., Chicago, IL, USA). All the data
are expressed as the mean ± standard deviation. The Student's
t-test was used for comparisons between 2 groups. The 3 groups were
compared using a single factor analysis of variance. The Student
Newman-Keuls method was used to compare between each group if the
difference was statistically significant. P<0.05 was considered
to indicate a statistically significant difference.
Results
KNDC1 expression increases with aging
of HUVECs
In a previous study (7), it was observed that with increasing
passage number, the number of aging HUVEC cells increases, as
demonstrated by SA-β-gal staining. In the 1–2nd generation of the
endothelial cells cell morphology was observed to be polygonal,
intercalated with transparent cytoplasm, a blurred cell outline and
a unilateral cobblestone arrangement. With an increase in
generation number, cell morphological alterations occurred,
including a flattened morphology, greater volume, lower
proliferative rates and slower growth. The 4–5th generation of the
endothelial cells were not of uniform size, exhibited cytoplasm
turbidity, a clear cell outline, an increased volume, slow growth,
a sparse arrangement and difficulty forming a monolayer of cells
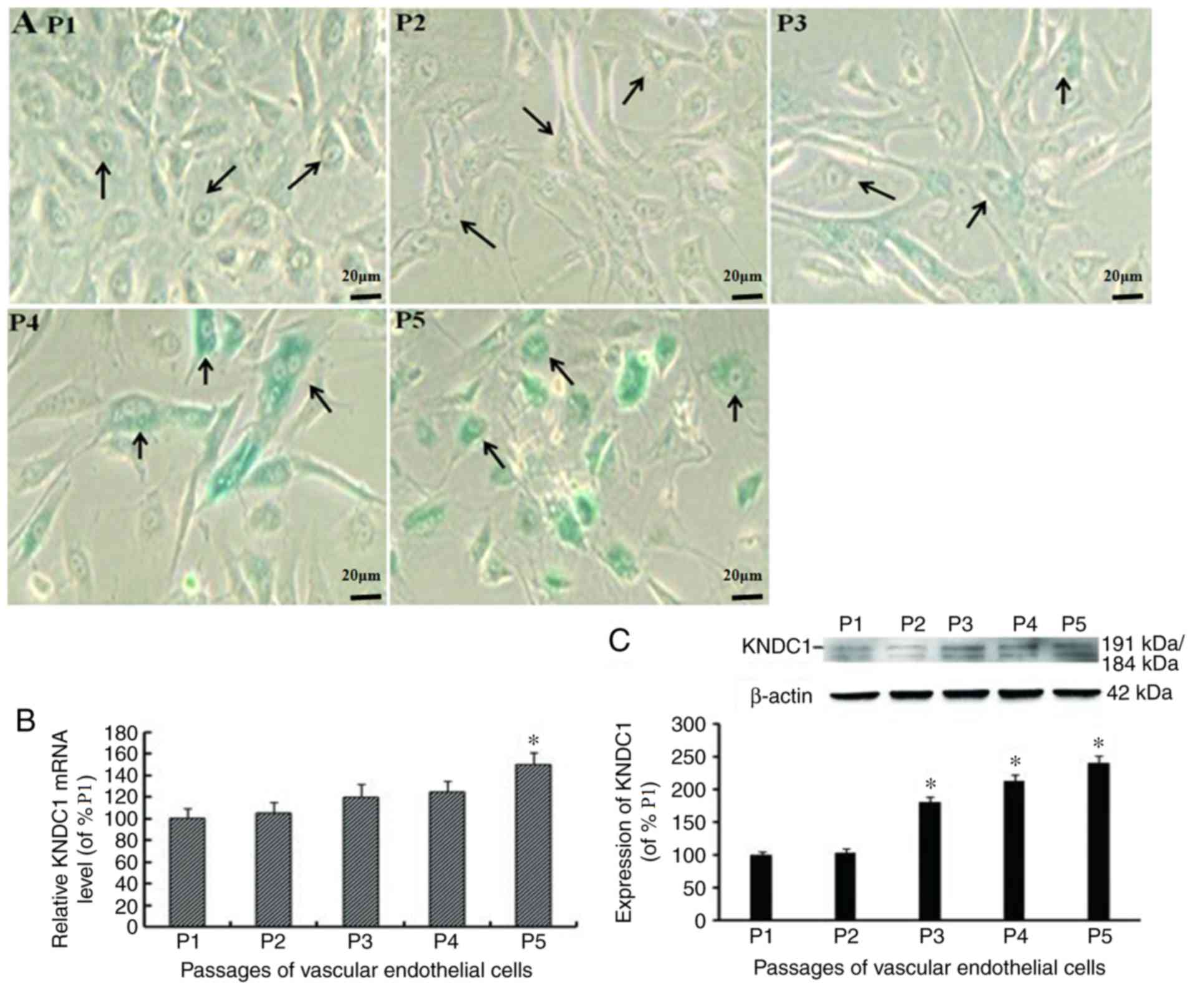
(Fig. 1A) (7). The present study investigated whether
KNDC1 was associated with the senescence of normal cells. The level
of KNDC1 transcription and expression in the HUVECs was examined at
different passages by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blot analysis. The results
demonstrated that the transcription and expression levels of KNDC1
increased gradually with the aging of the HUVECs with the mRNA
level significantly increasing at P5 whereas the protein expression
level was significantly increased from P3 (P<0.05; Fig. 1B and C) (7).
