Introduction
Osteoporosis is among the most common health
problems in the elderly (1).
Normal calcium homeostasis and supplementation are associated with
good bone health (2). However, it
has been demonstrated that calcium supplementation may cause a
variety of adverse conditions, including cardiovascular disease,
constipation and kidney stone development (3,4).
Therefore, the discovery of novel osteoporosis therapies is of high
importance.
Cellular apoptosis may be induced by a number of
factors (5,6). Among the most important factors is
oxidative stress (7). Oxidative
stress has been confirmed to be associated with osteonecrosis
(8,9). Hydrogen peroxide
(H2O2) is a strong oxidizer which is able to
induce cells to produce large amounts of reactive oxygen species
(ROS) (10). ROS are produced in
mitochondria as normal products of cellular metabolism. Elevated
ROS is related to oxidative stress, causing cellular dysfunction
and apoptosis (11,12). ROS may damage double-stranded DNA,
leading to abnormal variation (13,14)
and may additionally induce oxidative stress-induced apoptosis in
tissues (15,16). H2O2 is a key
metabolite of oxidation reactions and, therefore, has critical
involvement in the pathology of diseases mediated by oxidative
stress (15,16). Accumulating evidence has indicated
that H2O2 may regulate cell function and
induce cell death (17).
H2O2 may additionally penetrate the cell
membrane and act as a secondary messenger in signal transduction
pathways (18). A previous study
indicated that the manner of H2O2-induced
cell death is associated with cell type, H2O2
concentration and the type of stimulation received (19).
Parthenolide is a traditional medicine with
immunomodulatory effects (20). It
has additionally been demonstrated to have the potential to treat
certain types of cancer, including leukemia, and hepatic and breast
cancer (21–24). Parthenolide is able to regulate
multiple cellular and molecular signals in order to induce tumor
cell apoptosis (25). It may
additionally regulate the expression of the apoptosis regulator
Bcl-2 (Bcl-2) protein family and caspases (25), and promote the loss of
mitochondrial function (26). This
evidence highlights the potential use of parthenolide in tumor
therapy.
The specific effects of parthenolide on osteoblasts
are not completely understood. Therefore, the purpose of the
present study was to investigate the effect of parthenolide on
osteoblast proliferation and apoptosis. First, the effects of
parthenolide on osteoblast viability and
H2O2-induced apoptosis were demonstrated. The
influence of parthenolide on the expression of ROS, malondialdehyde
(MDA), lactate dehydrogenase (LDH), alkaline phosphatase (ALP),
superoxide dismutase (SOD) and glutathione peroxidase (GPX) in
H2O2-inducedosteoblastswas additionally
investigated. The regulation of oxidative stress-associated and
osteogenesis-associated gene expression by parthenolide was
subsequently analyzed. The results of the present study indicated
that parthenolide may be used as a therapy for osteoporosis.
Materials and methods
Osteoblast sample
Osteoblasts were acquired from an 8-year-oldpatient
with developmental dislocation of the hip. The operation was
performed in department of orthopedics, the Fifth People's Hospital
of Yuhang District, Hangzhou, Zhejiang in August, 2016. Informed
consent was obtained from the patient and the patient's guardian.
The present study was approved by The Ethics Committee of The Fifth
People's Hospital of Yuhang District (Hangzhou, China). During the
surgery, ~1.5 cm2 of the cancellous bone of the iliac
crest was removed and placed in Dulbecco's modified Eagle's
medium/F12 (DMEM/F12; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 15% newborn calf serum (Thermo Fisher Scientific, Inc.)
in sterile conditions.
Separation and cultivation of
osteoblasts
The bone surface-attached connective tissues were
eliminated and repeatedly washed with PBS. The bone tissue was cut
(1 mm3) and washed with PBS. The bone tissue was
subsequently digested with 0.25% trypsin (Thermo Fisher Scientific,
Inc.) for 30 min at 37°C, and 0.1% collagenase type II (Thermo
Fisher Scientific, Inc.) for 1 h at 37°C. The supernatant was
collected and centrifuged at 1,000 × g for 10 min at 4°C. Cell
deposits were placed in DMEM/F12 (Thermo Fisher Scientific, Inc.)
with 15% newborn calf serum (Thermo Fisher Scientific, Inc.).
Cell treatment
Osteoblasts at a density of 10,000 cells were
treated with PBS (control) and increasing concentrations of
parthenolide (0, 5, 10, 15, 20, 25 and 30 µM) for 24 and 48 h.
Osteoblasts were seeded into 6-well plates at a concentration of
3×105 cells/ml and treated with PBS, 0.8 mmol/l
H2O2 or 0.8 mmol/l H2O2
and parthenolide (5, 10 and 20 µM) for 24 h.
ALP staining
Osteoblasts (50,000 cells/well) were seeded in
24-well plates and induced in a humidified incubator for 24 h at
37°C. ALP activity was detected using the ALP staining kit (Merck
KGaA, Darmstadt, Germany), according to the manufacturer's
protocol. Osteoblasts were observed under a Wild Heerbrugg M400
Zoom Makroskop (Wild Heerbrugg, Heerbrugg, Switzerland) at ×200
magnification.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was obtained using TRIzol (Thermo Fisher
Scientific, Inc.), and RNA was reverse-transcribed to cDNA using a
Revert Aid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) with random primers, according to the
manufacturer's protocol. SYBR-Green PCR Master Mix kit (Takara
Biotechnology Co., Ltd., Dalian, China) was used to detect the mRNA
expression levels, and the assay was performed on an ABI 7500
system (Thermo Fisher Scientific, Inc.). Thermocycling conditions
were: 95°C for 15 sec; 40 cycles at 95°C for 10 sec, 59°C for 10
sec and 72°C for 15 sec. The relative mRNA expression levels were
normalized to GAPDH. The data were analyzed using the
2−ΔΔCt method (27).
