Introduction
Running is one of the most common weight-bearing
exercises and promotes general health and well-being (1,2).
However, the effect of running on bone mass is poorly understood.
In a review, Iwamoto et al (3) demonstrated that treadmill running may
increase bone mass in young and adult rats, particularly within the
long bones at weight-bearing sites. However, Bourrin et al
(4) reported that excessive
treadmill running may cause the opposite effect on rat tibia. In
parallel, the deleterious effects of endurance running on bone have
also been reported in populations exposed to high-intensity
exercise (5,6). These inconsistent findings clearly
imply that intensity may be an important factor influencing the
effect of running on bone mass. However, the association between
running intensity and bone mass is poorly understood.
It is well documented that, at the tissue level,
bone adapts to changes in mechanical loading. Bone formation in
healthy adults is mainly determined by the mechanical load history
(7). In addition, animal models
have demonstrated that bone remodeling is associated with a number
of loading parameters, including the magnitude of the load
(8,9). The mechanisms involved in bone
adaptation involve a multistep process of cellular
mechanotransduction that stimulates bone remodeling resulting in
either bone formation or resorption (10). However, the cellular basis whereby
bone adapts to mechanical loads remains unclear (10). According to the mechanostat theory,
bone mass adjusts when a typical load diverges from a physiological
‘set-point’, similar to a thermostat (7). The resident cells within bone tissue
detect and subsequently respond to changes in the mechanical load.
It is, therefore, hypothesized that treadmill running at different
intensities would lead to different cellular responses, and,
consequently, different bone adaptations.
Although osteocytes (which comprise >90% of bone
cells) are most likely responsible for sensing mechanical loads
(11), evidence suggests that
mechanical signals are influenced by the resident bone cell
population and by their progenitors (12). Bone marrow stromal cells (BMSCs)
are a type of self-renewing multipotential stem cell which can
differentiate into a number of lineages, including osteoblasts,
chondroblasts, and adipocytes (13). A body of evidence also suggests
that bone adaptation is associated with either osteogenic or
adipogenic differentiation of BMSCs (12,14–17).
Specifically, mechanical stimuli may have an important role in
influencing the osteogenic differentiation process of BMSCs and
their production of mineralized matrix in vivo and in
vitro (18). In addition,
increased adipogenesis was observed at the expense of osteogenesis
under conditions of hind limb unloading (19) and joint immobilization (20), whereas, mechanical loading
(21) and climbing exercises
(22), upregulated osteogenesis
and downregulated adipogenesis of BMSCs. However, the method
whereby BMSCs respond to mechanical signals induced by treadmill
running remains unclear, and, in particular, how such a response is
associated with to running intensity.
It is thought that cartilage and bone may respond to
mechanical loading in an intensity-dependent manner (23). Using a rat model, we previously
demonstrated that treadmill running with low-to-moderate intensity
maintains cartilage homeostasis, whereas, high-intensity running
may cause cartilage degradation (24). In the present study, the same
animal model was used to examine the effect of different
intensities of treadmill running on bone mass. To further gain
insight into the mechanisms responsible for the differentiation
potential of BMSCs, both osteogenic and adipogenic lineages were
investigated under various mechanical loading conditions.
Materials and methods
Experimental animals and exercise
protocols
This study was approved by the animal ethics
committee of Nanfang Hospital, Southern Medical University
(Guangzhou, China). The methods were performed out in accordance
with the approved guidelines. A total of 24 specific pathogen-free
grade adult male Wistar rats (13–14 weeks old, 180–220 g) were
randomized into four even groups (n=6 per group) as follows:
Control (CON); low-intensity running (LIR); moderate-intensity
running (MIR); and high-intensity running (HIR). All animals were
housed in cages with a temperature of 22±1°C, 40–70% humidity and a
controlled light/dark cycle of 12/12 h. Furthermore, food and water
were provided ad libitum. Animals in the Con group
maintained a sedentary lifestyle. Those in LIR, MIR, and HIR groups
were subjected to treadmill running according to the running
protocols described previously (24). The constant speed and inclination
varied as follows: LIR, 15.2 m/min with 0° incline for 60 min, 5
days/week; MIR, 19.3 m/min with 5° incline for 60 min, 5 days/week;
and HIR, 26.8 m/min with 10° incline for 60 min, 5 days/week.
After 8 weeks, all animals were sacrificed under
anesthesia by cervical dislocation. Bilateral femora from each
animal were dissected free of soft tissues and maintained in cold
phosphate-buffered saline (PBS) on ice. The bones were cut open at
both ends and bone marrow was collected from their central shafts.
The distal end of the left femur from each rat was fixed in 10%
formalin for histological and immunohistochemistry examinations,
while the distal end of the right femur was used for micro-computed
tomography (CT) scanning.
Histological and immunohistochemistry
examinations
Following dissection of the distal end of the left
femur from each rat, it was immediately immersed in fixative
solution (10% formalin at pH 7.4) for 24 h for histological,
morphological and immunohistochemistry examinations.
Decalcification was accomplished in 10% ethylenediaminetetraacetic
acid solution prior to embedding of the samples in paraffin wax.
They were then transversally sectioned in the diaphyseal region at
a thickness of 5 µm.
To assess adipocyte density, sections were stained
with hematoxylin and eosin (H&E). Density of adipocytes
(adipocyte number per mm2 marrow area, excluding
trabeculae) was measured and quantified with image analysis
software (Nikon H600L microscope and image analysis system; Nikon
Corporation, Tokyo, Japan) by calculating the mean value of three
sequential images from each of the six animal specimens. β-catenin
was immunostained using the two-step immunohistochemistry method as
previously described (25). The
areas of interest (AOI) were selected via the irregular AOI tools
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA). The ratio of integrated optical density (IOD)
to area in each region was also calculated using Image-Pro Plus 6.0
software (Media Cybernetics, Inc.), and subsequently averaged for
β-catenin content in each section.
Micro-CT measurements
Within ~1 week after fixation, the distal ends of
the right femurs were scanned using a micro-CT scanner (SkyScan
1076 Micro-CT system; Bruker Corporation, Billerica, MA, USA). The
scanner was set at a voltage 88 kV, a current of 100 µA and a
resolution of 18 µm per size. The region of interest was the area
1.0 mm below the lower end of the growth plate extending 3.2 mm
distally. A global threshold 90 to 255 was used for all samples to
identify mineralized tissue/soft tissue. For analysis of trabecular
bone, a cube of trabecular bone with a size of 1.04×1.04×1.04
mm3 in the ROI was selected. Three-dimensional
structural parameters measured included trabecular bone volume
(BV/TV), bone mineral density (BMD), trabecular number (Tb.N),
trabecular thickness (Tb.Th), trabecular separation (Tb.Sp),
structure model index (SMI) and the degree of anisotropy (DA).
Rat BMSCs cultures
Immediately after the distal ends of both femora
were excised, the bone marrow was flushed into a 15 ml sterile
centrifuge tube using L-Dulbecco's modified Eagle's medium (L-DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
10% fetal bovine serum stromal medium. The marrow isolate was
centrifuged at 179 × g for 5 min at room temperature, and the
pellet was resuspended in 4 ml L-DMEM supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
Colony-forming unit (CFU) assay
For CFU-fibroblast (CFU-F) assay, bone marrow femora
were flushed into L-DMEM and centrifuged at 179 × g for 5 min at
room temperature. The medium was then suspended in L-DMEM
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
Following 7 days of incubation at 37°C, non-adherent cells were
removed by rinsing with PBS. Adherent stromal cells were plated on
a 35 mm sterile culture dish (2×106 cells/dish) and
incubated in L-DMEM supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). At day 10, the cultures were fixed with 4%
paraformaldehyde for 20 min at room temperature and stained with
0.1% crystal violet for 30 min. The number of colonies was counted
under a light microscope.
For CFU-osteoblast (CFU-Ob) assay, BMSCs were
initially cultured in stromal medium. After 7 days, cells were
cultured in osteogenic induction medium consisting of DMEM, 10%
FBS, 20 mmol/l dexamethasone, 10 mmol/l β-glycerophosphate and 50
µg/ml sodium 2-phosphate ascorbate. At day 14, the plates were
stained with an alkaline phosphatase (ALP) staining kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) for 30 min.