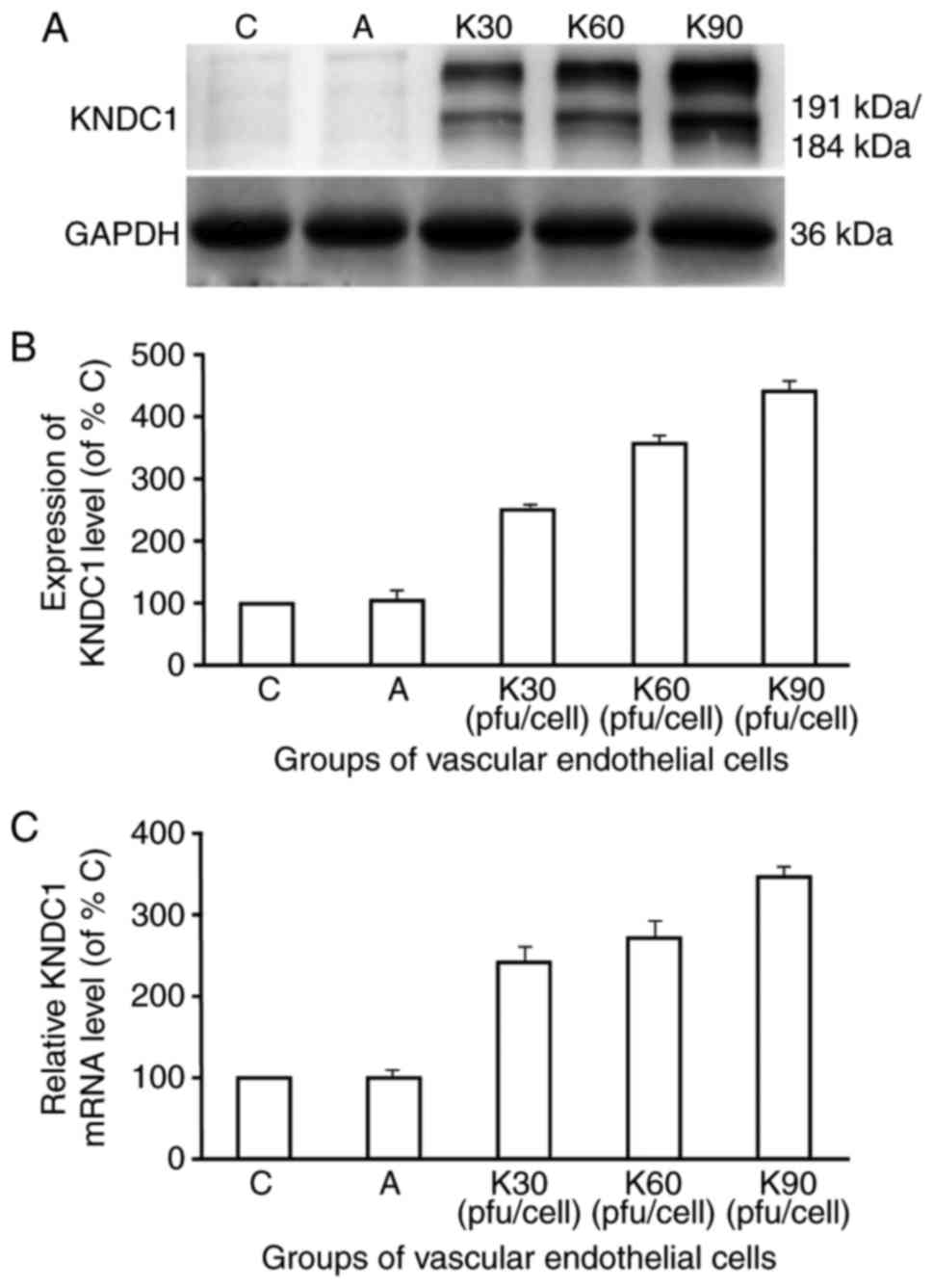
KNDC1-adenovirus vector increases
KNDC1 mRNA and protein expression levels
In order to investigate the association between
KNDC1 and the HUVEC aging process, the expression levels of KNDC1
were upregulated in P6 HUVECs by transfecting them with the
KNDC1-adenovirus vector (Fig. 2).
As exhibited in Fig. 2C,
transfection with the KNDC1-adenovirus vector resulted in a
239–344% increase in KNDC1 mRNA levels. Similarly, KNDC1 protein
levels among the groups also demonstrated a statistically
significant increase in a dose-dependent manner (Fig. 2B).
