Introduction
Myocardial ischemia is a direct result of coronary
artery stenosis or occlusion, which is characterized by high
morbidity and mortality worldwide (1). Myocardial ischemia, especially acute
myocardial ischemia, has been estimated by the World Health
Organization to be the primary cause of death in the world by the
year 2020 (2). Except sudden
death, myocardial ischemia causes cardiomyocyte hypertrophy,
cardiomyocyte apoptosis, cardiac fibrosis and cardiac dysfunction
(3–5). Therefore, interventions and
mechanisms involved in myocardial ischemia have been explored. For
example, rapid reperfusion is an active treatment for acute
myocardial ischemia, which attenuates myocardial infarction and
relieves cardiac dysfunction (6).
However, there remains a requirement for more active and more
convenient treatments for myocardial ischemia.
(−)-Epicatechin (EPI) belongs to the group of
flavanols, which is primarily contained in flavonoid-rich foods,
such as green tea and dark chocolate (7). It is believed that EPI contributes to
the beneficial effects of flavonoid-rich foods on the
cardiovascular system. Research has indicated that EPI protects
hearts from myocardial ischemia reperfusion injury, cardiac
hypertrophy, oxidative stress injury and coronary occlusion
(8–11). Catechin, a conformational isomer of
EPI, was demonstrated to attenuate chronic ventricular remodeling
induced by myocardial ischemia in rats (12). Epigallocatechin gallate, an
analogue of EPI, also inhibits telomere attrition induced
cardiomyocyte apoptosis (11).
However, it is still unclear whether EPI has an effect on
myocardial ischemia in vivo and cardiomyocyte apoptosis
in vitro.
Dysregulation of numerous signaling pathways has
been demonstrated to be involved in myocardial ischemia induced
cardiac injury, especially the phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT) pathway (13,14).
This pathway is an intracellular signaling pathway, which serves a
role in modulating cell proliferation, differentiation, autophagy
and apoptosis under physiological and pathological conditions
(15). The activation of the
PI3K/AKT pathway interacts with complexed downstream target
proteins including B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X
protein (Bax) to promote cell survival, which is negatively
regulated by phosphatase and tensin homolog (PTEN) (16). The PI3K/AKT pathway was shown to be
a promising therapy target for myocardial ischemia. For example, Ke
et al (13) revealed that
the activation of the PI3K/AKT pathway protects against
isoproterenol-induced myocardial ischemic injury. EPI was
demonstrated to induce physiological cardiac growth by activating
the PI3K/AKT pathway (17). The
aforementioned results suggested that EPI may exhibit an
anti-myocardial ischemia function via activation of the PI3K/AKT
pathway.
Therefore, the present study aimed to investigate
the effect of EPI on mouse myocardial ischemic injury induced by
coronary occlusion in vivo and by anoxia cultivation in
vitro. Furthermore, the present study also investigated the
mechanism underlying cardiac protection of EPI, which promotes the
activation of the PTEN/PI3K/AKT signaling pathway to inhibit
apoptosis of cardiomyocytes.
Materials and methods
Animals
A total of 24 healthy male C57BL/6 mice at the age
of eight weeks, ~25–30 g in weight, were obtained from the
experimental Animal Center of the Harbin Medical University
(Harbin, China). The mice were randomly divided into the sham
group, myocardial ischemia (MI) group, MI+EPI group and
MI+EPI+LY294002 group, and n=6 in each group. Subsequently,
according to the different groups, the mice were subjected to a
sham or MI operation and the mice that underwent MI surgery were
administered EPI or EPI+LY294002. The animals were kept under
standard animal room conditions (atmosphere 0.03% CO2;
temperature 21±1°C; humidity 55–60%; 12-h light-dark cycle) with
laboratory pellet food and autoclaved water freely available. All
experimental procedures were performed in accordance with the
Guidelines for the Care and Use of Laboratory Animals set by the US
National Institutes of Health (Bethesda, MD, USA), which was
approved by the Institutional Animal Care and Use Committee of the
Harbin Medical University.
Myocardial ischemia model
establishment and drug treatments in vivo
To induce myocardial ischemia, mice were
anesthetized with Avertin (160 mg/kg; intraperitoneal injection;
Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and their chests
were opened to expose the hearts. The left descending coronary
artery was ligated with a 7/0 nylon suture at 2 mm below the border
between the left atrium and ventricle to induce myocardial
ischemia. Ischemia was confirmed by visual observation (cyanosis)
and by observing ST segment elevation and QRS widen on
electrocardiogram. The sham group mice underwent the same
experimental procedures as the MI group but without ligation of
left descending coronary artery. Prior to the myocardial ischemia
operation, the mice in the MI+EPI (cat. no. 68097; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and MI+EPI+LY294002 (cat. no.
L9908; Sigma-Aldrich, Merck KGaA) groups were treated with 1 mg EPI
per kg body weight by oral gavage every day for 10 days.
Additionally, the mice in the MI+EPI+LY294002 group were treated
with LY294002 at 10 mg/kg body weight by intraperitoneal injection
30 min before treatment of EPI every day for 10 days. The mice in
the sham group were administered with an equal dosage of saline.
All mice were sacrificed at day 7 after the myocardial ischemia
operation.
Treatment of cardiomyocytes
Cardiomyocytes were isolated from neonatal C57BL/6
mice (aged 1–2 days, weight 1–3 g, a random mix of male or female;
purchased from the experimental Animal Center of Harbin Medical
University) as previously described, from mice that were sacrificed
following purchase (18). Briefly,
the heart was digested by pancreatin (cat no. C0201; Beyotime
Institute of Biotechnology, Haimen, China) and maintained in
Dulbecco's modified Eagle's medium (DMEM; cat no. 11965084; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
100 U/ml penicillin and 10% fetal bovine serum (FBS; cat no.
04-001-1A; Biological Industries, Kibbutz Beit Haemek, Israel)
(18). Cardiomyocytes were
purified by differential adherence and 0.1 mM
5-bromo-2-deoxyuridine (cat no. B23151; Thermo Fisher Scientific,
Inc.) was added to suppress cardiac fibroblasts for 48 h in a 5%
CO2 and 37°C humidified atmosphere. Then, as the anoxia
(ANO) group, ANO+EPI group or ANO+EPI+LY group, the purified
cardiomyocytes were treated with an equal amount of DMEM or EPI (5
µM) or EPI (5 µM) + LY294002 (20 µM) separately in a 5%
CO2 and 37°C humidified atmosphere for 1 h and then
incubated under anoxia condition at 37°C for 12 h. As the control
group, the purified cells were treated with an equal amount of DMEM
in a 5% CO2 and 37°C humidified atmosphere for 13 h.
Echocardiograph
Transthoracic echocardiography was performed to
measure alterations to the left ventricular function using a
Vevo2100 system (VisualSonics, Inc., Toronto, ON, Canada) equipped
with a 10-MH2 phased-array transducer with the M-mode recordings.