Cell proliferation assay
For a proliferation assay, cells were cultured in
96-well plates at a concentration of 5×104 cells/well
for 10 days. The culture medium (L-DMEM supplemented with 10% FBS)
was changed every 2–3 days. Cell proliferation was detected using
methyl thiazolyl tetrazolium (MTT) assay. Briefly, 20 µl MTT (5
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
each well for 4 h at 37°C. Subsequently, the supernatant was
replaced with 150 ml DMSO. After 15 min oscillation, the optical
density (OD) was quantified at 490 nm using a SpectraMAX M2
microplate reader.
Osteogenic differentiation
Cells were plated at 5×105 cells/well in
12-well plates and cultured in DMEM (Sigma-Aldrich; Merck KGaA). At
day 10, the medium was replaced with fresh osteogenic induction
medium containing 10 mmol/l β-glycerophosphate, 50 µg/ml sodium
2-phosphate ascorbate, 20 mmol/l dexamethasone (Sigma-Aldrich;
Merck KGaA). Medium was changed every 3 days. Osteogenic
differentiation of BMSCs was assessed on day 14 using ALP activity
assay and staining, and on day 21 using Alizarin red S
staining.
ALP activity and mineralization
analysis
Following culture of cells in osteogenic induction
medium for 14 days, rat BMSCs were fixed with 4% paraformaldehyde
at room temperature for 10 min. ALP activity assay was performed
using a p-nitrophenyl phosphate assay according to a previously
described protocol (26). For
mineralization analysis, cells were cultured in osteogenic medium
for 21 days. The extent of matrix mineralization was measured by
Alizarin red S (Sigma-Aldrich; Merck KGaA) staining which was then
quantified using 0.5 N HCl and 5% SDS as previously described
(26). The OD was then quantified
at 490 nm using a SpectraMAX M2 microplate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Primary stromal cells were cultured for 10 days as
described above. Total RNA was isolated from BMSCs using the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. RT was performed on 0.5 mg total
RNA using a Prime Script RT reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd., Dalian, China). The qPCR assay was
performed using the SYBR-Green PCR master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The 2−ΔΔCq relative
quantification method (27) was
used to calculate gene expression levels relative to the CON group.
Values were normalized to GAPDH expression. The primer sequences
for qPCR are presented in Table I.
The reported data represent the mean expression from three
experiments.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward | Reverse | Product size
(bp) |
|---|
| GAPDH |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ | 143 |
| RUNX2 |
5′-GGCCACTTACCACAGAGCTA-3′ |
5′-GAGGCGGTCAGAGAACAAAC-3′ | 109 |
| SP7 |
5′-GTCCTCTCTGCTTGAGGAAG-3′ |
5′-CTGTTGAGTCTCGCAGAGG-3′ | 107 |
| Collagen I |
5′-CATGTTCAGCTTTGTGGACC-3′ |
5′-TTAGGGACCCTTAGGCCATT-3′ | 120 |
| ALP |
5′-AACAACCTGACTGACCCTTC-3′ |
5′-TCCACTAGCAAGAAGAAGCC-3′ | 92 |
| PPARγ2 |
5′-GATCCTCCTGTTGACCCAGA-3′ |
5′-CTGATTCCGAAGTTGGTGGG-3′ | 119 |
| β-catenin |
5′-TTGTACGAGCACATCAGGAC-3′ |
5′-GCACCCTTCAACTATCTCCTC-3′ | 101 |
| Osteocalcin |
5′-GACTGCATTCTGCCTCTCTG-3′ |
5′-ATTCACCACCTTACTGCCCT-3′ | 100 |
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analysis was performed using a one-way
analysis of variance and Tukey's test for post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MIR has an anabolic effect on
trabecular bone
To determine bone adaptation to running at different
intensities, the metaphyseal femur was examined by micro-CT.
Compared with the Con group, denser and better-organized trabeculae
were observed in the MIR group (Fig.
1A-D). Micro-CT analysis of 3D microarchitecture parameters
from trabecular bone harvested from the distal rat femora are
summarized in Fig. 1E. The results
suggested that MIR significantly affected the amount and structural
organization of trabecular bone. MIR led to significantly higher
BV/TV than in the controls (P=0.002), indicating a stimulatory
effect on trabecular bone mass. Although MIR failed to
significantly affect the Tb.Th (thickness) and DA of trabecular
bone, MIR significantly decreased the value of Tb.Sp (P=0.006) and
SMI (P=0.043) compared with the Con group, suggesting a reduction
in trabecular separation with a more plate-like architecture. Thus,
the trabeculae became denser following MIR. Notably, there were no
obvious changes in either the LIR or HIR groups, except for a
significant increase in DA in the LIR group compared with the Con
group (P=0.012). These data indicate that running affects
trabecular bone in an intensity-dependent manner, and MIR can
enhance trabecular bone mass to improve structural
organization.
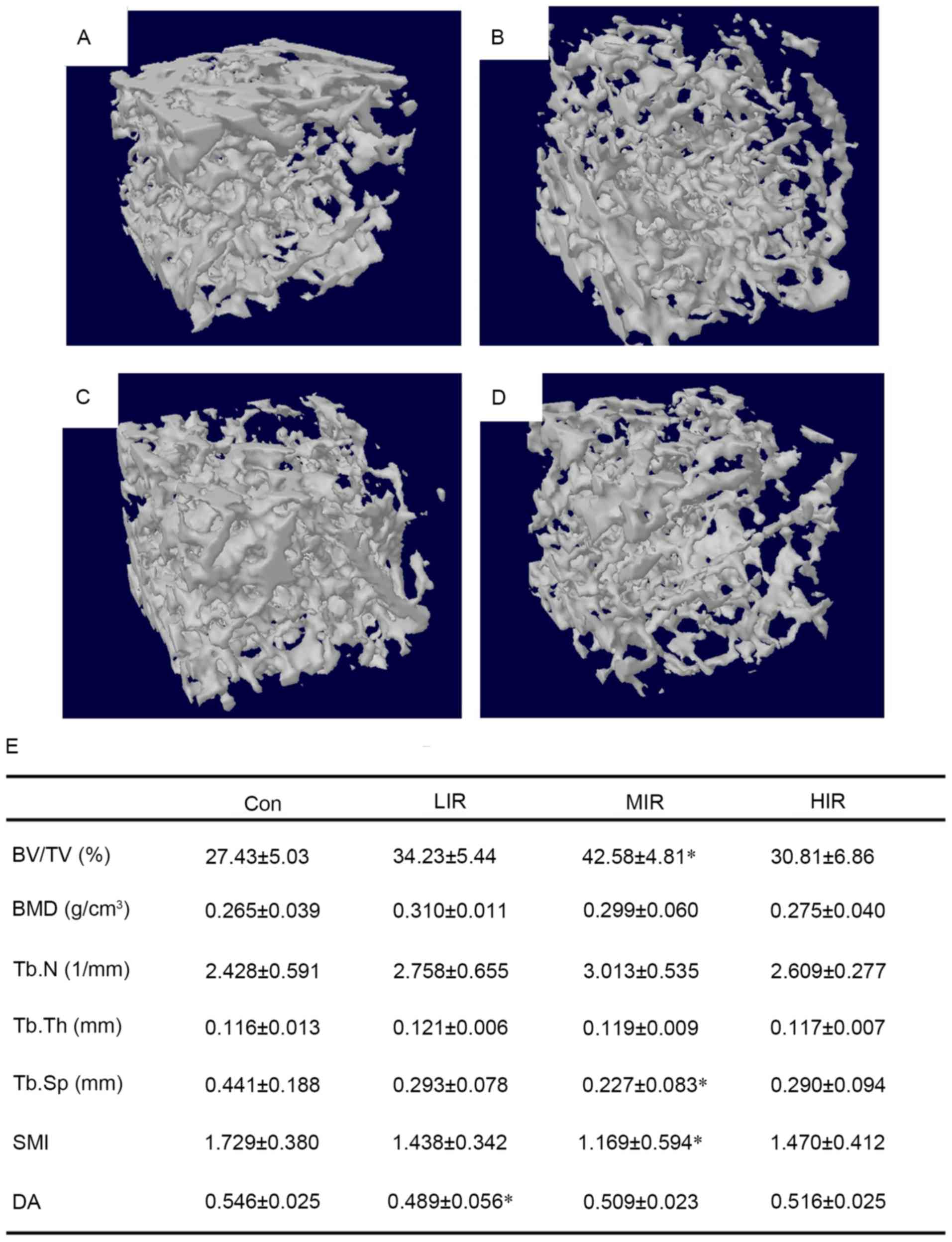 | Figure 1.3D images of right femoral
metaphyseal from (A) Con, (B) LIR, (C) MIR and (D) HIR groups. As
compared with Con group, denser and better-organized trabeculae
were observed in the MIR group. In addition, microarchitecture
parameters of trabecular bone from distal femur in four groups were
also presented, and (E) the results demonstrated that MIR
significantly affected the amount and structural organization of
trabecular bone (n=6 per group). *P<0.05 vs. Con group. Con,
control; LIR, low-intensity running; MIR, moderate-intensity
running; HIR, high-intensity running; BV/TV, trabecular bone
volume; BMD, bone mineral density; Tb.N, trabecular number; Tb.Th,
trabecular thickness; Tb.Sp, trabecular separation; SMI, structure
model index; DA, degree of anisotropy. |
MIR enhances the osteogenic potential
of BMSCs
CFU-F (Fig. 2A) and
CFU-Ob (Fig. 2B) assays were
performed to elucidate the differentiation potential of BMSCs. It
was observed that the number of bone marrow cells that were capable
of forming colonies were similar to the CON group for all three
running groups (Fig. 2C). This
result suggests that, regardless of the intensity, running failed
to affect the total number of progenitor cells present. By
contrast, in comparison with the Con group, significantly higher
numbers of ALP-positive colonies were observed in the MIR group
(P=0.001), but not in LIR or HIR groups (Fig. 2C), indicating that MIR, but not LIR
or HIR, may enhance the osteogenic potential of BMSCs.