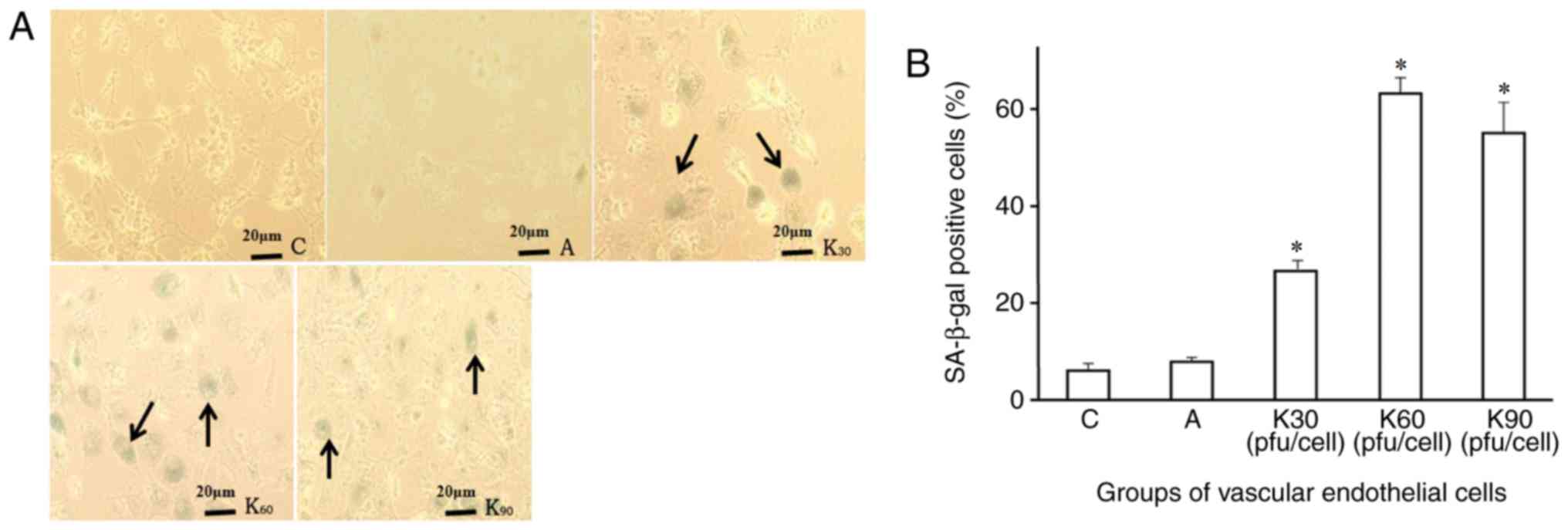
Overexpressing KNDC1 enhances
senescent phenotype
Furthermore, KNDC1 overexpression notably promoted
the senescent phenotype of HUVECs. The number of positive SA-β-Gal
staining observed in the HUVECs following the transfection of the
KNDC1-adenovirus vector significantly increased when compared with
that in the control group (Fig.
3A). The positive rate of SA-β-gal staining in the HUVECs also
increased in a dose-dependent manner over a 24 h incubation period
i.e., with increasing transfection doses of the KNDC1-adenovirus
vector from 30–60 pfu/cell. However, the morphological difference
of endothelial cells between the groups with different transfection
doses was not obvious, only the positive rate of SA-β-gal staining
increased. Morphological alterations may require long-term
observation. The positive rate of SA-β-gal staining in each group
(Fig. 3A) was group C 6.2±1.3%,
group A 8.2±0.3%, K30 26.3±2.5%, K60, 62.9±2.8% and K90 55.3±5.8%.
No significant difference (P=0.054) was observed between the
positive rates in SA-β-gal staining at the K60 and K90 doses
(Fig. 3B), and the 60 pfu/cell
resulted in the highest level of positively stained cells.
Therefore, transfection was carried out at 60 pfu/cell in the
experimental group for further studies.
Phosphorylated (p)p53 expression
increases if KNDC1 is overexpressed
Furthermore, the genes associated with HUVEC
senescence were investigated by examining the activation of the
phosphorylated forms of their proteins by western blot analysis.
The results demonstrated no significant difference in p53 (an
important cell cycle inhibitory factor) expression in the
experimental HUVEC group compared with the blank and negative
control groups. However, a significant increase in the expression
of p-p53 (Fig. 4A) was observed in
HUVECs that overexpressed KNDC1 when compared with the
NT-adenovirus-transfected control cells (244.3±19.5 vs. 113.5±9.0;
Fig. 4B), which suggests that the
p53 signaling pathway probably serves an important role in the
senescence process of the HUVECs.