Functional parameters were evaluated and analyzed including heart
rate (HR), left ventricular end-diastolic dimensions (LVEDd), left
ventricular end-systolic dimensions (LVESd), left ventricular
ejection fraction (EF) and fractional shortening (FS).
Histological analysis
Hearts were collected and fixed with 4%
paraformaldehyde at 4°C for 48 h, embedded in paraffin, sliced into
5-µm sections and subjected to standard hematoxylin and eosin
(H&E) staining and Masson staining at room temperature for 4 h.
The sections were visualized using a light microscope (80i; Nikon
Corporation, Tokyo, Japan). A total of 10 fields of view each were
analyzed. Image J software (version 1.46r; National Institutes of
Health, USA) was used for quantitative analysis of the % area of
fibrosis..
Cell viability assay
Cell viability was measured by the mitochondrial
dependent reduction of MTT reagent (cat no. C0009; Beyotime
Biotechnology, Shanghai, China), as previously described (19). Neonatal mouse cardiomyocytes
(NMCMs) were plated onto a 96-well plate and treated with an equal
amount of DMEM or EPI (5 µM) or EPI (5 µM) + LY294002 (20 µM) in a
5% CO2 and 37°C humidified atmosphere for 1 h and then
incubated under anoxia condition at 37°C for 12 h. As control
group, NMCMs plated onto a 96-well plate treated with an equal
amount of DMEM in a 5% CO2 and 37°C humidified
atmosphere for 13 h, as designated. A total of 20 µl MTT solution
(Sigma-Aldrich, Merck KGaA) and 180 µl DMEM was added into each
well and the plates were incubated at 37°C for 4 h. Then, the
medium of each well was carefully removed and 200 µl DMSO was added
into each well to dissolve formazan. The absorbance values were
read at a wavelength of 490 nm using a microplate reader (Tecan
Group, Ltd., Mannedorf, Switzerland).
Western blot analysis
For Western blot analysis, protein samples extracted
from the left ventricles of mice or cultured NMCMs were used.
Briefly, protein was lysed with radioimmunoprecipitation assay
lysis buffer containing a complete protease inhibitor cocktail
(cat. no. 4693116001; Roche Molecular Diagnostics, Pleasanton, CA,
USA). The protein concentration was determined by BCA Protein Assay
kit (cat no. P0012S; Beyotime Institute of Biotechnology, Shanghai,
China). A total of 10 µg protein was separated by 12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The blots
were probed with primary antibodies overnight at 4°C. The primary
antibodies included Bcl-2 (1:1,000; cat no. 3498), Bax (1:1,000;
cat no. 14796), caspase-3 (1:500; cat no. 9665), cleaved caspase-3
(1:500; cat no. 9661), PTEN (1:1,000; cat no. 9559; all Cell
Signaling Technology, Inc., Danvers, MA, USA), total AKT (T-AKT;
1:200; cat no. sc-8312; Santa Cruz Biotechnology, Inc., Dallas TX,
USA), phosphorylated-AKT (p-AKT; 1:1,000; cat no. 13038) and GAPDH
(1:1,000; cat no. 2118; both Cell Signaling Technology, Inc.).
After washing, the membranes were incubated with a horse radish
peroxidase-conjugated goat-anti-rabbit immunoglobulin G (1:1,000;
cat no. A0208; Beyotime Institute of Biotechnology) for 1 h at room
temperature. The signal was detected by an Enhanced
Chemiluminescence reagent (cat no. 29050; Engreen Biosystem New
Zealand Ltd., Auckland, New Zealand), quantified by using Odyssey
Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) and
analyzed by Odyssey Infrared Imaging System matched application
software (version 3.0.16; LI-COR Biosciences, Lincoln, NE,
USA).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
Apoptosis was assessed by a TUNEL assay (Roche
Molecular Diagnostics), as previously described (20). Then, 6-mm frozen sections or cells
were also co-stained with DAPI (1:1,000; cat no. D3571; Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 5 mins to
visualize nuclei, and mouse monoclonal anti-sarcomeric α-actinin
antibody (1:1,000; cat no. A7811; Sigma-Aldrich; Merck KGaA) at 4°C
overnight. Then the sections or cells were incubated with
goat-anti-mouse IgG-Alexa Fluor 594 at 37°C for 2 h (1:300; cat no.
A-11032; Invitrogen; Thermo Fisher Scientific, Inc.). The average
of TUNEL-positive cell ratio in at least five representative
microscopic fields was calculated with ZEN2012 (version 1.1.2.0;
Carl Zeiss AG, Oberkochen, Germany) to compare the apoptosis ratio
within different groups.
Statistical analysis
All data was expressed as the mean ± standard error.
GraphPad Prism version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) was used for statistical analysis. For two group
comparisons, an unpaired Student t-test was used. For multiple
group comparisons, a one-way analysis of variance with Bonferroni
post-test was used for comparisons between selected two groups as
well as Dunnett post-test for comparisons among all other treatment
groups to the corresponding control. P<0.05 was considered to
indicate a statistically significant difference.
Results
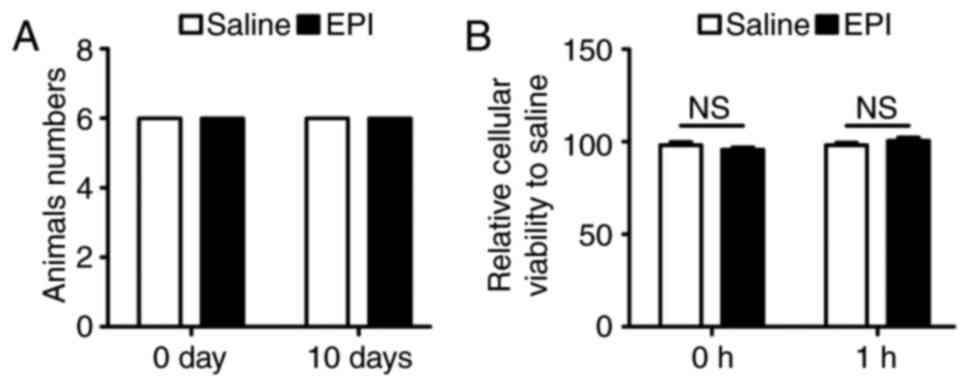
The safety dosage of EPI for treating
myocardial ischemia in vivo and in vitro
The present study confirmed the appropriate dosage
of EPI. In a previously published study, EPI was administered at 1
mg/kg/day via oral gavage for 10 days (21) to maintain a sufficient drug level.