The primer sequences were as follows: Bcl-2 forward,
5′-GCCTTCTTTGAGTTCGGTGG-3′ and reverse, 5′-GAAATCAAACAGAGGCCGCA-3′;
apoptosis regulator BAX (Bax) forward, 5′-GAGCTGCAGAGGATGATTGC-3′
and reverse, 5′-CCAATGTCCAGCCCATGATG-3′; runt related transcription
factor 2 (Runx2) forward, 5′-CTGTGGTTACTGTCATGGCG-3′ and reverse,
5′-AGGTAGCTACTTGGGGAGGA-3′; osteopontin (OPN) forward,
5′-ACTGATTTTCCCACGGACCT-3′ and reverse, 5′-CTCCTCGCTTTCCATGTGTG-3′;
osteocalcin (OCN) forward, 5′-CTCACACTCCTCGCCCTATT-3′ and reverse,
5′-AACTCGTCACAGTCCGGATT-3′; collagen 1 (Col-1) forward,
5′-TCATTCCGCAAACCCACTTG-3′ and reverse, 5′-CCCCAATCGAGAAGCCATTG-3′;
nuclear factor erythroid 2 like 2 (Nrf2) forward,
5′-GGTTGCCCACATTCCCAAAT-3′ and reverse, 5′-AGCAATGAAGACTGGGCTCT-3′;
heme oxygenase 1 (HO1) forward, 5′-TTCAGAAGGGTCAGGTGTCC-3′ and
reverse, 5′-CAGTGAGGCCCATACCAGAA-3′; NAD (P) H quinone
oxidoreductase 1 (NQO1) forward, 5′-CCTTCCGGAGTAAGAAGGCA-3′ and
reverse, 5′-TCTCCAGGCGTTTCTTCCAT-3′; and GAPDH forward,
5′-GCCATCACAGCAACACAGAA-3′ and reverse,
5′-GCCATACCAGTAAGCTTGCC-3′.
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) with a protease and phosphatase
inhibitor cocktail (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The concentration was measured using the Pierce Bicinchoninic
Acid Protein Assay kit (Thermo Fisher Scientific, Inc.) and
detected using a protein reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Proteins (30 µg) were separated on a 10%
SDS-PAGE gel and transferred to polyvinylidene difluoride membranes
(PerkinElmer Inc., Waltham, MA, USA). The membranes were blocked
with 5% low-fat milk (BD Biosciences, Franklin Lakes, NJ, USA) for
2 h at room temperature and subsequently incubated with the
following primary antibodies overnight at 4°C: Anti-GAPDH (1:2,000;
cat. no. ab8245; Abcam, Cambridge, UK), anti-Bax (1:1,000; cat. no.
ab32503; Abcam), anti-Bcl-2 (1:1,000; cat. no. ab32124; Abcam),
anti-Runx2 (1:1,500; cat. no. ab23981; Abcam), anti-OPN (1:1,500;
cat. no. ab166709; Abcam), anti-OCN (1:1,500; cat. no. ab93876;
Abcam), anti-Col-1 (1:1,000; cat. no. ab6308; Abcam), anti-Nrf2
(1:1,000; cat. no. ab62352; Abcam), anti-HO1 (1:1,200; cat. no.
ab69544; Abcam) and anti-NQO1 (1:1,000; cat. no. ab28947; Abcam).
The membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody (1:1,000; cat. no. ab97165;
Abcam) for 2 h at room temperature. The protein expression levels
were detected using the enhanced chemiluminescence substrate kit
(Thermo Fisher Scientific, Inc.) and the enhanced chemiluminescence
detection system (GE Healthcare, Chicago, IL, USA). The
densitometric analysis was performed by Labworks Software (version
4.5; UVP, Inc., Upland CA, USA).
ROS, SOD, MDA, GPX and LDH activity
detection
Osteoblasts were treated with PBS, 0.8 mmol/l
H2O2 or 0.8 mmol/l H2O2
and parthenolide (5, 10 and20 µM) for 12, 24 and 48 h. The
fluorescent dye dihydroethidium (DHE; cat. 309800; Merck KGaA,
Darmstadt, Germany) was used to measure ROS activity, as described
previously (28). Treated cells
were incubate d with 2.5 mmol/l DHE for 25 min at 37°C. Cells were
subsequently washed with PBS, digested with 0.25% trypsin
(Sigma-Aldrich; Merck KGaA) and incubated with DHE. The results
were detected by using flow cytometry equipped with an argon laser
and Cell Quest™ software (version 3.3; BD Biosciences, Franklin
Lakes, NJ, USA). The experiment was repeated at least three times.
SOD and MDA activity was detected using a commercial kit
(Sigma-Aldrich; Merck KGaA) (29)
and a MDA activity assay kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China), respectively, according to the
manufacturers' protocols. LDH activity was detected using a
diagnostic kit (cat. no. GL2623; Randox Laboratories Ltd., Crumlin,
UK), according to the method of Cabaud and Wroblewski (30). GPX enzyme activity was detected
using the cellular GPX assay kit (Sigma-Aldrich; Merck KGaA) as
described previously (31). The
colorimetric reactions of LDH and GPX were determined using a
microplate spectrophotometer system (BioRad-680, Bio-Rad
Laboratories, Inc.) at 440 and 412 nm, respectively.
ELISA analysis
Osteoblasts were treated with PBS (control), 0.8
mmol/l H2O2 or 0.8 mmol/l
H2O2 and parthenolide (5, 10 and 20 µM) for
12, 24 and 48 h. Treated osteoblasts were subsequently washed with
PBS and centrifuged at 1,000 × g for 5 min at 4°C. The culture
supernatant (500 µl) was harvested and ALP activity was measured
using an ELISA kit (cat. AR001, R&D Systems, Minneapolis, MN,
USA), according to the manufacturer's protocol. A 96-well plate was
incubated with anti-ALP (1:1,000, cat. no. ab83259; Abcam) for 1 h
at 37°Cand subsequently incubated with HRP-conjugated secondary
antibody (1:5,000; cat. no. ab97165; Abcam) for 1 h at 37°C. The
absorbance was measured at 450 nm using a Multiskan GO microplate
spectrophotometer (Thermo Fisher Scientific, Inc.).
Cell viability assay
Treated osteoblasts (2×103 cells/well)
were seeded into a 96-well plate and maintained at 37°C for 12, 24
and 48 h. At the indicated time-points (12, 24 and 48 h), 15 µl MTT
solution (5 mg/ml) was added into each well, respectively.
Following incubation for 4 h, cells were treated with 150 µl
dimethylsulfoxide. The absorbance was measured using a microplate
reader at 490 nm.
Flow cytometer analysis
Osteoblasts (1×106 cells/well) were
seeded into 6-well plates and treated with 0.8 mmol/l
H2O2 or 0.8 mmol/l H2O2
and parthenolide (5, 10 and 20 µM) for 24 h. Treated cells were
washed with PBS and centrifuged at 1,000 × g for 5mins at 4°C. The
precipitate was re-suspended in0.5 ml binding buffer, including 5
µl annexin V-fluoresce in isothiocyanate and propidium iodide (BD
Biosciences) for 20 min in the dark. The apoptotic rate was
examined using a FACSCalibur flow cytometer (BD Biosciences) with
CXP Analysis software version 2.2 (Beckman Coulter, Brea, CA, USA).