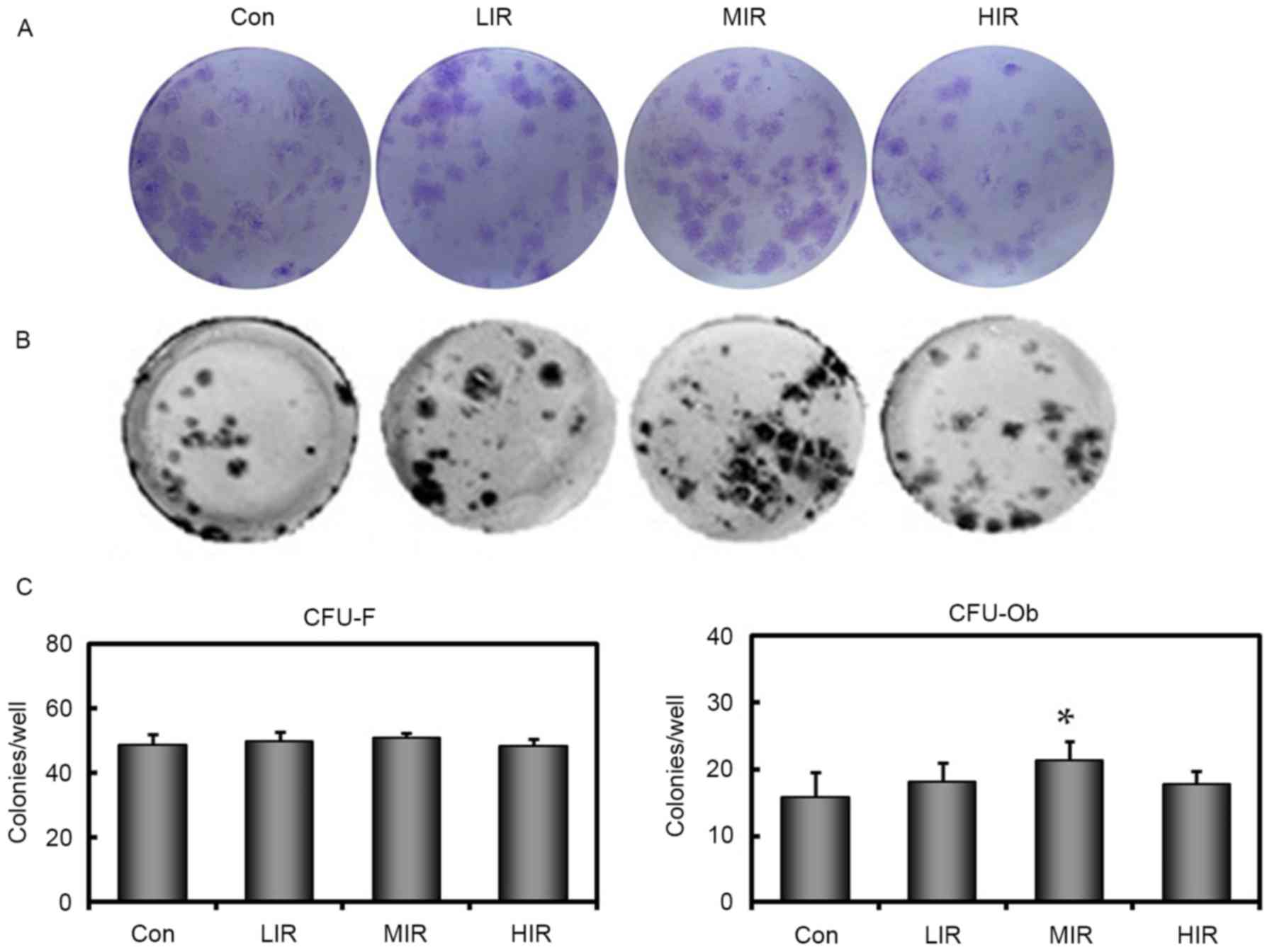 | Figure 2.Assays for the number of CFU for
fibroblasts and osteoblasts. (A) CFU-F and (B) CFU-Ob assays were
performed in duplicate in 6-well plates with 5×105
nucleated cells/well. A representative well is presented for each
group. (C) Quantitative results for these assays are also presented
as bar graphs. In all groups, the number of bone marrow cells
capable of forming fibroblast colonies is similar. Compared with
the Con group, a significantly higher number of ALP-positive
colonies were present in the MIR, but not in LIR and HIR groups
(n=6 per group). *P<0.05 vs. Con group. Con, control; LIR,
low-intensity running; MIR, moderate-intensity running; HIR,
high-intensity running; CFU, colony forming units; CFU-F,
CFU-fibroblasts; CFU-Ob, CFU-osteoblasts. |
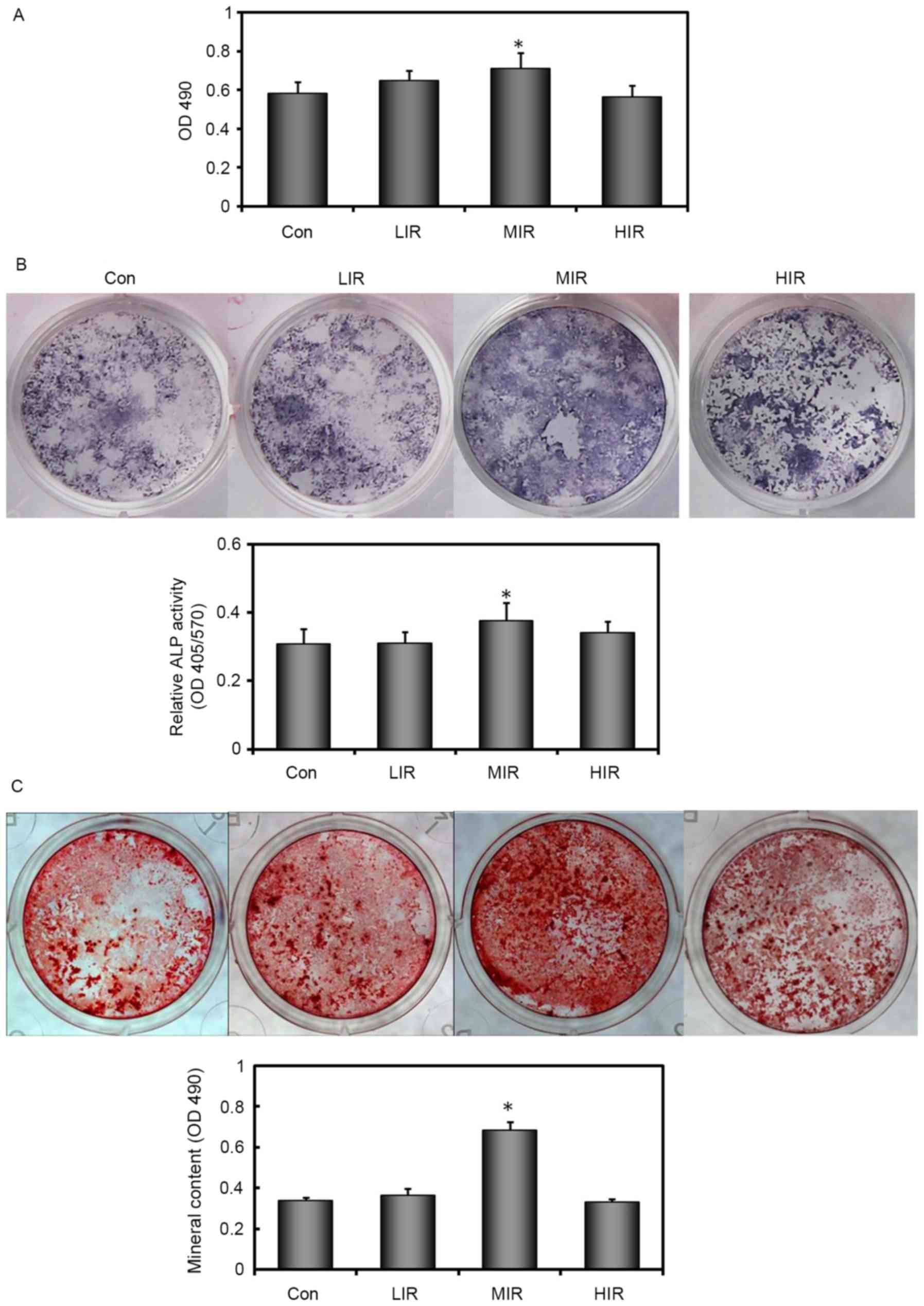
MIR enhances the capacity for
osteogenic differentiation and maturation of BMSCs
Changes in BMSC proliferation were evaluated using
an in vitro colorimetric assay. As compared with untreated
Con cells, cell proliferation was significantly enhanced in
MIR-treated BMSCs, but not in LIR-or HIR-treated BMSCs (Fig. 3A). In addition, the BMSC ability
for osteogenic differentiation was examined by ALP staining and
Alizarin red S staining. The number of ALP-positive cells changed
in an intensity-dependent manner (Fig.
3B). In a parallel experiment, ALP activity was found to change
in a similar manner (Fig. 3B). The
most intensive ALP staining and highest ALP activity was observed
in MIR-treated BMSCs. A significant difference in ALP activity
between MIR-treated and untreated BMSCs was detected (P=0.020).
To further investigate the terminal differentiation
state of BMSCs in each group, cells were cultured in osteogenic
induction medium for 21 days and stained with Alizarin red S to
visualize mineralized bone nodules. Results demonstrated that BMSCs