Therefore, the present study administered EPI (1 mg/kg daily) to
25–30 g male C57BL/6 mice for 10 days before left descending
coronary artery ligation for in vivo experiments. By paying
monitoring the overall survival of the mice, it was confirmed that
the drug dosage (1 mg/kg daily) was not lethal (Fig. 1A). Additionally, the eating
behavior of the mice was observed daily and documented the weight
of them over the 10 days. However, there was no significant
difference between the two group and therefore it was concluded
that the dose was safe (data not shown). According to previous
studies for in vitro experiments (21–23),
the present study pretreated NMCMs with 5 µM EPI for 1 h in 5%
CO2 and 37°C and then incubated cells under anoxia
conditions for 12 h. The possible toxicity effect of EPI on NMCMs
was determined by an MTT assay. As shown in Fig. 1B, the dosage of EPI was safe for
cardiomyocytes.
EPI attenuates myocardial
ischemia-induced cardiac fibrosis and apoptosis which is weakened
by the specific PI3K inhibitor, LY294002
C57BL/6 mice underwent left descending coronary
artery occlusion operation to induce myocardial ischemic, or sham
operation for negative control. H&E and Masson staining
analysis demonstrated that the areas of cardiomyocytes and cardiac
fibrosis of the MI group mice were significantly increased 1 week
following MI operation, when compared with the hearts of the sham
group mice. EPI treatment suppressed MI-induced cardiac hypertrophy
and fibrosis, as evidenced by decreased areas of cardiomyocytes and
cardiac fibrosis in the MI+EPI group mice when compared with the MI
group (Fig. 2A). TUNEL assay
revealed that cardiomyocyte apoptosis increased in the MI group
when compared with the sham group, which was significantly
inhibited by EPI administration in the MI+EPI group mice (Fig. 2B). Considering the crucial role of
EPI in myocardial ischemia, the underlying molecular mechanism
through which EPI exerts its protective effect was further
investigated. The PI3K/AKT signaling pathway mediates cell
apoptosis, which may be a potential treatment target for
ameliorating myocardial ischemia. Therefore, this study used
LY294002, a specific PI3K inhibitor, to confirm whether EPI
inhibits the myocardial ischemia-induced cardiac injury through the
PI3K/AKT pathway. The protective effect of EPI for myocardial
ischemia was abolished by LY294002 (Fig. 2A and B). Therefore, these results
illustrated that the inhibition of the PI3K/AKT pathway offsets the
cardiac protective effect of EPI.
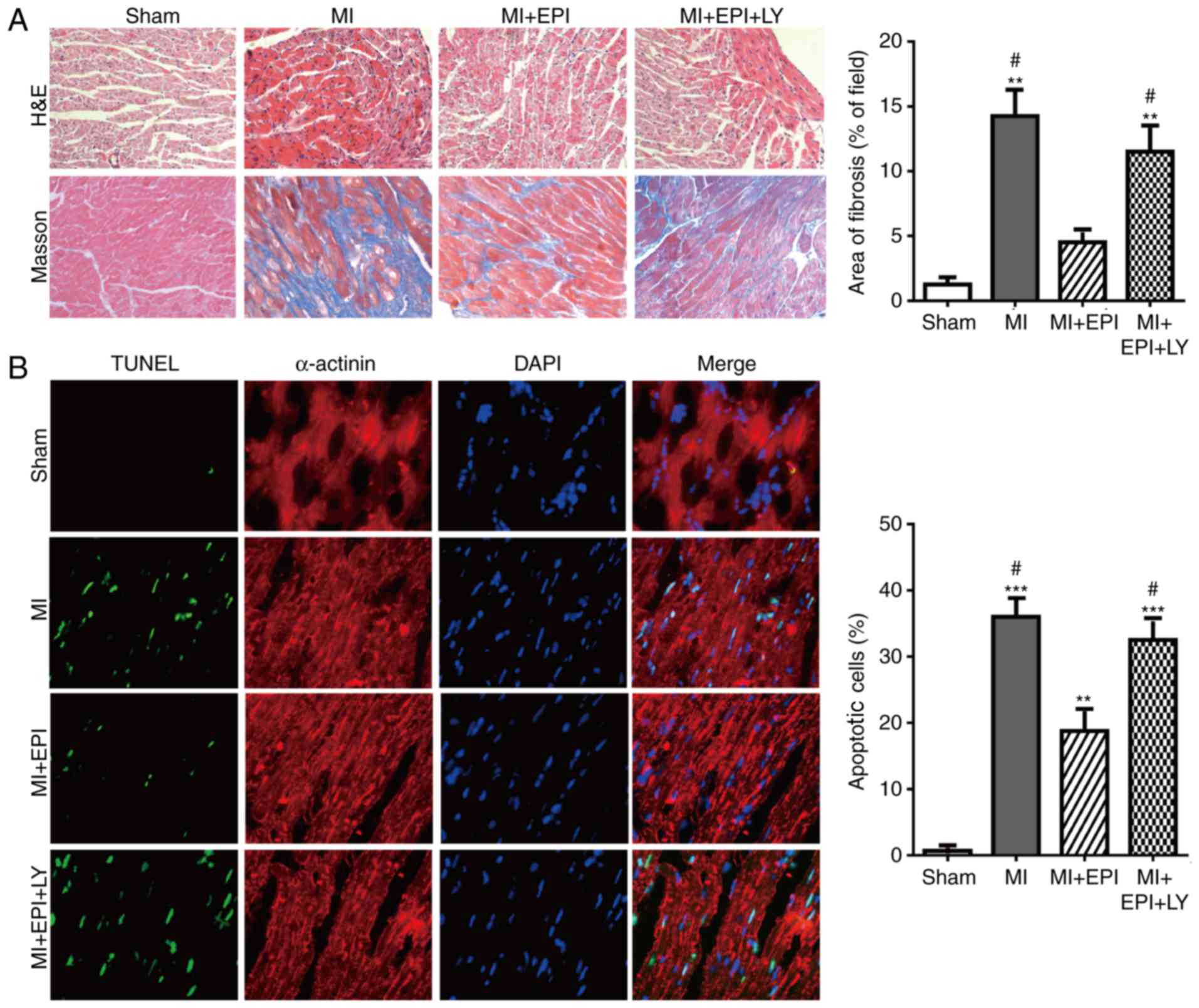 | Figure 2.PI3K specific inhibitor LY294002
abolishes the effect of EPI against ischemia-induced mouse cardiac
hypertrophy, fibrosis and apoptosis. (A) Microscopic examination of
histological sections detected by hematoxylin and eosin and Masson
staining (magnification, ×200) of mouse hearts from the sham group,
MI group, MI+EPI group and MI+EPI+LY294002 group. EPI suppressed
the increase of the areas of cardiomyocyte and fibers induced by
myocardial ischemia operation, which were counteracted by LY294002.
(B) Terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling staining of mouse hearts from the sham group, MI group,
MI+EPI group and MI+EPI+LY294002 group. EPI suppressed cellular
apoptosis induced by MI operation, which was abolished by LY294002.