Cells in the lower left quadrant of each picture correspond to
normal cells (Annexin V/PI). Cells in right lower quadrant
correspond to early apoptotic cells (Annexin V+/PI-). Cells in the
right upper quadrant correspond to late apoptotic/dead cells
(Annexin V+/PI+).
Statistical analysis
Results were analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA), and the statistical significance was calculated
using Student's paired t-test and one-way and/or
multiple-comparison analysis of variance followed by Tukey's test.
All data are presented as the mean ± standard deviation of three
repeated experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of parthenolide on osteoblast
viability
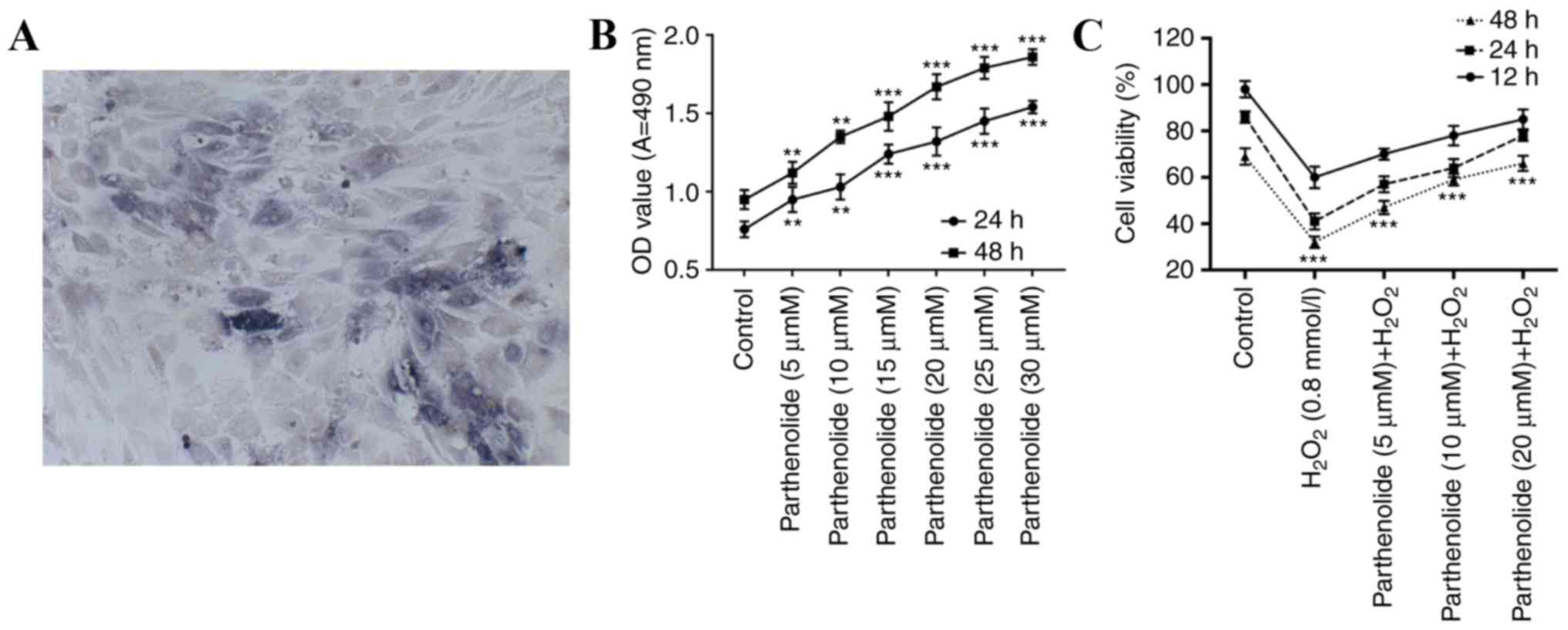
Under the microscope, control osteoblasts were
observed to be triangular, polygonal or irregular. The nuclei were
elliptical with one or two nucleoli and black cytoplasmic particles
were present. The dark nuclei in the cells were positive for ALP
(Fig. 1A). Osteoblasts were
treated with different concentrations of parthenolide (0, 5, 10,
15, 20, 25 and 30 µM) for 24 and 48 h. MTT assays were performed to
examine osteoblast viability. The results indicated that
parthenolide significantly improved osteoblast viability in a dose-
and time-dependent manner (**P<0.01; ***P<0.001; Fig. 1B).
Effect of parthenolide on
H2O2-induced osteoblasts
To examine the possible biological functions of
parthenolide and H2O2 in osteoblasts,
osteoblasts were treated with 0.8 mmol/l H2O2
or 0.8 mmol/l H2O2 and parthenolide (5, 10
and 20 µM) for 12, 24 and 48 h. An MTT assay was performed to
examine osteoblast viability in a H2O2
environment. Results indicated that H2O2
significantly decreased osteoblast viability, and this decrease was
rescued by parthenolide in a dose- and time-dependent manner
(***P<0.001, Fig. 1C).
