Introduction
Rhegmatogenous retinal detachment (RRD) refers to
the detachment of the retinal neuroepithelial fiber layer from the
retinal pigment epithelium (RPE) layer caused by the positioning of
the vitreous body underneath the retinal neuroepithelial layer
(1–3). The vitreous body may cause retinal
tearing and the resulting tension may separate the retina from the
RPE (4,5). RRD is a common ophthalmological
disease that usually affects middle-aged men with myopia (6,7). RRD
prevents the outer retina layer from obtaining nutrition from
choroid, which may lead to atrophy of the detached retina and its
subsequent degeneration (8,9).
Untreated RRD may lead to impairment or loss of vision, and
recovery is unlikely (10). The
human eye is made up of millions of non-renewable RPE cells
organized into a single-cell layer (11).
Following stimulation with human vitreous or
fibronectin, RPE cells differentiate and migrate to the vitreous
body (12,13). RPE cells proliferate and secrete
molecules into the extracellular matrix (ECM), resulting in
formation of periretinal membrane with contraction ability, leading
to retinal detachment (14,15).
Inflammatory cytokines may be involved in ECM function and cell
proliferation; therefore, it was hypothesized that inflammatory
cytokines affect ECM, induce abnormal proliferation of RPE cells
and contribute to the development of RRD (16,17).
Interleukin (IL)-10 is a multifunctional negative regulatory factor
that is mainly produced by T helper cells, activated B cells,
mononuclear cells and macrophages (18,19).
It participates in the regulation of immune, inflammatory and tumor
cells and therefore serves a crucial role in transplantation
immunity and in a variety of diseases, including autoimmune,
infectious and tumors (20,21).
However, the role of IL-10 as an RPE-secreted negative immune
regulator of RRD remains to be elucidated.
Materials and methods
Experimental animals and ethical
approval
A total of 20 healthy male Wistar rats (age, 2
months; weight, 250±20 g) were purchased from Shanghai SLAC
laboratory Animal Co., Ltd. (Shanghai, China) and raised in the
experimental animal center in Yan'an University (Yan'an, China) at
a temperature of 21±1°C with 50–70% humidity, 5% CO2 and
a 12 h day-night cycle. All rats had free access to food and water.
The present study was approved by the Ethics Committee of the
Affiliated Hospital of Yan'an University.
Materials and reagents
Atropine eye drops (0.1%), ofloxacin eye drops
(0.3%) and mydrin-P were supplied by Merck (Merck & Co., Inc.,
Whitehouse Station, NJ, USA). Pentobarbital sodium and lidocaine
were purchased from Zhpharma (Shanghai Zhonghua Pharmaceutical Co.,
Ltd., Shanghai, China). IL-10 was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). High glucose Dulbecco's modified
Eagle medium (DMEM), fetal bovine serum (FBS) and
penicillin-streptomycin were obtained from HyClone (GE Healthcare
Life Sciences, Logan, UT, USA). Dimethyl sulfoxide (DMSO) and MTT
were supplied by Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Trypsin was obtained from Sigma-Aldrich (Merck KGaA).
Polyvinylidene fluoride (PVDF) membrane was purchased from Pall
Life Sciences (Port Washington, NY, USA). Western blotting reagents
including polyvinylidene fluoride (PVDF) membrane and Tween-20 were
obtained from Beyotime Institute of Biotechnology (Shanghai,
China). Peroxidase substrate for Enhanced Chemiluminescence (ECL)
reagent was purchased from Amersham (GE Healthcare, Chicago, IL,
USA). Rabbit anti-rat vascular endothelial growth factor (VEGF)
monoclonal antibody (cat. no. 9698) and horseradish peroxidase
(HRP)-conjugated immunoglobulin G goat anti-rabbit secondary
antibody (cat. no. 7074) were purchased from Cell Signaling
Technology, Inc., Danvers, MA, USA). IL-1β (cat. no. RLB00) and
IL-6-specific (cat. no. R6000B) ELISA kits were supplied by R&D
Systems, Inc. (Minneapolis, MN, USA). Caspase-3 activity detection
kit was purchased from Cell Signaling Technology, Inc.
SYBR®-Green Quantitative RT-qPCR kit was from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). A Transwell chamber
was purchased from Corning Incorporated (Corning, NY, USA). The
microplate reader was purchased from Labsystems Diagnostics Oy
(Vantaa, Finland). ABI 7700 Fast Real Time PCR was supplied by
Thermo Fisher Scientific, Inc. Benchtop was purchased from Suzhou
Purification Engineering Installation Co., Ltd. (Suzhou, China). A
Forma incubator was purchased from Thermo Fisher Scientific,
Inc.
Rat RRD model establishment
The rat RRD model was established as previously
described (22). Briefly,
following 0.1% pentobarbital sodium intraperitoneal anesthesia
injection, rats were placed in supine position. Atropine eye drops
(0.1%) and ofloxacin eye drops (0.3%) were administered to rats for
3 days (1 drop daily) prior to the surgery. At the same time, 100
µl 0.5% Mydrin-P was used to induce mydriasis 3 days prior to
surgery. Lidocaine (2%) and bupivacaine (0.75%) (Sigma-Aldrich,
Merck KGaA) were applied subconjunctivally for local anesthesia.