from all groups formed mineralized nodules in a pattern similar to
that seen in ALP staining (Fig.
3C). Mineral content was also quantified in parallel. The most
intense ARS staining and the highest mineral content were observed
in MIR-treated BMSCs. There was a significant difference in mineral
content between the MIR-treated and untreated BMSCs (Fig. 3C). These data suggested that MIR
enhanced the capacity for osteogenic differentiation and maturation
of BMSCs, whereas, LIR or HIR did not.
MIR upregulates mRNA expression of
osteogenic genes
The effect of running on osteogenic differentiation
was also investigated by assessing mRNA expression of a number of
osteogenesis-associated genes in cultured BMSCs, including RUNX2,
SP7, ALP, osteocalcin, and collagen I (Fig. 4). As compared with the Con group,
MIR was observed to significantly enhance mRNA expression of all
the genes analyzed, which are involved in various stages of
osteogenic differentiation of BMSCs. No significant difference in
mRNA expression of these genes was detected between LIR and the Con
group. Compared with the Con group, HIR produced a significant
decrease in mRNA expression of RUNX2 and collagen I, and a
significant increase in the mRNA expression of osteocalcin. These
results indicated that the effect of running on mRNA expression of
osteogenic genes varied with intensity. In addition, MIR may affect
various steps of osteogenic differentiation of BMSCs, and
upregulate mRNA expression of multiple osteogenic genes.
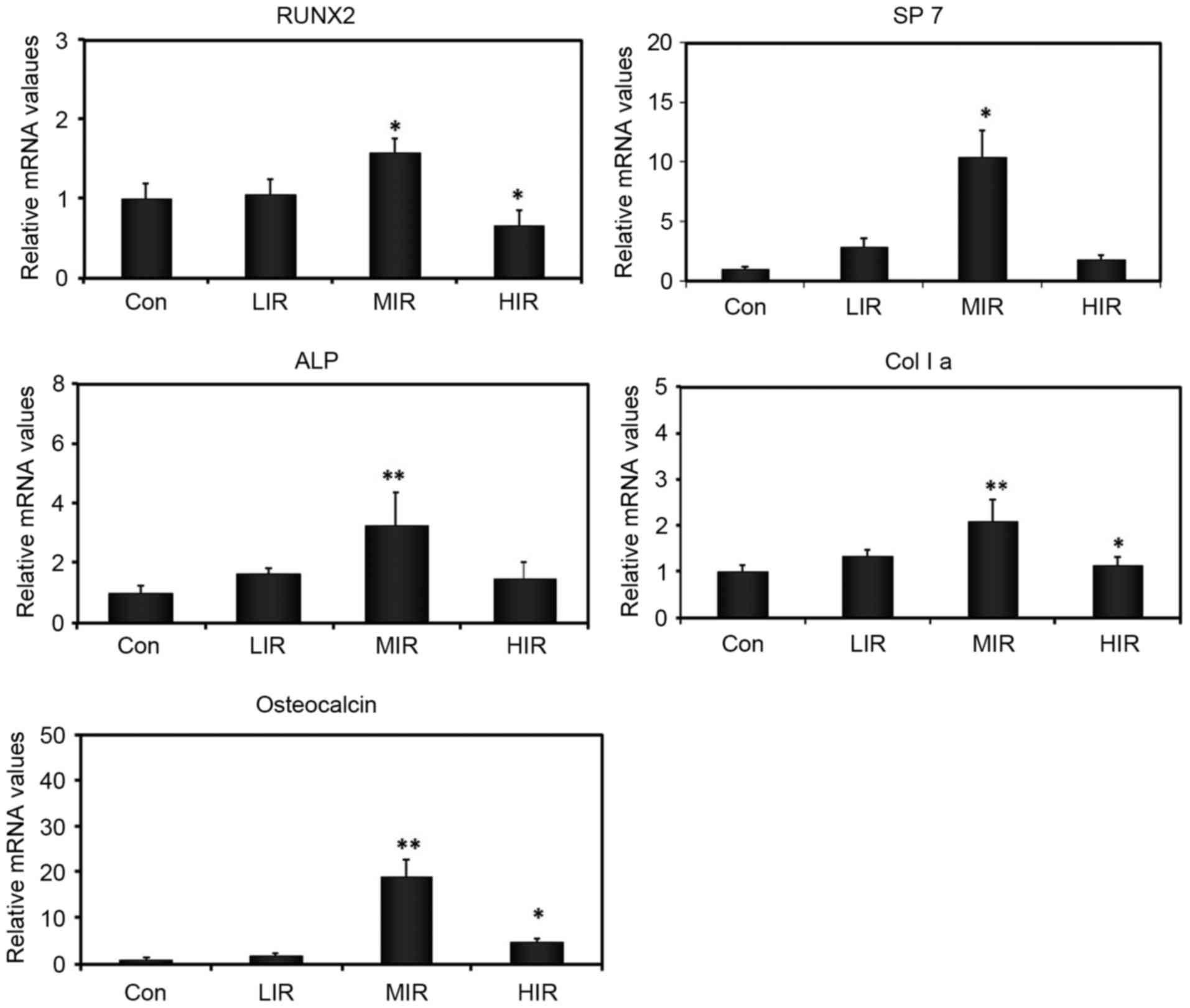 | Figure 4.Effect of running on the relative
expression of osteogenesis-associated genes in cultured BMSCs
analyzed by reverse transcription-quantitative polymerase chain
reaction, including RUNX2, SP7, ALP, osteocalcin, and Col I a (n=6
per group). *P<0.05 vs. Con group; **P<0.001 vs. Con group.