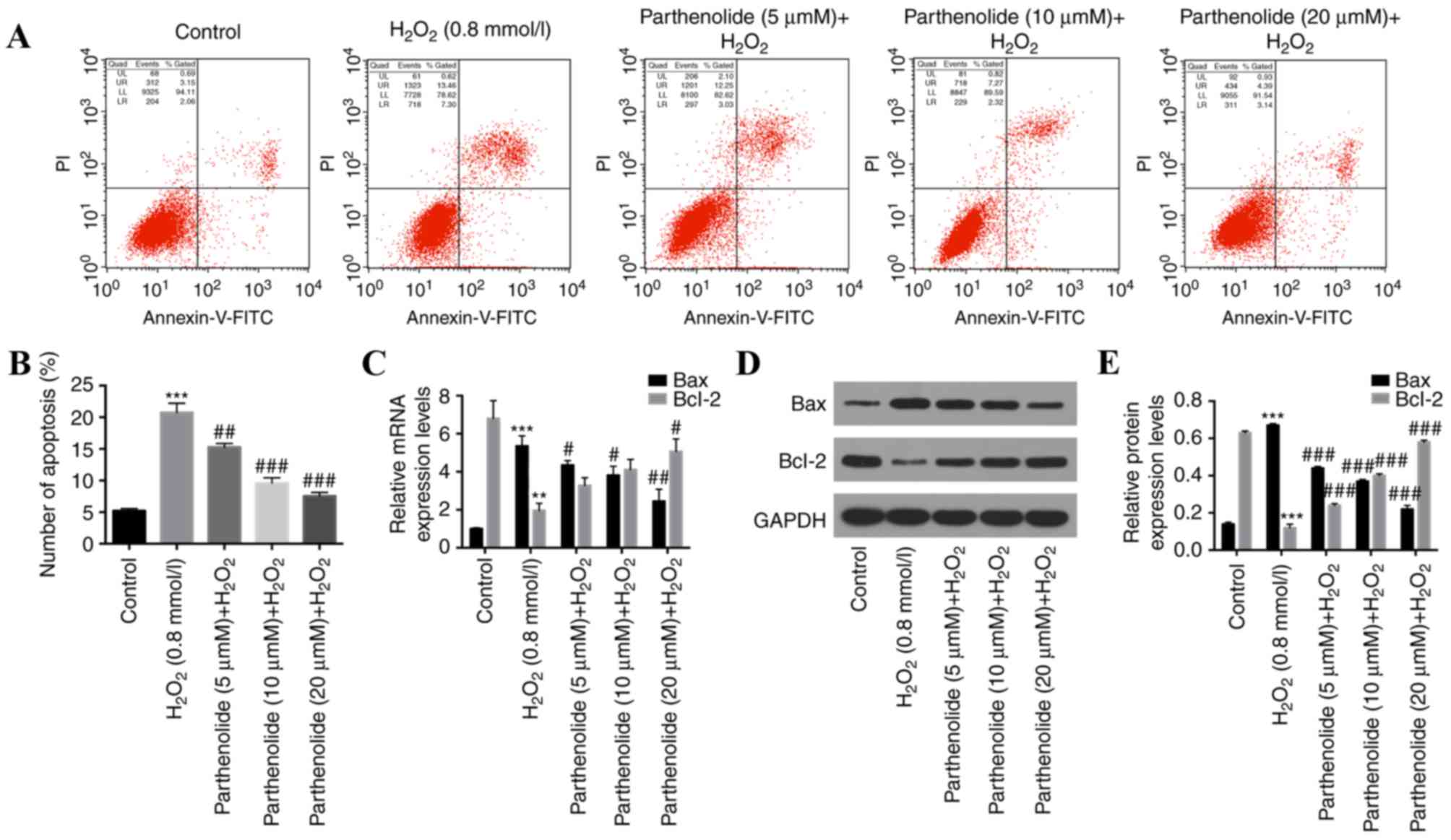
Parthenolide inhibits apoptosis in
H2O2-induced osteoblasts
To further investigate the effect of parthenolide on
H2O2-induced osteoblast apoptosis, flow
cytometry was performed. H2O2 significantly
increased the apoptotic rate in osteoblasts, and parthenolide
significantly decreased the H2O2-induced
increase in osteoblast apoptosis (***P<0.001 vs. control group;
##P<0.01, ###P<0.001 vs.
H2O2 group; Fig.
2A and B). To further understand the molecular mechanism of the
function of parthenolide, RT-qPCR and western blot analyses were
used to analyze Bax and Bcl-2 expression. The results revealed that
H2O2 significantly increased the expression
of Bax, and significantly decreased the expression of Bcl-2.
Parthenolide significantly reversed the alterations observed in
H2O2-induced osteoblasts (***P<0.001 vs.
control group, ###P<0.001 vs.
H2O2 group, Fig.
2C-E).
Parthenolide decreases the levels of
ROS, MDA, LDH and ALP, and increases SOD and GPX levels in
H2O2-inducedosteoblasts
Cellular apoptosis and proliferation are two
important events affected by oxidative stress (32–35).
Numerous studies have demonstrated that ROS levels are closely
associated with the induction of apoptosis in a number of
pathophysiological conditions (36,37).
Oxidative stress may cause an imbalance between ROS and activity of
antioxidative enzymes, including SOD and GPX (38). The influence of parthenolide and
H2O2 on serum marker enzymes MDA, LDH and ALP
was additionally investigated (39). Osteoblasts were treated with PBS,
0.8 mmol/l H2O2 or 0.8 mmol/l
H2O2 and parthenolide (5, 10 and 20 µM) for
24 h. The results indicated that H2O2
significantly increased ROS (Fig.
3A), MDA (Fig. 3C), LDH
(Fig. 3D) and ALP (Fig. 3F) levels. SOD (Fig. 3B) and GPX (Fig. 3E) levels were significantly
decreased. Parthenolide significantly reversed the alterations
observed in H2O2-induced osteoblasts
(***P<0.001 vs. control group; #P<0.05,
##P<0.01, ###P<0.001 vs.
H2O2 group).
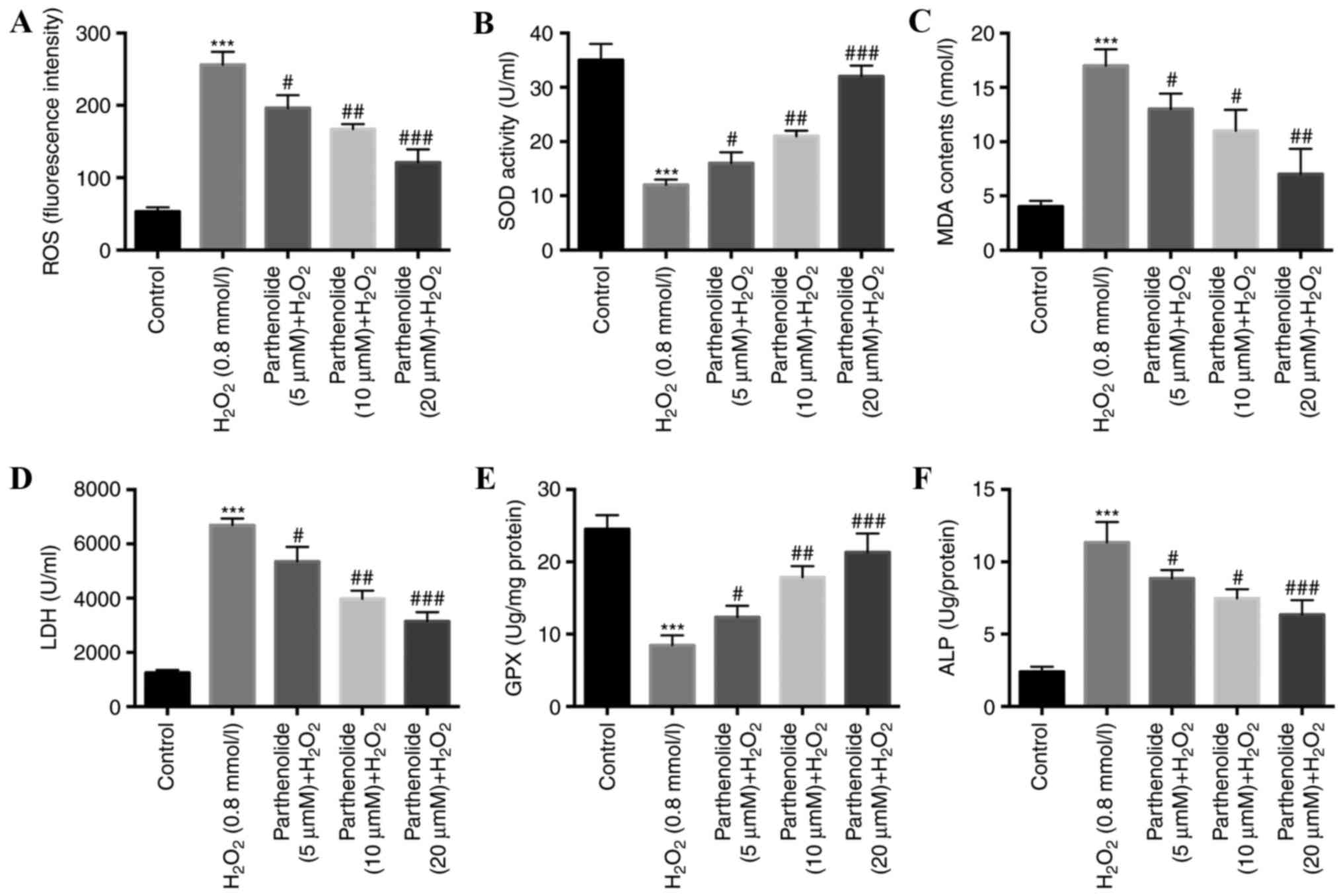 | Figure 3.Parthenolide decreased the levels of
ROS, MDA, LDH and ALP, and increased the levels of SOD and GPX in