Needles (30 gauge) were used to mechanically induce retinal
detachment of ~2 mm under the rim of the right eye on the sclera
and retina. Needles were then inserted back into the subretinal
space followed by injection of 70% sodium hyaluronate
(Sigma-Aldrich, Merck KGaA) until successful induction of retinal
detachment was achieved. A total of 10,000 units of gentamicin and
dexamethasone (Sigma-Aldrich, Merck KGaA) were injected locally and
chloramphenicol (Sigma-Aldrich, Merck KGaA) eye drops were used
postoperatively to prevent infection. Eye examination was performed
with an operating microscope to evaluate whether RRD was
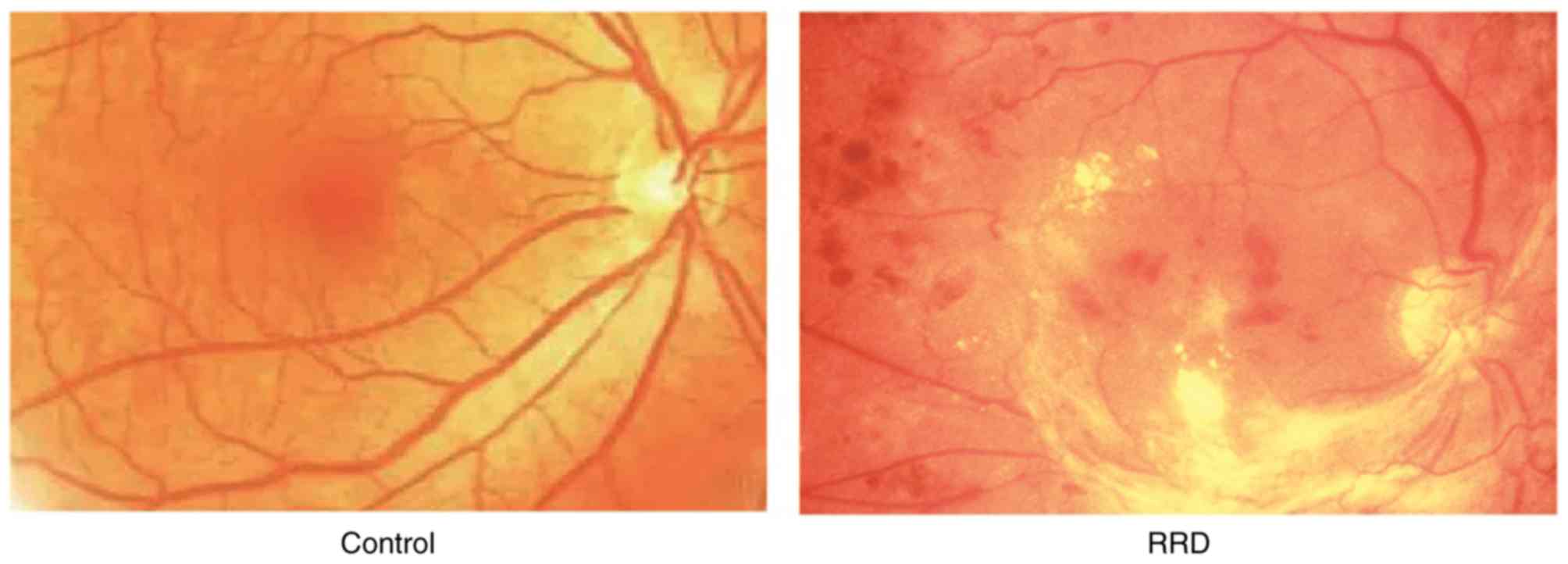
successfully induced (Fig. 1).
RPE cell culture
Rats were anesthetized with retrobulbar injections
of lidocaine (2%) and the eyeballs were subsequently removed. Rats
were subsequently sacrificed using cervical dislocation method. The
eyeballs were washed with gentamycin and placed in DMEM. The
eyeballs were cut open 3 mm posterior to the corneoscleral limbus,
and the anterior segment and vitreous body were removed and
discarded. The remaining tissue was digested with trypsin (0.25%)
at room temperature for 10 min. The retinal neurepithelium layer
was separated, removed and discarded. The retinal neurepithelium
tissue was further digested with trypsin (0.25%) at 37°C for 30
min. The resulting cell suspension was centrifuged at 125 × g for
10 min at room temperature and cells (purity >90%) were cultured
in DMEM medium containing 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C. RPE cells in the 2nd-8th generation and
logarithmic phase were used in subsequent experiments. Cells were
randomly divided into four groups: One untreated Model group and
three IL-10 groups, which received different concentrations of
IL-10 (100, 50, and 20 mM) for 48 h at room temperature.
MTT assay
RPE cells in logarithmic phase were seeded onto a
96-well plate at a density of 5×l03 cells/well and
cultured for 24 h at 37°C. The cells were divided into the four
aforementioned groups and treated accordingly. Following 48 h of
culture, MTT (20 µl) was added to each well and incubated for 4 h
at 37°C. Subsequently, DMSO (150 µl/well) was added to the plate,
which was then shaken for 10 min. The absorbance value was measured
at a wavelength of 570 nm. Each experiment was run in
triplicate.
ELISA
A total of 5×l03 RPE cells in logarithmic
phase were collected followed by centrifugation at 500 × g for 10
min at room temperature to obtain the supernatant which was used to
measure IL-1β and IL-6 levels by ELISA kits according to
manufacturer's instructions. A total of 50 µl diluted standard
solution was added to the 96-well plate to obtain the standard
curve. A total of 50 µl supernatant sample was added to each well.
Following washing five times using PBS, conjugate reagent (50
µl/well) was added to the wells and incubated at 37°C for 30 min.
Subsequently, reagent A (50 µl/well) and reagent B (50 µl/well)
were added and the plate was incubated at 37°C for 10 min. The stop
buffer (50 µl/well) was added and the absorbance value was measured
at a wavelength of 450 nm. The linear regression equation of
standard curve was calculated relative to the concentration of the
standard substance. The sample concentration was determined from
the absorbance value.