Con group. Con, control; LIR, low-intensity running; MIR,
moderate-intensity running; HIR, high-intensity running; RUNX2,
runt related transcription factor 2; Sp7, Sp7 transcription factor;
ALP, alkaline phosphatase; Col I a, collagen I. |
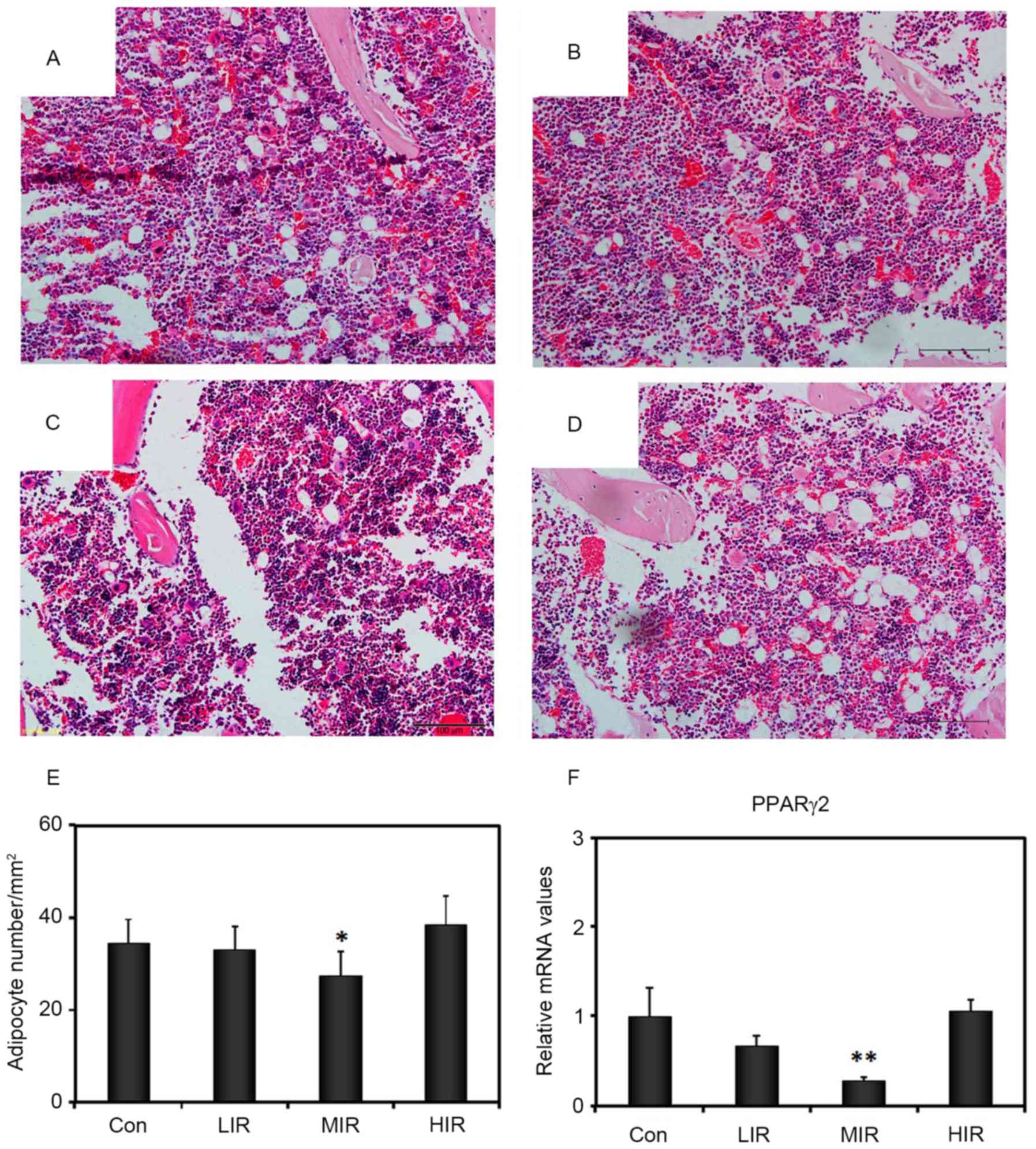
MIR inhibits the adipogenesis of
BMSCs
The marrow adipocyte density was assessed with
H&E staining of the femoral diaphyseal section (Fig. 5A-D). Compared with the Con group
(34.50±5.16), a significant decrease in adipocyte density was
observed in the MIR group (27.50±5.00; P=0.035), but not in the LIR
(33.00±4.97) or HIR (38.50±6.22) groups (Fig. 5E). Consistent with the
intensity-dependent effect on adipocyte density in bone marrow and
adipogenic differentiation of isolated BMSCs from treadmill-treated
rats, RT-qPCR analyses revealed an intensity-dependent effect on
expression of peroxisome proliferator activated receptor γ (PPARγ).
In comparison to the Con group (1.00±0.27), MIR BMSC samples
exhibited significantly reduced expression of PPARγ (0.27±0.03;
P=0.001), whereas, LIR (0.67±0.10) and HIR (1.05±0.11) were not
significantly different to the control (Fig. 5F). These observations suggested
that, in addition to the increase in osteogenesis, MIR also induced
a reduction in adipogenesis in BMSCs.