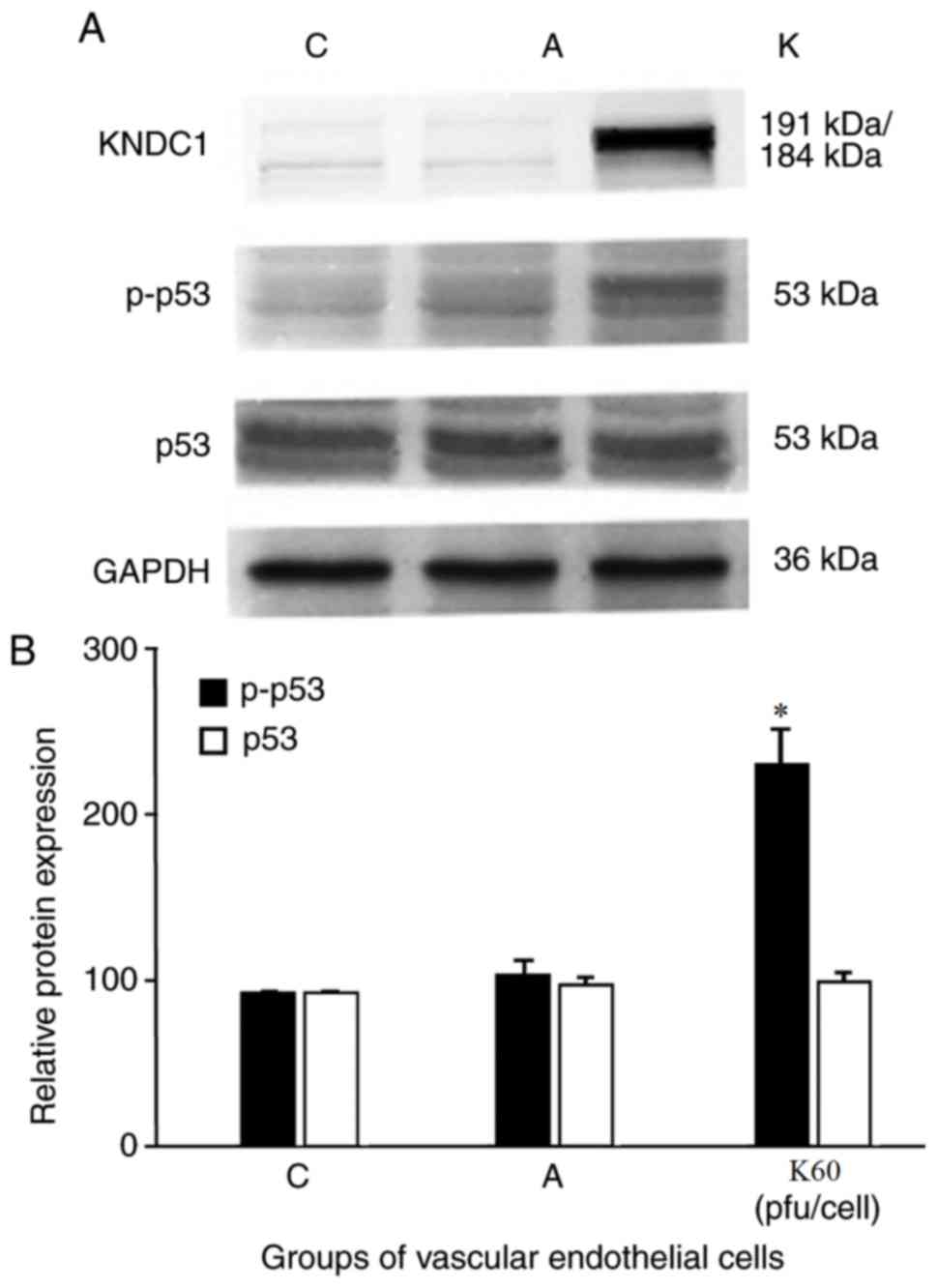 | Figure 4.Effect of KNDC1 overexpression on p53
and p-p53 activity in HUVECs. (A) Typical western blots for total
p53, p-p53 are shown. GAPDH served as the loading control. (B)
Summary of quantification of densitometric measurement of the
immunoblot data. Data are presented as the mean of three replicate
determinations (n=3) ± standard deviation. Bars having different
letters are significantly different (*P<0.05 vs. C and A
groups). C, blank control group; A, negative control group, HUVECs
were transfected with the adenovirus; K60, experimental group,
HUVECs were transfected with 60 pfu/cell KNDC1-adenovirus. KNDC1,
kinase noncatalytic C-lobe domain containing 1; HUVECs, human
umbilical vein endothelial cells; pfu, plaque forming units; p,
phosphorylated. |
ROS activity increases with KNDC1
overexpression
The author's previous study demonstrated that KNDC1
knockdown delayed HUVEC senescence by decreasing intracellular ROS
(7). In the present study, this
alteration was further verified. After 24 h of transfection with
KNDC1, the activity of antioxidant enzymes in HUVECs significantly
decreased. The antioxidase content in the experimental group was
decreased compared with the control group (SOD: 83.3±5.1 vs.
98.0±3.0; GPx: 83.0±5.6 vs. 95.0±6.0; P=NS; Fig. 5). These results suggested that
KNDC1 overexpression accelerated the senescence process in cells by
inhibiting the antioxidants (SOD and GPx) and activating ROS
generation.