Caspase-3 activity detection
RPE cells (5×103/ml) were digested with
trypsin and centrifuged at 600 × g and 4°C for 5 min. Subsequently,
lysis buffer was added into PRE cells and incubated on ice for 15
min. Following centrifugation (20,000 × g; 4°C; 5 min), 2 mM
Ac-DEVD-pNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was
added to the suspension and the absorbance value was measured at a
wavelength of 405 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from RPE cells
(5×103/ml) using TRIzol (Thermo Fisher Scientific,
Inc.); and reverse transcribed to cDNA using SYBR®-Green
Quantitative RT-qPCR kit (Sigma-Aldrich; Merck KGaA) according to
manufacturer's protocol. Primers were designed using Primer Premier
software version 6.0 (Premier Biosoft International, Palo Alto, CA,
USA) and synthetized by Invitrogen (Thermo Fisher Scientific,
Inc.); primer sequences are presented in Table I. qPCR reactions were performed at
95°C for 1 min, followed by 35 cycles at 92°C for 30 sec, 58°C for
45 sec, and 72°C for 35 sec. GAPDH was selected as an internal
reference gene. The relative expression level was calculated using
the 2−∆∆Cq method (23)
and represented as a fold changes relative to GAPDH.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
ACCAGGTATCTTGGTTG |
|
| R:
TAACCATGTCAGCGTGGT |
| VEGF | F:
AGGTCTAACAGTCTCGCCAC |
|
| R:
TCATTAGCGTAGCCATCTAACC |
Western blotting
Total protein was extracted from RPE cells
(5×103/ml) on ice using the radioimmunoprecipitation
assay buffer for 15–30 min. Following ultrasonications 4 times at
37°C, 5 sec each, and centrifugation at 4°C and 10,000 × g for 15
min, the protein solution was transferred to a new Eppendorf tube.
RPE proteins were stored at −20°C following quantification using
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Proteins were loaded into 10% SDS-PAGE (20 µg/well) at 100 mA for
1.5 h and transferred to a PVDF membrane. Following blocking with
5% skim milk at room temperature for 2 h, the membrane was
incubated with primary antibodies against VEGF (1:1,000) and
β-actin (1:2,000) at 4°C overnight. The membrane was washed with
PBS with 0.05% Tween-20 and further incubated with HRP-conjugated
goat anti-rabbit secondary antibody at 1:2,000 for 30 min at room
temperature. Protein bands were visualized with ECL reagent for 1
min and developed. β-actin used as a loading control and to
normalize expression in densitometric analysis. The membrane was
scanned with an image processing system and analyzed using Quantity
One 1-D Analysis Software version 4.6.5 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Each experiment was repeated four
times.
Cell migration assay
RPE cell suspensions were collected at the
logarithmic phase (density of cells, 5×103/ml) under 12
h starvation culture in cell starvation medium (Sigma-Aldrich;
Merck KGaA) following treatments with different concentrations of
IL-10 and subsequently added into upper part of the Transwell
chamber containing DMEM medium. 10% FBS was added into the lower
part of the chamber. Following incubation at 37°C for 24 h, cells
in the upper membrane were removed using a pipette tip or cotton
tipped applicator and RPE cells were stained with 0.1% crystal
violet at room temperature for 10 min and observed under an
inverted microscope. Five fields were selected for each staining
and cell numbers were counted.
Statistical analysis
SPSS software (version 19.0; IBM Corp., Armonk, NY,
USA) was used for data analysis. All data were presented as the
mean ± standard deviation. Intergroup differences were analyzed by
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of IL-10 on RPE cell
proliferation in RRD
Eye examination was performed with an operating
microscope to demonstrate the interruption in retinal
neurepithelium layer, apophysis and detachment of RPE, and opaque
dark area of fluid, suggesting that RRD was successfully induced
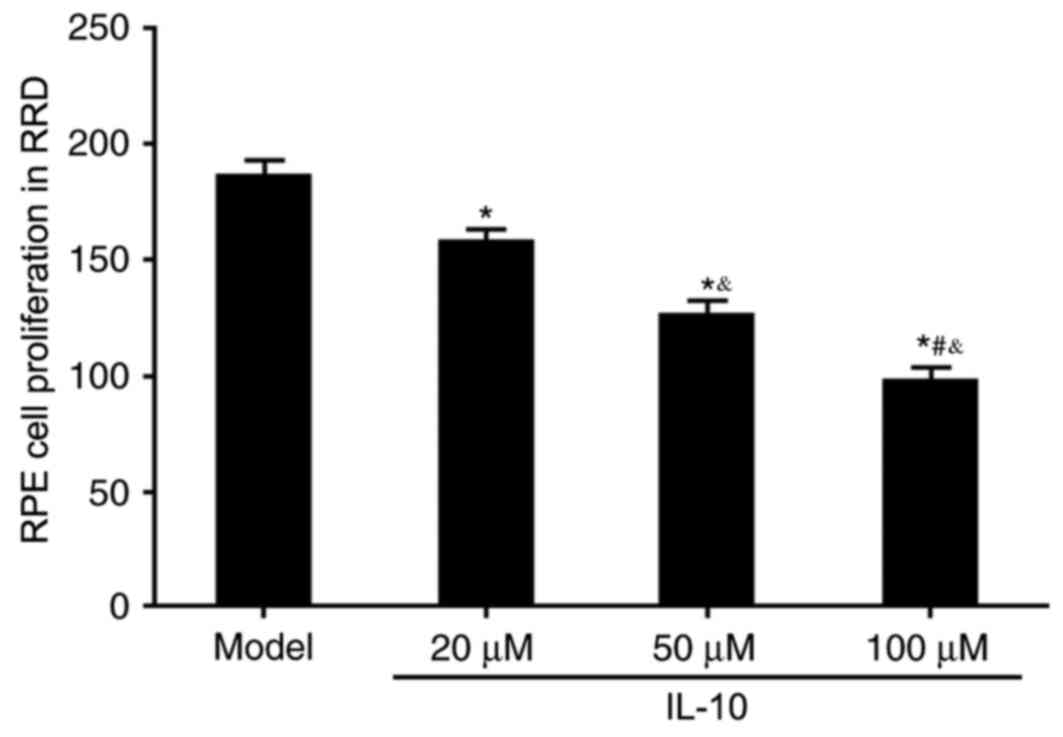
(Fig. 1). An MTT assay was used to
evaluate the effects of IL-10 on RPE cell proliferation in RRD. All
three concentrations of IL-10 (20, 50 and 100 µM) significantly
inhibited RPE cell proliferation compared with the untreated Model
group (P<0.05; Fig. 2); this
inhibitory effect on cell proliferation appeared to be in a
dose-dependent manner.