H2O2-induced osteoblasts. Osteoblasts were
treated with 0.8 mmol/l H2O2 or 0.8 mmol/l
H2O2 and parthenolide at increasing
concentrations for 24 h (A) ROS fluorescence intensity was detected
by flow cytometry with a fluorescence dye DHE. (B) SOD activity was
determined with a colorimetric assay. (C) MDA activity was
determined with a colorimetric assay. (D) LDH activity was
determined with a cytotoxicity assay. (E) GPX activity was
determined with a glutathione peroxidase cellular activity assay.
(F) ALP activity was determined with an ELISA kit. ***P<0.001
vs. control; #P<0.05, ##P<0.01,
###P<0.001 vs. H2O2 group. ROS,
reactive oxygen species; MDA, malondialdehyde; LDH, lactate
dehydrogenase; ALP, alkaline phosphatase; SOD, superoxide
dismutase; GPX, glutathione peroxidase. |
Parthenolide upregulates
osteogenesis-associated genes (Runx2, OPN, OCN, and Col-1) in
osteoblasts, mediated by H2O2
It has been reported that Runx2, OPN, OCN, and Col-1
are key genes associated with osteogenesis (40). Therefore, the impact of
parthenolide and H2O2 on the expression of
these genes in osteoblasts was investigated. RT-qPCR and western
blot analyses were performed to detect Runx2, OPN, OCN and Col-1
expression in treated osteoblasts. H2O2 was
demonstrated to significantly inhibit Runx2, OPN, OCN, and Col-1
expression. Parthenolide rescued this
H2O2-mediated inhibition (***P<0.001 vs.
control group; #P<0.05, ##P<0.01,
###P<0.001 vs. H2O2 group;
Fig. 4).
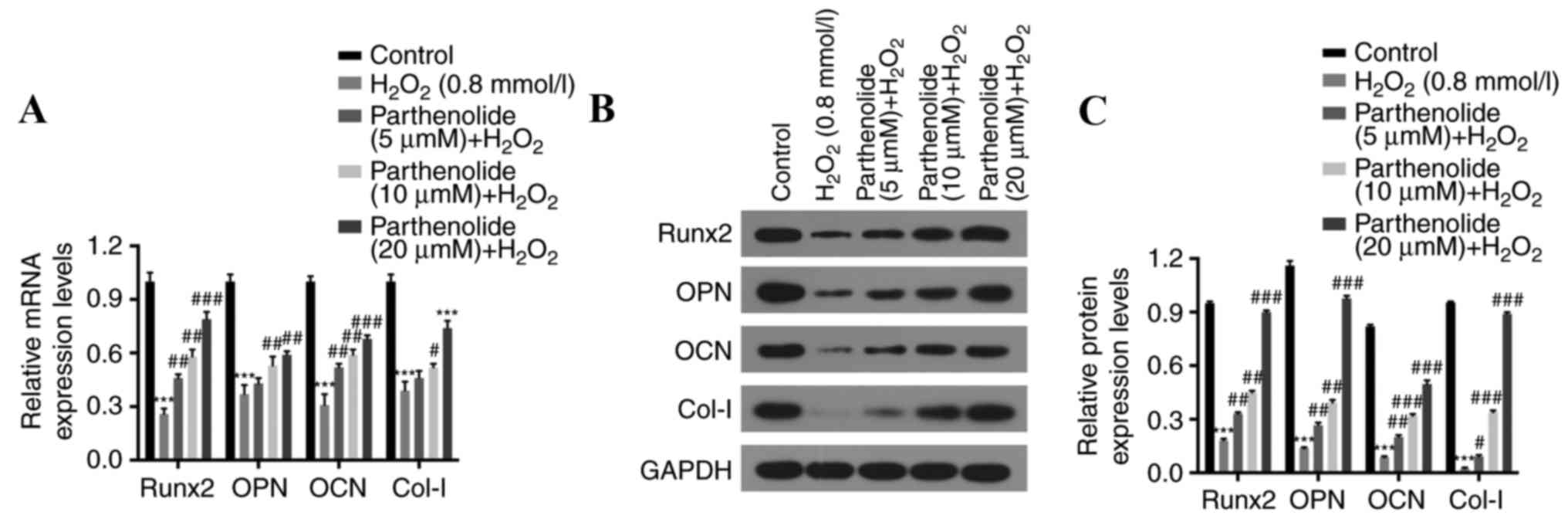 | Figure 4.Parthenolide upregulates the
osteogenesis-associated genes Runx2, OPN, OCN and Col-1 in
H2O2-induced osteoblasts. Osteoblasts were
treated with 0.8 mmol/l H2O2 or 0.8 mmol/l
H2O2 and increasing concentrations of
parthenolide for 24 h. (A) Reverse transcription
quantitative-polymerase chain reaction analysis was used to analyze
Runx2, OPN, OCN, and Col-1 mRNA expression levels. (B) Protein
expression levels were detected by western blot analysis and (C)
the relative quantification of proteins was calculated.
***P<0.001 vs. control group; #P<0.05,
##P<0.01, ###P<0.001 vs.