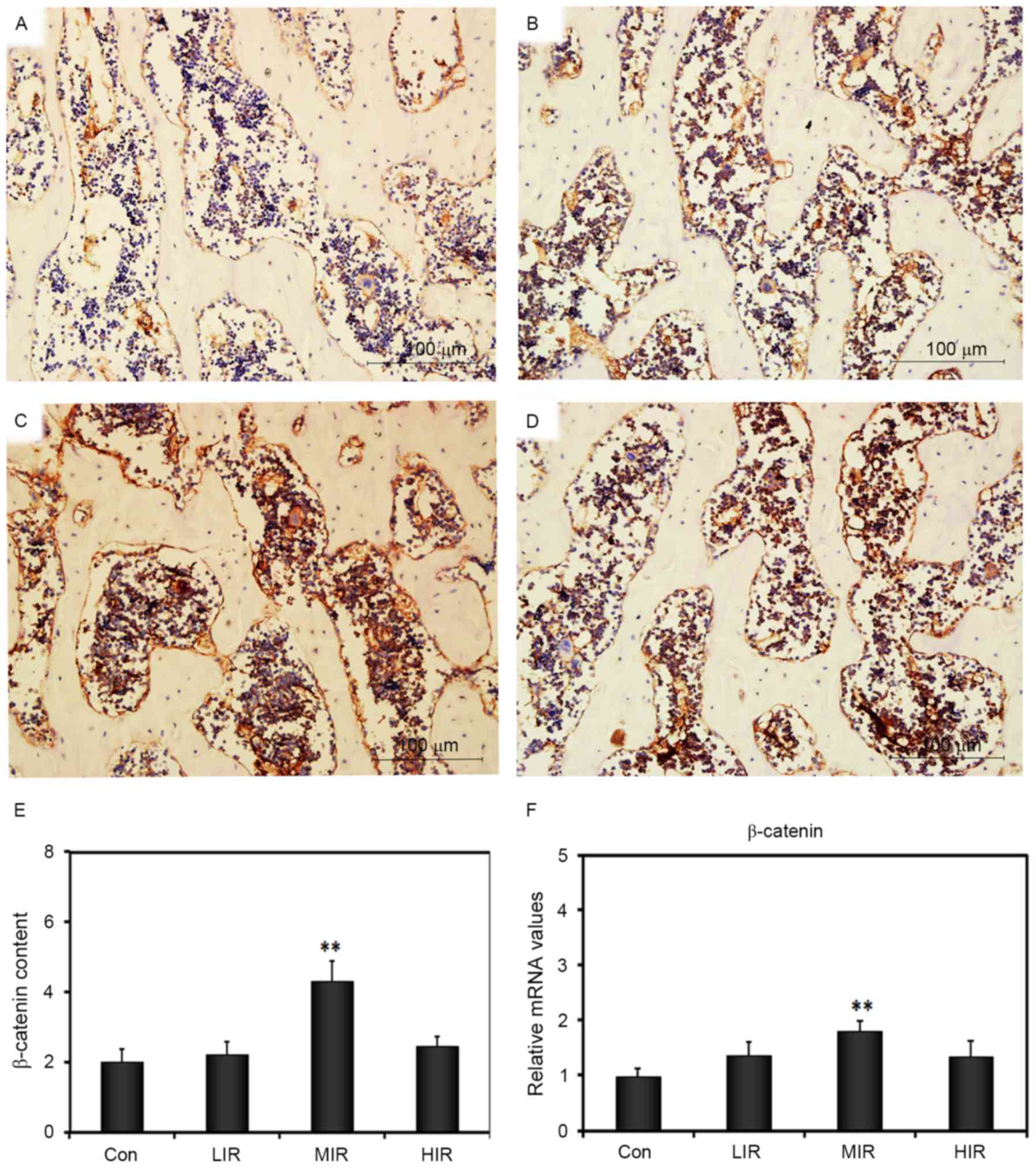
MIR stimulates β-catenin protein
expression in vivo and gene expression in BMSCs
We investigated whether running could enhance the
expression of β-catenin in the femoral diaphysis of rats. There was
low β-catenin staining in sections from the Con group (Fig. 6); however, β-catenin signals were
observed on the cells located in the extracellular matrix (ECM) in
the treadmill-treated groups; the most intense and concentrated
β-catenin signals appeared in the MIR group (Fig. 6A-D). The β-catenin content in each
group was quantified, and the results demonstrated a significant
increase in MIR (4.46±0.84; P<0.001), but not in LIR (2.42±0.64)
and HIR groups (2.79±0.29), as compared with Con group (2.07±0.60;
Fig. 6E). In addition, RT-qPCR was
used to analyze the relative mRNA expression of β-catenin, which is
part of the Wnt signaling pathway (25). Compared with the Con group
(1.00±0.13), a significant difference in mRNA expression of
β-catenin was observed in MIR (1.81±0.17; P=0.001), but not in LIR
(1.37±0.18) and HIR groups (1.36±0.21; Fig. 6F). These data demonstrated that MIR
stimulates β-catenin protein expression in vivo and
β-catenin gene expression in BMSCs.
Discussion
The present study investigated the effects of three
different running intensities (LIR, MIR, and HIR) on trabecular
bone remodeling using a rat model. The differentiation of BMSCs was
also examined under different mechanical conditions to gain insight
into the mechanisms responsible for bone homeostasis. By regulating
the osteogenic and adipogenic differentiation of the BMSCs, an
intensity-dependent effect of running on bone remodeling was noted.
This effect may occur by activating the Wnt/β-catenin
signaling.
Although a number of studies have examined the
effect of treadmill running on bone mass in normal and also
ovariectomized rats (3,25,28),
few studies have attempted to compare variations in running
intensity on bone mass. As assessed by micro-CT, the results of the
current study indicated that running affects trabecular bone in an
intensity-dependent manner. MIR was able to enhance trabecular bone
mass with better structural organization compared with either LIR
or HIR. Similar intensity-dependent effects from squatting
exercises have been reported in an ovariectomized rat model
(29). The intensity-dependent
effect observed in the present study is likely associated with the
different mechanical loads placed on rat femora using the three
different running intensity protocols (LIR, MIR and HIR).
In the LIR group, no beneficial effect was observed
on bone mass, which is consistent with a number of human and animal
studies (30,31). Animal studies have reported that
the induced mechanical strain must reach a ‘set point’ threshold
before an osteogenic effect is initiated. Once this threshold is
exceeded, bone formation is positively correlated with peak strain
magnitude (32,33). In this study, LIR may have induced
a mechanical strain that was below the ‘set point’ threshold,
whereas, the induced mechanical strain by MIR exceeded the
threshold. As excessive loading, or prolonged unloading, may have
detrimental effects on bone (10),
the finding that HIR had little effect on either bone amount or
structure in the present study suggests that HIR may induce a
mechanical load close to the microdamage threshold.
Notably, using the same animal model, we previously
demonstrated that running with low-to-moderate intensity can
maintain the integrity of cartilage, whereas, high-intensity
running damages articular cartilage (24). Similar to cartilage, there is
likely a biomechanical ‘window’ that is required to maintain
optimal bone homeostasis. The running-induced strain signals within
this biomechanical ‘window’ could result in proliferation and
osteogenic differentiation of BMSCs. This may partially explain the
observation of intensity-dependent bone adaptation in rat
femora.
Bone has a remarkable ability to adjust its mass and
architecture in response to mechanical loading, which may be
associated with the ability of bone cells to sense tiny strains
within the bone matrix. The effect of mechanical stimuli on bone
cell metabolism may be modulated by a number of variables, such as
the type of bone cell (34) and
the amount of mechanical stimuli (35). It appears that the magnitude of the
load has a crucial role in the response of bone cells to mechanical
loading. Jagodzinski et al (36) demonstrated that 8% cyclic
stretching in silicone-dishes significantly increased the secretion
of differentiation markers by both osteoblasts and BMSCs compared
with either 2% cyclic stretching or no stimulation. Bacabac et
al (34) overstimulated bone
cells with noisy fluid shear stress and suggested that noise
enhances the molecular response through a threshold-activated
mechanism. Our results indicated that mechanical strain must reach
a ‘set point’ threshold before the proliferation and osteogenic
differentiation of BMSCs can be activated. On the other hand, too
much strain can induce microdamage in the matrix and exacerbate the
death of cells adjacent to the damaged matrix (37). Excessive cycles may have an
inhibitory (rather than a stimulatory) effect on osteoblast
proliferation (38). The results
of the current study suggested that HIR may exert too much strain,
which may fail to activate (or even suppress) the proliferation and
osteogenic differentiation of BMSCs.
In the present study, the proliferation and
osteogenic differentiation potential of BMSCs were investigated in
an attempt to delineate the underlying mechanism responsible for
the adaptation of trabecular bone to treadmill running at different
intensities. From the results of MTT assays, MIR was demonstrated
to stimulate the proliferation of BMSCs, whereas, LIR or HIR did
not. The results also revealed that running did not induce
alteration in CFU-F. Similar results were previously reported in
rats following climbing exercises (22) and unloading (39), indicating that mechanical loading
had no impact on the total number of progenitor cells present. By
contrast, MIR was found to increase CFU-Ob, while LIR and HIR did
not. It was reported previously that climbing exercises (22) and unloading (39) may lead to an increase and decrease
in CFU-Ob, respectively. As such, there is likely a biomechanical
‘window’, in which the running-induced strain signals increase the
number of BMSCs and progenitor cells specific to the osteoblast
lineage.