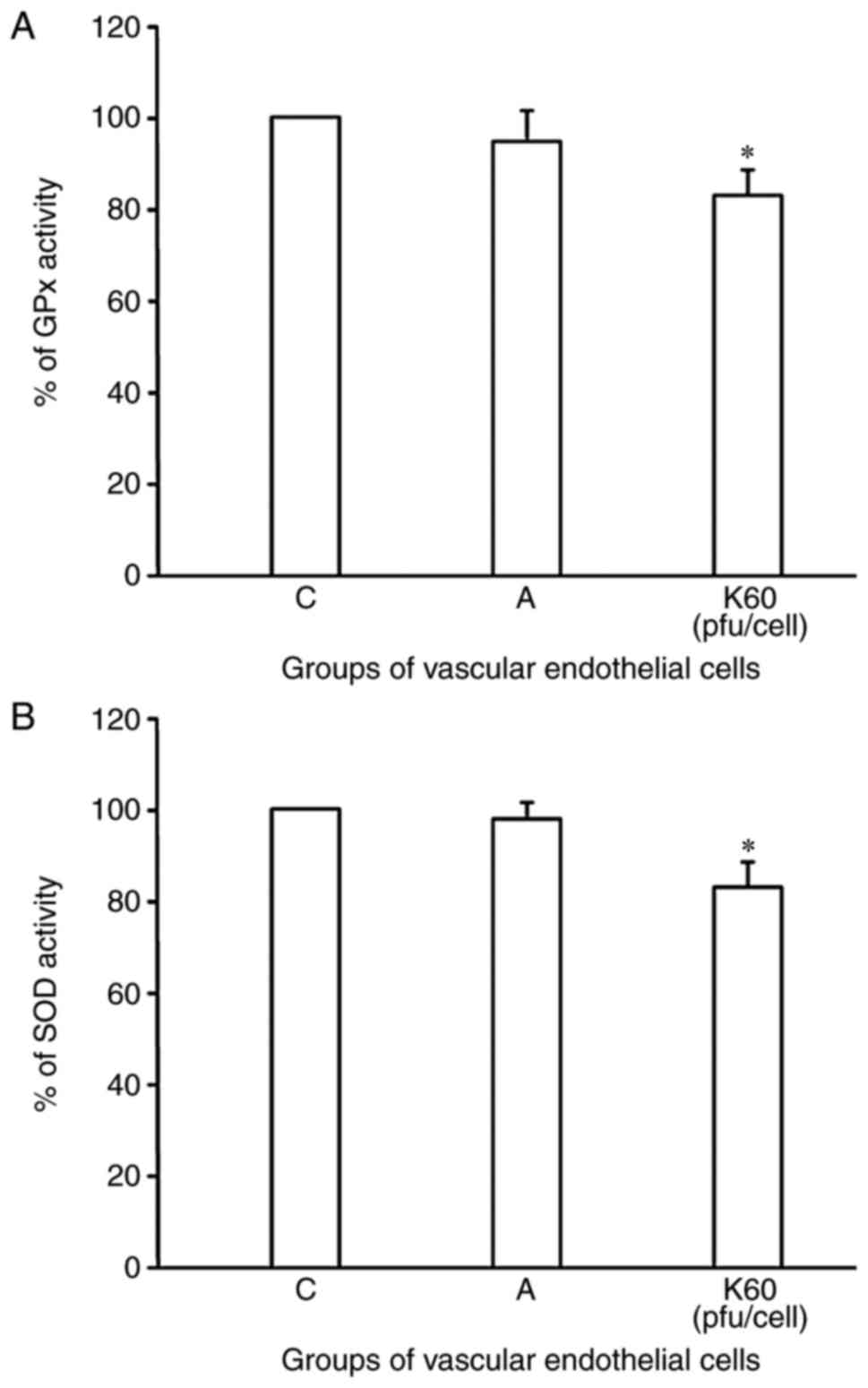 | Figure 5.Effect of KNDC1 overexpression on
antioxidant activity. (A) SOD and (B) GPx activity in HUVECs. Data
are presented as the mean of three replicate determinations (n=3) ±
standard deviation. Bars having different letters are significantly
different (*P<0.05 vs. C and A groups). C, blank control group;
A, negative control group, HUVECs were transfected with adenovirus;
K60, experimental group, HUVECs were transfected with 60 pfu/cell
KNDC1-adenovirus. KNDC1, kinase noncatalytic C-lobe domain
containing 1; HUVECs, human umbilical vein endothelial cells; pfu,
plaque forming units; SOD, superoxide dismutase; GPx, glutathione
peroxidase. |
Discussion
Aging or cell senescence is an important risk factor
for CVD. The development of vascular endothelial cellular
senescence serves an important role in several biological processes
(13). Senescent cells exhibit
several characteristic features including positive staining for
SA-β-gal, enlarged and flat cellular morphology and specific gene
expression patterns (14–16).