Effects of IL-10 on caspase-3 activity
in RPE cells from RRD model rats
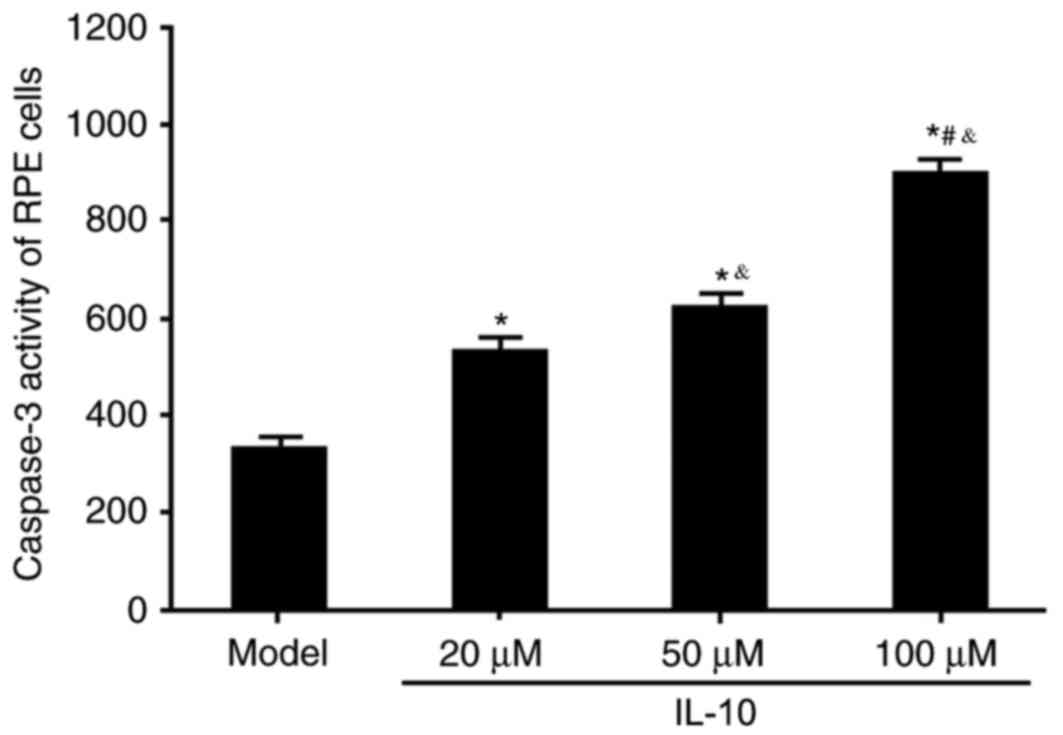
Caspase-3 activity was measured to evaluate the
effects of varying concentrations of IL-10 on apoptotic activity in
RPE cells from RRD. IL-10 treatment significantly increased
caspase-3 activity compared with the Model group (P<0.05;
Fig. 3), and this effect was in a
dose-dependent manner. These results indicated that IL-10 may
facilitate RPE cell apoptosis, which may subsequently serve an
inhibitory role on RRD progression.
Effects of IL-10 on inflammatory
cytokine expressions in RPE cells from RRD model rats
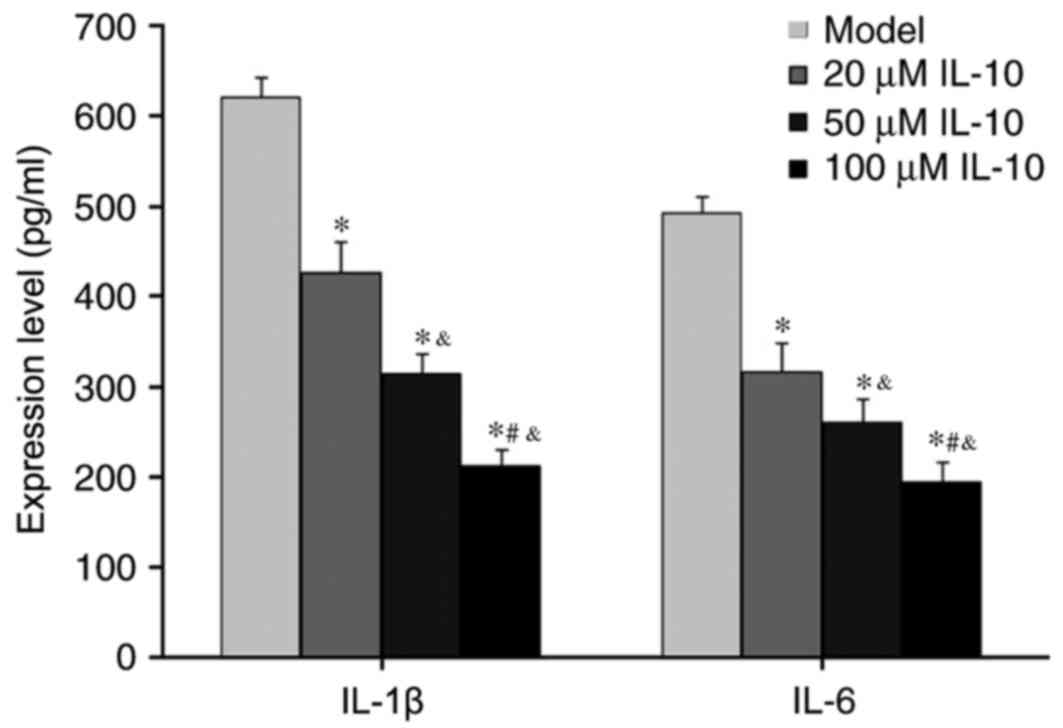
ELISA was used to measure the abundance of
inflammatory cytokines IL-1β and IL-6 in the supernatant of RPE
cells from RRD. IL-10 treatments significantly suppressed
expression of IL-1β and IL-6 in RPE cells derived from RRD
(P<0.05 vs. Model; Fig. 4), and
this reduction was dose-dependent. These results suggested that
IL-10 inhibited inflammatory cytokine secretion from RPE.