Data are expressed as the mean ± standard error (n=6). **P<0.01
and ***P<0.001 vs. sham. #P<0.05 vs. MI+EPI. MI,
myocardial ischemia; EPI, (−)-epicatechin; LY, LY294002. AKT,
protein kinase B; EPI, (−)-epicatechin; MI, myocardial injury. |
The EPI-mediated improvement of
cardiac left ventricular functions is reduced by the PI3k inhibitor
LY294002
The present study used two-dimensional
echocardiography and M-mode tracings to examine left ventricular
functions at day 7 of myocardial ischemic surgery. The left
ventricular wall thickness was increased in MI group, which was
suppressed by EPI. The effect of EPI on left ventricular wall
thickness was attenuated by LY294002 (Fig. 3A). HR, LVEDd, LVESd, EF and
fractional shortening FS were detected by transthoracic
echocardiography. There was no significant difference of HR between
any group (Fig. 3B). EPI
significantly inhibited the increase of LVEDd and LVESd induced by
the myocardial ischemic operation (Fig. 3C, D). In addition, EPI
administration increased FS and EF levels in the MI+EPI group
compared with the MI group (Fig. 3E
and F). As shown in Fig. 3C and
D, LVEDd and the LVESd in the MI+LY294002+EPI group were
significantly increased when compared with EPI-treated myocardial
ischemic mice. FS and EF were decreased in the MI+LY294002+EPI
group after co-treatment with EPI and LY294002 (Fig. 3E, F), compared with the MI+EPI
group. These results demonstrated that EPI protects left
ventricular function from myocardial ischemic injury via the AKT
pathway.
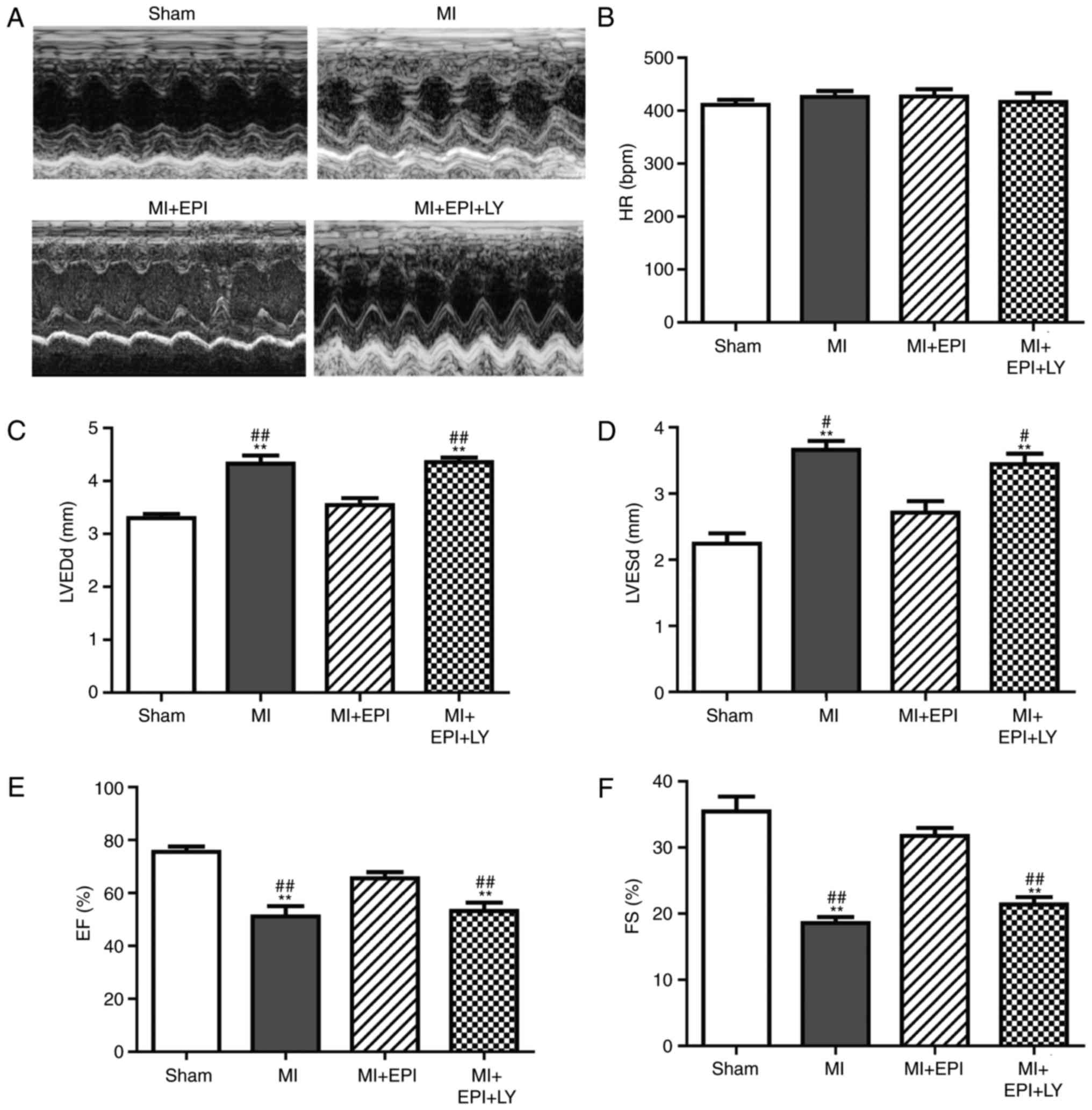 | Figure 3.Improvement effect of EPI on cardiac
function is counteracted by LY294002. Mice were randomly selected
from each group (sham, MI, MI+EPI and MI+EPI+LY294002) and
echocardiography was performed. (A) Representative M-mode
echocardiographs of the left ventricular wall thickness from the
four groups of mice. Each representative image illustrates the
continuous changes of left ventricular wall thickness during six to
seven mouse cardiac cycles. (B) HR, (C) LVEDd, (D) LVESd, (E) EF
and (F) FS. Data are expressed as the mean ± standard error (n=6).
**P<0.01 vs. sham; #P<0.05 and
##P<0.01 vs. MI+EPI. MI, myocardial ischemia; EPI,
(−)-epicatechin; LY, LY294002; HR, heart rate; LVEDd, left
ventricular end-diastolic dimensions; LVESd, left ventricular
end-systolic dimensions; EF, ejection fraction; FS, fractional
shortening. |
EPI inhibits myocardial ischemic
induced cardiomyocyte apoptosis via the PTEN/AKT signaling
pathway
Next, the present study further identified the
underlying molecular mechanism by which EPI safeguards cardiac
functions. As earlier work demonstrated, PTEN is a primary negative
regulator of the PI3K/AKT signaling pathway (24). To examine whether PTEN mediates the
cardiac protective action of EPI, this study used western blot
analysis to compare protein levels of components of the PTEN/AKT
pathway in each group. As shown in Fig. 4A, compared with the sham group,
myocardial ischemia significantly induced the decrease of Bcl-2 and
the increase of Bax and cleaved caspase-3 in MI group, which were
rescued by the administration of EPI. However, in the
MI+EPI+LY294002 group, LY294002 significantly abolished the rescue
effect of EPI. Furthermore, Fig.