The results of the current also suggested that
running affects the osteogenic differentiation of BMSCs in an
intensity-dependent manner. Osteoblastic differentiation is a
complex process that involves differentiation of mesenchymal cells
into pre-osteoblasts and osteoblasts, ultimately leading to
synthesis and deposition of bone matrix proteins. This process is
initially driven by RUNX2, and then by SP7. It is characterized by
expression of ALP and osteocalcin, and mineralization of the ECM
(21). RUNX2 is a key
transcription factor required for osteoblast differentiation. SP7
is a late bone marker required for differentiation of
pre-osteoblasts into functioning osteoblasts (40). ALP is a marker of early osteoblast
differentiation, associated with synthesis of organic bone matrix
before mineralization of the organic bone matrix. Collagen I is the
most abundant protein in the bone ECM with a high level of
expression in osteoblasts. The expression of collagen I gene occurs
at all stages during bone development (41). Osteocalcin is expressed by mature
osteoblasts in association with matrix mineralization (42). The increased mRNA expression of
these transcription factors, together with the enhanced capacity
for osteogenic differentiation and maturation of BMSCs (as
reflected by ALP activity and Alizarin red S staining), indicated
that MIR may stimulate the osteoblastic differentiation of BMSCs at
various stages of this process. However, no significant change in
mRNA expression of these transcription factors (nor in osteogenic
differentiation and maturation of BMSCs) was observed in the LIR
group. A significant decrease in mRNA expression of RUNX2 and
collagen I was observed in the HIR group, with a significant
increase in osteocalcin mRNA expression.
In addition to osteogenic differentiation, running
also affects the adipogenic differentiation of BMSCs in an
intensity-dependent manner. MIR led to a dramatic decrease in
adipocyte density and expression of PPARγ, whereas, LIR or HIR did
not. As adipocytes and osteoblasts share a common stromal precursor
cell pool in the bone marrow, the inverse relationship between
osteogenic and adipogenic differentiation of BMSCs has been
extensively studied (12,16,20–22).
Consistent with these reports, the results of the current study
demonstrated that MIR induces a decrease in marrow adiposity, which
accompanied an observed increase in overall bone volume. In support
of the histological increase in marrow adiposity, MIR-treated BMSCs
had a significantly lower adipogenic potential, suggesting that MIR
treatment alters the microenvironment to support bone formation and
inhibit fat formation within the bone marrow. Concordantly,
expression of PPARγ in cultured BMSCs decreased. PPARγ causes bone
loss in animals and humans, at least in part due to the suppression
of osteoblast differentiation from BMSCs (21,43,44).
Previously, treadmill running with moderate intensity led to
positive inhibition effect on bone marrow adipogenesis and
expression of PPARγ in ovariectomized rats (28). The in vivo findings of the
present study are supported by the in vitro observation that
MIR-treated BMSCs exhibited a decrease in adipogenic
differentiation potential, illustrating that MIR may directly
inhibit adipogenic commitment of BMSCs.
Mechanical loading is crucial for bone cells to
adjust bone architecture in order to maintain bone strength
(45). It is believed that the
Wnt/β-catenin signaling pathway is involved in this response, since
Wnt/β-catenin signaling is not only a normal physiological response
of bone to mechanical loading (46,47),
but is also a component of the early response of osteoblastic bone
cells to load-bearing (44). In
the current study, the enhancement of protein and gene expression
of β-catenin induced by MIR may have mediated the activation of
transcription factors such as RUNX2 (16), and the downregulation of PPARγ
(48), thus biasing BMSC
differentiation away from adipogenesis and towards
osteoblastogenesis (49). The
results of the present study suggested that the effect of MIR on
decreasing fat mass and increasing bone mass may function through
the regulation of BMSC differentiation via inhibiting PPARγ and
stimulating β-catenin. Since the level of β-catenin is an
indication of the Wnt/β-catenin signaling activation (50), the results support an involvement
of the Wnt/β-catenin signaling pathway in MIR-induced increase in
bone mass in rats.
In conclusion, the present study demonstrated that
treadmill running appears to affect trabecular bone mass in an
intensity-dependent manner. In addition, a biomechanical ‘window’
may exist that maintains optimal bone homeostasis. By regulating
the osteogenic and adipogenic differentiation of the BMSCs, an
intensity-dependent effect of running on bone remodeling was
observed. This result may provide insight into the molecular and
cellular mechanisms responsible for bone adaptation by activating
the Wnt/β-catenin signaling.
Acknowledgements
We gratefully acknowledge Mr. PR Zhaoat from the Key
Laboratory of Bone and Cartilage Regenerative Medicine in Southern
Medical University (Guangdong, China) for his technical assistance.
We also thank Professor Allen P. Liangat from the Department of
Orthopedics and Traumatology, Nanfang Hospital, Southern Medical
University for revision of this manuscript.
Funding
This study was supported by National Natural Science
Foundation of China (grant no. 81572219) and Guangdong Natural
Science Foundation (grant no. 2014A030313307).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GXN and MY designed the study. SYL, ZL, and SYX
performed the experiments. SYL and ZL collected the data. SYL and
LX analyzed the data. SYL, ZL, and SYX interpreted the data. GXN,
MY, SYL, ZL, SYX, and LX wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the animal ethics
committee of Nanfang Hospital, Southern Medical University
(application no. NFYY-2013-26).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Williams PT: Lower prevalence of
hypertension, hypercholesterolemia, and diabetes in marathoners.
Med Sci Sports Exerc. 41:523–529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schneider S, Askew CD, Diehl J, Mierau A,
Kleinert J, Abel T, Carnahan H and Strüder HK: EEG activity and
mood in health orientated runners after different exercise
intensities. Physiol Behav. 96:709–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwamoto J, Takeda T and Sato Y: Effect of
treadmill exercise on bone mass in female rats. Exp Anim. 54:1–6.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bourrin S, Genty C, Palle S, Gharib C and
Alexandre C: Adverse effects of strenuous exercise: a densitometric
and histomorphometric study in the rat. J Appl Physiol (1985).
76:1999–2005. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hind K, Truscott JG and Evans JA: Low
lumbar spine bone mineral density in both male and female endurance
runners. Bone. 39:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lappe J, Cullen D, Haynatzki G, Recker R,
Ahlf R and Thompson K: Calcium and vitamin d supplementation
decreases incidence of stress fractures in female navy recruits. J
Bone Miner Res. 23:741–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frost HM: Bone's mechanostat: A 2003
update. Anat Rec A Discov Mol Cell Evol Biol. 275:1081–1101. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozcivici E, Luu YK, Adler B, Qin YX, Rubin
J, Judex S and Rubin CT: Mechanical signals as anabolic agents in
bone. Nat Rev Rheumatol. 6:50–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubin CT and Lanyon LE: Regulation of bone
mass by mechanical strain magnitude. Calcif Tissue Int. 37:411–417.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scott A, Khan KM, Duronio V and Hart DA:
Mechanotransduction in human bone: In vitro cellular physiology
that underpins bone changes with exercise. Sports Med. 38:139–160.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klein-Nulend J, Bacabac RG and Bakker AD:
Mechanical loading and how it affects bone cells: The role of the
osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater.
24:278–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Georgiou KR, Scherer MA, Fan CM, Cool JC,
King TJ, Foster BK and Xian CJ: Methotrexate chemotherapy reduces
osteogenesis but increases adipogenic potential in the bone marrow.
J Cell Physiol. 227:909–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bianco P and Robey Gehron P: Marrow
stromal stem cells. J Clin Invest. 105:1663–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kajkenova O, Lecka-Czernik B, Gubrij I,
Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC and
Lipschitz DA: Increased adipogenesis and myelopoiesis in the bone
marrow of SAMP6, a murine model of defective osteoblastogenesis and
low turnover osteopenia. J Bone Miner Res. 12:1772–1779. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nuttall ME and Gimble JM: Is there a
therapeutic opportunity to either prevent or treat osteopenic
disorders by inhibiting marrow adipogenesis? Bone. 27:177–184.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nuttall ME and Gimble JM: Controlling the
balance between osteoblastogenesis and adipogenesis and the
consequent therapeutic implications. Curr Opin Pharmacol.