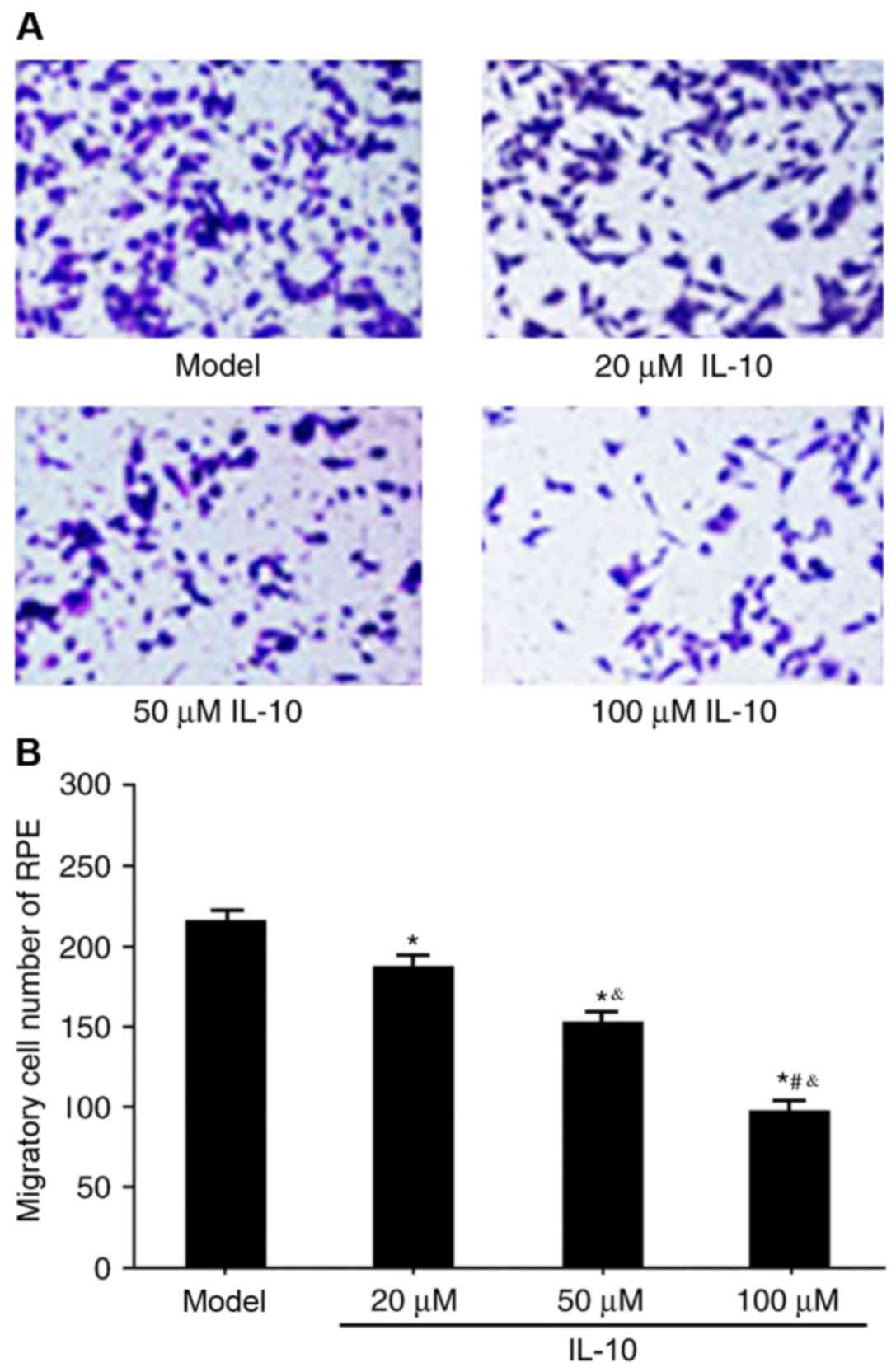
Effects of IL-10 on RPE cell migration
in RRD
The Transwell assay was used to determine the
effects of IL-10 on RPE cell migration in RRD. IL-10 expression
significantly restrained RPE migration in RRD compared with the
untreated model group (P<0.05), and the effect was dose
dependent (Fig. 5A and B). These
results suggested that IL-10 may regulate RPE cell migration in
RRD.