4B indicated that PTEN and the p-AKT/T-AKT ratio were
upregulated and downregulated, respectively, in the MI group
compared with the sham group. PTEN and p-AKT/T-AKT ratio were
decreased and increased, respectively, in the MI+EPI group compared
with the MI group, which were both significantly reversed by
LY294002 co-treatment in the MI+EPI+LY294002 group. Taken together,
these results demonstrated that EPI exerts an anti-apoptotic
function during myocardial ischemia in a PTEN/AKT pathway dependent
manner.
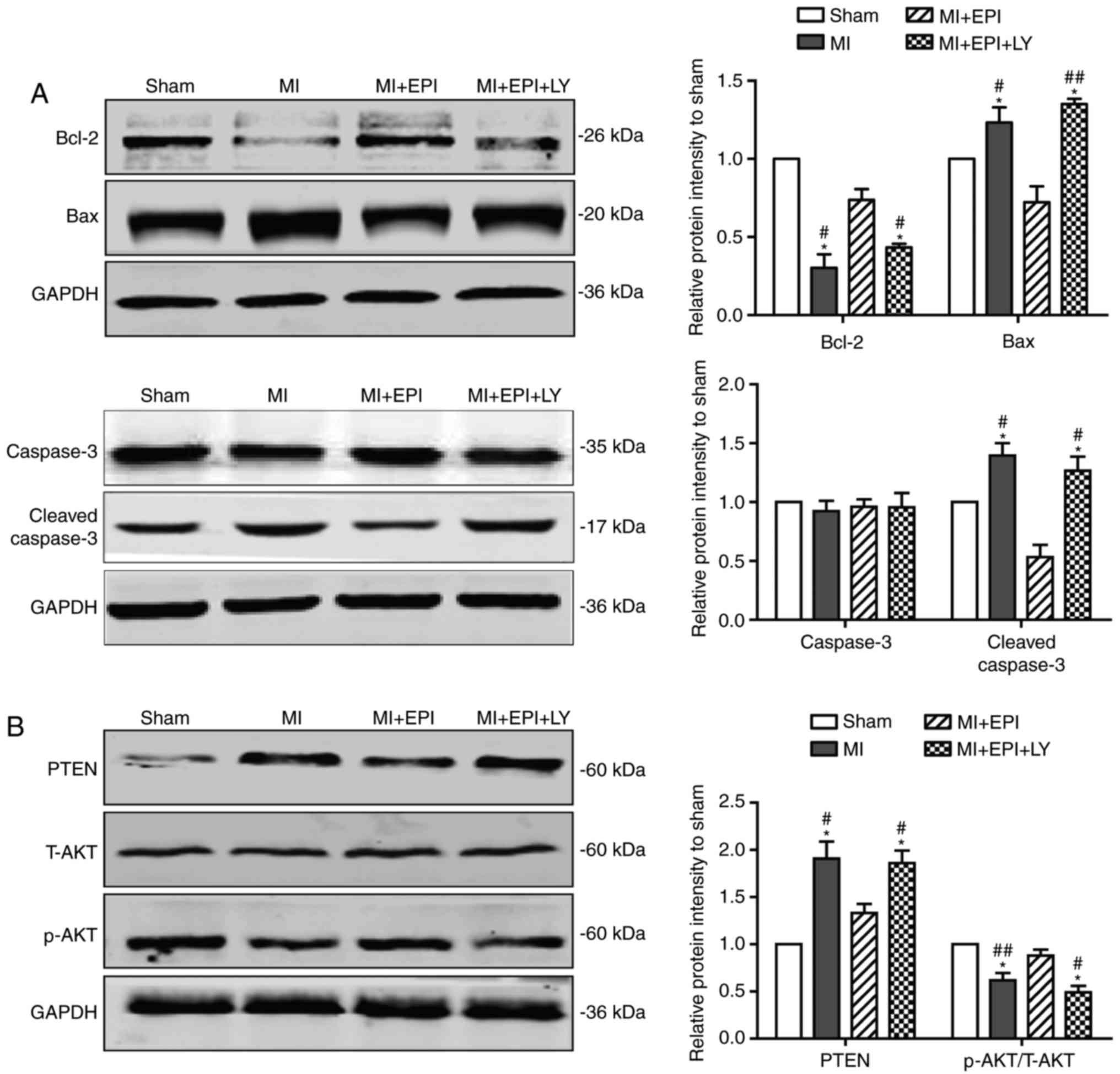 | Figure 4.EPI inhibits myocardial
ischemia-induced cardiomyocyte apoptosis, which is reversed by
LY294002. (A) Western blot analysis for Bcl-2, Bax, caspase-3 and
cleaved caspase-3 in mouse hearts from sham group, MI group, MI+EPI
group and MI+EPI+LY294002 group. EPI upregulated the expression of
Bcl-2 and downregulated the expression of Bax-3 and cleaved
caspase-3 in ischemic hearts, which were reversed by LY294002. (B)
Western blot analysis for PTEN, T-AKT and p-AKT. EPI upregulated
the expression ratio of p-AKT/T-AKT and downregulated the
expression of PTEN in ischemic hearts, which were reversed by
LY294002. Data are expressed as the mean ± standard error (n=6).
*P<0.05 and **P<0.01 vs. sham; #P<0.05 and
##P<0.01 vs. MI+EPI. MI, myocardial ischemia; EPI,
(−)-epicatechin; LY, LY294002; Bcl-2, B-cell lymphoma-2; Bax,
B-cell lymphoma-2-associated X protein; PTEN, phosphatase and
tensin homolog; T-AKT, total protein kinase B; p-AKT,
phosphorylated protein kinase B. |
EPI protects cardiomyocytes from
hypoxia-induced apoptosis via the PTEN/AKT signaling pathway
The above results indicated that EPI protects
myocardial ischemia-induced heart injury through the PTEN/AKT
signaling pathway in vivo. To further confirm the effect of
EPI on cardiomyocytes, NMCMs were incubated under normal or hypoxic
condition in vitro. As shown in Fig. 5A, cell viability was decreased in
ANO group when compared with the control group. Treatment with EPI
rescued the decrease of cell viability induced by hypoxia, which
was significantly counteracted by LY294002 co-treatment (Fig. 5A). In agreement with the previous
results in vivo, hypoxia induced the decrease of Bcl-2 and
the increase of Bax and cleaved caspase-3 in the NMCMs of ANO group
in vitro. EPI treatment significantly rescued the decrease
of Bcl-2 and suppressed the increase of Bax and cleaved caspase-3
in hypoxia-treated NMCMs when compared to the level of the control
group, which was abolished by the addition of LY294002 (Fig. 5B). In addition, treatment with EPI
significantly attenuated hypoxia-induced upregulation of PTEN and
downregulation of p-AKT when compared with the MI group. Similarly,
LY294002 treatment reversed the effect of EPI on the PTEN and the
p-AKT. The level of T-AKT was not affected in each group (Fig. 5C). Taken together, these results
indicated that EPI protects cardiomyocytes from hypoxia-induced
apoptosis through the PTEN/AKT pathway in vitro.