H2O2 group. Runx2, runt related transcription
factor 2; OPN, osteopontin; OCN, osteocalcin; Col-1, collagen
1. |
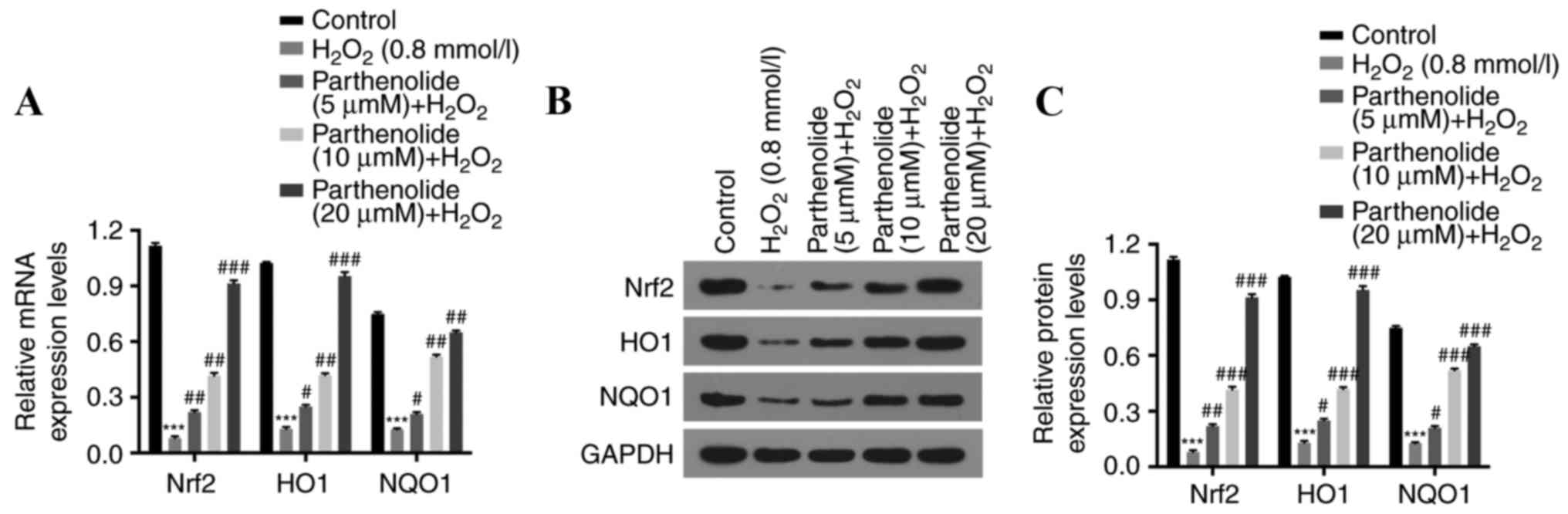
Parthenolide increases Nrf2, HO-1 and
NQO1 expression in osteoblasts, mediated by
H2O2
The expression of Nrf2, HO-1 and NQO1 was measured
by RT-qPCR and western blot analyses, to further elucidate the
molecular mechanisms of action of parthenolide and
H2O2 in osteoblasts.
H2O2 was demonstrated to significantly
decrease the expression of Nrf2, HO-1 and NQO1. Parthenolide
reversed this decrease (***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. H2O2 group;
Fig. 5).
Discussion
Large-scale and multi-directional osteoporosis
research has made significant progress worldwide. The study of the
effect of Chinese medicine in the process of bone collagen
formation remains in its infancy, particularly in regards to the
underlying molecular mechanisms (41). Tanacetum vulgare is an herbage used
as a traditional Chinese medicine for its analgesic, antibacterial
and anti-tumor properties; it is used to treat fever, migraine and
joint pain (42). Tanacetum
vulgare contains a variety of medicinal ingredients, including
parthenolide, which is an active component of the sesquiterpene
lactones (43). Recent studies
have demonstrated that parthenolide has strong anti-tumor activity
and may enhance the sensitivity of tumor cells to apoptosis
signaling (44). The molecular
mechanism of action of parthenolide in osteoporosis is unclear.
Oxidative stress is an inevitable condition in all
organisms. A series of adaptive mechanisms exist to protect cells
from damage, although various harmful stimuli may cause an
imbalance in the equilibrium of oxidation, leading to the induction
of apoptosis and even pathological damage (45). Oxidative stress may induce
apoptosis through the mitochondrial, death receptor and endoplasmic
reticulum stress pathways (46),
in addition to through the activation of the protein kinase,
nuclear factor-κB and caspase pathways (47). It has been demonstrated that
oxidative stress may be a risk factor associated with the
development and progression of osteoporosis (48). Increasing evidence additionally
suggests that ROS accumulation leads to oxidative stress under
conditions of aging and certain illnesses or medicines,
contributing to the development and progression of osteoporosis
(49).
The present study demonstrated that parthenolide
inhibited the decrease in osteoblast viability and the increase in
apoptosis mediated by H2O2. Parthenolide
additionally decreased Bax expression and increased Bcl-2
expression in osteoblasts induced by H2O2.
The expression of osteogenesis-associated genes Runx2, OPN, OCN and
Col-1 was increased in H2O2-induced
osteoblasts. Additionally, previous studies (38,50)
revealed that oxidative stress affects the activity of the
antioxidant enzymes SOD and GPX and serum marker enzymes MDA, LDH
and ALP (38,50). The present study demonstrated that
parthenolide decreased the H2O2-induced
increase in ROS, MDA, LDH, and ALP levels. SOD and GPX levels were
also rescued in H2O2-induced osteoblasts.
A previous study indicated that parthenolide, a
sesquiterpene lactone obtained from Tanacetum pathenium, has high
antineoplastic activity (44).
Parthenolide has been demonstrated to inhibit the growth and induce
the death in vitro of numerous tumor cell types, including
liver cancer, cholangiocarcinoma and multiple myeloma (44,51–53).
Furthermore, parthenolide has been demonstrated to enhance the
sensitivity of cancer cells to therapy, including liver cancer cell
sensitivity to cisplatin (54,55).
The present study revealed that H2O2
increased Bax expression and decreased Bcl-2 expression.
Parthenolide significantly prevented these
H2O2-mediated alterations in osteoblasts. A
direct interaction may exist between parthenolide and
H2O2 in the culture medium, and parthenolide
may inhibit H2O2-induced apoptosis in
osteoblasts.