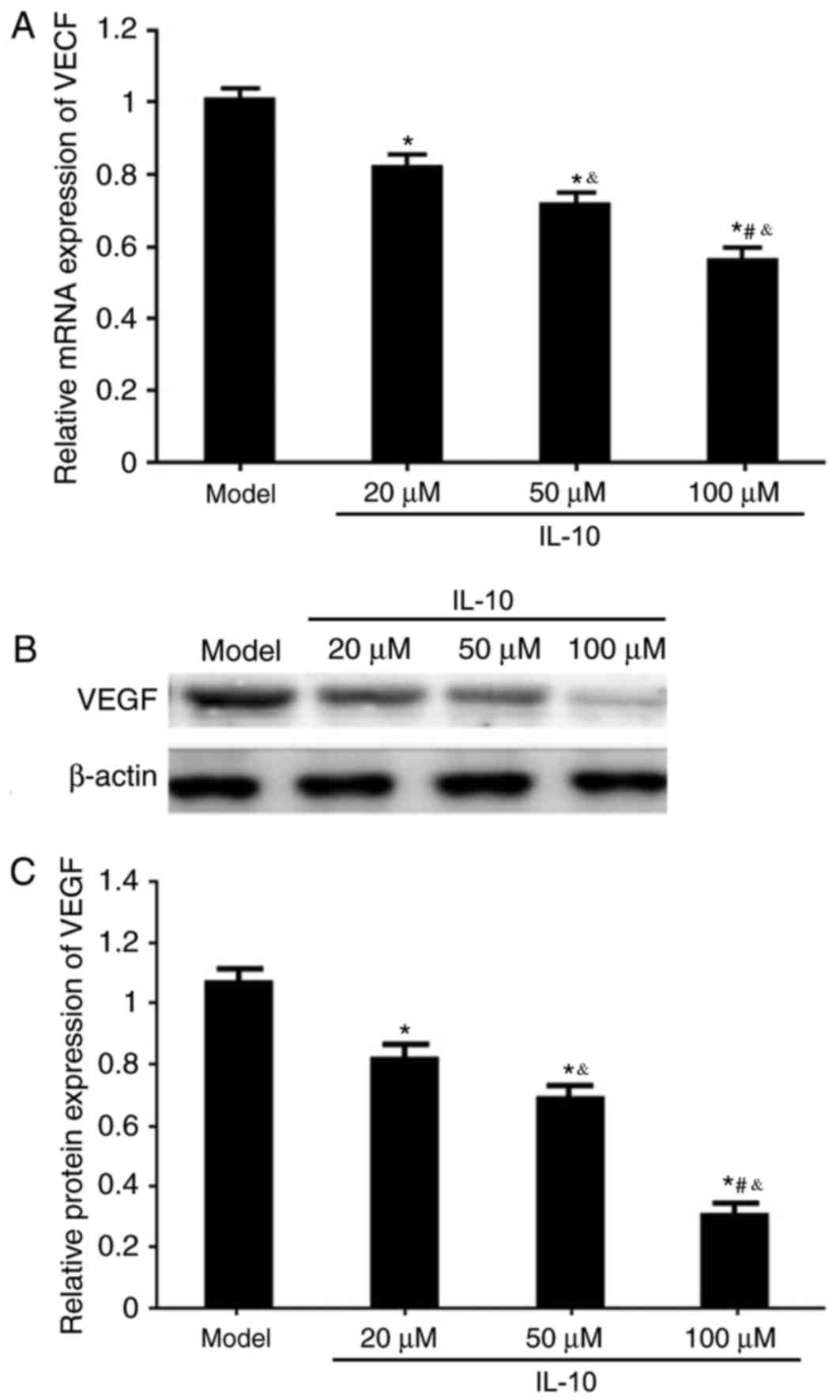
Effects of IL-10 on VEGF mRNA and
protein expression in RPE from RRD model rats
RT-qPCR and western blotting were used to analyze
the effects of different concentrations of IL-10 on VEGF mRNA and
protein expression in RPE cells from RRD. It was demonstrated that
the IL-10 treatments significantly downregulated VEGF mRNA and
protein expressions in RPE cells from RRD in a dose-dependent
manner, compared with untreated Model cells (P<0.05; Fig. 6).
Discussion
RPE cells migrate to the vitreous body, promote
secretion of a large amount of inflammatory cytokines and
facilitate the secretion of ECM, ultimately leading to ECM
reconstruction and the development of retinal detachment (24,25).
RRD is an inflammatory pathological process, in which a tear forms
on the lower surface of retina or at the interface between the
vitreous body and the retina, and leads to further secretion of
inflammatory cytokines, including IL-1β and IL-6. The tear promotes
ECM secretion to form a pathological deposition, which pulls on the
retina and causes retinal detachment (26). The risk factors of retinal
detachment include genetic factors, aging, reduction of immune
capacity and abnormal hyperplasia of RPE cells (27). Elevated secretion of inflammatory
cytokines IL-1β and IL-6 leads to inflammation and induces abnormal
growth and migration of RPE cells (28). Inflammation in RPE cells serves a
role in the development and pathogenesis of RRD; however, whether
IL-10, an anti-inflammatory cytokine, serves a role in RRD remains
to be elucidated. The present study demonstrated that IL-10
treatment inhibited proliferation and migration of RPE cells from
RRD model rats.
IL-10 was first described as a T helper 2-derived
cytokine; however, it is now considered that IL-10 may not be
restricted to a T cell subset, but instead is produced by most
leukocytes (29). The
immunosuppressive activity of IL-10 is mediated by heterodimeric
IL-10 receptors IL-10R1 and IL-10R2 (30,31).