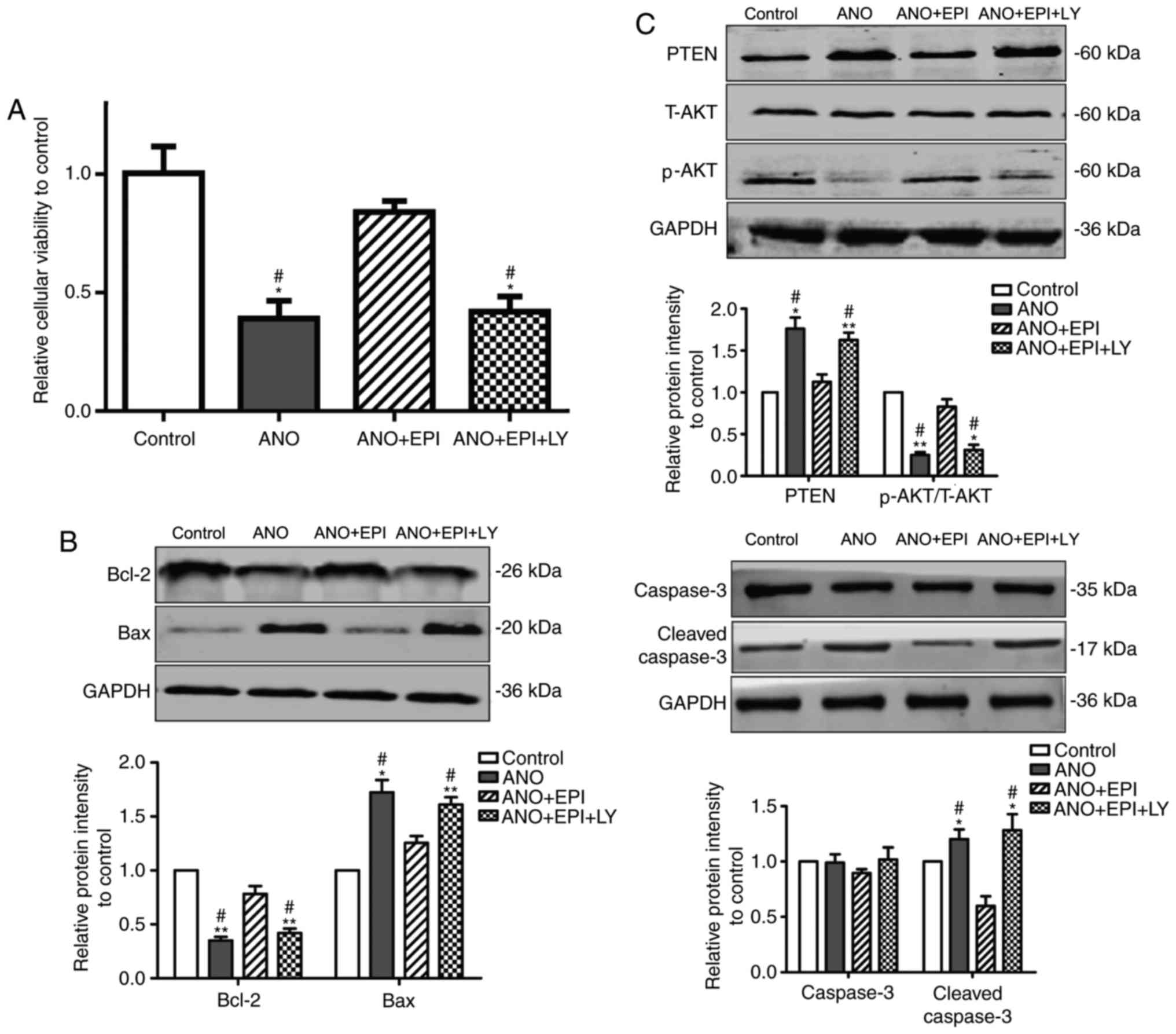 | Figure 5.EPI protects cardiomyocytes from
hypoxia-induced apoptosis, which is reversed by LY294002. (A) Cell
viability was detected by MTT. EPI increased cardiomyocyte
viability under hypoxia culture conditions, whereas LY294002
reversed this effect of EPI. (B) Western blot analysis for Bcl-2,
Bax, caspase-3 and cleaved caspase-3 in cardiomyocytes. EPI
upregulated the expression of Bcl-2 and downregulated the
expression levels of Bax-3 and cleaved caspase-3 in cardiomyocytes
cultured under hypoxia conditions, which were reversed by LY294002.
(C) Western blot analysis for PTEN, T-AKT and p-AKT. EPI
upregulated the expression ratio of P-AKT/T-AKT and downregulated
the expression of PTEN in cardiomyocytes cultured under anoxia
condition, which were reversed by LY294002. Data are expressed as
the mean ± standard error (n=6). *P<0.05 and **P<0.01 vs.
control; #P<0.05 vs. ANO+EPI. ANO, anoxia; EPI,
(−)-epicatechin; LY, LY294002; Bcl-2, B-cell lymphoma-2; Bax,
B-cell lymphoma-2-associated X protein; PTEN, phosphatase and
tensin homolog; T-AKT, total protein kinase B; p-AKT,
phosphorylated protein kinase B. |
Discussion
The present study demonstrated that EPI serves a
significant role in protecting against cardiac ischemia in
vivo and in vitro. In addition, the results revealed
that the cardiac protective effect of EPI was derived from its
anti-apoptotic ability and these effects were mediated through the
AKT-PTEN signaling pathway.
Myocardial ischemia is one of primary causes of
sudden death worldwide (25).
Although life science and medicine are improving, there is still no
effective method of prevention and treatment for myocardial
ischemia injury. Therefore, it is necessary to explore novel
therapeutics for this disease. For the past decade, microRNAs and
long non-coding (lnc)RNAs have been confirmed to exert significant
regulatory effect of myocardial ischemia (26). However, due to the limitation of
drug-delivery methods to expediently transport therapeutic
microRNAs or lncRNAs into patients' cardiomyocytes, therapy of
myocardial ischemia with microRNAs and lncRNAs are not effective
and widely used in clinical treatments. Therefore, exploring a
natural substance that may be easily applied is required for
treating myocardial ischemia.