As a novel Ras GEF protein, a study described KNDC1
as one of the genes associated with senescent cells (17). The preliminary study also
demonstrated that the inhibition and/or knockdown of KNDC1
expression promotes cerebellar granule cells and hippocampal neuron
dendritic growth in cultured cells, suggesting it is a signaling
molecule involved in the development, regulation, and restriction
of cell growth (18,19). However, there are few studies in
this field, which are insufficient to confirm the mechanism of
KNDC1 in promoting the aging of endothelial cells. The regulatory
functions and mechanisms of KNDC1 on vascular endothelial cells
have not been reported. HUVECs are frequently used as biological
senescent models due to their limited capacity to divide when
cultured in vitro (20,21).
Therefore, in the present study, a biological senescence model was
constructed by culturing HUVECs in vitro and then the effect
of KNDC1 on cell senescence was investigated. In the present study
KNDC1-adenovirus-transfected HUVECs were demonstrated to undergo
senescence over a short time-period (24 h). It was also
demonstrated that with an increased number of passages, SA-β-gal
staining demonstrated a gradual increase in the number of senescent
endothelial cells and that the transcription and expression levels
of KNDC1 also increased gradually.
To further investigate the effect of KNDC1 on
endothelial cell senescence, gene transfection techniques were
used. The recombinant KNDC1-adenovirus was constructed through a
homologous recombination of cells, followed by the KNDC1 gene being
transfected into HUVECs by the adenovirus vector. On comparing the
positive rates of KNDC1-adenovirus transfection at different doses,
the group dosed with 60 pfu/cell exhibited SA-β-gal staining
similar to the group dosed with 90 pfu/cell, however increased
activity compared with the group dosed with 30 pfu/cell. RT-qPCR
and western blotting results further demonstrated that the mRNA and
protein expression levels in the KNDC1-adenovirus vector group were
increased compared with the empty vector and blank group following
a 24 h incubation. Typical morphological alterations including cell
enlargement, multinucleation and the activity of the lysosomal
enzyme β-galactosidase were also observed to be increased in HUVECs
where the expression of KNDC1 was upregulated with the
KNDC1-adenovirus vector when compared with the
NT-adenovirus-transfected HUVECs of the same passage.
Following this, the mechanism of the functional
alterations in HUVECs following transfection with the
KNDC1-adenovirus vector was investigated. Consistent with previous
results, the overexpression of KNDC1 led to alterations in the
expression of specific genes closely associated with cell growth
regulation (p-p53) in the present study (22). Previous studies have confirmed p53
as a regulator of the DNA damage response and p53/p21 are thought
to serve a role in hydrogen peroxide induced cell growth,
inactivity, and replicative senescence (23,24).
Transcription factor p53 is one of the most important proteins that
inhibits the aging process and its multiple isoforms also serve a
role in maintaining genomic integrity (25). p53 performs specific biological
functions ranging from transient or permanent cell cycle arrest to
cell death (26,27). In the present study, the results of
the western blotting experiments demonstrated that although the p53
expression did not markedly alter in the endothelial cells
transfected with the KNDC1-adenovirus vector, p53 phosphorylation
and p-p53 expression increased. It was reported that p53 and its
binding partners may be modified (e.g., via phosphorylation) to
induce a conformational change in their protein structure or to
directly interfere with their interactive process (28). Therefore, the increase in p53
phosphorylation indicates that KNDC1 exerts an aging effect by
regulating p53 protein modification, increasing the expression of
functional p53 and then activating downstream genes (29).
A previous study demonstrated that p53 exerts its
antiproliferative function via the regulation of energy metabolism
and oxidative stress (30). One of
the stress factors closely associated with aging is an increase in
intracellular ROS levels (31). In
the present study, it was demonstrated that increased
phosphorylated p53 levels compared with the control led to an
increased production of ROS in a dose-dependent manner. Therefore,
the activation of the p53 pathway may be in response to the
ROS-induced DNA damage (32). p53
may downregulate the expression of antioxidant enzymes including
SOD and GPx under physiological conditions when acutely stressed.
Furthermore, p53 exhibits pro-oxidant activity and may exacerbate
oxidative stress responses to cell sustained stressors (33). ROS may also activate p53 and
initiate p53-dependent stress-response programs including cell
cycle arrest, senescence, and apoptosis (34,35).
Therefore, the positive feedback loops of p53 and ROS transform
stressed cells into apoptotic or senescent cells. Additionally, p53
activation initiates a series of downstream transcriptional events
that lead to cell growth inhibition and senescence. These results
suggest that KNDC1 may serve an important role in the aging process
of HUVECs and that the overexpression of KNDC1 promoted the aging
process.