A previous study demonstrated that human IL-10 inhibits the
secretion of interferon (IFN)-γ in peripheral blood mononuclear
cells (32), and in the presence
of accessory cells, IL-10 inhibits mitogen or anti-CD3 monoclonal
antibody-stimulated T cell proliferation (33). It has been also demonstrated that
IL-10-mediated inhibition of T cell growth was associated with
decreased production of IFN-γ and IL-2 (33). Consistent with the role of IL-10 as
an inhibitor of cell proliferation, the present study demonstrated
that IL-10 treatment significantly inhibited RPE cell proliferation
and migration in a dose-dependent manner. Furthermore, treatment of
RPE cells with IL-10 also reduced the secretion of pro-inflammatory
cytokines IL-1β and-6.
The angiogenic factor VEGF is expressed in vascular
endothelial cells, nerve cells and retinal cells; it binds to
transmembrane tyrosine kinase VEGF receptors on the plasma
membrane, which induces receptor dimerization and activation, and
the assembly of a membrane-proximal signaling complex (34). VEGF may promote cell proliferation,
differentiation, migration and movement by increasing the
permeability of blood vessels and degradation of extracellular
matrix (35). VEGF upregulation
may promote blood vessel growth, facilitate inflammatory factors
secretion and aggravate inflammation, leading to damage of vascular
integrity (36). In the present
study, a significant reduction in the expression levels of VEGF
mRNA and protein in RPE cells was observed following IL-10
treatment, which suggested that IL-10 may inhibit angiogenesis
through the downregulation of VEGF expression. These results are
consistent with a previous study, which demonstrated that mice with
a VEGF-producing ovarian cancer exhibited an IL-10-mediated
inhibition of angiogenesis and tumor growth (37). However, the exact mechanisms
underlying the IL-10-mediated regulation of VEGF expression in RPE
cells remain to be elucidated.
In conclusion, IL-10 may suppress inflammation,
facilitate RPE cell apoptosis and limit cell proliferation and
migration by regulating VEGF expression to delay RRD
progression.
References
|
1
|
Nemet A, Moshiri A, Yiu G, Loewenstein A
and Moisseiev E: A review of innovations in rhegmatogenous retinal
detachment surgical techniques. J Ophthalmol. 2017:43106432017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghazi NG and Green WR: Pathology and
pathogenesis of retinal detachment. Eye (Lond). 16:411–421. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang HK and Luff AJ: Management of retinal
detachment: A guide for non-ophthalmologists. BMJ. 336:1235–1240.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang C, Zhang T, Liu J, Ji Q and Tan R:
Changes in axial length, central cornea thickness and anterior
chamber depth after rhegmatogenous retinal detachment repair. BMC
Ophthalmol. 16:1212016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yumusak E, Ornek K and Ozkal F: Bilateral
simultaneous rhegmatogenous retinal detachment following laser in
situ keratomileusis. Case Rep Ophthalmol. 7:341–345. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kominami A, Ueno S, Kominami T, Nakanishi
A, Piao CH, Ra E, Yasuda S, Asami T and Terasaki H: Restoration of
cone interdigitation zone associated with improvement of focal
macular ERG after fovea-off rhegmatogenous retinal reattachment.
Invest Ophthalmol Vis Sci. 57:1604–1611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotzaridis S, Liazos E, Petrou P and
Georgalas I: 25-Gaugevitrectomy and incomplete drainage of
subretinal fluid for the treatment of primary rhegmatogenous
retinal detachment. Ophthalmic Surg Lasers Imaging Retina.
47:333–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi E, Fukushima A, Haga A, Inomata
Y, Ito Y, Fukushima M and Tanihara H: Effects of mechanical stress
and vitreous samples in retinal pigment epithelial cells. Biochem
Biophys Res Commun. 470:569–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heriot WJ: Thermofusion of the retina with
the RPE to seal tears during retinal detachment repair. Graefes
Arch Clin Exp Ophthalmol. 254:691–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sukseree S, Chen YT, Laggner M, Gruber F,
Petit V, Nagelreiter IM, Mlitz V, Rossiter H, Pollreisz A,
Schmidt-Erfurth U, et al: Tyrosinase-Cre-mediated deletion of the
autophagy gene atg7 leads to accumulation of the RPE65 Variant M450
in the retinal pigment epithelium of C57BL/6 mice. PLoS One.
11:e01616402016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koutsandrea C, Kanakis M, Papaconstantinou
D, Brouzas D, Ladas I, Petrou P and Georgalas I: Scleral buckling
versus vitrectomy for retinal detachment repair: Comparison of
visual fields and nerve fiber layer thickness. Ophthalmologica.
235:10–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hinton DR, He S, Graf K, Yang D, Hsueh WA,
Ryan SJ and Law RE: Mitogen-activated protein kinase activation
mediates PDGF-directed migration of RPE cells. Exp Cell Res.
239:11–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirchhof B, Kirchhof E, Ryan SJ and
Sorgente N: Vitreous modulation of migration and proliferation of
retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis
Sci. 30:1951–1957. 1989.PubMed/NCBI
|
|
14
|
Knickelbein JE, Liu B, Arakelyan A, Zicari
S, Hannes S, Chen P, Li Z, Grivel JC, Chaigne-Delalande B, Sen HN,
et al: Modulation of immune responses by extracellular vesicles
from retinal pigment epithelium. Invest Ophthalmol Vis Sci.