Nrf2 is a transcription factor that regulates the
intracellular metabolism of endogenous and exogenous substances in
the oxidative stress response. Nrf2 binds to the antioxidant
response element (1) and
up-regulates ARE-associated antioxidative genes, including
endogenous antioxidants, phase II detoxification enzymes and
transcripts encoding intracellular genes that determine cell
survival or death (56). The
sustained expression of Nrf2 has important functions in restoring
cellular homeostasis (57). A
number of natural or synthetic small molecule compounds have been
confirmed to activate the Nrf2/ARE signaling pathway, resulting in
protection of cells from toxic or carcinogenic material-induced
cell damage (58). Nrf2/ARE
pathway activation results in the expression of cytoprotective
enzymes, including NQO1 and HO-1 (58). Therefore, Nrf2 has an essential
role in protection against oxidative stress. The results of the
present study indicated that parthenolide increased Nrf2, HO-1 and
NQO1 expression in H2O2-induced osteoblasts.
Therefore, parthenolide may suppress osteoblast apoptosis by
reducing oxidative stress.
In conclusion, the results of the present study
confirmed that parthenolide increased viability and inhibited
apoptosis in H2O2-induced osteoblasts. The
decrease in apoptosis mediated by parthenolide may be via a
reduction in oxidative stress. Additionally, parthenolide was
demonstrated to increase the expression of the
osteogenesis-associated genes Runx2, OPN, OCN and Col-1 in
H2O2-induced osteoblasts. Therefore,
parthenolide may have potential as a therapeutic drug for
osteoporosis. Future studies are required to further validate the
key findings of the present study. The effects of parthenolide may
be verified in vivo in osteogenic tissues and a rat model
under oxidative stress, and additional cell lines may be used to
validate the important conclusions of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
WM wrote the main manuscript. WM and ZZ performed
the experiments. WM and ZZ designed the study. ZZ performed data
analysis. WM and ZZ contributed to manuscript revisions and all
authors reviewed the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the fifth People's Hospital of Yuhang District.
Consent for publication
Informed consent was approved by both the 8-year-old
patient and his guardian.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marengoni A, Rizzuto D, Wang HX, Winblad B
and Fratiglioni L: Patterns of chronic multimorbidity in the
elderly population. J Am Geriatr Soc. 57:225–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet G, Dermauw V and Bouillon R:
Vitamin D signaling in calcium and bone homeostasis: A delicate
balance. Best Pract Res Clin Endocrinol Metab. 29:621–631. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reid IR, Bristow SM and Bolland MJ:
Calcium supplements: Benefits and risks. J Int Med. 278:354–368.
2015. View Article : Google Scholar
|
|
4
|
Ströhle A, Hadji P and Hahn A: Calcium and
bone health-goodbye, calcium supplements? Climacteric. 18:702–714.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davidovich P, Kearney CJ and Martin SJ:
Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol
Chem. 395:1163–1171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamogashira T, Fujimoto C and Yamasoba T:
Reactive oxygen species, apoptosis and mitochondrial dysfunction in
hearing loss. Biomed Res Int. 2015:6172072015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baek KH, Oh KW, Lee WY, Lee SS, Kim MK,
Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, et al: Association of
oxidative stress with postmenopausal osteoporosis and the effects
of hydrogen peroxide on osteoclast formation in human bone marrow
cell cultures. Calcif Tissue Int. 87:226–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackinnon ES, Rao AV and Rao LG: Dietary
restriction of lycopene for a period of one month resulted in
significantly increased biomarkers of oxidative stress and bone
resorption in postmenopausal women. J Nutr Health Aging.
15:133–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Callaway DA and Jiang JX: Reactive oxygen
species and oxidative stress in osteoclastogenesis, skeletal aging
and bone diseases. J Bone Miner Metab. 33:359–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamada Y, Fujii H and Fukagawa M: Role of
oxidative stress in diabetic bone disorder. Bone. 45 Suppl
1:S35–S38. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vela L, Contel M, Palomera L, Azaceta G
and Marzo I: Iminophosphorane-organogold (III) complexes induce
cell death through mitochondrial ROS production. J Inorg Biochem.
105:1306–1313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Amico E, Factor-Litvak P, Santella RM
and Mitsumoto H: Clinical perspective on oxidative stress in
sporadic amyotrophic lateral sclerosis. Free Radic Biol Med.
65:509–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling F, Niu R, Hatakeyama H, Goto Y,
Shibata T and Yoshida M: Reactive oxygen species stimulate
mitochondrial allele segregation toward homoplasmy in human cells.
Mol Biol Cell. 27:1684–1693. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majid AS, Yin ZQ and Ji D: Sulphur
antioxidants inhibit oxidative stress induced retinal ganglion cell
death by scavenging reactive oxygen species but influence nuclear
factor (erythroid-derived 2)-like 2 signalling pathway differently.
Biol Pharm Bull. 36:1095–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romiszewska A and Nowak-Stępniowska A:
Photodynamic reaction and oxidative stress-influence of the
photodynamic effect on the activity antioxidant enzymes. Postepy
Biochemii. 60:355–364. 2014.PubMed/NCBI
|
|
17
|
She C, Zhu LQ, Zhen YF, Wang XD and Dong
QR: Activation of AMPK protects against hydrogen peroxide-induced
osteoblast apoptosis through autophagy induction and NADPH
maintenance: New implications for osteonecrosis treatment? Cell
Signal. 26:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harachikuma M, Watanabe S and Satooka H:
Involvement of aquaporin-3 in epidermal growth factor receptor
signaling via H2O2 transport in cancer cells.
Biochem Biophys Res Commun. 471:603–609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holmström KM and Finkel T: Cellular
mechanisms and physiological consequences of redox-dependent
signalling. Nat Rev Mol Cell Biol. 15:411–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yue Q, Gao G, Zou G, Yu H and Zheng X:
Natural products as adjunctive treatment for pancreatic cancer:
Recent trends and advancements. Biomed Res Int. 2017:84125082017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun J, Zhang C, Bao YL, Wu Y, Chen ZL, Yu
CL, Huang YX, Sun Y, Zheng LH, Wang X and Li YX:
Parthenolide-induced apoptosis, autophagy and suppression of
proliferation in HepG2 cells. Asian Pac J Cancer Prev.
15:4897–4902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchibori R, Tsukahara T, Ohmine K and
Ozawa K: Cancer gene therapy using mesenchymal stem cells. Int J
Hematol. 99:377–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J, Zhang H, Gu P, Bai J, Margolick JB
and Zhang Y: NF-kappaB pathway inhibitors preferentially inhibit
breast cancer stem-like cells. Breast Cancer Res Treat.
111:419–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diamanti P, Cox CV, Moppett JP and Blair
A: Parthenolide eliminates leukemia-initiating cell populations and
improves survival in xenografts of childhood acute lymphoblastic
leukemia. Blood. 121:1384–1393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al Fatlawi AA, Al-Fatlawi AA, Irshad M,
Rahisuddin and Ahmad A: Effect of parthenolide on growth and
apoptosis regulatory genes of human cancer cell lines. Pharm Biol.