The present study investigated the effects of EPI on
cardiac ischemia injury. It was observed that EPI suppressed mouse
myocardial hypertrophy and fibrosis induced by myocardial ischemia
in vivo. EPI is a common substance which is contained in
numerous daily foods and drinks. Therefore, it is worthy to explore
the anti-myocardial ischemia effects of EPI. Due to its
antioxidative effect, EPI has been demonstrated to inhibit cancer,
diabetes mellitus and insulin resistance (27–29).
In addition, EPI was predicted to decrease the possibility of
developing cardiovascular disease by increasing flow-mediated
dilation of endothelium and decreasing platelet aggregation
(30,31). Certain studies have demonstrated
that EPI is able to alleviate cardiomyocyte hypertrophy (11,32,33),
which is consistent with the results of the present study. The
protective effect of EPI on cardiomyocyte hypertrophy was shown to
occur via a reduction in oxidative stress and an improvement in
mitochondrial structure and function (8,9). The
present study revealed that EPI significantly decreased myocardial
ischemia-induced apoptosis of cardiomyocytes and increased levels
of cleaved caspase-3 and Bax which are considered to be apoptotic
molecular markers. Taken together, the in vivo results
indicated that EPI may prevent myocardial ischemia-induced cardiac
injury through inhibiting hypertrophy, fibrosis and apoptosis. This
hypothesis was further confirmed on NMCMs which were cultured under
hypoxic conditions to stimulate myocardial ischemia in
vitro.
Dysregulation of multiple signaling pathways has
been shown to be implicated in myocardial ischemia (34). As a primary intracellular signaling
pathway, the PI3K/AKT pathway modulates cell apoptosis,
proliferation, differentiation and autophagy under physiological
and pathological conditions. AKT serves an important role in
suppressing cellular apoptosis. It is able to decrease
pro-apoptotic Bad and Bax levels, but elevates anti-apoptotic Bcl-2
(35). Conversely, it diminishes
the release of p53, which promotes apoptosis in the presence of DNA
damage or other stress (36).
Therefore, the overall effect of the PI3K/AKT signaling pathway is
to promote cellular survival and to reduce apoptosis. As a major
negative regulator of the PI3K/AKT signaling pathway, PTEN is a key
molecule in the development of many cardiovascular diseases
especially in myocardial ischemia. This study demonstrated that the
administration of EPI suppressed the increase of PTEN induced by
myocardial ischemia and thus rescued the decrease of AKT signaling.
Therefore, it should be noted that the regulatory role of EPI on
the PTEN/AKT signaling pathway may at least in part to inhibit
cardiomyocyte apoptosis induced by myocardial ischemia.
In conclusion, the research of the present study
demonstrated the protective effect of EPI on mouse myocardial
ischemia in vivo and in vitro. The present work
revealed EPI as a novel anti-apoptotic reagent that attenuates
ischemic cardiac injury through targeting the PTEN/AKT signaling
pathway. These results indicated that EPI may be important for the
development of novel therapeutic strategies for protecting against
ischemic insults. Therefore, EPI may represent a novel approach for
cardiac ischemia disease prevention and treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL: Study concept and design, acquisition of data,
drafting of the manuscript, and statistical analysis; XW: Study
concept and design, analysis and interpretation of data, drafting
of the manuscript, and statistical analysis; XZ: Acquisition of
data, analysis and interpretation of data, and technical support;
LG and LW: Acquisition of data, and technical support; XY: Study
concept and design, critical revision of the manuscript for
important intellectual content, obtained funding, administrative,
and study supervision.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals set by the US National Institutes of Health (Bethesda, MD,
USA), which was approved by the Institutional Animal Care and Use
Committee of the Harbin Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steg PG, Greenlaw N, Tendera M, Tardif JC,
Ferrari R, Al-Zaibag M, Dorian P, Hu D, Shalnova S, Sokn FJ, et al:
Prevalence of anginal symptoms and myocardial ischemia and their
effect on clinical outcomes in outpatients with stable coronary
artery disease: Data from the international observational CLARIFY
registry. JAMA Intern Med. 174:1651–1659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng D, Zhu C, Cao J and Jiang W: The
protective effects of polyphenols from jujube peel (Ziziphus
Jujube Mill) on isoproterenol-induced myocardial ischemia and
aluminum-induced oxidative damage in rats. Food Chem Toxicol.
50:1302–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Misumida N, Kobayashi A, Saeed M, Fox JT
and Kanei Y: Electrocardiographic left ventricular hypertrophy as a
predictor for nonsignificant coronary artery disease in patients
with non-ST-segment elevation myocardial infarction. Angiology.
67:27–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung HK, Chan YK, Han M, Jahng JWS, Song
E, Danielson E, Berger T, Mak TW and Sweeney G: Lipocalin-2 (NGAL)
attenuates autophagy to exacerbate cardiac apoptosis induced by
myocardial ischemia. J Cell Physiol. 232:2125–2134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panza JA, Holly TA, Asch FM, She L,
Pellikka PA, Velazquez EJ, Lee KL, Borges-Neto S, Farsky PS, Jones
RH, et al: Inducible myocardial ischemia and outcomes in patients
with coronary artery disease and left ventricular dysfunction. J Am
Coll Cardiol. 61:1860–1870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel RD and Saver JL: Evolution of
reperfusion therapies for acute brain and acute myocardial
ischemia: A systematic, comparative analysis. Stroke. 44:94–98.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shay J, Elbaz HA, Lee I, Zielske SP, Malek
MH and Huttemann M: Molecular mechanisms and therapeutic effects of
(−)-epicatechin and other polyphenols in cancer, inflammation,
diabetes, and neurodegeneration. Oxidat Med Cell Longev.
2015:1812602015. View Article : Google Scholar
|
|
8
|
Quine SD and Raghu PS: Effects of
(−)-epicatechin, a flavonoid on lipid peroxidation and antioxidants
in streptozotocin-induced diabetic liver, kidney and heart.
Pharmacol Rep. 57:610–615. 2005.PubMed/NCBI
|
|
9
|
Yamazaki KG, Romero-Perez D,
Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, Ceballos G and
Villarreal F: Short- and long-term effects of (−)-epicatechin on
myocardial ischemia-reperfusion injury. Am J Physiol Heart Circulat
Physiol. 295:H761–H767. 2008. View Article : Google Scholar
|
|
10
|
Yamazaki KG, Taub PR, Barraza-Hidalgo M,
Rivas MM, Zambon AC, Ceballos G and Villarreal FJ: Effects of
(−)-epicatechin on myocardial infarct size and left ventricular
remodeling after permanent coronary occlusion. J Am Coll Cardiol.