In conclusion, the overexpression of KNDC1 inhibited
proliferation and promoted senescence of the HUVECs. With respect
to the mechanism of action, p53 proved to be an essential inducer
of KNDC1-mediated senescence. Furthermore, it was demonstrated that
the overexpression of KNDC1 increased the expression of ROS by
activating the p53 signaling pathway. The p53-ROS positive feedback
loop probably served an important role in the regulation of
KNDC1-induced cell senescence. KNDC1 also promoted the activity of
p53, the amplification of the p53-ROS feedback loop and then served
a catalytic role in the cell senescence process by increasing the
expression of the phosphorylated p53 protein. The present study
differs from previous research (17–19)
as it further demonstrated the role of KNDC1 overexpression in
promoting endothelial cell senescence and identified a novel
molecular mechanism (p53-ROS positive feedback loop). To the best
of the authors' knowledge this study is the first time that
KNDC1-adenovirus vector inhibition of HUVEC proliferation by
activating the p53 signaling pathway has been reported. Elucidating
the biological function and molecular mechanism of KNDC1 will
provide theoretical support for the study of cell senescence
mechanism. The authors' next step will be to carry out experiments
in vivo, to further invest the mechanism of the high
expression of KNDC1 induced senescence of endothelial cells and
potentially provide novel targets for anti-aging treatment.
Finally, the results depict KNDC1 as a promising candidate since it
delays the aging process and prolongs human life.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81671391)
and Henan Province Science and Technology Research Plan (grant no.
162102310002).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JJ and ZH designed the present study, conducted
experiments, analysis and interpretation of data, and drafting of
manuscripts. HL and YJL reviewed the concepts of the experiment.
YL, JL, CM and BL were involved in experimental analysis and data
acquisition. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study had obtained human research ethics
approval from the Ethics Committee of Beijing Hospital on April
15th, 2013. The informed consent of the subjects was obtained at
May 2nd, 2013. Human umbilical vein endothelial cells (HUVECs) used
in this study were isolated by Dr. Lin in 1st August 2013 (Beijing
Hospital).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seals DR: Edward F: Adolph distinguished
lecture: The remarkable anti-aging effects of aerobic exercise on
systemic arteries. J Appl Physiol (1985). 117:425–439. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hafner F, Kieninger A, Meinitzer A, Gary
T, Froehlich H, Haas E, Hackl G, Eller P, Brodmann M and Seinost G:
Endothelial dysfunction and brachial intima-media thickness: Long
term cardiovascular risk with claudication related to peripheral
arterial disease: A prospective analysis. PLoS One. 9:e933572014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sikora E, Bielak-Zmijewska A and Mosieniak
G: Cellular senescence in ageing, age-related disease and
longevity. Curr Vasc Pharmacol. 12:698–706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Shaer MH, Choueiri NE, Correia ML,
Sinkey CA, Barenz TA and Haynes WG: Effects of aging and
atherosclerosis on endothelial and vascular smooth muscle function
in humans. Int J Cardiol. 109:201–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erusalimsky JD: Vascular endothelial
senescence: From mechanisms to pathophysiology. J Appl Physiol
(1985). 106:326–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cardus A, Uryga AK, Walters G and
Erusalimsky JD: SIRT6 protects human endothelial cells from DNA
damage, telomere dysfunction, and senescence. Cardiovasc Res.
97:571–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Zhen YZ, Lin YJ, Liu J, Wei J, Xu
R and Hu G: KNDC1 knockdown protects human umbilical vein
endothelial cells from senescence. Mol Med Rep. 10:82–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dickinson RJ, Delavaine L, Cejudo-Marín R,
Stewart G, Staples CJ, Didmon MP, Trinidad AG, Alonso A, Pulido R
and Keyse SM: Phosphorylation of the kinase interaction motif in
mitogen-activated protein (MAP) kinasephosphatase-4 mediates
cross-talk between protein kinase A and MAP kinase signaling
pathways. J Biol Chem. 286:38018–38026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basu K, Mukhopadhyay A, Ghosh I and Datta
K: Nuclear morphology and c-Jun N-terminal kinase 1 expression
differentiate serum-starved oxidative stress signalling from
hydrogen peroxide-induced apoptosis in retinal neuronal cell line.
Cell Biol Int. 36:1021–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SK, Xiao L, Li J, Liu F, Sun L and
Kanwar YS: Role of guanin-nucleotide exchange factor Epac in renal
physiology and pathopysiology. Am J Physiol Renal Physiol.
304:F831–F839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J, Furuya A, Hayashi K and Furuichi
T: Interaction between very-KIND Ras guanine exchange factor and
microtubule-associated protein 2, and its role in dendrite
growth-structure and function of the second kinase noncatalytic
C-lobe domain. FEBS J. 278:1651–1661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rezk N, Elkotb SM and Naguib YM: Swimming
Exercise Ameliorates Elevated Blood Pressure and Vascular
Endothelial Dysfunction in Old Rats. Am J Med Med Sci. 4:192–202.