4:290–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai MY, Shyr CR, Kang HY, Chang YC, Weng
PL, Wang SY, Huang KE and Chang C: The reduced trabecular bone mass
of adult ARKO male mice results from the decreased osteogenic
differentiation of bone marrow stroma cells. Biochem Biophys Res
Commun. 411:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mauney JR, Sjostorm S, Blumberg J, Horan
R, O'Leary JP, Vunjak-Novakovic G, Volloch V and Kaplan DL:
Mechanical stimulation promotes osteogenic differentiation of human
bone marrow stromal cells on 3-D partially demineralized bone
scaffolds in vitro. Calcif Tissue Int. 74:458–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakata T, Sakai A, Tsurukami H, Okimoto N,
Okazaki Y, Ikeda S, Norimura T and Nakamura T: Trabecular bone
turnover and bone marrow cell development in tail-suspended mice. J
Bone Miner Res. 14:1596–1604. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahdjoudj S, Kaabeche K, Holy X, Fromigué
O, Modrowski D, Zérath E and Marie PJ: Transforming growth
factor-beta inhibits CCAAT/enhancer-binding protein expression and
PPARgamma activity in unloaded bone marrow stromal cells. Exp Cell
Res. 303:138–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
David V, Martin A, Lafage-Proust MH,
Malaval L, Peyroche S, Jones DB, Vico L and Guignandon A:
Mechanical loading down-regulates peroxisome proliferator-activated
receptor gamma in bone marrow stromal cells and favors
osteoblastogenesis at the expense of adipogenesis. Endocrinology.
148:2553–2562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mori T, Okimoto N, Sakai A, Okazaki Y,
Nakura N, Notomi T and Nakamura T: Climbing exercise increases bone
mass and trabecular bone turnover through transient regulation of
marrow osteogenic and osteoclastogenic potentials in mice. J Bone
Miner Res. 18:2002–2009. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yokota H, Leong DJ and Sun HB: Mechanical
loading: Bone remodeling and cartilage maintenance. Curr Osteoporos
Rep. 9:237–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni GX, Liu SY, Lei L, Li Z, Zhou YZ and
Zhan LQ: Intensity-dependent effect of treadmill running on knee
articular cartilage in a rat model. Biomed Res Int.
2013:1723922013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bu S, Chen Y, Wang S, Zhang F and Ji G:
Treadmill training regulates β-catenin signaling through
phosphorylation of GSK-3β in lumbar vertebrae of ovariectomized
rats. Eur J Appl Physiol. 112:3295–3304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su N, Sun Q, Li C, Lu X, Qi H, Chen S,
Yang J, Du X, Zhao L, He Q, et al: Gain-of-function mutation in
FGFR3 in mice leads to decreased bone mass by affecting both
osteoblastogenesis and osteoclastogenesis. Hum Mol Genet.
19:1199–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Wang S, Bu S, Wang Y, Duan Y and
Yang S: Treadmill training prevents bone loss by inhibition of
PPARγ expression but not promoting of Runx2 expression in
ovariectomized rats. Eur J Appl Physiol. 111:1759–1767. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiuchi A, Shimegi S, Tanaka I, Izumo N,
Fukuyama R, Nakamuta H and Koida M: Dose-response effects of
exercise intensity on bone in ovariectomized rats. Int J Sports
Health Sci. 4:10–18. 2006. View Article : Google Scholar
|
|
30
|
Huang TH, Lin SC, Chang FL, Hsieh SS, Liu
SH and Yang RS: Effects of different exercise modes on
mineralization, structure, and biomechanical properties of growing
bone. J Appl Physiol (1985). 95:300–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sööt T, Jürimäe T, Jürimäe J, Gapeyeva H
and Pääsuke M: Relationship between leg bone mineral values and
muscle strength in women with different physical activity. J Bone
Miner Metab. 23:401–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turner CH, Forwood MR, Rho JY and
Yoshikawa T: Mechanical loading thresholds for lamellar and woven
bone formation. J Bone Miner Res. 9:87–97. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chow JW, Jagger CJ and Chambers TJ:
Characterization of osteogenic response to mechanical stimulation
in cancellous bone of rat caudal vertebrae. Am J Physiol.
265:E340–E347. 1993.PubMed/NCBI
|
|
34
|
Bacabac RG, Van Loon JJ, Smit TH and
Klein-Nulend J: Noise enhances the rapid nitric oxide production by
bone cells in response to fluid shear stress. Technol Health Care.
17:57–65. 2009.PubMed/NCBI
|
|
35
|
Li YJ, Batra NN, You L, Meier SC, Coe IA,
Yellowley CE and Jacobs CR: Oscillatory fluid flow affects human
marrow stromal cell proliferation and differentiation. J Orthop
Res. 22:1283–1289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jagodzinski M, Drescher M, Zeichen J,
Hankemeier S, Krettek C, Bosch U and van Griensven M: Effects of
cyclic longitudinal mechanical strain and dexamethasone on
osteogenic differentiation of human bone marrow stromal cells. Eur
Cell Mater. 7:35–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verborgt O, Gibson GJ and Schaffler MB:
Loss of osteocyte integrity in association with microdamage and
bone remodeling after fatigue in vivo. J Bone Miner Res. 15:60–67.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stanford CM, Welsch F, Kastner N, Thomas
G, Zaharias R, Holtman K and Brand RA: Primary human bone cultures
from older patients do not respond at continuum levels of in vivo
strain magnitudes. J Biomech. 33:63–71. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Basso N, Jia Y, Bellows CG and Heersche
JN: The effect of reloading on bone volume, osteoblast number, and
osteoprogenitor characteristics: Studies in hind limb unloaded
rats. Bone. 37:370–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao Y, Zhou Z, de Crombrugghe B, Nakashima
K, Guan H, Duan X, Jia SF and Kleinerman ES: Osterix, a
transcription factor for osteoblast differentiation, mediates
antitumor activity in murine osteosarcoma. Cancer Res.
65:1124–1128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jikko A, Harris SE, Chen D, Mendrick DL
and Damsky CH: Collagen integrin receptors regulate early
osteoblast differentiation induced by BMP-2. J Bone Miner Res.
14:1075–1083. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pockwinse SM, Wilming LG, Conlon DM, Stein
GS and Lian JB: Expression of cell growth and bone specific genes
at single cell resolution during development of bone tissue-like
organization in primary osteoblast cultures. J Cell Biochem.
49:310–323. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shockley KR, Lazarenko OP, Czernik PJ,
Rosen CJ, Churchill GA and Lecka-Czernik B: PPARgamma2 nuclear
receptor controls multiple regulatory pathways of osteoblast
differentiation from marrow mesenchymal stem cells. J Cell Biochem.
106:232–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bruedigam C, Koedam M, Chiba H, Eijken M
and van Leeuwen JP: Evidence for multiple peroxisome
proliferator-activated receptor gamma transcripts in bone:
Fine-tuning by hormonal regulation and mRNA stability. FEBS Lett.
582:1618–1624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Armstrong VJ, Muzylak M, Sunters A, Zaman
G, Saxon LK, Price JS and Lanyon LE: Wnt/beta-catenin signaling is
a component of osteoblastic bone cell early responses to
load-bearing and requires estrogen receptor alpha. J Biol Chem.
282:20715–20727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bonewald LF and Johnson ML: Osteocytes,
mechanosensing and Wnt signaling. Bone. 42:606–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Robinson JA, Chatterjee-Kishore M,
Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P,
Brown EL, et al: Wnt/beta-catenin signaling is a normal
physiological response to mechanical loading in bone. J Biol Chem.
281:31720–31728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takada I, Kouzmenko AP and Kato S: Wnt and
PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev
Rheumatol. 5:442–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sen B, Xie Z, Case N, Ma M, Rubin C and
Rubin J: Mechanical strain inhibits adipogenesis in mesenchymal
stem cells by stimulating a durable beta-catenin signal.
Endocrinology. 149:6065–6075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Case N, Ma M, Sen B, Xie Z, Gross TS and
Rubin J: Beta-catenin levels influence rapid mechanical responses
in osteoblasts. J Biol Chem. 283:29196–29205. 2008. View Article : Google Scholar : PubMed/NCBI
|