53:104–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sang WK, Park ES and Lee CS: Parthenolide
induces apoptosis by activating the mitochondrial and death
receptor pathways and inhibits FAK-mediated cell invasion. Mol Cell
Biochem. 385:133–144. 2013.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Y, Guo W, Wang Z, Zhang Y, Zhong L
and Zhu Y: Protective Effects of hydrogen sulfide in hypoxic human
umbilical vein endothelial cells: A possible mitochondria-dependent
pathway. Int J Mol Sci. 14:13093–13108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mccord JM and Fridovich I: Superoxide
dismutase. An enzymic function for erythrocuprein (hemocuprein). J
Biol Chem. 244:6049–6055. 1969.PubMed/NCBI
|
|
30
|
Cabaud PG and Wroblewski F: Colorimetric
measurement of lactic dehydrogenase activity of body fluids. Am J
Clin Pathol. 30:234–236. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maseko T, Howell K, Dunshea FR and Ng K:
Selenium-enriched Agaricus bisporus increases expression and
activity of glutathione peroxidase-1 and expression of glutathione
peroxidase-2 in rat colon. Food Chem. 146:327–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Can M, Guven B, Bektas S and Arikan I:
Oxidative stress and apoptosis in preeclampsia. Tissue Cell.
46:477–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jain S, Webster TJ, Sharma A and Basu B:
Intracellular reactive oxidative stress, cell proliferation and
apoptosis of Schwann cells on carbon nanofibrous substrates.
Biomaterials. 34:4891–4901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vaz CV, Marques R, Maia CJ and Socorro S:
Aging-associated changes in oxidative stress, cell proliferation
and apoptosis are prevented in the prostate of transgenic rats
overexpressing regucalcin. Transl Res. 166:693–705. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yen YH, Farooqi AA, Li KT, Butt G, Tang
JY, Wu CY, Cheng YB, Hou MF and Chang HW: Methanolic extracts of
solieria robusta inhibits proliferation of oral cancer ca9-22 cells
via apoptosis and oxidative stress. Molecules. 19:18721–18732.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simon HU, Hajyehia A and Levischaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yee C, Yang W and Hekimi S: The intrinsic
apoptosis pathway mediates the pro-longevity response to
mitochondrial ROS in C. elegans. Cell. 157:8972014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma SS and Dietz KJ: The relationship
between metal toxicity and cellular redox imbalance. Trends Plant
Sci. 14:43–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sivaramakrishnan V, Shilpa PN, Kumar VR
and Devaraj SN: Attenuation of N-nitrosodiethylamine-induced
hepatocellular carcinogenesis by a novel flavonol-Morin. Chem Biol
Interact. 171:79–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Feng Z, Wu G, Bai S, Yan D and
Zhao Y: In vitro studies on human periodontal ligament stem cell
sheets enhanced by enamel matrix derivative. Colloids Surf B
Biointerfaces. 141:102–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rosen CJ and Bouxsein ML: Mechanisms of
Disease: is osteoporosis the obesity of bone? Nat Clin Pract
Rheumatol. 2:35–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie G, Schepetkin IA and Quinn MT:
Immunomodulatory activity of acidic polysaccharides isolated from
Tanacetum vulgare L. Int Immunopharmacol. 7:1639–1650. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tiuman TS, Uedanakamura T, DA Cortez
Garcia, Filho Dias BP, Morgado-Díaz JA, de Souza W and Nakamura CV:
Antileishmanial activity of parthenolide, a sesquiterpene lactone
isolated from tanacetum parthenium. Antimicrob Agents Chemother.
49:176–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mathema VB, Koh YS, Thakuri BC and
Sillanpää M: Parthenolide, a sesquiterpene lactone, expresses
multiple anti-cancer and anti-inflammatory activities.
Inflammation. 35:560–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valko M, Morris H and Cronin MT: Metals,
toxicity and oxidative stress. Curr Med Chem. 12:1161–1208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Manna SK, Sarkar S, Barr J, Wise K,
Barrera EV, Jejelowo O, Rice-Ficht AC and Ramesh GT: Single-walled
carbon nanotube induces oxidative stress and activates nuclear
transcription factor-κB in human keratinocytes. Nano Lett.
5:1676–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Manolagas SC: From estrogen-centric to
aging and oxidative stress: A revised perspective of the
pathogenesis of osteoporosis. Endocr Rev. 31:266–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Doshi SB and Agarwal A: The role of
oxidative stress in menopause. J Midlife Health. 4:140–146.
2013.PubMed/NCBI
|
|
50
|
Kim Y, You Y, Yoon HG, Lee YH, Kim K, Lee
J, Kim MS, Kim JC and Jun W: Hepatoprotective effects of fermented
Curcuma longa L. on carbon tetrachloride-induced oxidative stress
in rats. Food Chem. 151:148–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pajak B, Gajkowska B and Orzechowski A:
Molecular basis of parthenolide-dependent proapoptotic activity in
cancer cells. Folia Histochem Cytobiol. 46:129–135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wyrębska A, Gach K and Janecka A: Combined
effect of parthenolide and various anti-cancer drugs or anticancer
candidate substances on malignant cells in vitro and in vivo. Mini
Rev Med Chem. 14:222–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim JH, Liu L, Lee SO, Kim YT, You KR and
Kim DG: Susceptibility of cholangiocarcinoma cells to
parthenolide-induced apoptosis. Cancer Res. 65:6312–6320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fang LJ, Shao XT, Wang S, Lu GH, Tao X and
Zhou JY: Sesquiterpene lactone parthenolide markedly enhances
sensitivity of human A549 cells to low-dose oxaliplatin via
inhibition of NF-κB activation and induction of apoptosis. Planta
Med. 76:258–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim SL, Liu YC, Park YR, Seo SY, Kim SH,
Kim IH, Lee SO, Lee ST, Kim DG and Kim SW: Parthenolide enhances
sensitivity of colorectal cancer cells to TRAIL by inducing death
receptor 5 and promotes TRAIL-induced apoptosis. Int J Oncol.
46:1121–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Michaeloudes C, Mercado N, Clarke C,
Bhavsar PK, Adcock IM, Barnes PJ and Chung KF: Bromodomain and
extraterminal proteins suppress NF-E2-related factor 2-mediated
antioxidant gene expression. J Immunol. 192:4913–4920. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Paul MK, Bisht B, Darmawan DO, Chiou R, Ha
VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al:
Dynamic changes in intracellular ROS levels regulate airway basal
stem cell homeostasis through Nrf2-dependent Notch signaling. Cell
Stem Cell. 15:199–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|