55:2869–2876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheng R, Gu ZL and Xie ML:
Epigallocatechin gallate, the major component of polyphenols in
green tea, inhibits telomere attrition mediated cardiomyocyte
apoptosis in cardiac hypertrophy. Int J Cardiol. 162:199–209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki J, Ogawa M, Maejima Y, Isobe K,
Tanaka H, Sagesaka YM and Isobe M: Tea catechins attenuate chronic
ventricular remodeling after myocardial ischemia in rats. J Mol
Cell Cardiol. 42:432–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ke Z, Wang G, Yang L, Qiu H, Wu H, Du M,
Chen J, Song J, Jia X and Feng L: Crude terpene glycoside component
from Radix paeoniae rubra protects against isoproterenol-induced
myocardial ischemic injury via activation of the PI3K/AKT/mTOR
signaling pathway. J Ethnopharmacol. 206:160–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui G, Shan L, Hung M, Lei S, Choi I,
Zhang Z, Yu P, Hoi P, Wang Y and Lee SM: A novel Danshensu
derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways.
Int J Cardiol. 168:1349–1359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouyang ZH, Wang WJ, Yan YG, Wang B and Lv
GH: The PI3K/Akt pathway: A critical player in intervertebral disc
degeneration. Oncotarget. 8:57870–57881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Los Santos S, García-Pérez V,
Hernández-Reséndiz S, Palma-Flores C, González-Gutiérrez CJ,
Zazueta C, Canto P and Coral-Vázquez RM: (−)-Epicatechin induces
physiological cardiac growth by activation of the PI3K/Akt pathway
in mice. Mol Nutri Food Res. 61:2017.
|
|
18
|
Li C, Li X, Gao X, Zhang R, Zhang Y, Liang
H, Xu C, Du W, Zhang Y, Liu X, et al: MicroRNA-328 as a regulator
of cardiac hypertrophy. Int J Cardiol. 173:268–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Z, Sun X, Shan H, Wang N, Wang J, Ren
J, Feng S, Xie L, Lu C, Yuan Y, et al: MicroRNA-101 inhibited
postinfarct cardiac fibrosis and improved left ventricular
compliance via the FBJ osteosarcoma oncogene/transforming growth
factor-β1 pathway. Circulation. 126:840–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abbate A, Salloum FN, Van Tassell BW,
Vecile E, Toldo S, Seropian I, Mezzaroma E and Dobrina A:
Alterations in the interleukin-1/interleukin-1 receptor antagonist
balance modulate cardiac remodeling following myocardial infarction
in the mouse. PLoS One. 6:e279232011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Panneerselvam M, Tsutsumi YM, Bonds JA,
Horikawa YT, Saldana M, Dalton ND, Head BP, Patel PM, Roth DM and
Patel HH: Dark chocolate receptors: Epicatechin-induced cardiac
protection is dependent on delta-opioid receptor stimulation. Am J
Physiol Heart Circ Physiol. 299:H1604–H1609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu AZ, Loh SH, Cheng TH, Lu HH and Lin CI:
Antiarrhythmic effects of (−)-epicatechin-3-gallate, a novel sodium
channel agonist in cultured neonatal rat ventricular myocytes.
Biochem Pharmacol. 85:69–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng W, Hwang HS, Kryshtal DO, Yang T,
Padilla IT, Tiwary AK, Puschner B, Pessah IN and Knollmann BC:
Coordinated regulation of murine cardiomyocyte contractility by
nanomolar (−)-epigallocatechin-3-gallate, the major green tea
catechin. Mol Pharmacol. 82:993–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddall HK, Warrell CE, Yellon DM and
Mocanu MM: Ischemia-reperfusion injury and cardioprotection:
Investigating PTEN, the phosphatase that negatively regulates PI3K,
using a congenital model of PTEN haploinsufficiency. Basic Res
Cardiol. 103:560–568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ounzain S, Crippa S and Pedrazzini T:
Small and long non-coding RNAs in cardiac homeostasis and
regeneration. Biochim Biophys Acta. 1833:923–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y,
Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, et al: MicroRNA-103/107
regulate programmed necrosis and myocardial ischemia/reperfusion
injury through targeting FADD. Circ Res. 117:352–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rein D, Lotito S, Holt RR, Keen CL,
Schmitz HH and Fraga CG: Epicatechin in human plasma: In vivo
determination and effect of chocolate consumption on plasma
oxidation status. J Nutri. 130 8S Suppl:S2109–S2114. 2000.
View Article : Google Scholar
|
|
28
|
Del Rio D, Rodriguez-Mateos A, Spencer JP,
Tognolini M, Borges G and Crozier A: Dietary (poly)phenolics in
human health: Structures, bioavailability, and evidence of
protective effects against chronic diseases. Antioxidants Redox
Signal. 18:1818–1892. 2013. View Article : Google Scholar
|
|
29
|
Cremonini E, Bettaieb A, Haj FG, Fraga CG
and Oteiza PI: (−)-Epicatechin improves insulin sensitivity in high
fat diet-fed mice. Arch Biochem Biophys. 599:13–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drouin A, Bolduc V, Thorin-Trescases N,
Bélanger É, Fernandes P, Baraghis E, Lesage F, Gillis MA,
Villeneuve L, Hamel E, et al: Catechin treatment improves
cerebrovascular flow-mediated dilation and learning abilities in
atherosclerotic mice. Am J Physiol Heart Circ Physiol.
300:H1032–H1043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lotito SB and Frei B: Consumption of
flavonoid-rich foods and increased plasma antioxidant capacity in
humans: Cause, consequence, or epiphenomenon? Free Radic Biol Med.
41:1727–1746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen DD, Dong YG, Liu D and He JG:
Epigallocatechin-3-gallate attenuates cardiac hypertrophy in
hypertensive rats in part by modulation of mitogen-activated
protein kinase signals. Clin Exp Pharmacol Physiol. 36:925–932.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hao J, Kim CH, Ha TS and Ahn HY:
Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by
pressure overload in rats. J Vet Sci. 8:121–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lau E: Complex disease: Piecing together
the puzzle of coronary artery disease. Nat Rev Genet. 15:572–573.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Atif F, Yousuf S and Stein DG: Anti-tumor
effects of progesterone in human glioblastoma multiforme: Role of
PI3K/Akt/mTOR signaling. J Steroid Biochem Mol Biol. 146:62–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji H, Ding Z, Hawke D, Xing D, Jiang BH,
Mills GB and Lu Z: AKT-dependent phosphorylation of Niban regulates
nucleophosmin- and MDM2-mediated p53 stability and cell apoptosis.
EMBO Rep. 13:554–560. 2012. View Article : Google Scholar : PubMed/NCBI
|