57:4101–4107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mones J, Leiva M, Peña T, Martínez G,
Biarnés M, Garcia M, Serrano A and Fernandez E: A swine model of
selective geographic atrophy of outer retinal layers mimicking
atrophic AMD: A phase i escalating dose of subretinal sodium
iodate. Invest Ophthalmol Vis Sci. 57:3974–3983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fields MA, Cai H, Bowrey HE, Moreira EF,
Beck Gooz M, Kunchithapautham K, Gong J, Vought E and Del Priore
LV: Nitrite modification of extracellular matrix alters CD46
expression and VEGF release in human retinal pigment epithelium.
Invest Ophthalmol Vis Sci. 56:4231–4238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaw PX, Stiles T, Douglas C, Ho D, Fan W,
Du H and Xiao X: Oxidative stress, innate immunity and age-related
macular degeneration. AIMS Mol Sci. 3:196–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fernandez-Godino R, Pierce EA and Garland
DL: Extracellular matrix alterations and deposit formation in AMD.
Adv Exp Med Biol. 854:53–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamawaki T, Ito E, Mukai A, Ueno M, Yamada
J, Sotozono C, Kinoshita S and Hamuro J: The ingenious interactions
between macrophages and functionally plastic retinal pigment
epithelium cells. Invest Ophthalmol Vis Sci. 57:5945–5953. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tenconi PE, Giusto NM, Salvador GA and
Mateos MV: Phospholipase D1 modulates protein kinase C-epsilon in
retinal pigment epithelium cells during inflammatory response. Int
J Biochem Cell Biol. 81:67–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du H, Xiao X, Stiles T, Douglas C, Ho D
and Shaw PX: Novel mechanistic interplay between products of
oxidative stress and components of the complement system in AMD
pathogenesis. Open J Ophthalmol. 6:43–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang RF, Liu ZR, Chen B and Zhang JJ: The
impact of miR-26b on retinal pigment epithelium cells in
rhegmatogenous retinal detachment model. Int J Clin Exp Patho.
10:8141–8147. 2017.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dardik R, Livnat T, Halpert G, Jawad S,
Nisgav Y, Azar-Avivi S, Liu B, Nussenblatt RB, Weinberger D and
Sredni B: The small tellurium-based compound SAS suppresses
inflammation in human retinal pigment epithelium. Mol Vis.
22:548–562. 2016.PubMed/NCBI
|
|
25
|
Fu D, Yu JY, Connell AR, Yang S, Hookham
MB, McLeese R and Lyons TJ: Beneficial effects of berberine on
oxidized LDL-induced cytotoxicity to human retinal muller cells.
Invest Ophthalmol Vis Sci. 57:3369–3379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sreekantam S, Macdonald T, Keane PA, Sim
DA, Murray PI and Denniston AK: Quantitative analysis of vitreous
inflammation using optical coherence tomography in patients
receiving sub-Tenon's triamcinolone acetonide for uveitic cystoid
macular oedema. Br J Ophthalmol. 101:175–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Estrago-Franco MF, Moustafa MT,
Riazi-Esfahani M, Sapkal AU, Piche-Lopez R, Patil AJ, Sharma A,
Falatoonzadeh P, Chwa M, Luczy-Bachman G, et al: Effects of
benzo(e)pyrene on reactive oxygen/nitrogen species and inflammatory
cytokines induction in human RPE cells and attenuation by
mitochondrial-involved mechanism. J Ophthalmic Vis Res. 11:385–393.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keir LS, Firth R, Aponik L, Feitelberg D,
Sakimoto S, Aguilar E, Welsh GI, Richards A, Usui Y, Satchell SC,
et al: VEGF regulates local inhibitory complement proteins in the
eye and kidney. J Clin Invest. 127:199–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuhn R, Löhler J, Rennick D, Rajewsky K
and Müller W: Interleukin-10-deficient mice develop chronic
enterocolitis. Cell. 75:263–274. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walter MR: The molecular basis of IL-10
function: From receptor structure to the onset of signaling. Curr
Top Microbiol Immunol. 380:191–212. 2014.PubMed/NCBI
|
|
31
|
Mosser DM and Zhang X: Interleukin-10: New
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naundorf S, Schroder M, Hoflich C, Suman
N, Volk HD and Grutz G: IL-10 interferes directly with TCR-induced
IFN-gamma but not IL-17 production in memory T cells. Eur J
Immunol. 39:1066–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taga K and Tosato G: IL-10 inhibits human
T cell proliferation and IL-2 production. J Immunol. 148:1143–1148.
1992.PubMed/NCBI
|
|
34
|
Sen P, Vinay Kumar S, Bhende M and Sharma
T: Combined argon laser photocoagulation and antivascular
endothelial growth factor for management of macular polypoidal
choroidal vasculopathy. Oman J Ophthalmol. 9:139–143. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Corydon TJ, Mann V, Slumstrup L, Kopp S,
Sahana J, Askou AL, Magnusson NE, Echegoyen D, Bek T, Sundaresan A,
et al: Reduced expression of cytoskeletal and extracellular matrix
genes in human adult retinal pigment epithelium cells exposed to
simulated microgravity. Cell Physiol Biochem. 40:1–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SH, Kim H, Ku HJ, Park JH, Cha H, Lee
S, Lee JH and Park JW: Oxalomalate reduces expression and secretion
of vascular endothelial growth factor in the retinal pigment
epithelium and inhibits angiogenesis: Implications for age-related
macular degeneration. Redox Biol. 10:211–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kohno T, Mizukami H, Suzuki M, Saga Y,
Takei Y, Shimpo M, Matsushita T, Okada T, Hanazono Y, Kume A, et
al: Interleukin-10-mediated inhibition of angiogenesis and tumor
growth in mice bearing VEGF-producing ovarian cancer. Cancer Res.
63:5091–5094. 2003.PubMed/NCBI
|