2014.
|
|
14
|
Zhao X, Zhao Q, Luo Z, Yu Y, Xiao N, Sun X
and Cheng L: Spontaneous immortalization of mouse liver sinusoidal
endothelial cells. Int J Mol Med. 35:617–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sosińska P, Mikuła-Pietrasik J, Ryżek M,
Naumowicz E and Książek K: Specificity of cytochemical and
fluorescence methods of senescence-associated β-galactosidase
detection for ageing driven by replication and time.
Biogerontology. 15:407–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vo NT, Mikhaeil MS, Lee LE, Pham PH and
Bols NC: Senescence-associated β-galactosidase staining in fish
cell lines and primary cultures from several tissues and species,
including rainbow trout coelomic fluid and milt. In Vitro Cell Dev
Biol Anim. 51:361–371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mees A, Rock R, Ciccarelli FD, Leberfinger
CB, Borawski JM, Bork P, Wiese S, Gessler M and Kerkhoff E:
Very-KIND is a novel nervous system specific guanine nucleotide
exchange factor for Ras GTPases. Gene Expr Patterns. 6:79–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang J, Furuya A and Furuichi T:
Very-KIND, a KIND domain containing RasGEF, controls dendrite
growth by linking Ras small GTPases and MAP2. J Cell Biol.
179:539–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayashi K, Furuya A, Sakamaki Y, Akagi T,
Shinoda Y, Sadakata T, Hashikawa T, Shimizu K, Minami H, Sano Y, et
al: The brain-specific RasGEF very-KIND is required for normal
dendritic growth in cerebellar granule cells and proper motor
coordination. PLoS One. 12:e01731752017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levine EM and Mueller SN: Cultured
vascular endothelial cells as a model system for the study of
cellular senescence. Int Rev Cytol. Suppl:67–76. 1979.
|
|
21
|
Muck C, Micutkova L, Zwerschke W and
Jansen-Durr P: Role of insulin-like growth factor binding protein-3
in human umbilical vein endothelial cell senescence. Rejuvenation
Res. 11:449–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madden SL, Galella EA, Riley D, Bertelsen
AH and Beaudry GA: Induction of cell growth regulatory genes by
p53. Cancer Res. 56:5384–5390. 1996.PubMed/NCBI
|
|
23
|
Speidel D: The role of DNA damage
responses in p53 biology. Arch Toxicol. 89:501–517. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyauchi H, Minamino T, Tateno K, Kunieda
T, Toko H and Komuro I: Akt negatively regulates human endothelial
cell lifespan via the p53/p21 dependent pathway. EMBO J.
23:212–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KS, Kang KW, Seu YB, Baek SH and Kim
JR: Interferon-gamma induces cellular senescence through
p53-dependent DNA damage signaling in human endothelial cells. Mech
Ageing Dev. 130:179–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carstens MJ, Krempler A, Triplett AA, Van
Lohuizen M and Wagner KU: Cell cycle arrest and cell death are
controlled by p53-dependent and p53-independent mechanisms in
Tsg101-deficient cells. J Biol Chem. 279:35984–35994. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T,
He TC, Du W and Yuan CS: Genistein induces G2/M cell cycle arrest
and apoptosis via ATM/p53-dependent pathway in human colon cancer
cells. Int J Oncol. 43:289–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamura S, Roth JA and Mukhopadhyay T:
Multiple lysine mutations in the C-terminal domain of p53 interfere
with MDM2-dependent protein degradation and ubiquitination. Mol
Cell Biol. 20:9391–9398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu W and Roeder RG: Activation of p53
sequence-specific DNA binding by acetylation of the p53 C-terminal
domain. Cell. 90:595–606. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hernández-Reséndiz I, Román-Rosales A,
García-Villa E, López-Macay A, Pineda E, Saavedra E, Gallardo-Pérez
JC, Alvarez-Ríos E, Gariglio P, Moreno-Sánchez R and
Rodríguez-Enríquez S: Dual regulation of energy metabolism by p53
in human cervix and breast cancer cells. Biochim Biophys Acta.
1853:3266–3278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong SG and Cho GW: Endogenous ROS levels
are increased in replicativesenescence in human bone marrow
mesenchymal stromal cells. Biochem Biophys Res Commun. 460:971–976.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Valent L and Strasser A: Distinct target
genes and effector processes appear to be critical for
p53-activated responses to acute DNA damage versus p53-mediated
tumour suppression. Biodiscovery. 8:1–16. 2013.
|
|
33
|
Chatoo W, Abdouh M and Bernier G: p53
pro-oxidant activity in the centralnervous system: Implication in
aging and neurodegenerative diseases. Antioxid Redox Signal.
15:1729–1737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinha VC, Qin L and Li Y: A
p53/ARF-dependent anticancer barrier activates senescence and
blocks tumorigenesis without impacting apoptosis. Mol Cancer Res.
13:231–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu J, Song T, Liu S, Li X, Li G and Xu J:
Icariside II inhibits cell proliferation and induces cell cycle
arrest through the ROS-p38-p53 signaling pathway in A375 human
melanoma cells. Mol Med Rep. 11:410–416. 2015. View Article : Google Scholar : PubMed/NCBI
|